Hot-Corrosion Behaviour of NiCoCrW Superalloy Fabricated by Selective Laser Melting
Abstract
:1. Introduction
2. Materials and Methods
3. Results
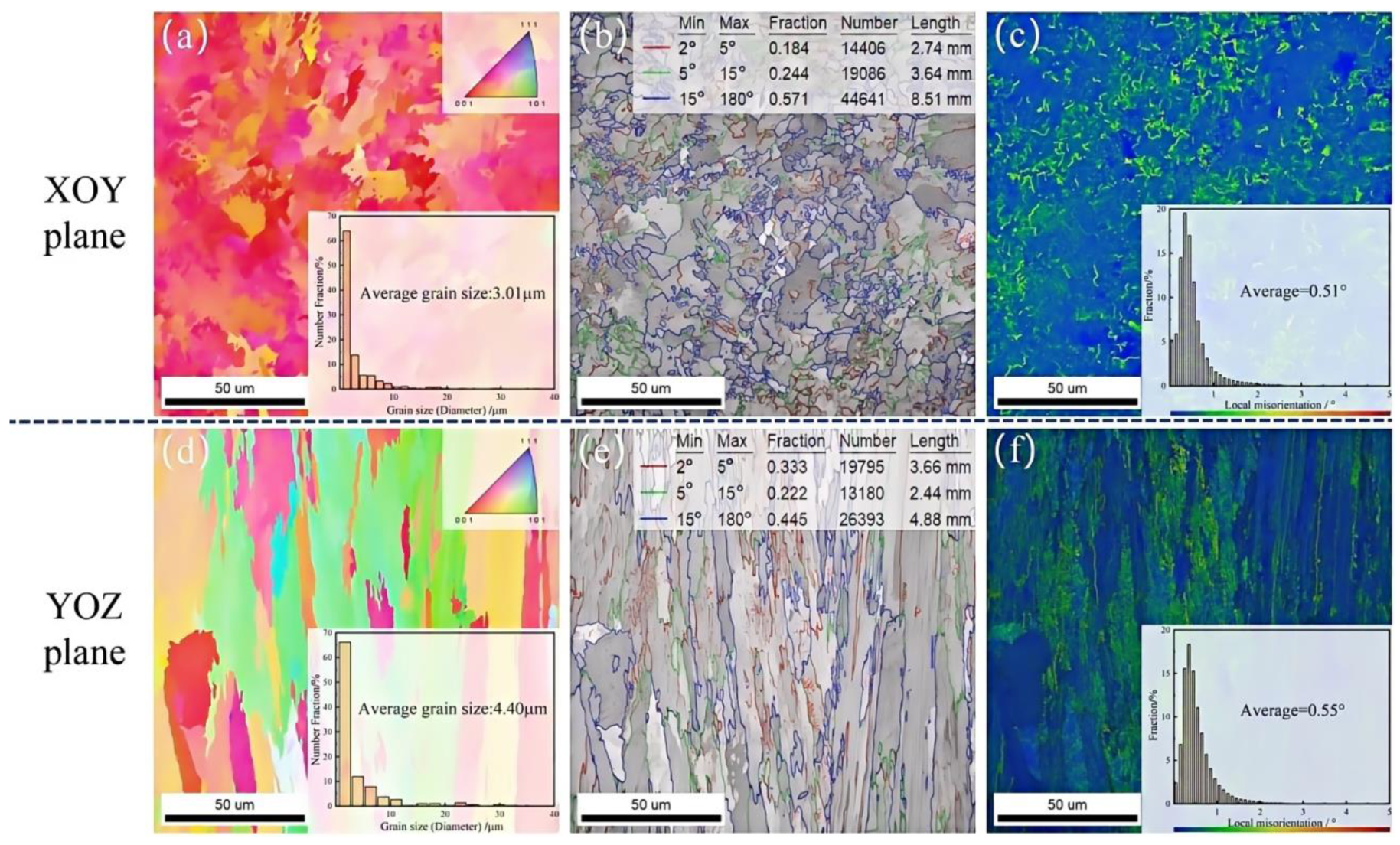
3.1. Microstructural Characterisation
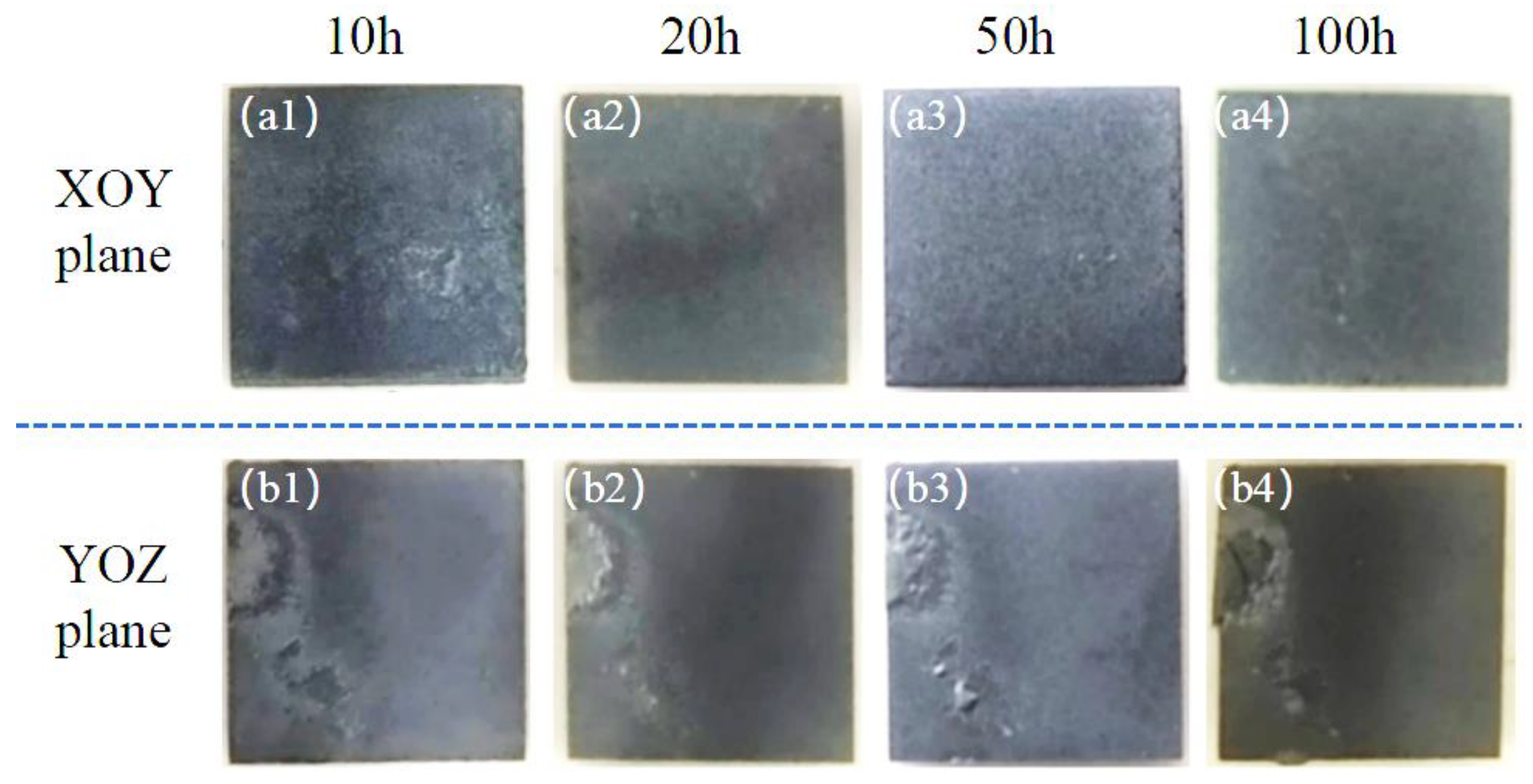
3.2. Hot-Corrosion Test Results
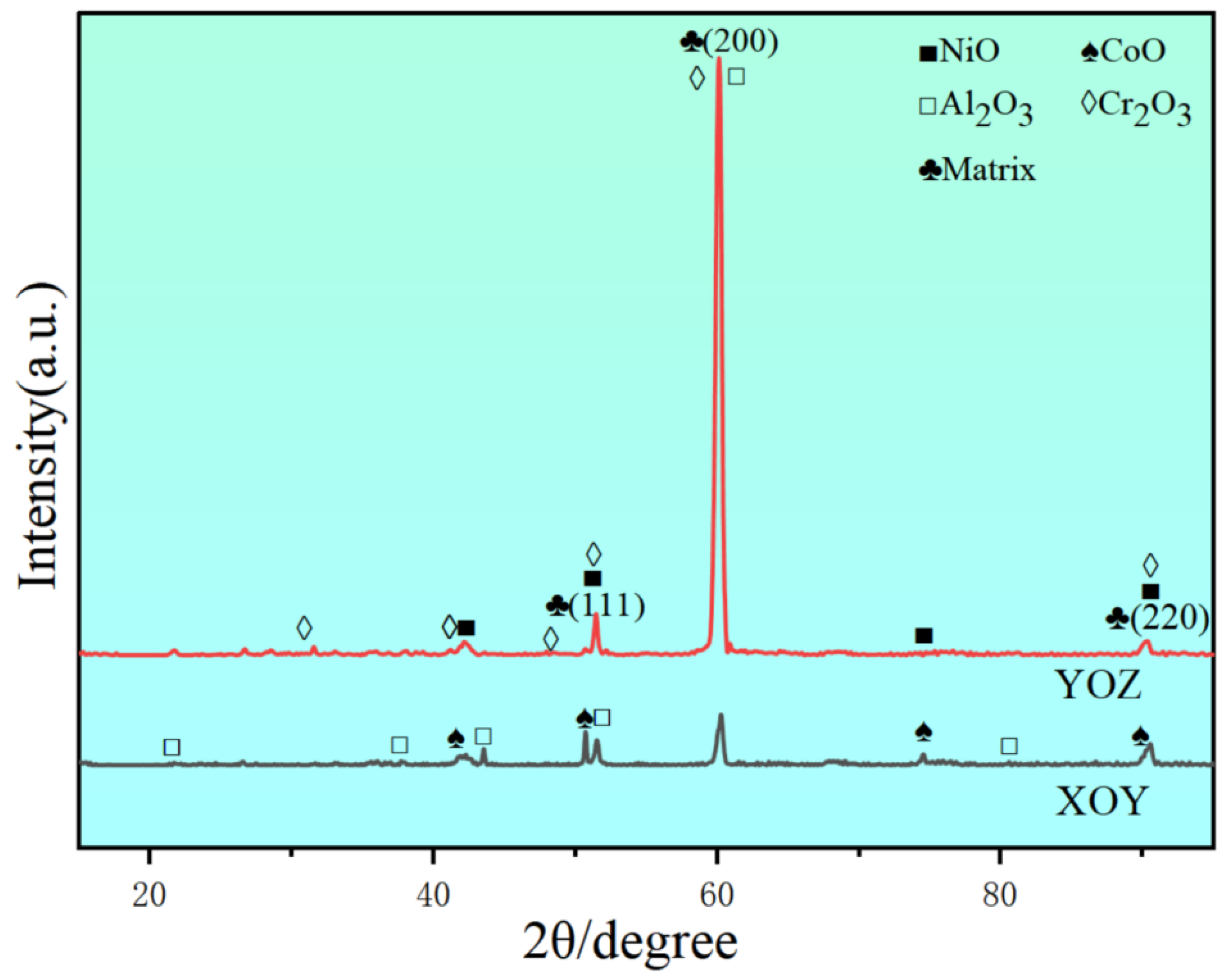
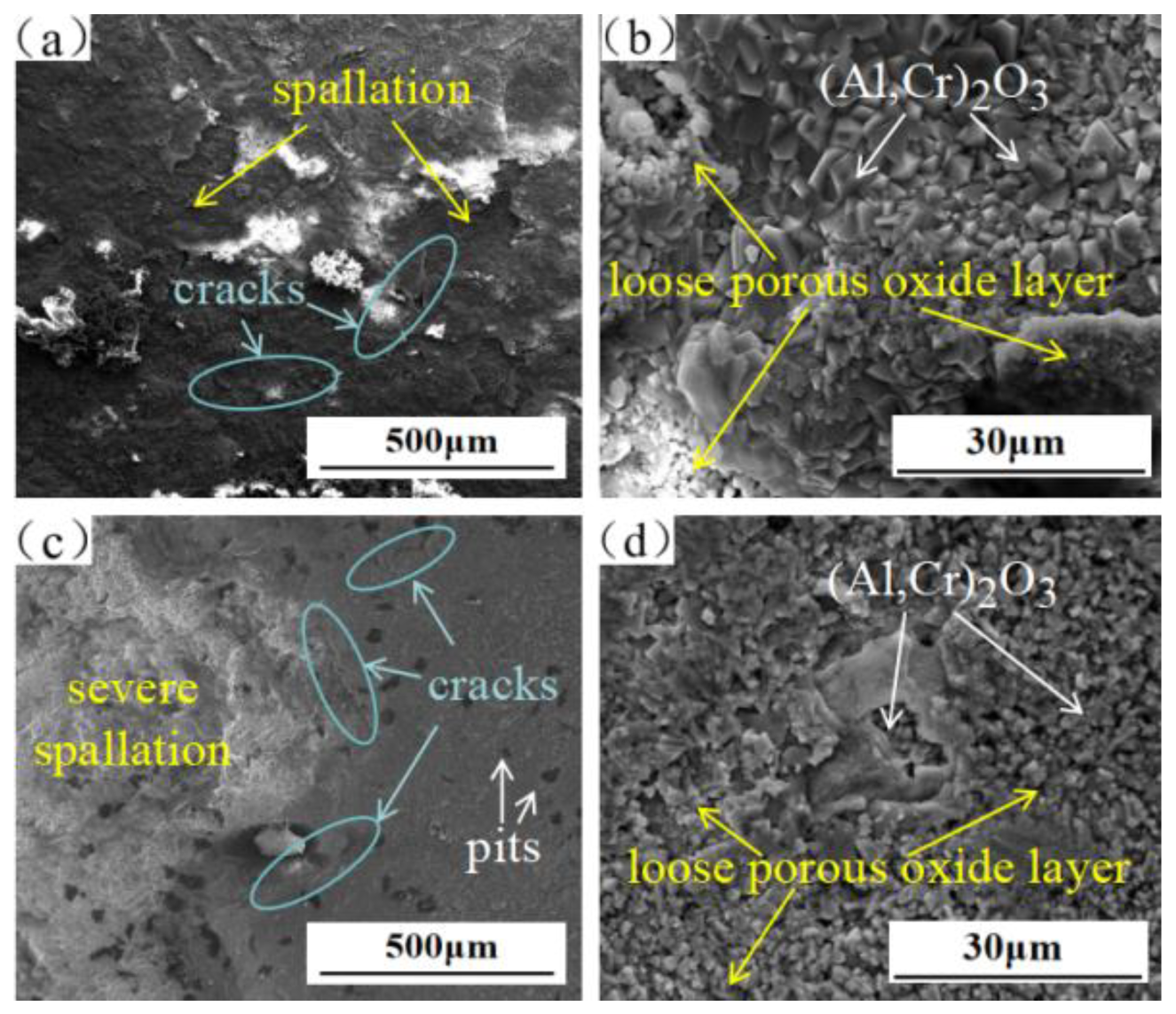
3.3. Characterisation of Hot-Corrosion Products
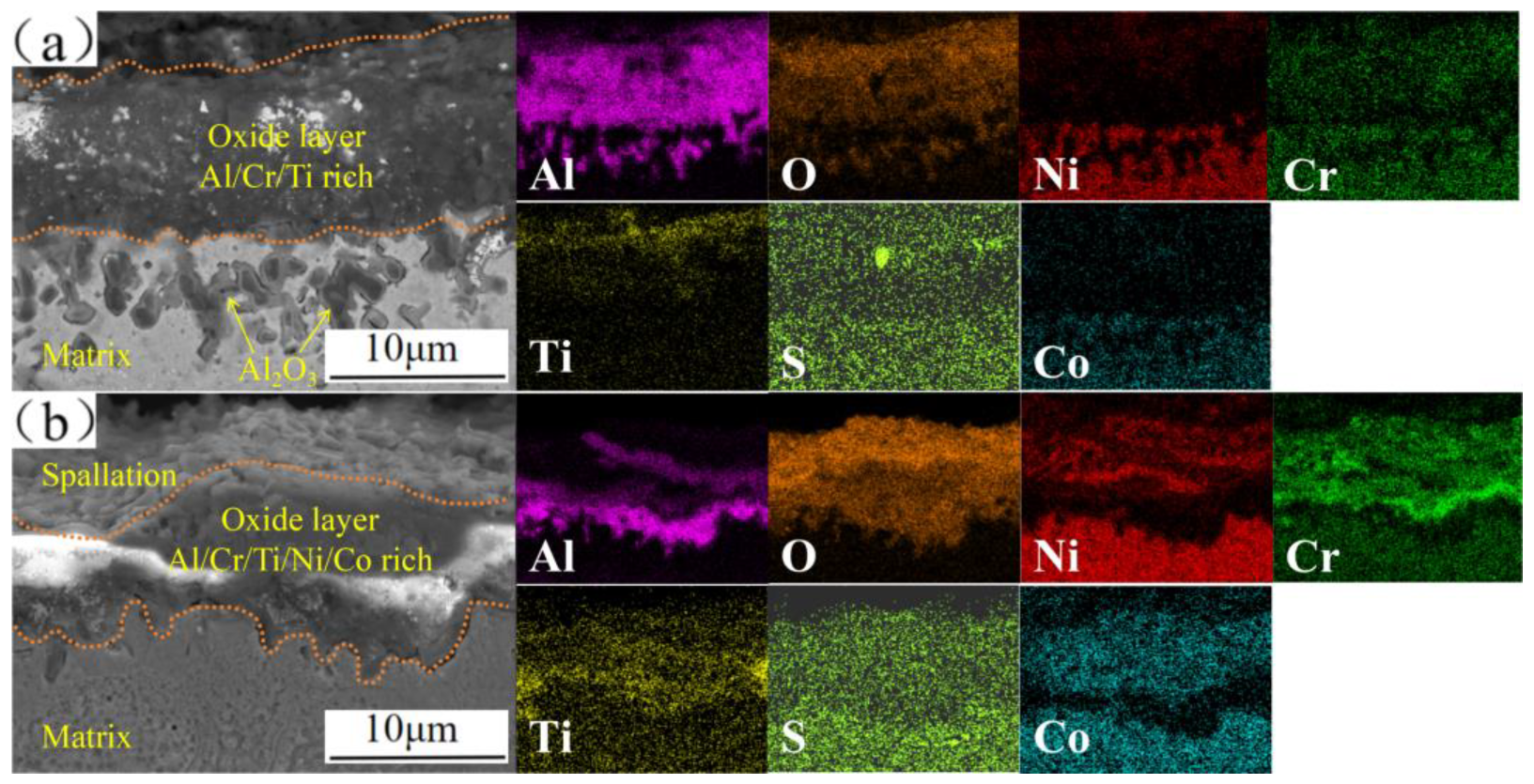
3.4. Cross-Sectional Morphologies of Corrosion Layer
4. Discussion
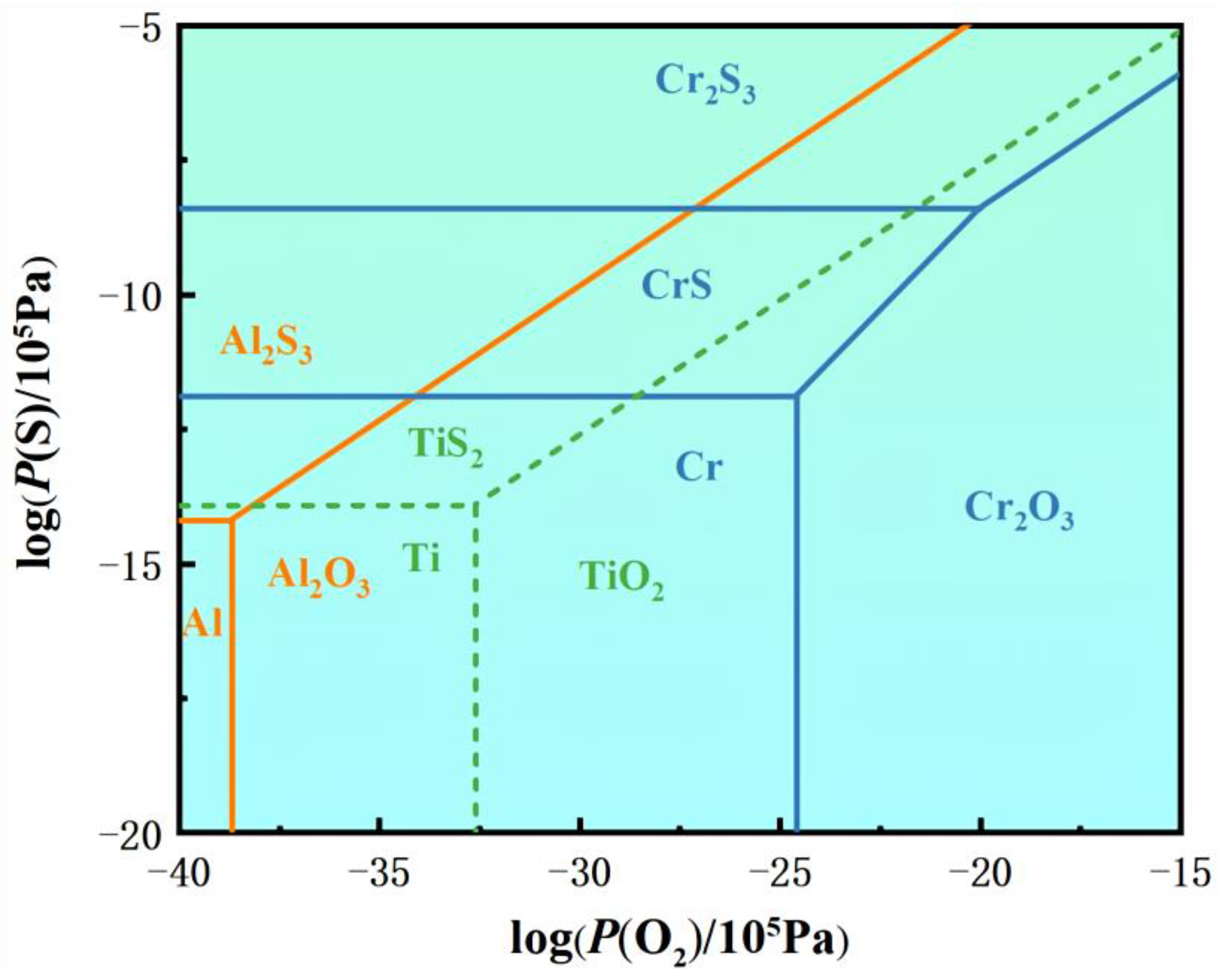
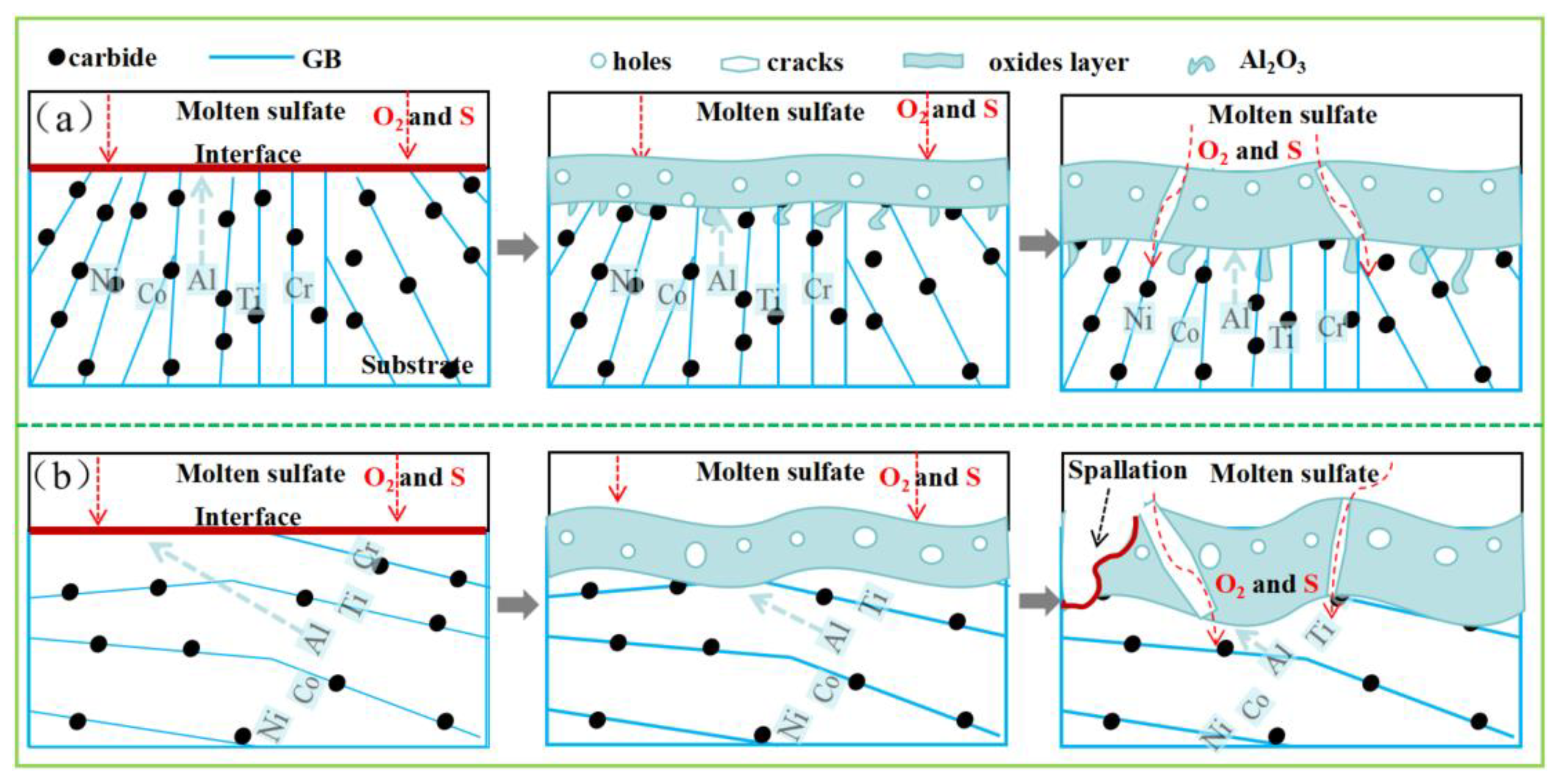
4.1. Hot-Corrosion Mechanisms
4.2. Microstructure Anisotropy Influence on Hot Corrosion
5. Conclusions
- The hot corrosion of the SLM-fabricated NiCoCrW (K447A) superalloy exhibits anisotropy in different build directions. The XOY plane (parallel to the build direction) exhibits superior hot-corrosion resistance compared to that in the YOZ plane (perpendicular to the build direction).
- The microstructure of the NiCoCrW (K447A) superalloy shows significant anisotropy. The XOY plane has finer grains, a more uniform distribution of carbides, and a lower local stress concentration.
- The excellent hot-corrosion resistance of the XOY plane is attributed to the larger area fraction and more uniform distribution of carbides, which contribute to the stability of the oxide film. The oxide film formed on the XOY surface is more stable, providing better protection for the underlying matrix.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Dahmen, T.; Henriksen, N.G. Densification, microstructure, and mechanical properties of heat-treated MAR-M247 fabricated by Binder Jetting. Addit. Manuf. 2021, 39, 101912. [Google Scholar] [CrossRef]
- Yi-Lung, T.; Sea-Fue, W. Effects of alloy elements on microstructure and creep properties of fine-grained nickel-based superalloys at moderate temperatures. Mater. Sci. Eng. A 2013, 571, 155–160. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhao, Y. The role of the pulsed-wave laser characteristics on restraining hot cracking in laser cladding non-weldable nickel-based superalloy. Mater. Des. 2021, 198, 109346. [Google Scholar] [CrossRef]
- Pan, C.; Yao, Z. Solidification microstructure characteristics and their formation mechanism of K447A nickel-based superalloy for dual-performance blisk. Mater. Charact. 2023, 203, 113155. [Google Scholar] [CrossRef]
- Baldan, R.; Rocha, P.L.R. Solutioning and Aging of MAR-M247 Nickel-Based Superalloy. J. Mater. Eng. Perform. 2013, 22, 2574–2579. [Google Scholar] [CrossRef]
- Navneet, K.; Kishan, Z. Review on machining of additively manufactured nickel and titanium alloys. J. Mater. Res. Technol. 2021, 15, 3192–3221. [Google Scholar] [CrossRef]
- Tan, J.H.K.; Sing, S.L. Microstructure modelling for metallic additive manufacturing: A review. Virtual Phys. Prototyp. 2020, 15, 87–105. [Google Scholar] [CrossRef]
- Sumner, J.; Encinas-Oropesa, A. Type II hot corrosion: Behavior of CMSX -4 and IN 738 LC as a function of corrosion environment. Mater. Corros. 2014, 65, 188–196. [Google Scholar] [CrossRef]
- Pei, Y.; Zhou, C. Improved hot corrosion resistance of Dy-Co-modified aluminide coating by pack cementation process on nickel base superalloys. Corros. Sci. 2016, 112, 710–717. [Google Scholar] [CrossRef]
- Qiao, M.; Zhou, C. Hot corrosion behavior of Co modified NiAl coating on nickel base superalloys. Corros. Sci. 2012, 63, 239–245. [Google Scholar] [CrossRef]
- Koike, R.; Unotoro, I. Evaluation for mechanical characteristics of Inconel625–SUS316L joint produced with direct energy deposition. Procedia Manuf. 2017, 14, 105–110. [Google Scholar] [CrossRef]
- Yuan, K.; Guo, W. Influence of process parameters and heat treatments on the microstructures and dynamic mechanical behaviors of Inconel 718 superalloy manufactured by laser metal deposition. Mater. Sci. Eng. A 2018, 721, 215–225. [Google Scholar] [CrossRef]
- Popovich, V.A.; Borisov, E.V. Functionally graded Inconel 718 processed by additive manufacturing: Crystallographic texture, anisotropy of microstructure and mechanical properties. Mater. Des. 2017, 114, 441–449. [Google Scholar] [CrossRef]
- Wilson-Heid, E.A.; Wang, Z. Quantitative relationship between anisotropic strain to failure and grain morphology in additively manufactured Ti-6Al-4V. Mater. Sci. Eng. A 2017, 706, 287–294. [Google Scholar] [CrossRef]
- Kouraytem, N.; Chanut, A.R. Dynamic-loading behavior and anisotropic deformation of pre- and post-heat-treated IN718 fabricated by laser powder bed fusion. Addit. Manuf. 2020, 33, 101083. [Google Scholar] [CrossRef]
- Sridar, S.; Pizano, L.F.L. Achieving High Strength and Creep Resistance in Inconel 740H Superalloy through Wire-Arc Additive Manufacturing and Thermodynamic-Guided Heat Treatment. Materials 2023, 16, 6388. [Google Scholar] [CrossRef]
- Tang, W.; Yang, X. Effect of rotation speed on microstructure and mechanical anisotropy of Al-5083 alloy builds fabricated by friction extrusion additive manufacturing. Mater. Sci. Eng. A 2022, 860, 144237. [Google Scholar] [CrossRef]
- Montero, X.; Ishida, A. Effect of surface treatment and crystal orientation on hot corrosion of a Ni-based single-crystal superalloy. Corros. Sci. 2020, 166, 108472. [Google Scholar] [CrossRef]
- Wei, B.; Chen, C. Comparing the hot corrosion of (100), (210) and (110) Ni-based superalloys exposed to the mixed salt of Na2SO4-NaCl at 750 °C: Experimental study and first-principles calculation. Corros. Sci. 2022, 195, 109996. [Google Scholar] [CrossRef]
- Wu, J.; Jiang, X. Anisotropy of interface characteristics between NiCoCrAlY coating and a hot corrosion resistant Ni-Based single crystal superalloy during thermal exposure at different temperatures. Appl. Surf. Sci. 2020, 532, 147405. [Google Scholar] [CrossRef]
- Na, G.; Meng, L. High-temperature oxidation and hot corrosion of Ni-based single crystal superalloy in the incubation stage. Corros. Sci. 2023, 214, 111026. [Google Scholar] [CrossRef]
- Visibile, A.; Gunduz, O.K. High temperature oxidation of inconel 939 produced by additive manufacturing. Corros. Sci. 2024, 233, 112067. [Google Scholar] [CrossRef]
- Liu, B.; Zhang, H. Improving the hot corrosion resistance of additively manufactured Inconel 718 via recrystallization-based grain boundary engineering induced by its residual stress. Mater. Today Commun. 2024, 39, 108792. [Google Scholar] [CrossRef]
- Lv, Y.; Liu, Y. Hot corrosion behavior of a novel TiC/GTD222 nickel-based composite prepared by selective laser melting. Mater. Charact. 2023, 205, 113245. [Google Scholar] [CrossRef]
- Yang, R.; Han, S. Hot corrosion mechanism of laser metal deposited Ni-based single crystal superalloy under fuel gas atmosphere. Surf. Coat. Technol. 2023, 474, 130057. [Google Scholar] [CrossRef]
- Kang, Q.; Wang, G. Experimental and theoretical study for hot corrosion behavior of network structured TiBw/TA15 composite with NaCl film at 800 °C. Corros. Sci. 2022, 206, 110540. [Google Scholar] [CrossRef]
- Abbaszadeh, S.; Pakseresht, A. Investigation of the High-Temperature Oxidation Behavior of the Al 0.5 CoCrFeNi High Entropy Alloy. Surf. Interfaces 2020, 21, 100724. [Google Scholar] [CrossRef]
- Sun, L.; Jiang, F. Microstructure and Mechanical Properties of Low-Carbon High-Strength Steel Fabricated by Wire and Arc Additive Manufacturing. Metals 2020, 10, 216. [Google Scholar] [CrossRef]
- Kang, Q.; Xu, X. Hot corrosion behavior of network structured TiBw/TA15 composite with Na2SO4 film at 800 °C. Mater. Charact. 2023, 195, 112499. [Google Scholar] [CrossRef]
- Dong, R.; Liu, D. Hot-corrosion behavior associated with the evolution of corrosion scales of a Ni-based superalloy in molten salts. Prog. Nat. Sci. Mater. Int. 2021, 31, 486–492. [Google Scholar] [CrossRef]
- Grégoire, B.; Montero, X. Correlations between the kinetics and the mechanisms of hot corrosion of pure nickel at 700 °C. Corros. Sci. 2019, 155, 134–145. [Google Scholar] [CrossRef]
- Chang, J.X.; Wang, D. Effect of Rhenium Addition on Hot Corrosion Resistance of Ni-Based Single Crystal Superalloys. Metall. Mater. Trans. 2018, 49, 4343–4352. [Google Scholar] [CrossRef]
- Pettit, F. Hot Corrosion of Metals and Alloys. Oxid. Met. 2011, 76, 1–21. [Google Scholar] [CrossRef]
- Liu, H.; Tan, C.K.I. Hot corrosion and internal spallation of laser-cladded inconel 625 superalloy coatings in molten sulfate salts. Corros. Sci. 2021, 193, 109869. [Google Scholar] [CrossRef]

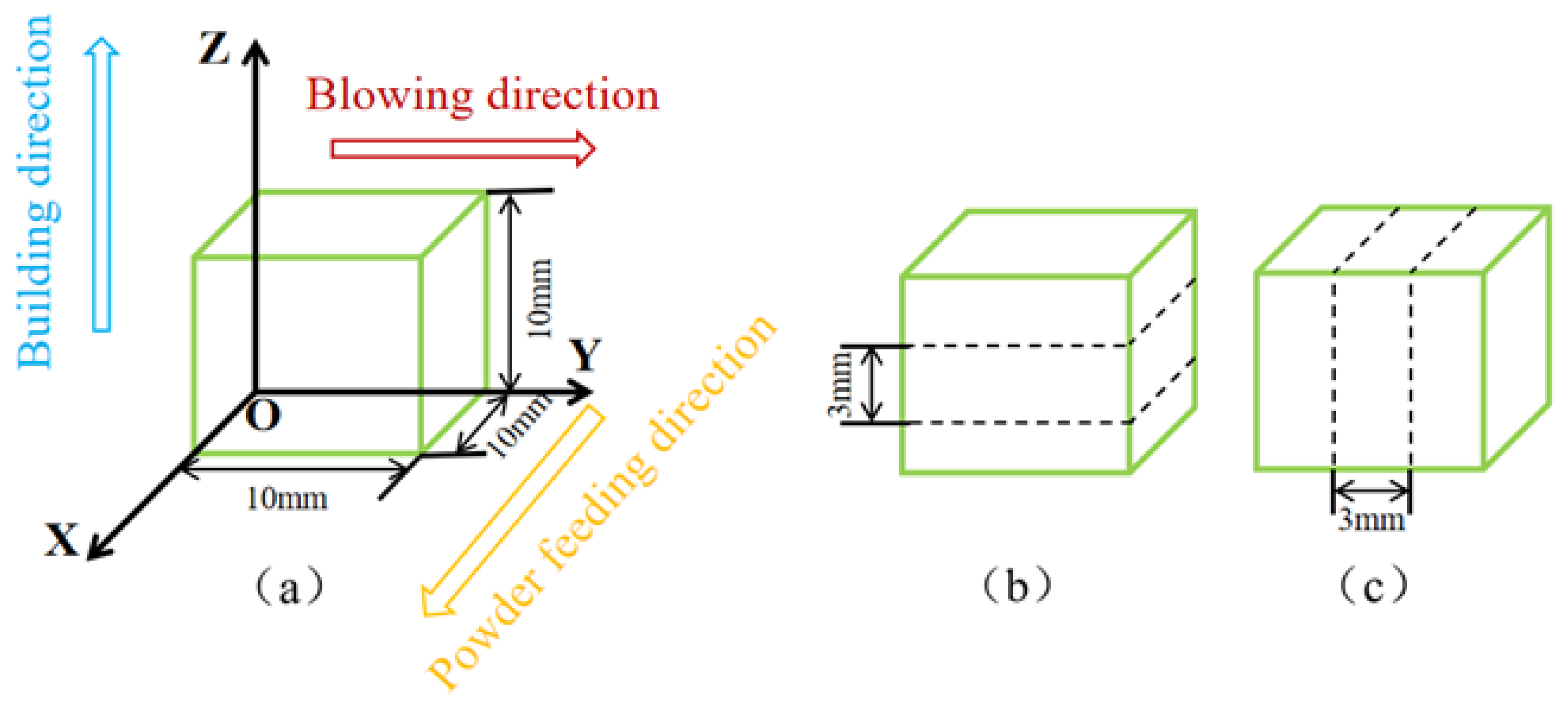




| Elements | Co | Cr | Mo | Al | Ti | C | Ta | Hf | W | Ni |
|---|---|---|---|---|---|---|---|---|---|---|
| wt.% | 9.70 | 8.41 | 0.70 | 5.46 | 1.18 | 0.16 | 3.13 | 1.46 | 10.50 | Bal. |
| Spectrum | C | Cr | Mo | Al | Ti | Ta | Hf | W | Ni |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 13.73 | 8.07 | 1.01 | 3.53 | 2.38 | 6.78 | 7.17 | 14.45 | 42.88 |
| 2 | 13.12 | 8.84 | 1.27 | 3.76 | 2.66 | 8.42 | 2.30 | 14.88 | 44.75 |
| 3 | 10.90 | 8.68 | 0.86 | 4.41 | 1.20 | 4.72 | 5.05 | 9.99 | 54.19 |
| 4 | 12.87 | 8.01 | 0.62 | 3.75 | 1.93 | 8.21 | 3.99 | 13.05 | 47.57 |
| 5 | 12.25 | 7.30 | 0.61 | 3.62 | 2.71 | 11.03 | 5.38 | 14.28 | 42.82 |
| 6 | 11.63 | 7.47 | 1.41 | 3.63 | 2.84 | 8.96 | 10.07 | 10.88 | 43.11 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, B.; Dong, T.; Liu, G.; Su, J.; Yu, W.; Wang, A. Hot-Corrosion Behaviour of NiCoCrW Superalloy Fabricated by Selective Laser Melting. Metals 2025, 15, 338. https://doi.org/10.3390/met15040338
Han B, Dong T, Liu G, Su J, Yu W, Wang A. Hot-Corrosion Behaviour of NiCoCrW Superalloy Fabricated by Selective Laser Melting. Metals. 2025; 15(4):338. https://doi.org/10.3390/met15040338
Chicago/Turabian StyleHan, Bo, Tao Dong, Geng Liu, Jie Su, Wenchao Yu, and Ao Wang. 2025. "Hot-Corrosion Behaviour of NiCoCrW Superalloy Fabricated by Selective Laser Melting" Metals 15, no. 4: 338. https://doi.org/10.3390/met15040338
APA StyleHan, B., Dong, T., Liu, G., Su, J., Yu, W., & Wang, A. (2025). Hot-Corrosion Behaviour of NiCoCrW Superalloy Fabricated by Selective Laser Melting. Metals, 15(4), 338. https://doi.org/10.3390/met15040338






