Abstract
The history of metallic orthopedic materials spans a few centuries, from the use of carbon steel to the widespread adoption of titanium and its alloys. This paper explores the evolution of these materials, emphasizing their mechanical properties, biocompatibility, and the roles that they have played in improving orthopedic care. Key developments include the discovery of titanium’s osseointegration capability, the advent of porous coatings for osseointegration, surface modifications, and the rise of additive manufacturing for patient-specific implants. Beyond titanium, emerging materials such as biodegradable alloys, tantalum, zirconium, and amorphous metals are creating a completely new field of application for orthopedic metals. These innovations address longstanding challenges, including stress shielding, corrosion, and implant longevity, while leading the way for bioresorbable and 3D-printed patient-specific solutions. This paper concludes by examining future trends and their potential for industrial application. By understanding the historical developments in metallic orthopedic materials, this review highlights how past advancements have laid the foundation for both current and future innovations, guiding research towards solutions that better mimic the properties of biological tissues, offer higher reliability in vivo, and enable patient-specific treatments.
1. Introduction
Orthopedic materials are critical to the field of medicine, particularly in the treatment of musculoskeletal injuries and disorders [1,2]. These materials are used in the production of implants, prosthetics, and surgical devices that restore or enhance the function of bones, joints, and soft tissues. Unlike materials used in tissue engineering, the primary objective of orthopedic materials is not to mimic the mechanical properties of the tissues they replace, but instead to restore mobility and reduce pain, while ensuring compatibility with the human body [3].
Materials for orthopedic applications must meet several essential criteria, including mechanical strength to withstand the body’s loads [4], biocompatibility to minimize rejection or inflammation [5], wear resistance to endure prolonged use [6], and corrosion resistance to maintain integrity over time [7]. Common materials used in orthopedic devices include metals [8], polymers [9], ceramics [10], and composites [11], each offering distinct advantages and limitations depending on the application. The continuous development and improvement of these materials are key to advancing surgical techniques, enhancing patient outcomes, and reducing the incidence of complications, such as implant failure or infection. As the demand for more effective and durable orthopedic solutions increases, ongoing research into novel materials and fabrication techniques is crucial to meeting the challenges of an aging society [12].
The development of metallic materials for orthopedic applications represents an important chapter in the advancement of biomedical science and engineering. Their primary goal is to restore functionality to the musculoskeletal system, typically through implants that replace or support damaged bones or joints. Among these materials, metals have played a critical role due to their exceptional combination of mechanical strength, durability, and biocompatibility [13].
In the early 20th century, surgeons sought materials that could withstand the mechanical demands of the human body while minimizing biological rejection. Initial choices, such as stainless steel and cobalt–chromium alloys, provided sufficient strength and corrosion resistance for load-bearing implants at the time [14,15]. These materials marked the foundation of metallic orthopedics and demonstrated the potential for long-term use in vivo. However, they were not without challenges, including wear, ion release, and limited integration with surrounding biological tissues [16].
Over time, the scope of orthopedic metallurgy has expanded to address these limitations, driven by advancements in materials science and manufacturing techniques. Titanium and its alloys emerged as game-changers in the mid-20th century, offering a combination of high strength-to-weight ratio, excellent corrosion resistance, and superior biocompatibility [17]. These materials led to a shift toward implants with reduced systemic impact and improved patient outcomes [18].
Contemporary metallic implants have further evolved with surface modifications and coatings aimed at enhancing osseointegration and reducing the chances for the formation of wear debris. For example, hydroxyapatite coatings [19] and bioactive glass [20] are now used to mimic the properties of natural bone, encouraging cellular adhesion and bone growth. Moreover, the advent of additive manufacturing technologies, such as electron beam melting and selective laser melting, has enabled the fabrication of complex porous structures that closely replicate the mechanical and biological behavior of bone [21].
Despite these advances, challenges remain in the field of metallic orthopedic materials. Issues such as implant-related infections, metal ion toxicity, and long-term wear continue to motivate research into next-generation alloys and hybrid systems. Additionally, the integration of multi-material designs, including metallic–ceramic and metallic–polymer composites, aims to achieve a balance between mechanical strength and biological compatibility.
This review synthesizes information drawn from an extensive literature survey. Relevant papers were identified using major scientific databases, including PubMed, Scopus, and Web of Science, with a focus on peer-reviewed articles published in the last 30 years. The selection criteria prioritized studies addressing the historical development, material properties, clinical performance, and advancements in fabrication techniques for metallic orthopedic materials. Keywords such as “metallic implants”, “orthopaedic materials”, “biocompatibility”, “corrosion resistance”, and “additive manufacturing” were employed in the search process. In total, over 800 documents were reviewed, and the most impactful studies were included based on their citation metrics, relevance, and contribution to the field. This selection process ensured that the review would provide a comprehensive and balanced understanding of the topic.
1.1. Objectives and Scope of the Review
This review aims to provide a comprehensive historical overview of metallic materials used in orthopedics, with a focus on their evolution, key achievements, and challenges over time. The scope includes metals such as stainless steel, titanium, cobalt–chromium, tantalum, and zirconium, along with emerging materials like biodegradable alloys and the role of additive manufacturing in improving design and production.
1.2. Structure of the Paper
This paper is structured as follows:
Historical Overview (Section 2, Section 3, Section 4, Section 5 and Section 6)—The development and key milestones of metallic materials for orthopedic applications.
Material Properties and Challenges (Section 7, Section 8 and Section 9)—A detailed discussion on the mechanical, biological, and corrosion properties of metallic biomaterials, as well as the challenges that they address.
Advancements in Production Techniques (Section 10, Section 11 and Section 12)—Exploration of traditional and emerging fabrication methods, including surface treatments, coatings, and additive manufacturing.
Conclusions (Section 13)—Summary of the key findings, gaps in knowledge, and suggestions for future research.
To facilitate consultation, the chronological development, methods, and key achievements of the various metallic biomaterials discussed in this review are listed in Table 1.

Table 1.
Chronological development, methods, and key achievements of metallic biomaterials in orthopedic applications.
2. Ancient History and Myths
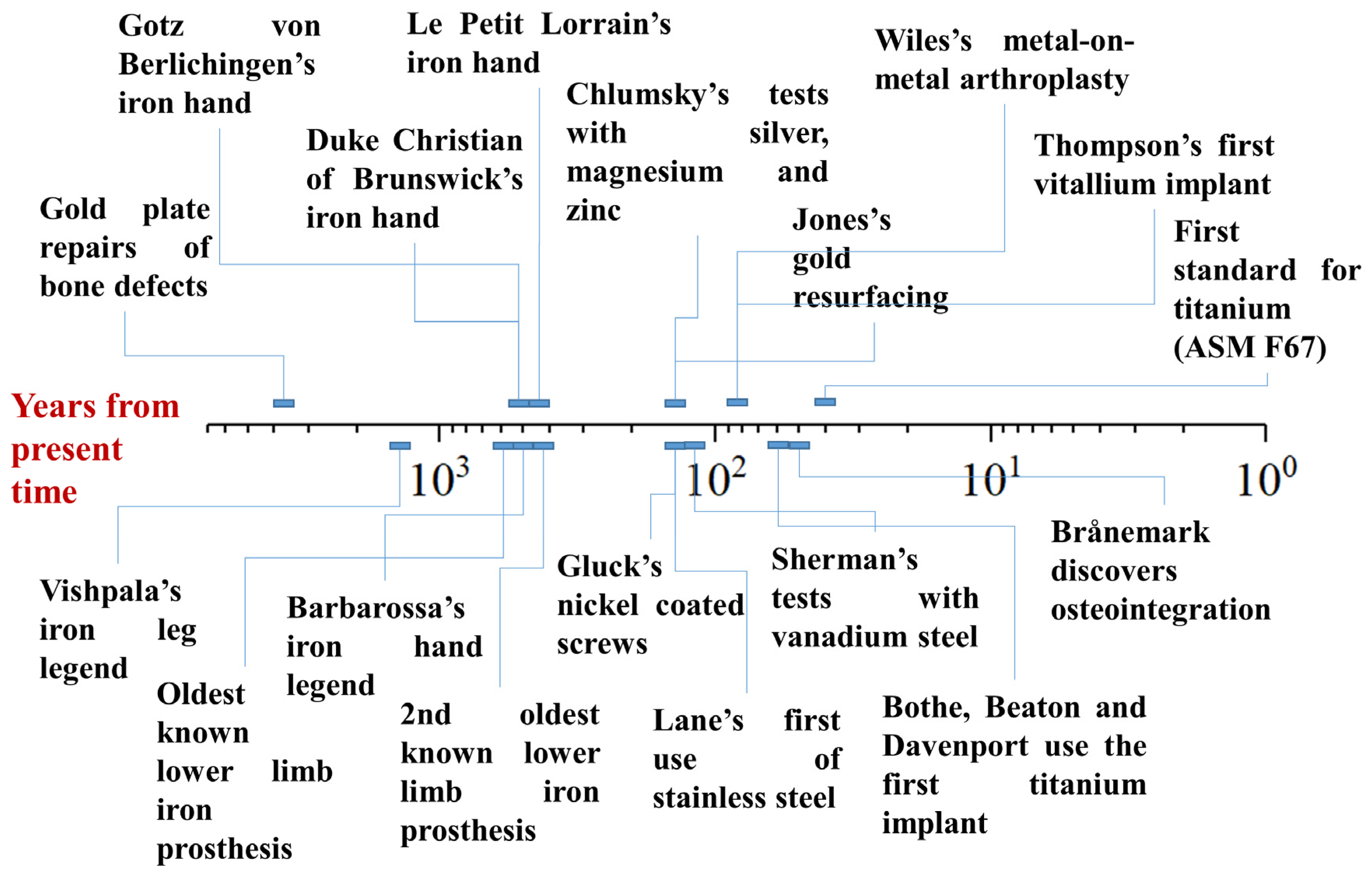
The first metal to be used as a biomaterial was gold, primarily for bone reconstruction, particularly in the form of skull repair or for orthodontics (Figure 1). One of the earliest examples of this can be traced back to South America, around 2000–1500 BC, where a frontal bone defect was repaired with a hammered gold plate [22]. Similarly, the ancient Incas used gold in a variety of medical applications, including the production of surgical blades and other objects, although its use in medical procedures was likely driven by its high workability and spiritual significance rather than its intrinsic biocompatibility [23]. Gold’s use as a material for dental applications also has a long history, with the Etruscans, Greeks, and Romans employing gold wires to stabilize teeth, a practice that was later adopted and refined during the Middle Ages and the Renaissance [16]. Although its role in medical devices, particularly for skull and dental repair, was well established, gold’s use in other orthopedic applications remained limited overall, as the focus remained on dental applications and aesthetics [24].
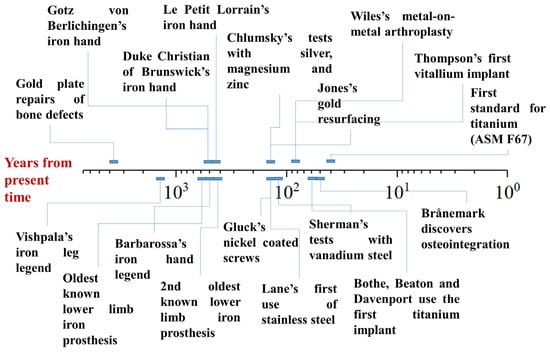
Figure 1.
Timeline of the myths and evolutions in the field of metallic orthopedic materials, expressed in years from the present time (logarithmic scale).
The use of silver in medical applications, particularly for cranioplasty, has been less commonly reported compared to gold, likely due to its higher reactivity and softness. However, in the 16th century, silver was used in some facial reconstruction procedures, such as the application of silver clips [25]. Silver was also employed in sutures for hernia surgery, and as foils intended to prevent postoperative infections. In the 19th century, the use of silver expanded further, with silver wire sutures being utilized for the treatment of vesico-vaginal fistula [26].
The earliest known reference to the medical use of iron appears in the ancient Rig-Veda, a Sanskrit text from India dating back to around 1500–1200 BC [27]. The poem tells the story of Vishpala, a mythical warrior queen (or, according to Karl Friedrich Geldner’s interpretation, a horse [16]) who loses her leg in battle (or a race) and is subsequently fitted with an iron prosthesis, enabling her to return to battle (or run again). While the interpretation of this text is not entirely clear, it is widely regarded as one of the earliest mentions of a limb prosthesis. However, it is important to note that the Vishpala story is mythological, and the actual historical existence of the prosthesis or the events surrounding it is not verifiable. The date of the poem aligns with the early development and spread of iron-smelting technology in India [28], making the story of Vishpala one of the first references to iron crafting in the region. The use of iron in the prosthesis is likely symbolic, reflecting the material’s newfound significance, as a functional iron leg prosthetic would have been too heavy to use comfortably at the time.
The biomedical use of iron remained largely unreported until the 16th century, but the Renaissance marked a significant period in the evolution of iron prosthetics. One of the earliest accounts comes from the historian and physician Paolo Giovio (1483–1552 AD), who recounted the story of the Turkish pirate Horuk Barbarossa, who lost his hand during the Battle of Bugia and was subsequently fitted with an iron prosthesis [29]. A more notable example involves the German mercenary Gotz von Berlichingen, who, in 1505 AD, had his hand severed by a cannonball during battle. A Bavarian artisan crafted him an iron hand with movable fingers, allowing Gotz to hold reins, grip weapons, and return to the battlefield [30,31]. The practice of iron prosthetics continued, with Duke Christian of Brunswick receiving a similar iron prosthetic after losing his hand in the Battle of Fleury in 1622 AD [32]. One of the most intricate examples of 16th-century iron prosthetics comes from Ambroise Paré, who described a prosthetic hand, made around 1550 AD by the craftsman “Le Petit Lorrain”, featuring mechanisms that allowed for controlled finger movements [32]. Paré also proposed several ideas for articulated metallic prosthetics for the lower limbs, advancing the field further [33]. While two examples of iron lower-limb prostheses from the 15th and 17th centuries are held in the Stibbert Museum’s collection, these devices were less complex than the prosthetic hands and likely served as static supports, possibly for horse riding or standing [34].
Starting in the 18th century, the field of orthopedics saw significant advancements, particularly in the area of hip joint surgery. Henry Park (1744–1831) was one of the early proponents of radical interventions in joint diseases. He proposed a method that involved the total removal of the articulating surfaces, suggesting “The resource I mean is the total extirpation of the articulation, or the entire removal of the extremities of all the bones which form the joints, with the whole, or as much as possible of the Capsular Ligament; thereby obtaining a cure by means of Callus”. This approach laid the groundwork for further developments in joint reconstruction and limb function restoration [35].
Anthony White (1782–1849) is credited with a pioneering approach in hip joint surgery, particularly excising the head, neck, and trochanters of the femur. It is said that “He who first excised the head, neck and trochanters of the femur, the patient surviving the operation twelve years, and then dying consumptive … Mr. White had been unable, from his extensive practice, to contribute any literary work to the advancement of medical science … although he did deliver an Hunterian Oration before the Royal Society”. White’s work highlighted the importance of surgical intervention in the treatment of hip joint diseases, albeit with limited understanding of the long-term outcomes [36].
Another significant figure in orthopedic history was John Rhea Barton (1794–1871), whose innovative methods in bone division contributed to joint surgery. Barton stated “to divide the bone through the great trochanter and part of the neck of the bone … to extend the [adducted] limb and dress the wound. After the irritation from the operation shall have passed away, to prevent, if possible by gentle and daily movement of the limb, the formation of bony union, and to establish an attachment by ligament only, as in cases of ununited (sic) fractures, or artificial joints as they are called”. His techniques reflected an evolving understanding of joint treatment, where the focus shifted towards promoting ligament healing over rigid bone union [37].
These innovative concepts paved the way for further progress in joint repair: Auguste S. Verneuil (1823–1895) made a crucial step forward by recognizing the potential of interposed materials to improve joint function. Verneuil was the first to “intuit” that the introduction of human or animal soft tissues between the articulating surfaces could not only enhance movement but also reduce pain, marking the early conceptualization of what would later become joint replacement techniques [38].
Together, the works of these early pioneers illustrate the gradual evolution of orthopedic practices, progressing from basic bone removal to the understanding of the importance of interposition of soft tissues in preserving function and alleviating pain. Their contributions laid the foundation for the modern approach to joint repair, which incorporates both the mechanical restoration of the joint and the incorporation of biologically compatible materials.
3. From Early Developments to Titanium
In the early development of orthopedics, metals began to be explored as materials for joint resurfacing and replacement. Resurfacing involves replacing only the damaged surface of a joint, leaving the underlying bone intact [39], while replacement entails removing the entire joint and replacing it with an artificial implant [40]. These concepts, though often interchanged in casual use, represent distinct approaches to treating joint diseases.
Henry S. Levert (1804–1864) was possibly one of the earliest researchers to investigate the biocompatibility of metals. In 1829, he began testing the efficacy and safety of various metallic sutures on dogs. Levert reported the superior performance of platinum sutures over those made of gold, silver, and lead [41]. During the second half of the 19th century, Joseph Lister (1827–1912) began fixing bone fractures with silver wire [42], and just a few years later, Carl Hansmann (1852–1917) performed the first internal plate fixation using a removable steel plate and nickel-plated screws [43]. Today, we are well aware of the cytotoxicity associated with both silver [44] and nickel [45], but at the time, these materials offered the best possible alternatives, particularly thanks to their durability in the biological environment.
In the early 20th century, several other pioneers experimented with various metals and materials for both resurfacing and replacement. Vitezslav Chlumsky (1867–1943), for example, conducted tests using silver, magnesium, and zinc as potential materials for resurfacing, with the intention of restoring function to damaged joints while preserving the integrity of the surrounding bone [46]. Meanwhile, Themistocles Glück (1853–1942) took a more innovative approach by using nickel-coated screws and polymeric resins to fix an ivory hip replacement to the surrounding bone tissue [47,48]. When a replacement involves both the hip and the femur, these types of implants are often referred to as total hip replacements (THRs) or hip joint prostheses when describing the combination of femoral and acetabular components.
However, despite these early trials, the use of metals in orthopedics remained largely experimental and consisted mostly of individual cases. It was not until Robert Jones (1855–1933) reintroduced gold as a biomaterial for resurfacing that some meaningful clinical results emerged. His work demonstrated good long-term clinical follow-ups, with patients experiencing positive outcomes for up to 20 years [49].
It was ultimately William Arbuthnot Lane (1859–1943) who developed stainless steel screws, wires, and plates to repair bone fractures [50] (Figure 2a), most of which failed due to design flaws. In particular, he designed his plates so as to bury the least amounts of metal commensurate with secure fixation, as he believed that the tissue reaction was proportional to the amount of metal buried [51]. The transition from standard steel (often improperly referred to as “iron”) to stainless steel, which offers superior corrosion resistance, was made possible by a series of pivotal scientific discoveries of the 19th and early 20th centuries, including the identification of chromium’s corrosion-resistant properties by Michael Faraday in the 1820s, the subsequent work on iron–chromium alloys by Robert Bunsen and others, and the crucial understanding of the iron–chromium–carbon phase diagram. These advancements culminated in 1912 with the production of the first austenitic stainless steel by Eduard Maurer and Benno Strauss. But even if the interest towards steel was growing, its biological properties were still under scrutiny. In 1909, Elie (1856–1912) and Albin Lambotte (1866–1955) compared several metals and found gold- or nickel-plated steel to be the most satisfactory in terms of mechanical and corrosion resistance qualities [52]. A few years later, after an in vivo experiment on one hundred animals, Groves concluded that ordinary steel plates were instead well tolerated by the tissues, pointing out that fixation was a much bigger issue [53]. It was ultimately von Baeyer (1857–1925) who, in 1908, conducted pioneering research on the toxicity of implanted metals. He described the cellular reaction around pieces of metal that were left buried in tissues for varying periods. The metallic particles set free by corrosion were identified in the surrounding tissues, and the cellular responses were described in detail. Von Baeyer also observed that when two different metals, copper and zinc, were implanted close together, galvanic coupling occurred, leading to rhythmic contractions in the underlying muscles, while the connective tissue cells aligned in the direction of the electric current. In a subsequent paper, von Baeyer noted that when small rolls of zinc and copper were placed beneath the skin of a rabbit and left for several months, the copper remained shiny instead of corroding. The copper ions migrated toward and united with the zinc, instead of becoming oxidized or dissolved, and the copper was no longer toxic to the surrounding cells [54]. A few years later, Lenche and Policard observed the impregnation of tissue around steel implants with iron salts, confirming not only that the biological environment was corroding the steel in vivo but also that the corrosion products could migrate to the surrounding tissues [55]. After Von Baeyer, Ménégaux, Moyse, and Odiette [56] studied the impact of metals on cell growth in tissue cultures, finding copper, magnesium, aluminum, bronze, and brass to be highly toxic. In contrast, metals like V2A extra-stainless steel (18% Cr, 8% Ni, unspecified amounts of Mn and Ti) and platinostainless D steel (18% Cr, 8% Ni, unspecified amounts of W), both of which are chemically similar to the modern AISI 304, showed minimal toxicity and were recommended for bone fixation. Positive results were also noted for Nicral D (20% Cr, 16% Ni) and even Duralumin (4% Cu, 0.5% Mg, 0.2% Mn, 0.3–0.5% Si, Al bal.) [57].

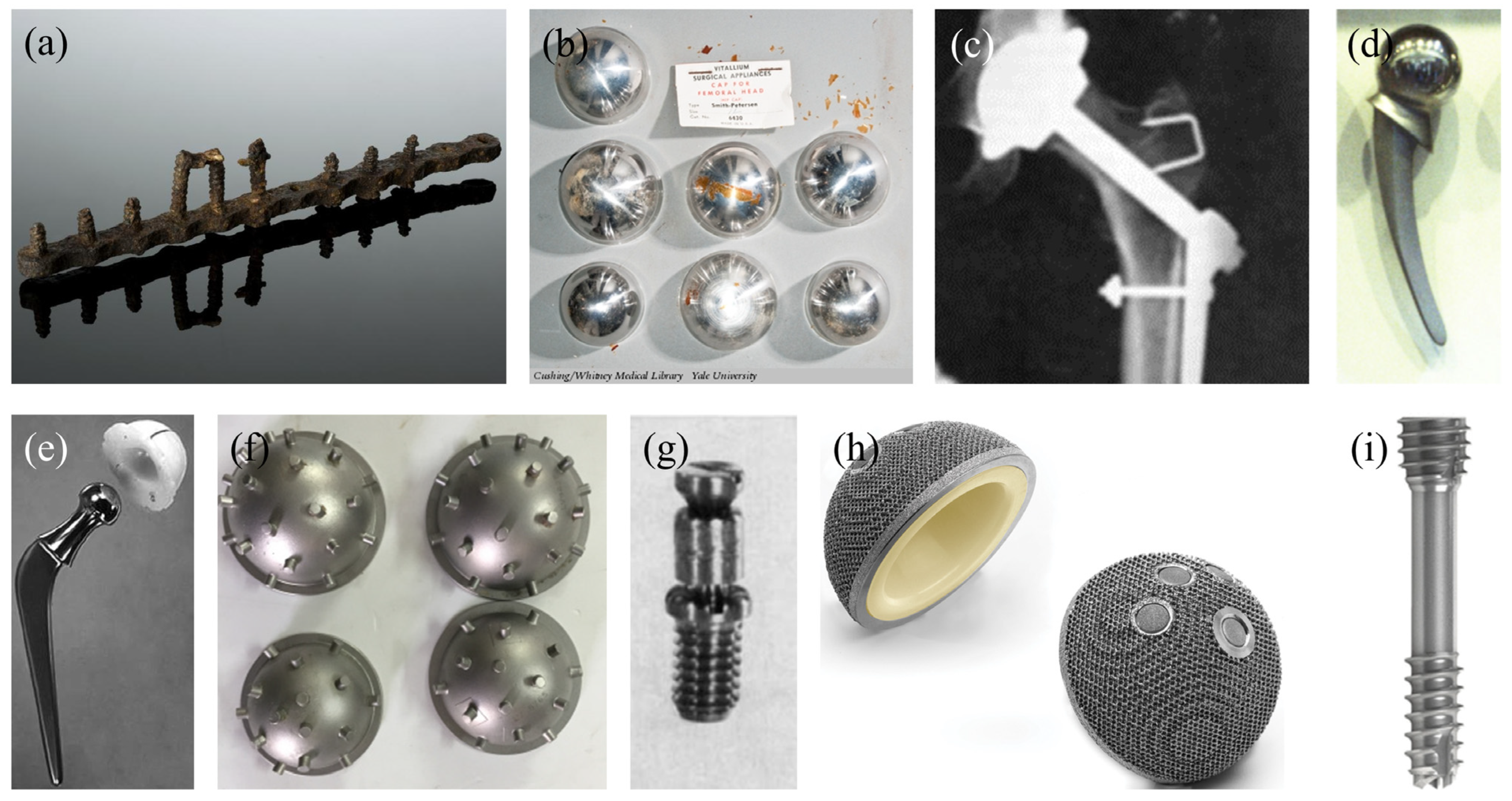
Figure 2.
Historically relevant metallic implants: (a) plate and screws developed by William Arbuthnot Lane. (b) Vitallium resurfacing cups developed by Marius Smith-Petersen. (c) X-ray of an early hip implant developed by Philip Wiles. (d) Vitallium femoral component developed by Frederick Thompson. (e) Femoral component developed by John Charnley. (f) Acetabular cups developed by Kenneth McKee. (g) Titanium screws developed by Marius Smith-Petersen. (h) First commercially available 3D-printed acetabular cup by LimaCorporate. (i) First commercially available resorbable magnesium screw by Syntellix.
Haas emphasized the variability in stainless steel compositions, noting that some corroded rapidly [58]. He illustrated this with a corroded VM rustless steel nail and recommended V2A steel for its superior corrosion resistance. Orsos, building on von Baeyer’s earlier observations, confirmed the generation of galvanic currents when two dissimilar metals were used for bone fixation. Using a more methodical approach, he measured these currents and demonstrated their effects, such as muscle contractions. Based on these findings, Orsos advocated for single-metal implants to avoid such complications [59], but even when single metals were used, a difference in potential could be observed between different parts. Perves and Damany reported a case in which there were pain and cyanosis in the forearm after fixation of a fracture plate, and the symptoms were relieved by removal of the plate. They expressed the belief that the symptoms were due to the electric current generated and showed that there was a difference in potential between the Lambotte plate and the screws (66 mV), between the Sherman vanadium steel plate and screws (44 mV), and between the Sherman screws and the Lambotte plate (52 mV). For comparison, they could not register any difference in potential between platinostainless steel and screws [60].
Masmonteil reported three cases in which were was serious corrosion of screws, measuring the differences in potential between the corroded and non-corroded parts. He expressed the belief that the electrolytic phenomenon was the prominent factor causing the unfavorable reaction of bone to the metals used for osteosynthesis, recommending the use of inert metals with a potential of about 0.21 mV at the isoelectric point of bone [61].
Apart from biocompatibility, concerns were still focused on mechanical strength. Stainless steel lacked the refined properties of modern alloys, which led William Sherman (1880–1954) to explore possible alternatives, such as vanadium steel [62]. Building upon Lane’s example, Sherman believed that vanadium steel, which had superior mechanical strength, could provide even better results for fracture fixation. He also made modifications to improve its application, such as rounding the edges of the plates to reduce irritation and improve fitting.
In 1930, the American College of Surgeons requested the assistance of the United States Bureau of Standards in the establishment of a standard Lane plate. As the result of their deliberations, a set of standards was adopted for the numerous plates of the Sherman type. They were to be made of a specific vanadium steel alloy known as Society of Automotive Engineers steel 6150 (C 0.45–0.55%, Mn 0.5–0.8%, Cr 0.8–1.1%, V nut under 0.15%, with a desired amount of 0.18%) [63]. It should be noted that no physiological tests were carried out, and the alloy was specifically selected following the recommendation of the engineers of the Carnegie Steel Company in Pittsburgh because of its surface hardness [57]. However, despite its mechanical advantages, vanadium steel suffered from significant corrosion in biological environments, limiting its effectiveness.
Hudack reviewed the clinical experience of the Presbyterian Hospital of New York with high-chromium, low-nickel stainless steel, while also testing bone plates of similar composition on dogs. He noticed that, after removal, the part showed a weight loss of about 0.1%, was well tolerated, and caused no reaction in bone. Microscopic sections showed that the bone was closely approximated to the metal and had grown into the threads of the screw, while remaining living. He then recommended that all stainless steel materials should be passivated in nitric acid before use, a practice that is still in use today [64].
Max Lange (1899–1975) [65] investigated the reactions of bone and soft tissues to Krupp stainless steel wire in experimental animals, comparing the results with similar experiments using wires made from iron and bronze. Based on microscopic and clinical observations, he concluded that Krupp steel wire was the optimal suture material for bone. It was strong, caused minimal tissue reaction, and interfered less with the formation of callus compared to iron or aluminum bronze wire. Like any other stainless steel [66], the alloy contained nickel and chromium, with the latter being mainly responsible for the positive clinical outcome. The high chromium content is especially important, as it forms a passive oxide layer on the surface of the wire when exposed to air. This oxide layer, primarily composed of chromium oxide (Cr2O3), acts as a protective barrier, preventing further corrosion and minimizing the risk of metal ion release into the surrounding tissues. This passivation of the surface is what makes Krupp steel wire particularly biocompatible. The formation of the stable oxide layer prevents the wire from reacting negatively with biological tissues, reducing inflammation and promoting a favorable healing environment for bone fractures.
Although resistant to corrosion, stainless steel soon proved to be far from the ideal metal for implantation. Jones and Lieberman [57] investigated the interactions between bone and various types of steel. They highlighted the diversity of stainless steels available at the time, noting significant differences in their compositions. Their study evaluated nickel-free rustless steel, high-nickel rustless steel, low-nickel rustless steel, and vanadium steel by embedding tacks made from these materials into the bones of experimental animals. After periods ranging from one to two-and-a-half months, they analyzed the weight loss of the implants and examined the reactions of the surrounding bone tissue. They concluded that the severity of the bone reaction was correlated directly with the extent of chemical changes—grouped under the general term “corrosion”—occurring in the implanted metal. Among the materials tested, high-chromium, low-nickel rustless steels were identified as the most favorable due to their minimal corrosion, although they were not entirely nonirritant to bone.
Nevertheless, by the 1940s, stainless steels were well established, and the choice was between using AISI 304 or 316 [55].
Arthur Adalbert Zierold (1886–1976) made a significant breakthrough in the field of orthopedic implants when he proposed the use of Stellite (now known by the ASTM F75 standard [67]) in orthopedic surgery, particularly for bone fractures [68]. Building upon Baeyer’s experience, Zierold’s research focused on the reaction of bone to various metals and revealed that the body was indifferent to the implantation of certain metals while being sensitive to others. Metals that were well tolerated by bone remained largely unchanged after being buried in tissues for extended periods, whereas metals that caused adverse reactions exhibited varying degrees of corrosion. Zierold explained that, in their bulk form, metals could modify other elements at the expense of their own substance. When placed in an electrolyte (such as tissue fluids), this modification occurred through the dissociation of metallic ions, forming a colloidal solution that followed the solution pressure of the metal. This process, he argued, underlay corrosion, explaining the degradation of metals like steel and iron when exposed to tissue fluids.
Zierold’s conclusion was that gold, aluminum, and Stellite were particularly well tolerated by bone and were capable of becoming encapsulated with minimal hindrance to the healing process. These metals appeared inert, unaffected by living cells and bodily fluids, making them ideal candidates for orthopedic implants. On the other hand, steel, which was poorly tolerated and highly soluble, was deemed the least suitable of all of the metals studied for bone fixation. Zierold’s identification of Stellite as the ideal metal for orthopedic implants was a turning point, offering the best balance of mechanical strength and corrosion resistance, making it a more durable and reliable option for implants. Before 1924, rustless (a less strict definition compared to stainless) steel was not generally employed in bone surgery, but the work of Zierold gave an immediate stimulus to its intensive use.
Although modern research on bioresorbable alloys has brought renewed attention to their potential, Erwin Payr (1871–1946) was actually among the first to apply magnesium in the orthopedic field. Payr experimented with magnesium in the form of inter-medullary wires and bars, marking an early attempt at using resorbable materials in surgical implants [69].
In 1932, Albert W. Merrick (1879–1942) revolutionized dental implants by developing a new dental cobalt alloy as a more affordable alternative to gold implants [70]. This material, known as Vitallium (now a trademark of Dentsply Sirona), demonstrated exceptional corrosion resistance and strong mechanical properties, making it an ideal material for long-lasting dental implants.
Marius Smith-Petersen (1886–1953) made an important observation that glass could induce the formation of a bio-inert membrane surrounding an implant. He became the first to experiment with stamped glass prosthetic implants for resurfacing. Unfortunately, these glass implants failed catastrophically, prompting Smith-Petersen to explore other materials, such as celluloid, Bakelite, and Pyrex. By 1937, after being advised by his dentist, he tested Vitallium, a material that would prove to be the first to provide more than 10 years of in vivo success [71] (Figure 2b).
Venable, Stuck, and Beach placed screws of Vitallium, copper, brass, galvanized iron, vanadium steel, plain steel and chromium-plated and silver-plated copper in animal models, checking the screws at intervals that varied between a few days and three months. They noticed that all screws except the ones made of Vitallium caused reactions and absorption in the bone, becoming loose [72,73,74,75].
In 1938, Philip Wiles (1899–1966) made another major advancement by performing the first complete hip arthroplasty, utilizing a “metal on metal” (MoM) solution, where both the femoral head and acetabular components were made of stainless steel and fixed with screws (Figure 2c). However, due to design flaws, the results were less than optimal, and the procedure did not become widely adopted at the time [76].
Later, Frederick R. Thompson (1907–1983) developed the first Vitallium prosthetic implant for joint replacement (Figure 2d), distinguishing his work from Smith-Petersen’s earlier use of Vitallium solely for resurfacing purposes [77].
Around the same time, Sir John Charnley (1911–1982) refined the design of femoral and acetabular components (Figure 2e), optimizing their geometry for better load distribution and long-term stability [78,79,80]. He introduced the concept of low-friction arthroplasty, pairing a small-diameter metallic femoral head with an ultra-high-molecular-weight polyethylene (UHMWPE) acetabular cup. This innovation significantly reduced wear, extending implant longevity and establishing the foundation for modern total hip arthroplasty.
Similarly, Kenneth McKee (1906–1991) played an important role in advancing joint prosthetics by developing the first successful metal-on-metal total hip replacement [81]. His work made use of the improved metallurgical techniques that enabled the production of highly polished, wear-resistant cobalt–chromium alloy components. McKee’s implants, often characterized by innovative and futuristic designs (Figure 2f), demonstrated superior durability compared to earlier designs [82], paving the way for further innovations in modularity and fixation methods, ultimately influencing contemporary implant strategies.
Around the same period, Bothe, Beaton, and Davenport conducted experiments with a relatively “new” material in 1940, demonstrating the potential of titanium as a biomaterial by observing its compatibility with bone [83]. These early findings provided critical insights into titanium’s suitability for implants. Subsequently, during micro-circulation experiments on rabbits, Per-Ingvar Brånemark (1929–2014) observed an unexpected finding: the titanium camera that he was using had partially integrated with the surrounding bone tissue [84]. This observation led Brånemark to investigate the use of titanium for prosthetic implants. His work culminated in the development of titanium implants, which, by 1982, demonstrated a remarkable 97% success rate in vivo, with bone integration and no adverse reactions, laying the foundation for modern dental and orthopedic implantology [85].
4. The Titanium Revolution
The post-Brånemark era in orthopedic implantology marked a period of rapid growth in the use of titanium and its alloys in medical applications, particularly in joint replacement and dental implants. Following Per-Ingvar Brånemark’s pioneering discovery of titanium’s unique ability to osseointegrate with bone tissue, the use of titanium in orthopedic surgery flourished (Figure 2g).
Although Brånemark’s findings in the 1960s were revolutionary, titanium was not immediately adopted as the standard material for orthopedic implants. The process of refining titanium for medical use involved overcoming numerous challenges, including its cost, material inconsistencies, and difficulties in manufacturing. However, as research into the biocompatibility and mechanical properties of titanium progressed, it became increasingly clear that titanium had the potential to transform the field of orthopedic implantology [86].
The early clinical applications of titanium implants, particularly in dental and hip prosthetics, were based on pure titanium (Grade 1 and Grade 2) [87]. However, pure titanium’s relatively low mechanical strength, which was suitable for dental applications but not ideal for load-bearing joint replacements, soon led to the development of titanium alloys [88]. These alloys combined titanium with other metals like aluminum and vanadium, improving its mechanical properties without sacrificing biocompatibility.
The use of titanium alloys in orthopedic implants became increasingly widespread in the 1970s and 1980s, with the introduction of Grade 5 titanium alloy, also known as Ti-6Al-4V (titanium-6% aluminum-4% vanadium). This alloy offered significant improvements in strength, fatigue resistance, and workability compared to pure titanium, making it an ideal candidate for joint replacements and other orthopedic devices [89].
However, as the use of titanium alloys in aerospace and medical applications grew, so did concerns about material properties, particularly in terms of corrosion resistance [90] and strength [91]. Ti-6Al-4V, the most commonly used alloy in both the aerospace and medical industries, has been widely used for hip and knee implants. Despite its strength, Ti-6Al-4V has also raised issues related to corrosion in physiological environments, as its surface oxide layer could degrade over time, leading to the release of metal ions into surrounding tissues [92,93]. This phenomenon raises concerns about potential inflammatory responses, allergic reactions, and long-term implant failure [94,95].
In response to these issues, the medical device industry began to focus on improving the manufacturing and treatment of titanium alloys to enhance their biocompatibility and durability. This included the development of new alloy compositions, as well as surface modification techniques to reduce the risk of corrosion and increase the osseointegration potential of titanium implants.
With the increasing use of titanium in orthopedic implants, the need for standardized testing and regulations became evident. The development of standards for medical use became crucial in ensuring the safety and efficacy of implants [96]. A turning point came in 1980 with the publication of ASTM F67, the first standard for commercially pure titanium for surgical implants, which defined the material’s requirements for use in medical devices [97].
Subsequently, ASTM F136, introduced in 1984, set standards for the composition and mechanical properties of titanium alloys, specifically Ti-6Al-4V, for use in surgical implants [98]. These standards helped regulate the quality of titanium used in the manufacturing of orthopedic implants, ensuring that the material met the necessary criteria for biocompatibility, strength, and durability.
The adoption of such standards by the medical device industry helped to increase the acceptance of titanium as the material of choice for orthopedic implants. It also led to the development of other titanium alloys, such as Ti-6Al-7Nb (ISO 5832), a vanadium-free alternative to Ti-6Al-4V, which addressed concerns over vanadium’s potential toxicity [99].
Moreover, researchers began to explore surface modification techniques to enhance the performance of titanium alloys, such as the incorporation of hydroxyapatite coatings to promote osseointegration, and anodizing to create a more stable oxide layer. These innovations helped mitigate the shortcomings of Grade 5 titanium and improved the long-term success of titanium implants.
In recent years, there has been a continued shift toward the use of highly specialized titanium alloys for specific orthopedic applications. One of the most significant developments has been the increasing use of titanium for spinal implants, where properties such as flexibility, fatigue resistance, and biocompatibility are critical. The medical industry has increasingly turned to titanium alloys such as Ti-15Mo (titanium-15% molybdenum) and Ti-10V-2Fe-3Al (titanium-10% vanadium-2% iron-3% aluminum) for these specialized applications, as they offer superior fatigue strength, corrosion resistance, and overall durability compared to earlier alloys like Ti-6Al-4V [100,101,102]. Still, Ti-6Al-4V still dominates the orthopedic and implant sectors, accounting for an estimated 60–70% of the titanium market due to its versatility, cost-effectiveness, and well-established properties [103].
Shape-Memory Alloys
Alongside the development of conventional titanium alloys for orthopedic applications, the introduction of shape-memory alloys (SMAs) has provided new possibilities for implant design and functionality. Shape-memory alloys, which can recover their original shape upon heating or under mechanical stress, have attracted interest in biomedical applications due to their unique mechanical properties, including superelasticity [104] and the shape-memory effect [105]. Among these materials, nickel–titanium (NiTi), commonly referred to as nitinol, is the most widely used SMA in the medical field [106].
Nitinol’s exceptional mechanical behavior allows it to exhibit pseudoelasticity, meaning that it can undergo large deformations and return to its original shape upon unloading. This property is particularly advantageous in applications where implants must conform to complex anatomical structures while maintaining flexibility and durability, such as in plates for repairing bone fractures [107], staples [108], and intramedullary pins [109]. The material’s low elastic modulus, which is closer to that of bone compared to conventional titanium alloys, helps reduce stress shielding effects, minimizing bone resorption and improving implant longevity [110].
The corrosion resistance and biocompatibility of NiTi have also been key factors in its adoption for medical applications. However, concerns regarding the release of nickel ions—known for their potential cytotoxicity and allergenic effects—have led to extensive surface modification strategies to enhance the material’s biocompatibility [111]. These include oxidation treatments, coatings, and surface passivation techniques to create a stable titanium oxide layer that minimizes nickel’s release.
The material’s ability to apply continuous compressive forces makes it particularly effective in fracture fixation and dynamic stabilization systems. Additionally, NiTi has been explored for applications in minimally invasive surgery, where its shape-memory properties enable the deployment of implants through small incisions before they expand into their functional form [112,113].
5. Beyond Titanium
While titanium and its alloys have long been the materials of choice in orthopedic implants due to their superior strength, corrosion resistance, and biocompatibility, research into alternative metals has expanded over the years. Zirconium [114], niobium [115], and tantalum [116] have emerged as promising candidates for orthopedic applications, offering unique properties that complement or surpass titanium in certain areas. These materials are gaining attention for their potential to improve the performance and longevity of implants, particularly reducing the elastic modulus, while addressing some of the biological limitations associated with titanium-based devices, such as the potential ion release.
5.1. Zirconium and Its Alloys
Zirconium is a transition metal known for its remarkable properties, including high strength, excellent corrosion resistance, and biocompatibility. While zirconium has been used in aerospace and nuclear [117,118] applications for decades, it has recently gained significant interest in the biomedical field [119], particularly in the development of joint replacements and dental implants.
The key advantage of zirconium over titanium is its ability to form an even more stable oxide layer on its surface [120,121]. This oxide layer is highly resistant to corrosion and wear, making zirconium a highly attractive material for use in the harsh physiological environment of the human body. Additionally, some zirconium alloys have a relatively low modulus of elasticity compared to titanium, which helps them to mimic the natural mechanical properties of bone tissue and provides better load transfer in certain applications [122].
When comparing wear resistance, zirconium alloys demonstrate superior performance to titanium alloys, particularly in articulating or load-bearing applications [123,124]. This is largely attributable to the transformation of the zirconium surface into a hard, ceramic-like oxide layer under wear conditions, which provides excellent abrasion resistance. Titanium alloys, while durable, are more prone to generating wear debris due to their relatively softer oxide layer and susceptibility to galling under certain conditions. This distinction makes zirconium alloys particularly suitable for joint replacements, where reducing wear particles is critical to minimizing inflammation and osteolysis.
In addition to wear resistance, zirconium is also valued for its biocompatibility [114,125,126]. Several studies have demonstrated that zirconium alloys are less likely to elicit an inflammatory response or cause adverse reactions in the body [127,128]. Furthermore, zirconium implants are less prone to metal ion release, which can be a concern with titanium-based alloys like Ti-6Al-4V.
5.2. Niobium and Its Alloys
Niobium, another transition metal, is increasingly being explored for use in biomedical applications due to its excellent biocompatibility, corrosion resistance, and potential for enhancing osseointegration [129,130]. Niobium has a relatively low modulus of elasticity, which makes it a suitable material for implants designed to integrate with bone tissue by providing a more natural load distribution, but so far it has mainly been studied for dental implants [129] or stents [115] due to being a soft metal [131].
Niobium is known for its low toxicity and bio-inertness, which make it less likely to provoke an immune response compared to other metals [132]. Additionally, niobium has shown good compatibility with bone tissue, enhancing its ability to osseointegrate without the need for additional surface coatings or treatments [133].
Due to its limited mechanical characteristics, niobium is mainly used as an additive in other transition-metal-based biocompatible alloys, such as titanium–niobium (Ti-Nb) and zirconium–niobium (Zr-Nb), which have been investigated for use in a variety of orthopedic applications, including joint replacements and bone fixation devices [134,135,136]. Niobium-containing alloys have demonstrated superior fatigue resistance and strength compared to pure titanium, making them promising candidates for applications in which high mechanical stress is anticipated, such as spinal implants and load-bearing joints.
Furthermore, niobium-coated implants have also shown improved resistance to corrosion in vitro [137], as well as osseointegration properties, which could be particularly useful in the orthopedic field.
5.3. Tantalum and Its Alloys
Tantalum is a rare and highly corrosion-resistant metal with unique properties that make it well suited for use in orthopedic implants [138]. One of the most significant applications of tantalum has been as porous foams, which can be controlled and tailored to mimic the architecture of human bone [139], thus stimulating secondary fixation [140] while also preventing stress shielding [141]. This property has made tantalum a particularly attractive material for bone scaffolds, spinal implants, and joint replacements, leading to the development of Trabecular Metal by Zimmer Biomet.
Trabecular Metal’s highly porous structure is considered to be ideal for enhancing osseointegration, as it allows for bone ingrowth and the formation of a biological bond between the implant and the surrounding bone tissue. This makes porous tantalum particularly useful in applications such as hip replacements and knee prostheses, where long-term fixation and stability are essential for successful outcomes. In these applications, tantalum implants promote faster healing and a stronger bond between bone and implant, reducing the risk of implant loosening and the need for revision surgeries [141,142,143].
As a material, the main advantage of tantalum is its biocompatibility. Like zirconium and niobium, tantalum has excellent compatibility with the human body and does not elicit significant immune or inflammatory responses [144]. Its high corrosion resistance [145] ensures that it will maintain its integrity over time without leaching harmful ions into the surrounding tissue, which can cause long-term complications with some other materials.
Porous tantalum is often used in the form of coatings for other implants, such as titanium-based prostheses, to improve their integration with bone. The development of tantalum-coated implants, which combine the strength and osseointegrative properties of tantalum [146,147] with the mechanical properties of other metals, like titanium [148], has led to improved implant longevity and clinical outcomes in many orthopedic procedures.
6. The End of the Era of Stainless Steel?
Stainless steel has been a key material in the field of orthopedic implants for many decades, primarily due to its strength, durability, and corrosion resistance [149]. However, over time, its usage has declined in favor of other materials, especially titanium and its alloys, which offer superior biocompatibility and performance in clinical settings [150]. Despite this decline, stainless steel continues to be used in specific orthopedic applications, especially where cost-efficiency and mechanical strength are of primary concern [151].
6.1. The Decline of Stainless Steel
Stainless steels, particularly the 304 L and 316 L grades, have historically been among the most commonly used materials for orthopedic implants, such as bone plates, screws, pins, and wires. As we discussed in Section 4, its use began in the early 20th century, when surgeons sought a material that combined strength and corrosion resistance. Stainless steel quickly gained acceptance due to its relatively low cost, ease of processing, and ability to withstand the mechanical loads associated with bone fixation. Additionally, stainless steel’s corrosion resistance helped it to resist degradation in the harsh environment of the human body, an important characteristic for implants that are intended to last for extended periods of time.
However, despite these initial advantages, the limitations of stainless steel in terms of biocompatibility and long-term performance became increasingly evident as the field of orthopedics progressed, particularly with the development of titanium and titanium alloys in the 1960s and 1970s.
Over time, several factors led to the decline of stainless steel’s dominance in orthopedic implants:
Biocompatibility Issues: Stainless steel, while corrosion-resistant, does not match the biocompatibility of titanium. Over time, the nickel content in certain grades of stainless steel (e.g., 316L) can cause allergic reactions and immune responses in some patients, leading to complications such as inflammation and implant failure. Titanium, by contrast, is known for its excellent biocompatibility, making it less likely to cause these reactions. This has made titanium the preferred material for implants that must remain in the body for extended periods [152,153,154].
Mechanical Properties: While stainless steel is strong and durable, it does not possess the same strength-to-weight ratio or flexibility as titanium. Titanium alloys, particularly Grade 5 titanium (Ti-6Al-4V), offer superior fatigue resistance and are less likely to break or fracture under stress. Stainless steel can be weaker than titanium alloys, particularly in environments where the material is subject to cyclic loading, such as with joint replacements or fracture fixation [98,155,156].
Corrosion Resistance: Although stainless steel is more corrosion-resistant than many other metals, it is still prone to stress corrosion cracking and can degrade over time in the body, particularly in joint replacements, where the implant is constantly subjected to wear and friction. Titanium, on the other hand, forms a protective oxide layer on its surface that makes it much more resistant to corrosion, especially in the harsh, acidic, and oxygen-poor conditions within the human body [157,158,159].
Radiopacity: Stainless steel is radiopaque, meaning that it shows up on X-ray images, which can be both an advantage and a disadvantage. In the case of some surgical procedures, titanium’s lower radiopacity is preferred, as it is less likely to interfere with medical imaging. This can be particularly important in spinal surgery or joint replacements, where clear imaging is critical to monitor postoperative healing and detect any complications [160,161].
Increased Focus on Patient Comfort: Modern orthopedic implants focus not only on the mechanical performance of the materials but also on the overall patient experience. The lightness and flexibility of titanium alloys have become increasingly important in reducing postoperative pain and discomfort for patients. As a result, many hospitals and surgeons have opted for titanium-based implants, which are more patient-friendly due to their lower weight and better fit within the body.
6.2. Present of Stainless Steel in Orthopedics
Despite its declining use, stainless steel is still employed in some specific orthopedic applications, particularly where cost-efficiency and high mechanical strength are essential. Its use remains prevalent in the following scenarios:
Fracture Fixation Devices: Stainless steel is still commonly used for fracture fixation devices such as plates, screws, and pins. These devices are often used in the treatment of bone fractures, particularly in less demanding, low-stress environments such as the upper limbs, or when the fracture is not expected to bear heavy loads during the healing process. As Laing had already noted in 1966, stainless steel can be a viable option for fixation devices, as once they have fulfilled their function they can be removed, before they can cause any adverse reaction [55].
Spinal Implants: Stainless steel is still used in some spinal implants, such as rods and screws, particularly in pediatric patients or in cases where cost is a major concern. Spinal fixation systems often require implants that are strong enough to support the weight of the body, and stainless steel’s high tensile strength makes it suitable for these applications.
Cost-Effective Applications: In certain regions or healthcare systems where cost is a significant consideration, stainless steel continues to be favored for its affordability compared to titanium. This is particularly the case in emergency settings or developing regions, where the cost of titanium-based implants might be prohibitive [162,163,164,165,166].
While stainless steel is no longer the material of choice for most permanent orthopedic implants, it still plays a significant role in the orthopedic field, especially for short-term applications and budget-conscious solutions. The lower material costs associated with stainless steel allow it to remain competitive, particularly in cases where the mechanical strength requirements are not as demanding as in more advanced applications like joint replacement or long-term bone fixation.
Advances in stainless steel alloys have improved their corrosion resistance, with newer grades of stainless steel offering better biocompatibility and stress corrosion resistance. Additionally, coatings such as polymeric or ceramic coatings can enhance the material’s resistance to corrosion and improve its overall performance in the body.
7. Bioresorbable Alloys
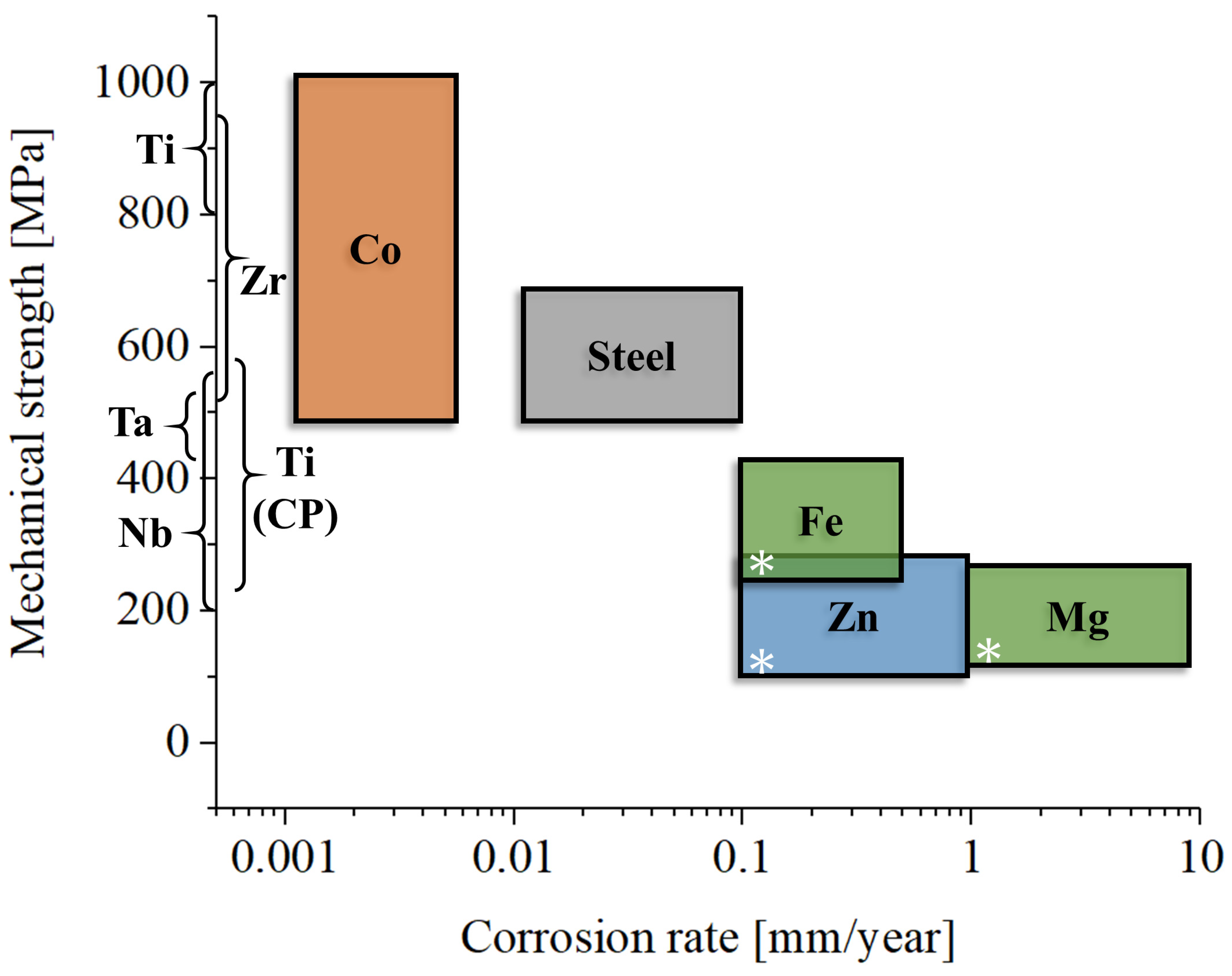
The growing field of bioresorbable alloys represents a promising direction in orthopedics, particularly for applications where temporary support is needed during healing, after which the material safely resorbs into the body without requiring removal [167,168]. Bioresorbable alloys have the potential to eliminate the need for secondary surgeries to remove hardware, making them highly attractive for bone fixation devices, fracture stabilization, and even some joint replacements [169]. Among the various bioresorbable metals under investigation, magnesium [170], iron [171], and zinc [172] alloys have shown the most promise, each offering a unique combination of mechanical properties, resorption rates, and biocompatibility.
It is important to acknowledge that magnesium, iron, and zinc alloys, while offering distinct mechanical properties suitable for various orthopedic applications, also exhibit significant variations in their biological responses, particularly concerning their resorption times. The rate at which these materials degrade within the physiological environment is a critical factor influencing their suitability for temporary implant applications, with magnesium being the fastest to dissolve in vivo and zinc the slowest. Alloying elements can further enhance or reduce dissolution rates over time, making quantitative comparisons not particularly relevant. To facilitate a qualitative comparative analysis of these materials, Table 2 presents a list of corrosion rate data extracted from the relevant literature. This table provides ranges of corrosion rates for pure and alloyed magnesium, iron, and zinc under simulated physiological conditions and in vivo.

Table 2.
Comparative corrosion rates of magnesium, iron, and zinc alloys in simulated physiological solutions and in vivo.
7.1. Magnesium-Based Bioresorbable Alloys
Magnesium has been widely studied as a bioresorbable alloy in orthopedic applications due to its natural biodegradability and mechanical properties that closely resemble those of human bone (Figure 2i). Magnesium is the lightest structural metal, and its high strength-to-weight ratio makes it an attractive candidate for use in implants that need to support bone during the healing process [189]. It is particularly useful for applications such as fracture fixation plates [190], pins [191], screws [190], and, outside of orthopedics, stents [173].
Other than its strength-to-weight ratio, the other main primary advantage of magnesium is its controllable biodegradability [192,193]. Once implanted in the body, magnesium alloys gradually dissolve through corrosion in a controlled manner, with the rate of degradation influenced by factors such as alloy composition, surface treatment, and environmental conditions. The rate of degradation can be theoretically adjusted to match the healing time of the bone, thus avoiding the need for removal. Magnesium also naturally supports osseointegration, as it plays various biological roles related to bone formation and remodeling, particularly in the regulation of osteoblast and osteoclast activity: magnesium promotes the activity of osteoblasts [194,195,196], enhancing their ability to synthesize bone matrix and deposit minerals like calcium and phosphate into the matrix [197]. Adequate magnesium levels also support the production of collagen [198], the primary organic component of the bone matrix.
Magnesium regulates osteoclast activity [199], meaning that it can contribute to maintaining the balance between bone formation and resorption. Moreover, magnesium is critical for the synthesis and activation of PTH [200], a hormone that regulates calcium and phosphate levels in the blood and bone. PTH acts by stimulating calcium’s release from bones (resorption) [201] and promoting calcium absorption in the intestines (mediated by active vitamin D) when needed. Moreover, magnesium ions (Mg2+) can substitute for calcium ions (Ca2+) in the crystal lattice of hydroxyapatite, influencing its stability and solubility [202]. This substitution affects the mechanical properties of the bone, making it more resistant to brittle fractures [203].
Chronic inflammation negatively impacts bone remodeling by disrupting the balance between osteoblast and osteoclast activity. Magnesium has anti-inflammatory properties and helps modulate the release of cytokines like interleukin-6 (IL-6) [204] and tumor necrosis factor-alpha (TNF-α), both of which are associated with bone resorption [205].
However, there are several challenges with magnesium-based alloys. The primary concern is that magnesium corrodes too quickly in the physiological environment, which can lead to a loss of mechanical integrity before the bone has fully healed [206]. This rapid corrosion can cause the implant to weaken prematurely and, in some cases, lead to adverse effects such as hydrogen gas formation at the implant site, which can interfere with bone healing. To address these issues, research has focused on creating magnesium alloys with added elements, such as calcium [207], zinc [208], and rare-earth metals [209], to slow down the corrosion rate and improve the material’s mechanical properties [210].
Surface coatings [211,212] and secondary phases, such as hydroxyapatite or polymers, are often applied to magnesium implants to reduce corrosion and enhance the implant’s biocompatibility. Although the use of magnesium alloys is still in its early stages, they are significantly more studied and developed compared to their iron- and zinc-based counterparts. Numerous clinical studies have reported promising results, positioning magnesium-based bioresorbable alloys as a potential mainstream solution for bone fixation in the future [213].
7.2. Iron-Based Bioresorbable Alloys
Iron is another promising metal for bioresorbable orthopedic implants. It has several inherent advantages, including good biocompatibility, abundant availability, and mechanical strength comparable to that of stainless steel [171]. Iron-based alloys are also bioabsorbable [214], and they degrade in the body in a controlled manner [215], which makes them suitable for use in bone fixation devices that provide temporary structural support during the healing process.
Iron alloys can be engineered to control the rate of degradation by adjusting their composition. Adding elements such as manganese, phosphorus, carbon, and silicon to iron can enhance its mechanical properties and corrosion resistance. For instance, the addition of manganese [216] improves the strength and ductility of iron, while the addition of phosphorus enhances its corrosion resistance [217]. Carbon increases the hardness and strength of iron by forming carbides [171], which also contribute to improving wear resistance. Silicon promotes the formation of a passivating oxide layer, further improving corrosion resistance and stability [218]. These modifications allow for more controlled and tailored degradation, making iron alloys more suitable for a variety of biomedical applications. Furthermore, if needed, both the biological response and the corrosion rate of iron alloys can be further controlled by manipulating the surface structure or by applying coatings [219,220,221].
Iron-based alloys could be particularly advantageous for bone fixation plates and screws [222], as they offer strength similar to that of titanium or stainless steel. Like magnesium, iron can also play several roles in bone remodeling and metabolism. It is involved in various signaling pathways and gene expression related to bone formation, and low iron levels can impair osteoblast function and bone formation [223]. Iron is also involved in osteoclast differentiation, and iron deficiency can lead to altered osteoclast activity [224]. While excessive iron may promote osteoclastogenesis and increase bone resorption [225], adequate levels are needed for balanced bone remodeling.
Iron influences bone mineralization by interacting with key factors like collagen, osteocalcin, and hydroxyapatite. Adequate iron levels can support proper mineralization of the bone matrix, contributing to bone density and strength [226]. As with magnesium, iron levels can modulate the activity of cytokines that regulate bone remodeling, such as tumor necrosis factor-alpha (TNF-α) [227], interleukin-6 (IL-6) [228], and others. These cytokines influence inflammatory responses and the activity of both osteoblasts and osteoclasts, playing a key role in bone resorption and formation.
The primary challenge for the use of iron-based bioresorbable implants remains their corrosion rates. Iron corrodes slowly compared to magnesium [215], which could result in the need for longer-term support than required for bone healing. However, when combined with other metals such as zinc [229] or manganese [230], iron can provide a more controlled degradation profile, making it a promising alternative for certain orthopedic applications, such as fracture fixation and spinal implants.
7.3. Zinc-Based Bioresorbable Alloys
Zinc, like magnesium and iron, is another metal that has shown promise as a bioresorbable material in orthopedic applications. Zinc is essential for bone formation, as it plays a crucial role in the function of osteoblasts (bone-forming cells) and in the mineralization of bone. Zinc-based alloys offer a unique balance of mechanical properties and biocompatibility, taking an intermediate position between iron- and magnesium-based alloys, and making them an attractive option for bone fixation devices and other temporary implants.
Zinc alloys exhibit excellent biocompatibility and low toxicity, and they corrode at a controlled rate that can be further optimized to match the healing process of bone. Like iron and magnesium, zinc’s corrosion rate can be regulated by adding small amounts of other transition metals, such as magnesium and iron [231]. In vivo, zinc undergoes uniform dissolution, leaving minimal byproducts. Additionally, zinc’s corrosion byproducts, such as zinc oxides, have been shown to have bioactive effects on their own. Similarly to magnesium and iron, zinc has been shown to stimulate osteoblastic activity, possibly by increasing the expression of growth factors such as insulin-like growth factor-1, while inhibiting the osteoclastic activity by downregulation of the receptor activator of nuclear factor kappa B ligand (RANKL). Zinc-dependent enzymes, such as lysyl oxidase, are also essential for crosslinking collagen fibers [232], providing tensile strength to the bone matrix [233]. Moreover, zinc is a component of superoxide dismutase (SOD), an antioxidant enzyme that protects bone cells from oxidative stress-induced damage [234], and like magnesium and iron it can help reduce the production of pro-inflammatory cytokines, such as tumor necrosis factor-alpha (TNF-α) and interleukin-6 (IL-6) [235].
One of the main challenges with zinc-based alloys is their relatively low mechanical strength compared to other metals, such as titanium and stainless steel. This can be an issue in load-bearing applications, especially when mechanical support is required for an extended period. However, recent advancements in zinc alloy design have improved their strength and mechanical performance, making them viable for use in bone screws, plates, and other temporary implants [236,237].
The development of zinc-based bioresorbable implants is ongoing, with promising results in animal studies and early human trials. Researchers are focusing on optimizing the degradation rates and mechanical properties of these alloys to ensure that they provide effective support during the critical healing phase without compromising the long-term health of the surrounding bone tissue.
8. Materials’ Properties
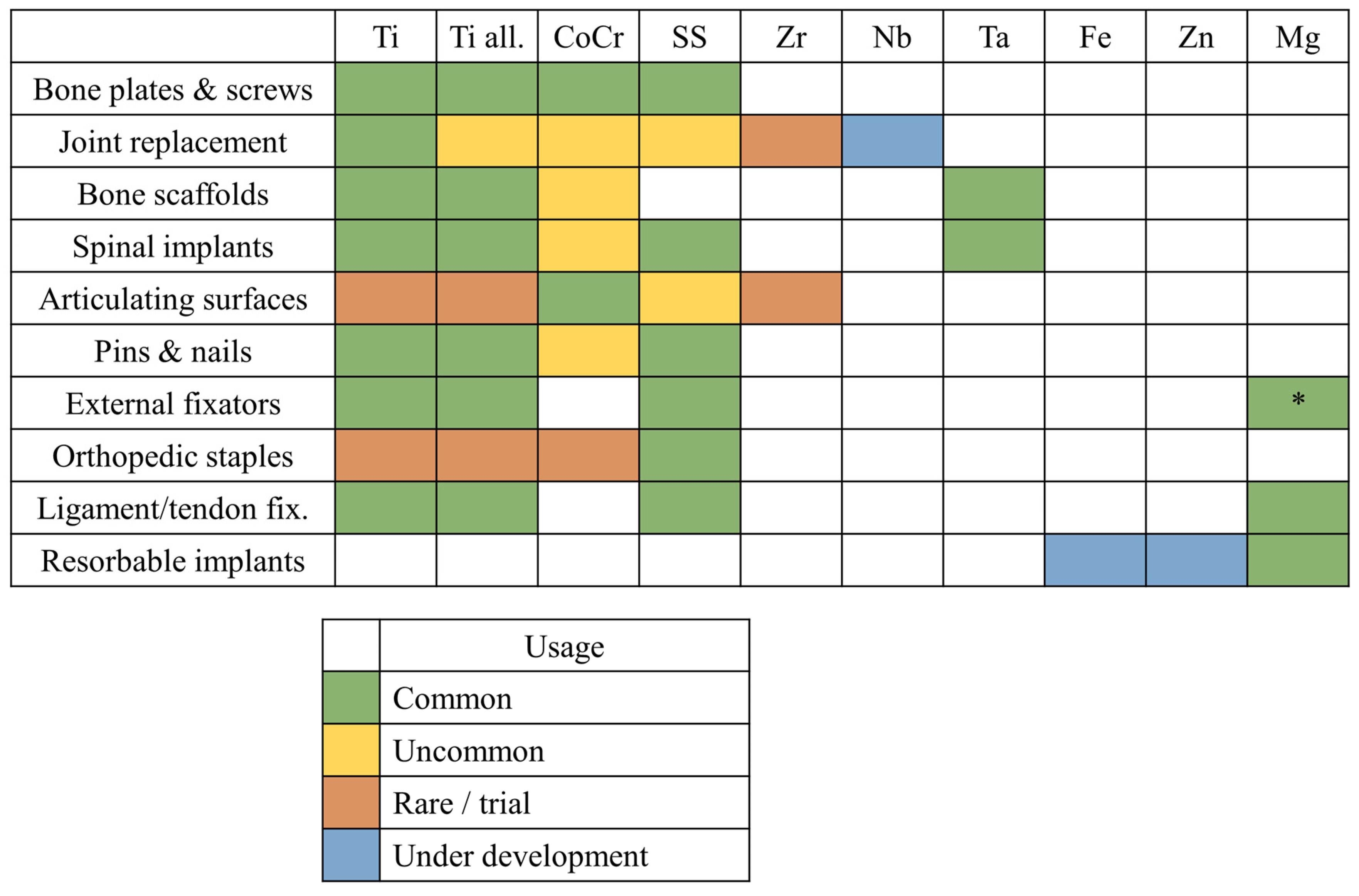
As we discussed in the previous paragraphs, metals used in orthopedics can be broadly categorized into two groups: bio-inert metals, which are mechanically strong and durable, and bioresorbable metals, which are mechanically weaker and degrade over time. These two classes of materials serve distinct purposes in the field of medical implants (Figure 3).
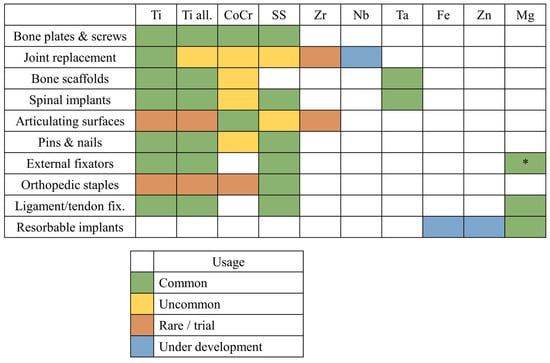
Figure 3.
Orthopedic applications for each class of metallic materials presented in this review: Ti, commercially pure titanium (Grades 1–4); Ti all., titanium alloys; CoCr, cobalt–chromium alloys; SS, stainless steels; Zr, zirconium alloys; Nb, niobium alloys; Ta, tantalum alloys; Fe, bioresorbable iron-based alloys; Zn, bioresorbable zinc-based alloys; Mg, bioresorbable magnesium-based alloys; * indicates magnesium alloys not intended for bioresorbable applications.
Bio-inert metals, such as titanium and cobalt–chromium alloys, are widely used for load-bearing implants. Examples include femoral stems in total hip replacements, orthopedic screws, and bone plates. These metals are selected for their high mechanical strength, excellent toughness, and resistance to corrosion in physiological environments, as well as in the presence of local inflammation, where the pH may vary. Their role is critical in withstanding significant mechanical stresses over long periods, where no viable alternative exists that matches their toughness and strength. For applications requiring exceptional wear resistance and hardness, such as femoral heads or dental implants, cobalt–chromium alloys are relatively common [238,239], although ceramic materials are often preferred due to their superior wear properties [240]. Conversely, when a lower elastic modulus and enhanced biocompatibility are critical, as in femoral stems or acetabular cups [241], titanium has become the most widely used material on the market.
Bioresorbable metals, such as magnesium and its alloys, are used in applications like temporary fixation devices (e.g., resorbable screws) or bone fillers. Although these materials can bear loads, particularly in the case of screws and plates, the applied stresses are generally less intense than those endured by bio-inert metals. Bioresorbable metals gradually degrade within the body and are absorbed over time, eliminating the need for a second surgery to remove the implant. However, this resorption process introduces variability in their mechanical behavior, making bioresorbable materials less predictable and, consequently, less secure for certain applications [242].
The applications of bioresorbable metals, in particular, partially overlap with those of certain ceramics and polymeric materials used in bone repair and regeneration, such as hydroxyapatite and bioactive glass, as well as biodegradable polymers like polylactic acid (PLA) and polycaprolactone (PCL). For large, load-bearing implants, metals remain irreplaceable due to their unique combination of higher toughness/tensile strength compared to ceramics and greater strength compared to polymers. This makes metals the materials of choice for implants that are subjected to moderate-to-intense mechanical loads, and they are the only reliable choice for components under tensile stresses.
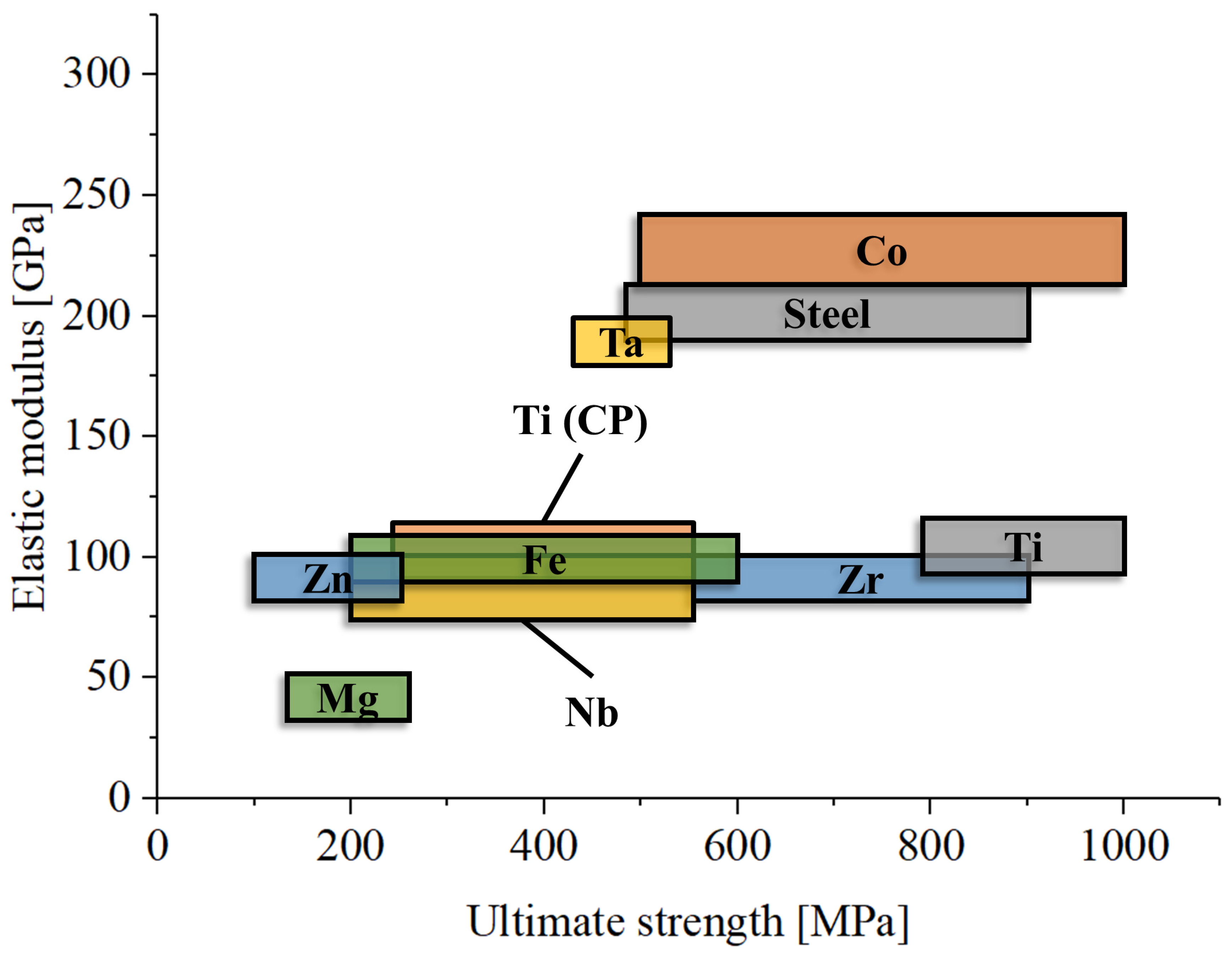
One major limitation of metals in orthopedics is their high elastic modulus (Figure 4), which is significantly greater than that of bone, including cortical bone. For example, titanium has an elastic modulus of about 110 GPa [243], cobalt–chromium alloys about 200 GPa [244], and magnesium 45 GPa [245], while the most dense cortical bone only reaches about 30 GPa [246]. Only dental enamel has an elastic modulus that is comparable at least with that of titanium (about 100 GPa) [247]. This mismatch can lead to a phenomenon known as stress shielding. Stress shielding occurs when the stiff metal implant bears most of the mechanical load, reducing stress transfer to the surrounding bone [248]. This lack of mechanical stimulation can result in bone resorption and weakening over time, compromising the long-term success of the implant [249]. One method taken to favor osseointegration and reduce the local Young’s modulus is the adoption of reticular microstructures [250].
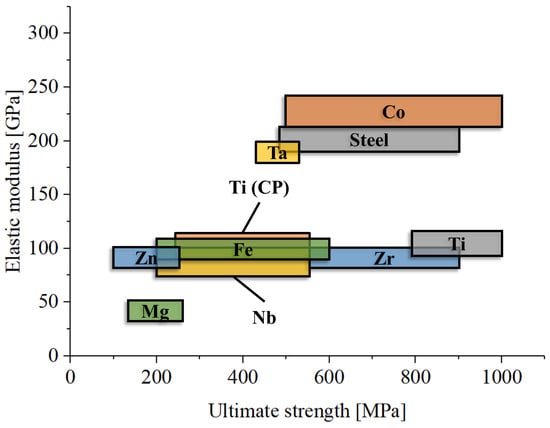
Figure 4.
Comparative graph of elastic modulus and ultimate strength for the main metallic materials used in orthopedics.
One key property to consider in this context is fracture toughness (K1C) [251], which measures a material’s resistance to crack propagation. Fracture toughness is a property in which metals consistently outperform ceramics [252,253], making them better suited for load-bearing applications where mechanical reliability is critical. Metals such as titanium (K1C~50–100 MPa·m1/2) [254], cobalt–chromium alloys (K1C~75–115 MPa·m1/2) [255], and stainless steel (K1C~50–200 MPa·m1/2) [256] exhibit far superior fracture toughness compared to biomedical ceramics. In contrast, ceramics like alumina (Al2O3, K1C~3–5 MPa·m1/2) [257], zirconia (ZrO2, K1C~7–10 MPa·m1/2) [258], and hydroxyapatite (K1C~0.7–1.2 MPa·m1/2) [259] are much more brittle, making them prone to catastrophic failure under high stress or the presence of flaws. It is known that the aforementioned parameter is strictly correlated with the microstructure of the material, and the advantage of using metals is that most of them can undergo heat treatment in order to change their microstructure and, thus, also their mechanical properties.
This fundamental difference provides metals with a significant advantage in load-bearing applications. Their ductility allows them to absorb and distribute stress more effectively, reducing the risk of sudden failure even in the presence of internal defects that act as local stressors. In that case, the toughness of metals is important to avoid the activation of local defects, which can be origin of unexpected failures. On the other hand, ceramics, while advantageous for their bioactivity (e.g., hydroxyapatite) or wear resistance (e.g., alumina and zirconia), are limited in applications where toughness and mechanical reliability under dynamic loading are critical. Thus, while ceramics are better suited for coatings or non-load-bearing components, metals remain the materials of choice for large implants exposed to high mechanical stresses, particularly where tensile stresses are present.
While biomedical ceramics often exhibit superior mechanical strength under compressive loads, this property does not translate well to tensile or flexural conditions. The brittle nature of ceramics makes them highly susceptible to failure under tensile stress, as any minor flaw or crack can act as a stress concentrator, rapidly propagating and leading to catastrophic failure. In flexural conditions, where tensile and compressive stresses coexist, the performance of ceramics is further compromised due to their low fracture toughness (K1C) and limited ability to redistribute stress around flaws.
For example, alumina (Al2O3) has an impressive compressive strength of ~4000 MPa but a much lower tensile strength of ~300 MPa [260]. Zirconia (ZrO2) [258], despite its higher toughness among ceramics, also shows a significant disparity, with its compressive strength reaching ~2000 MPa but its tensile strength often below ~400 MPa.
In contrast, metals such as titanium, cobalt–chromium alloys, and stainless steel provide consistent mechanical performance across all stress modes (compressive, tensile, and flexural). Their ductile nature allows them to undergo plastic deformation, absorbing and redistributing stress, including locally, to prevent crack propagation. For instance, titanium exhibits a compressive strength of ~1000 MPa and a tensile strength of ~900 MPa, with nearly equal performance across stress conditions [261].
This disparity makes metals the materials of choice for components subjected to complex stress fields, such as those encountered in load-bearing implants like femoral stems or bone plates. Under these conditions, ceramics are prone to unpredictable failures, whereas metals provide reliable performance and durability, ensuring better clinical outcomes.
This difference is further magnified under cyclic loading conditions, where fatigue plays a crucial role, particularly when the applied stresses are also partially working in the tensile regime. Fatigue failure occurs when a material is subjected to repeated cyclic stresses below its ultimate tensile strength, leading to crack initiation and growth over time. The crack initiation usually occurs in the presence of internal discontinuities, as inclusions or pores, or surface alterations, as roughness [262], while also being induced by the local environment (corrosion fatigue) [263]. The fatigue limit (or endurance limit) is the stress level below which a material can endure infinite cycles without failing (no activating any internal or surface defect). Metals are particularly advantageous in this regard:
Austenitic stainless steel typically has a fatigue (R = −1) limit around 180–300 MPa, depending on its grade (e.g., 316 L used in implants), making it reliable for long-term cyclic loading in applications like orthopedic screws or plates. The limit can vary from 180 to 400 MPa as a function of R [264,265,266].
Titanium alloys (e.g., Ti-6Al-4V) have a slightly higher fatigue limit (400–500 MPa) [267], combined with lower density and excellent corrosion resistance in biological environments. The fatigue limit is strongly influenced by the R ratio and the microstructure of the material.
Cobalt–chromium alloys are highly resistant to fatigue, with fatigue limits ranging from 400 to 800 MPa, making them ideal for demanding applications like joint replacements and dental implants [263,268,269].
These fatigue properties highlight another critical advantage of metals over ceramics. Ceramics lack a well-defined fatigue limit due to their brittleness and susceptibility to crack growth, even under low cyclic stresses, due to both a lack of tensile stress resistance and the activation of dislocation motion on long-range paths. This makes ceramics unsuitable for load-bearing components that are subjected to dynamic stress, while metals remain the preferred choice for applications requiring long-term durability and resistance to cyclic loading.
The discussion thus far has focused primarily on bio-inert, load-bearing materials such as titanium, stainless steel, and cobalt–chromium alloys, which excel in applications requiring high strength, toughness, and fatigue resistance. However, the situation is markedly different for bioresorbable alloys, which are designed to degrade over time within the body.
Bioresorbable alloys, such as magnesium-based and iron-based alloys, offer unique advantages in specific applications, such as temporary fixation devices or scaffolds. These materials are meant to provide mechanical support during the healing process and then degrade, eliminating the need for secondary surgeries. However, their mechanical properties fall short when compared to bio-inert metals, which limits their use in high-load applications:
Ultimate Load: The ultimate tensile strength of bioresorbable alloys is considerably lower. For example, the magnesium alloys commonly used in resorbable implants have an ultimate tensile strength of 150–250 MPa, much lower than that of titanium (~900 MPa) or stainless steel (~500–700 MPa). This limits their use in applications subjected to high or complex loading [270,271].
Toughness: Bioresorbable alloys also have lower fracture toughness, making them more prone to brittle failure, especially in critical regions of stress concentration. For example, magnesium alloys have a fracture toughness of ~10–20 MPa·m0.5, compared to titanium (~50–100 MPa·m0.5), making them unsuitable for applications where toughness is critical, as in presence of notches or stressors [272].
Fatigue Limit: The fatigue resistance of bioresorbable alloys is significantly lower. Cyclic loading accelerates the degradation process, further compromising their mechanical reliability. For instance, magnesium alloys typically exhibit fatigue limits below 100 MPa, making them unsuitable for load-bearing implants like femoral stems or joint replacements, where fatigue strength is critical for long-term performance [273,274].
These limitations mean that bioresorbable alloys are not viable for applications where catastrophic failure poses significant risk, such as femoral stems, load-bearing plates, or major joint replacements. Instead, they are used in roles where the following can be saide:
The mechanical load is relatively low (e.g., bone fillers, resorbable screws for minor fractures).
The degradation process aligns with the healing timeline, ensuring that mechanical support diminishes as the natural tissue regains strength [273,274].
9. Metal Biocompatibility
Defining biocompatibility is a complex challenge, as it is not an absolute concept. Biocompatibility depends on many factors, including anatomical location, patient characteristics, surgeon skill, and, most importantly, time. In the case of metals applied in the orthopedic field, we can essentially divide them into two groups that should be evaluated separately: bio-inert materials, and bioresorbable alloys [16,22].
On the one hand, we have bio-inert materials, such as cobalt–chromium, titanium, and stainless steel. With the exception of titanium—which Brånemark demonstrated to be capable of osseointegration—as we previously discussed, these metals generally exhibit little or no biological interaction with the surrounding environment. They are often “separated” from the surrounding tissues by an interlayer that forms over time, similar to how a cyst might form in response to foreign material [16]. These metals do not dissolve over time or only dissolve in negligible amounts, ensuring that once a stable separating layer forms, they minimally interact with the biological environment. However, titanium stands out in this category due to its unique ability to bond with bone through osseointegration, providing structural support and facilitating tissue integration. Despite this advantage, the level of interaction remains negligible compared to materials that are now classified as “bioactive” [275,276].
A notable clinical example of the challenges associated with bio-inert materials is the use of metal-on-metal hip implants, which primarily use cobalt–chromium alloys. These implants, once widely used for their durability, have been linked to significant adverse effects due to the generation of metal wear debris [277]. In many cases, patients have developed local tissue damage, inflammation, and even pseudotumors [278], which are masses formed due to the accumulation of debris and immune response. These reactions have led to the revision of many implants and prompted the decline of metal-on-metal designs in favor of ceramic or polyethylene components for hip replacements [279]. The high wear rate and biological reactivity of the debris have significantly influenced material selection for future orthopedic implants.
On the other hand, bioresorbable alloys [167] operate on the opposite principle: they interact with the biological environment, resulting in the release of ions over time. For these materials, both the bulk metal and the ions released must not cause adverse reactions [13,173]. They should not bioaccumulate in the body [280] or hinder the metabolism of surrounding cells and tissues. Furthermore, they should be progressively disposed of through natural bodily excretion processes, such as via urine. In some cases, such as with magnesium alloys, there are additional risks associated with the dissolution process. One notable concern is the formation of hydrogen gas bubbles during the degradation, which can accumulate inside the body and potentially lead to local tissue damage, pressure buildup, or inflammation [281,282].
A clinical example of this is the use of magnesium-based implants for fracture fixation. Magnesium alloys, while beneficial for their biodegradability and lack of need for removal surgery, have been associated with hydrogen gas formation during their degradation [283]. In some cases, this led to localized tissue inflammation and gas-related complications, such as subcutaneous swelling or painful reactions [284]. These challenges have led to increased research into alloying techniques and surface coatings that can control the degradation rate of magnesium alloys, allowing them to be safely used in clinical practice while minimizing adverse reactions.
Given that metals are generally not as hard as ceramics, they are typically avoided for use in sliding surfaces, such as in the femoral head and the acetabular cup, where wear resistance is crucial. The primary exception to this rule is cobalt–chromium alloys, which are selected for their higher hardness and wear resistance [285]. Metal-on-metal (MoM) implants are becoming increasingly less common due to the risks associated with the generation of dangerous wear debris [286]. Wear debris can also form at any interface between metallic components, such as in the joints of modular implants [287], or at the interface between different components in modular implants [288]. This debris can not only cause adverse biological reactions but also compromise the mechanical stability of the implant, leading to potential mechanical failure [289].
Metallic wear debris can interact with the biological environment in various ways and is generally considered to be more biologically hazardous than ceramic particles due to its higher reactivity. Potential effects include the following:
Inducing Inflammation: Particles can trigger the immune system, leading to inflammation and soft tissue damage. Metal debris and released ions can be recognized by the body as foreign or harmful, activating immune cells like macrophages [290]. Macrophages engulf the debris via phagocytosis, but their inability to degrade metal particles leads to a chronic inflammatory response. Activated macrophages release cytokines [291] such as tumor necrosis factor-alpha (TNF-α), interleukin-1β (IL-1β), and interleukin-6 (IL-6), which recruit more immune cells and sustain inflammation [292].
Inducing Granuloma Formation: Wear particles can lead to the formation of granulomas, a type of inflammatory tissue response. As macrophages continue to try to degrade the metallic particles, the constant activation of the immune system leads to chronic inflammation. Other immune cells, such as neutrophils, lymphocytes, and fibroblasts, are recruited to the site of the foreign body [293,294]. Over time, the persistent inflammatory environment causes macrophages to differentiate into multinucleated giant cells [295], which are large cells that are formed when several macrophages fuse together to try to absorb the debris more effectively. Granulomas form as a result of the aggregation of these multinucleated giant cells, macrophages, and surrounding fibroblasts. These cells surround the metallic debris, creating a protective barrier around the foreign material. The granuloma attempts to isolate the harmful material from surrounding healthy tissue, attempting to contain the debris and prevent it from spreading or causing more widespread damage [296,297].
Catalyzing Osteolysis: The release of metal ions and wear particles can promote the resorption of bone tissue, resulting in osteolysis [298,299,300], which may compromise the implant’s fixation and bone health.
Toxicity: Prolonged exposure to high levels of certain metal ions (e.g., chromium, cobalt) can be toxic to the surrounding cells and even organs, particularly in the liver or kidneys, where they tend to accumulate [301,302,303].
Allergic Reactions: Some patients may develop hypersensitivity or allergic reactions to certain metals, especially cobalt and nickel [304,305,306,307], leading to local or systemic effects.
As previously discussed, ions released from metallic implants, particularly bioresorbable ones, can have a positive effect on the surrounding biological environment. It should be noted that, unlike wear debris—which can cause chronic inflammation, tissue damage, and adverse biological reactions due to its persistent and inert nature—the ions released from bioresorbable metals are typically absorbed by the body and gradually integrate into physiological processes.
These ions, such as magnesium (Mg2+), zinc (Zn2+), and iron (Fe2+), can influence various cellular activities in a beneficial way. Despite each ion having unique interactions with the biological environment, there are several common pathways through which these ions exert their effects. Ions released from bioresorbable implants tend to have a direct effect on osteoblastic and osteoclastic differentiation and activity [308,309,310], promoting bone formation while modulating bone resorption. Specifically, magnesium has been shown to stimulate osteoblast differentiation and mineralization, while zinc plays a critical role in collagen synthesis, which is essential for bone matrix formation. Iron, in low concentrations, can promote osteoblast activity and angiogenesis, facilitating bone tissue regeneration [311]. Moreover, these ions can substitute calcium in hydroxyapatite crystals, directly participating in the mineralization process and enhancing the strength, elasticity, and density of newly formed bone tissue [202,312,313,314]. Furthermore, these ions can help modulate the inflammatory response, reducing excessive inflammation and promoting a more favorable healing environment [205,315,316]. For instance, magnesium and zinc ions have anti-inflammatory effects, which can mitigate the chronic inflammatory reactions that are often associated with traditional metal implants and improve overall tissue integration. These multifaceted actions make bioresorbable metals highly attractive for use in bone repair, as they actively support both the healing process and the integration of the implant with the surrounding tissue. The controlled release of these ions can also contribute to the prevention of infection and accelerate tissue repair, making bioresorbable metallic implants highly attractive for use in orthopedic and dental applications. Moreover, as the material gradually resorbs, it avoids the need for a second surgery to remove the implant, further reducing the risk of complications.
In contrast, ions released from bio-inert alloys tend to be seen as a danger to avoid. In most cases, elements like aluminum (Al) and vanadium (V) in titanium alloys (such as Ti6Al4V) or nickel (Ni) in stainless steel alloys (such as AISI 316L) have shown cytotoxic effects, triggering immune responses and potentially causing tissue damage. These ions often accumulate in tissues, creating long-term, adverse biological impacts. This starkly contrasts with bioresorbable metals, where the entire implant is designed to release ions that either have no harmful interaction with the body or interact positively, promoting healing and regeneration. The ability of bioresorbable alloys to release ions that are absorbed and expelled from the body or have beneficial effects further distances these materials from bio-inert alloys. This makes bioresorbable implants a promising solution in the medical field, as they avoid the risks associated with the release of toxic ions from non-degradable metals while actively contributing to tissue healing and promoting biocompatibility.
10. Coatings and Surface Modifications for Metallic Orthopedic Materials
Surface modification techniques have been developed to enhance the interactions between metallic implants and the surrounding tissues, promoting osseointegration and minimizing the risk of infection. Several surface treatments are commonly employed:
Plasma Spray Coatings: A common method for improving the biocompatibility of implants, plasma spraying involves coating the metal with materials like hydroxyapatite (HA). This mimics the composition of the mineral fraction of bone and encourages better integration with the surrounding tissues [317].
Hard Anodizing: This is a treatment that is applied mainly to titanium and titanium alloys. The process creates a thick, hard oxide layer on the surface of the metal, which increases wear resistance and provides a protective barrier against corrosion. This is especially useful in knee and hip joint replacements, where wear resistance is critical [318].
Nitriding: The process of nitriding involves introducing nitrogen into the surface layer of a metal, usually titanium or steel, to form titanium nitride (TiN) or steel nitrides. This enhances corrosion resistance, fatigue strength, and wear resistance [319,320].
Plasma-Immersion Ion Implantation (PIII): This technique uses plasma to introduce ions such as nitrogen or carbon into the surface of metals, resulting in improved surface hardness, wear resistance, and corrosion resistance [321,322].
Laser Surface Treatment: A laser beam is used to modify the surface of metals like titanium, enhancing their mechanical properties and biocompatibility. This process can also be used to create textured surfaces that improve cell adhesion and osseointegration.
Physical Vapor Deposition (PVD) Coatings: PVD is a widely used technique for applying thin, durable coatings on metallic surfaces, such as titanium, stainless steel, and cobalt–chromium alloys. This process involves the vaporization of a material in a vacuum environment, which is then deposited onto the implant surface. PVD coatings, such as titanium nitride (TiN), zirconium, and diamond-like carbon (DLC) [323], improve the wear resistance, corrosion resistance, and biocompatibility of the implant. These coatings also provide a smooth, non-porous surface that reduces friction and enhances tissue integration [319,324].
Sol–Gel Coating: A wet chemical process where a colloidal solution (sol) undergoes gelation and is deposited onto the implant. This method allows for the incorporation of bioactive ceramics like hydroxyapatite and bioactive glass at low temperatures, forming uniform, thin coatings that enhance biocompatibility and osseointegration [325,326].
Electrophoretic Deposition (EPD): This electrochemical method applies a charged suspension of bioactive particles, such as hydroxyapatite or biopolymers, onto a conductive implant under an electric field. EPD enables the formation of uniform, porous coatings with precise thickness control, enhancing surface bioactivity and facilitating drug or growth factor delivery [327,328].
Chemical Vapor Deposition (CVD) Coatings: A process in which gaseous precursors react on the implant surface to form a thin, uniform coating. CVD is commonly used for diamond-like carbon (DLC) and titanium dioxide (TiO2) coatings, which significantly improve surface hardness, wear resistance, and hemocompatibility, making them ideal for cardiovascular and orthopedic applications [329,330].
Hydrothermal Coating: A method where implants are immersed in a high-temperature, high-pressure aqueous solution to deposit bioactive layers such as hydroxyapatite. This technique results in well-adhered, highly crystalline coatings that enhance bone bonding while avoiding the thermal degradation associated with plasma spraying [331,332].
Dip Coating: A simple and cost-effective technique where the implant is submerged in a coating solution and withdrawn at a controlled rate to form a thin, uniform film. Dip coating is often combined with sol–gel processing or polymer-based coatings to create multilayered bioactive surfaces [333,334].
Laser Cladding: A surface modification technique that uses a high-power laser to fuse coating materials, such as hydroxyapatite or titanium alloys, onto the implant surface. This process produces strong, wear-resistant coatings with excellent adhesion while preserving the bulk properties of the implant [335,336].
Coatings, in particular, play a crucial role in enhancing the performance of metallic orthopedic materials, addressing challenges such as wear resistance, biocompatibility, and osseointegration. Broadly, coating strategies can be categorized into two main types: hard coatings, which improve wear resistance and durability, and bioactive coatings, which promote bone integration and enhance biocompatibility (Table 3). Each category has specific applications and challenges, largely dependent on the substrate material and the mechanical and chemical interactions between the coating and the surrounding biological environment.

Table 3.
Coating strategies for metallic biomaterials in orthopedic applications, categorized into hard coatings for tribological applications and bioactive coatings for enhancing biocompatibility.
10.1. Hard Coatings for Wear Resistance
Hard coatings are primarily used to enhance the tribological performance of metallic implants by reducing friction, wear, and surface degradation. These coatings are commonly applied to load-bearing components such as joint replacements, where mechanical wear can lead to implant failure over time. Key materials used for hard coatings include titanium nitride (TiN), chromium nitride (CrN), diamond-like carbon (DLC), titanium oxide, and zirconium oxide coatings, although a wider range of transition metal carbides, nitrides, and oxides can also be utilized for this purpose, as long as they are hard, chemically stable, and adhere to the substrate they are applied to.
Different metallic substrates, such as stainless steel, titanium alloys, cobalt–chromium–molybdenum (CoCrMo) alloys, and zirconium, benefit from hard coatings:
Stainless Steel: Hard coatings are employed to enhance wear resistance, particularly in load-bearing implants. However, achieving long-term durability and ensuring biocompatibility remain challenges, and these implants are avoided when possible.
Titanium Alloys: Various coatings can be used to improve wear resistance in joint replacements by reducing friction, but titanium cannot be used in metal-on-metal articular joints, due to its low elastic modulus.
CoCrMo Alloys: Used extensively in hip and knee implants, these alloys benefit from hard coatings that further enhance wear resistance and reduce friction. However, long-term performance depends on the adhesion of the coating to the substrate.
Zirconium: Zirconium oxide and other hard coatings are applied to enhance durability; however, their high cost and the relative rarity of zirconium in orthopedic applications limit their widespread use.
A critical challenge for hard coatings, particularly on titanium, is the mismatch in elastic modulus between the coating and the underlying metal. If the coating is significantly stiffer than the substrate, mechanical stresses can cause delamination, cracking, or early failure of the coating. This is particularly relevant for ceramic-based coatings, which, despite their high wear resistance, are prone to fracture under repetitive loading when applied to metals with lower stiffness.
While hard coatings are often used to improve wear resistance in orthopedic applications, they are also employed in non-orthopedic implants, such as stents and artificial heart valves, to prevent unwanted biological interactions. However, as these applications fall outside the scope of this review, they will not be discussed further here.
10.2. Bioactive Coatings for Enhancing Osseointegration
Unlike hard coatings, bioactive coatings are designed to interact favorably with biological tissues, promoting bone integration and improving implant stability. The most commonly used bioactive coatings include hydroxyapatite (HA), calcium phosphate (CaP), bioactive glass, magnesium hydroxide, zinc oxide, and other biodegradable materials, and their choice is independent of the material used for the substrate. These coatings are particularly important for implants where osseointegration is critical, such as bone fixation devices and load-bearing orthopedic implants.
A key limitation of bioactive coatings is adhesion strength, as coatings must remain attached to the implant surface while undergoing degradation in vivo. Strategies such as plasma spraying, sol–gel deposition, and biomimetic mineralization have been developed to improve adhesion and ensure controlled release of bioactive ions to enhance bone healing.
11. Additive Manufacturing
One of the most important developments in orthopedic implants is the use of 3D printing (additive manufacturing) to create metal components with precisely controlled morphology and mechanical properties. This technology allows for the fabrication of complex structures that enhance osseointegration, reduce stress shielding, and improve overall implant performance. Metals like titanium, cobalt–chromium, and even bioresorbable alloys can be processed to create porous implants optimized for specific anatomical and functional requirements.
The introduction of additive manufacturing has revolutionized the ways in which implants are designed and fabricated, offering several key advantages:
Customizability [364]: Implants can be tailored to the specific anatomy of the patient, ensuring a better fit and improved surgical outcomes.
Porosity Control [365]: The ability to create highly porous structures mimicking the bone architecture promotes osseointegration, enhances mechanical stability, and allows for nutrient flow.
Optimized Material Distribution [366]: Three-dimensional (3D) printing enables weight reduction without compromising mechanical strength, making implants lighter and more efficient.
Geometric Freedom [367]: Unlike conventional manufacturing, additive techniques allow for the creation of intricate and functionally graded structures that would be impossible to achieve through subtractive or formative methods.
Before the advent of 3D printing, porous materials for orthopedic applications were created by sintering metal beads [368] or by foaming processes [369], but these techniques often resulted in limited control over the pore structure, porosity, interconnectivity, and uniformity, which are crucial for optimizing tissue integration and mechanical properties in implants.
While these methods have been effective, they lack the precision and design flexibility afforded by 3D printing. Additive manufacturing allows for the optimization of pore size, shape, and interconnectivity, leading to improved mechanical and biological performance.
A major advancement enabled by 3D printing is the use of sophisticated geometric structures that optimize mechanical properties and biological performance. Among these, lattice structures and Triply Periodic Minimal Surface (TPMS) designs have garnered significant attention.
Lattice structures are periodic arrangements of struts and nodes that provide tailored mechanical properties and enhanced osseointegration. Despite these advantages, lattice structures can exhibit limitations, such as stress concentrations at node junctions, which may lead to fatigue failure [370].
An alternative to traditional strut-based lattices is the use of Triply Periodic Minimal Surfaces (TPMSs) [371]. TPMSs are mathematically defined surfaces that exhibit zero mean curvature throughout their structure, resulting in smooth, interconnected networks. This inherent smoothness offers several advantages over conventional lattice structures, particularly for biomedical applications. The continuous, curvature-driven nature of TPMSs minimizes sharp corners and node junctions, thereby significantly reducing stress concentrations and improving fatigue resistance, which is crucial for load-bearing implants, where long-term durability is essential. Furthermore, the interconnected pore network of TPMSs facilitates improved fluid flow and nutrient transport, which are vital for tissue ingrowth and vascularization, and especially relevant for bone scaffolds, where efficient nutrient delivery promotes osseointegration [372]. TPMS structures also offer precise control over surface area and pore size distribution, allowing for the optimization of cell adhesion, proliferation, and differentiation. This tailored porosity is essential for mimicking the natural bone microenvironment. The smooth, continuous morphology of TPMSs can better mimic the trabecular bone structure, leading to improved biomechanical compatibility and reduced stress shielding. Finally, TPMS structures can be designed to exhibit more isotropic mechanical properties compared to traditional lattice structures, which often display anisotropic behavior due to their directional struts. This isotropy is beneficial for ensuring uniform load distribution and minimizing the risk of implant failure. Therefore, while lattice structures offer valuable benefits, TPMS structures provide a promising alternative with enhanced performance characteristics for biomedical applications, particularly in terms of reducing stress concentrations, improving fluid flow, and promoting tissue integration.
12. Novel Alloys’ Composition
Over recent decades, there have been significant advancements in the use of metals for orthopedic implants, driven by innovations in materials science and fabrication techniques. Modern trends aim to improve the performance, longevity, and biocompatibility of orthopedic devices, addressing some of the limitations seen with traditional materials. Key developments include the use of advanced manufacturing methods such as additive manufacturing, advanced surface treatments, and novel alloy compositions, all of which contribute to enhanced implant functionality and patient outcomes.
Advances in alloy development have led to the creation of new metal compositions that offer superior properties for orthopedic applications:
Beta-Titanium Alloys: These alloys, which contain less aluminum than traditional Ti6Al4V, have a lower elastic modulus more similar to that of bone. This reduces the risk of stress shielding, where the implant takes on too much of the load, leading to bone resorption. Some attempts have been made to achieve a Young’s modulus very close to that of bone [373].
CoCr Alloys: Some studies have been carried out to add Cu or Ag as an alloying element to increase the antibacterial properties of the alloy and, thus, reduce the risk of inflammation of implanted prosthetics, particularly for dental implants [374,375].
Mg Alloys: Mg alloys have been studied with the addition of RE (rare-earth) elements in order to decrease the degradation kinetics, which is one of the limiting properties of these alloys [376]. Some studies have also been conducted by adding Yb, Au, and B for the aforementioned purposes [377].
Despite these advancements, several challenges remain in the field of metal-based orthopedic implants. While research continues to improve the performance of metals and alloys, certain issues persist, including the difficulty in eliminating harmful elements, understanding biological responses to metals, and achieving optimal mechanical properties for implants.
Substitution of Potentially Harmful Elements (e.g., Ti6Al4V): Ti6Al4V, one of the most widely used titanium alloys in orthopedic implants, contains aluminum and vanadium, both of which can have toxic effects on the body, particularly with long-term exposure. There is ongoing research into titanium alloys that minimize or eliminate these potentially harmful elements, using substitutes such as niobium or zirconium, which are believed to offer safer, more biocompatible alternatives. However, finding alloys that match the strength, biocompatibility, and manufacturing feasibility of Ti6Al4V remains a significant challenge.
Unpredictable Allergic Responses: While metals like nickel and cobalt have long been associated with allergic reactions, there is still limited understanding of why some patients exhibit hypersensitivity to metals used in orthopedic implants. This is particularly concerning for cobalt–chromium alloys, which, despite their excellent mechanical properties, can trigger immune responses in a subset of patients. More research is needed to better understand these allergic reactions, improve diagnostic techniques, and develop metal alloys that are less likely to cause such responses.
Reducing the Elastic Modulus to Match That of Bone: One of the persistent issues with metal-based implants is the stiffness mismatch between the implant and bone. Most metals, such as titanium and cobalt–chromium alloys, have a much higher elastic modulus compared to natural bone, leading to stress shielding—a phenomenon where the bone around the implant becomes weaker due to reduced mechanical loading. Efforts to develop alloys with lower elastic moduli (e.g., beta-titanium alloys) have made some progress, but a perfect match to bone stiffness is still elusive.
Fretting and Wear: Fretting is the damage that occurs at the interface between two metal surfaces that are subjected to small, oscillatory movements. It is particularly problematic in modular implants, where parts of the implant rub against each other, particularly for Ti alloys. The wear caused by fretting can lead to corrosion, the release of metal ions, and the formation of harmful particles that can lead to tissue damage and implant failure, usually due to fatigue assisted by corrosion. This issue is difficult to predict and mitigate, as even small movements can cause significant damage over time. Solutions such as surface coatings and better joint design are being explored to reduce fretting wear.
Corrosion and Ion Release: Corrosion is another significant challenge for metallic implants, especially in the case of long-term implant survival (Figure 5). Localized corrosion, such as pitting or crevice corrosion, can occur in implants made from materials like stainless steel or cobalt–chromium alloys. The release of metal ions into the body can cause local or systemic effects, such as inflammation, allergic reactions, or even toxicity. Advanced coating techniques, such as plasma spraying or anodizing, are being developed to improve the corrosion resistance of metals, but ensuring long-term stability remains a challenge. Some attempts are also being made to increase the antibacterial properties of the implants by means of the use of alloying elements such as Ag or Cu.
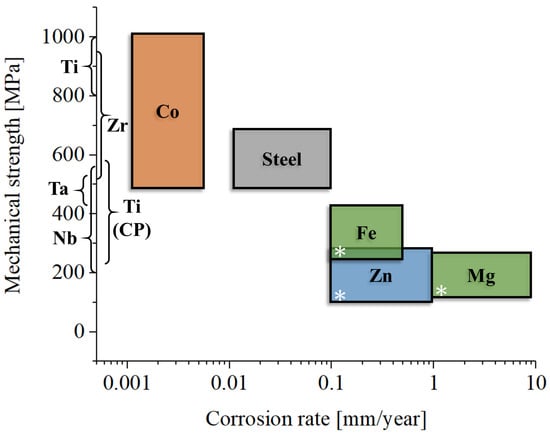
Figure 5.
Mechanical strength as a function of representative corrosion rate for the different metallic materials used in orthopedics.
Implant Longevity and Fatigue Failure: Implants are subjected to continuous loading over time, leading to fatigue failure. While metals like titanium and cobalt–chromium alloys are designed to withstand high stress, over time, small cracks can form and propagate, eventually leading to implant fracture. Improving the fatigue resistance of these materials, particularly in dynamic load-bearing applications, remains an important area of research. Some attempts to increase the fatigue life are being researched in terms of the modification of mechanical properties, with the main target being to increase the tensile strength or corrosion resistance of the material, due to the fact that fatigue failure in implants usually originates from a previous corrosion process. Lastly, surface modification processes are used to increase both the corrosion resistance and fatigue properties of materials.
13. Conclusions
The development of metallic materials for orthopedic implants has advanced significantly, driven by innovations in materials science and an improved understanding of biological interactions. Traditional metals such as titanium, stainless steel, and cobalt–chromium alloys continue to dominate the field, but emerging technologies, including 3D printing, bioresorbable alloys, and smart materials, hold the potential to revolutionize implant design and function. These innovations offer promising solutions to improve implant performance, enhance osseointegration, reduce wear debris, and minimize adverse biological responses.
However, several challenges remain that need to be addressed for continued advancement in this field:
Material Optimization: The optimization of material properties, particularly in matching the elastic modulus of bone, remains a critical challenge. While some alloys have been developed to reduce this mismatch, achieving a perfect balance between strength, flexibility, and osseointegration is still elusive.
Toxicity and Biocompatibility: There is an ongoing concern regarding the potential toxicity of alloying elements in metallic biomaterials. The elimination of potentially harmful elements, such as nickel or aluminum, is crucial for improving the long-term biocompatibility of implants.
Biological Responses: Our understanding of the complex biological responses to metal exposure is still incomplete. This knowledge gap hinders the development of more effective materials that can interact favorably with the body, promoting healing while minimizing adverse effects.
Coatings and Surface Treatments: The development of novel coatings and surface treatments is essential for enhancing the performance of metallic implants. Future research should focus on the development of coatings that not only improve wear resistance but also promote better biocompatibility and long-term stability.
Despite the evolution of metallic orthopedic materials over the last few decades, a few key questions are still partially unanswered:
How can we better understand and control the biological responses to the metal ions and particles released from implants, particularly with respect to inflammation, immune response, and long-term tissue integration?
What are the most effective strategies for reducing the elastic modulus of metallic implants without compromising mechanical strength and durability?
How can we balance the customizability options offered by additive manufacturing with the need for standardization to ensure both personalized, patient-specific implants and consistent clinical outcomes?
What strategies could we utilize to discover and test bioresorbable alloys, given that the current approaches are often inconsistent, to ensure that their mechanical integrity and controlled degradation align with tissue healing rates?
Future research should prioritize tissue regeneration rather than substitution. However, research on bioresorbable alloys is still in its early stages, with limited applicability to real-world clinical situations.
Author Contributions
Conceptualization, E.M.; methodology, E.M. and A.L.; validation, E.M. and A.L.; resources, E.M. and A.L.; data curation, E.M. and A.L.; writing—original draft preparation, E.M.; writing—review and editing, E.M. and A.L.; visualization, A.L.; supervision, E.M. and A.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
The original contributions presented in the study are included in the article, further inquiries can be directed to the corresponding authors.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Szczęsny, G.; Kopec, M.; Politis, D.J.; Kowalewski, Z.L.; Łazarski, A.; Szolc, T. A review on biomaterials for orthopaedic surgery and traumatology: From past to present. Materials 2022, 15, 3622. [Google Scholar] [CrossRef] [PubMed]
- Barreto, M.; Srivastava, S.; Mittal, H. Design, materials and biomechanics of orthopaedic implants: A narrative review. J. Clin. Diagn. Res. 2024, 18, 1. [Google Scholar] [CrossRef]
- Mahyudin, F.; Widhiyanto, L.; Hermawan, H. Biomaterials in Orthopaedics. In Advanced Structured Materials; Springer International Publishing: Cham, Switzerland, 2016; pp. 161–181. [Google Scholar]
- Zieliński, A.; Sobieszczyk, S.; Seramak, T.; Serbiński, W.; Świeczko-Żurek, B.; Ossowska, A. Biocompatibility and bioactivity of load-bearing metallic implants. Adv. Mater. Sci. 2010, 10, 21–31. [Google Scholar] [CrossRef]
- Cacopardo, L. Biomaterials and Biocompatibility. In Human Orthopaedic Biomechanics; Elsevier: Amsterdam, The Netherlands, 2022; pp. 341–359. [Google Scholar]
- Geringer, J.; Forest, B.; Combrade, P. Wear analysis of materials used as orthopaedic implants. Wear 2006, 261, 971–979. [Google Scholar] [CrossRef]
- Geringer, J.; Forest, B.; Combrade, P. Fretting-corrosion of materials used as orthopaedic implants. Wear 2005, 259, 943–951. [Google Scholar] [CrossRef]
- Gibon, E.; Amanatullah, D.F.; Loi, F.; Pajarinen, J.; Nabeshima, A.; Yao, Z.; Hamadouche, M.; Goodman, S.B. The biological response to orthopaedic implants for joint replacement: Part I: Metals. J. Biomed. Mater. Res. B Appl. Biomater. 2017, 105, 2162–2173. [Google Scholar] [CrossRef]
- Ambrose, C.G.; Hartline, B.E.; Clanton, T.O.; Lowe, W.R.; McGarvey, W.C. Polymers in Orthopaedic Surgery. In Advanced Polymers in Medicine; Springer International Publishing: Cham, Switzerland, 2015; pp. 129–145. [Google Scholar]
- Hannouche, D.; Hamadouche, M.; Nizard, R.; Bizot, P.; Meunier, A.; Sedel, L. Ceramics in total hip replacement. Clin. Orthop. Relat. Res. 2005, 430, 62–71. [Google Scholar] [CrossRef]
- Evans, S.L.; Gregson, P.J. Composite technology in load-bearing orthopaedic implants. Biomaterials 1998, 19, 1329–1342. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, V.M.; Buckwalter, J.A.; Hayes, W.C.; Koval, K.J. Orthopaedic challenges in an aging population. Instr. Course Lect. 1997, 46, 417–422. [Google Scholar]
- Chen, Q.; Thouas, G.A. Metallic implant biomaterials. Mater. Sci. Eng. R. Rep. 2015, 87, 1–57. [Google Scholar] [CrossRef]
- Swenson, S.; Neto, M.Q.; Hall, D.J.; Pourzal, R.; Kohler, J.; Buckwalter, J. Retrieval of a Lane plate 82 years after implantation: Case report, metallurgical analysis, and historical review. Iowa Orthop. J. 2023, 43, 37–43. [Google Scholar] [PubMed]
- Mahalingam, K.; Reidy, D. Smith-Petersen vitallium mould arthroplasty: A 45-year follow-up. J. Bone Jt. Surg. Br. Vol. 1996, 78, 496–497. [Google Scholar] [CrossRef]
- Marin, E.; Boschetto, F.; Pezzotti, G. Biomaterials and biocompatibility: An historical overview. J. Biomed. Mater. Res. A 2020, 108, 1617–1633. [Google Scholar] [CrossRef] [PubMed]
- Linder, L.; Carlsson, A.; Marsal, L.; Bjursten, L.M.; Branemark, P.I. Clinical aspects of osseointegration in joint replacement: A histological study of titanium implants. J. Bone Jt. Surg. Br. 1988, 70-B, 550–555. [Google Scholar] [CrossRef] [PubMed]
- Shega, F.D.; Zhang, H.; Manini, D.R.; Tang, M.; Liu, S. Comparison of effectiveness between cobalt chromium rods versus titanium rods for treatment of patients with spinal deformity: A systematic review and meta-analysis. Adv. Orthop. 2020, 2020, 8475910. [Google Scholar] [CrossRef] [PubMed]
- Shepperd, J.A.N.; Apthorp, H. A contemporary snapshot of the use of hydroxyapatite coating in orthopaedic surgery. J. Bone Jt. Surg. Br. 2005, 87, 1046–1049. [Google Scholar] [CrossRef]
- Drnovšek, N.; Novak, S.; Dragin, U.; Čeh, M.; Gorenšek, M.; Gradišar, M. Bioactive glass enhances bone ingrowth into the porous titanium coating on orthopaedic implants. Int. Orthop. 2012, 36, 1739–1745. [Google Scholar] [CrossRef] [PubMed]
- Marin, E. Forged to heal: The role of metallic cellular solids in bone tissue engineering. Mater. Today Bio 2023, 23, 100777. [Google Scholar] [CrossRef]
- Marin, E.; Basoli, V. From Ancient Remedies to Modern Contraptions: Tracing the Evolution of Biocompatible Materials. In Engineering Methodologies for Medicine and Sports; Springer Nature: Cham, Switzerland, 2024; pp. 313–326. [Google Scholar]
- Marino, R., Jr. Preconquest Peruvian neurosurgeons: A study of Inca and pre-Columbian trephination and the art of medicine in ancient Peru. Neurosurgery 2001, 49, 477–478. [Google Scholar]
- Demann, E.T.K.; Stein, P.S.; Haubenreich, J.E. Gold as an implant in medicine and dentistry. J. Long. Term. Eff. Med. Implants 2005, 15, 687–698. [Google Scholar] [CrossRef]
- Lansdown, A.B.G. Silver in health care: Antimicrobial effects and safety in use. Curr. Probl. Dermatol. 2006, 33, 17–34. [Google Scholar] [PubMed]
- Muffly, T.M.; Tizzano, A.P.; Walters, M.D. The history and evolution of sutures in pelvic surgery. J. R. Soc. Med. 2011, 104, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Flood, G. Introduction. In The Oxford History of Hinduism; Oxford University Press: Oxford, UK, 2020; pp. 1–32. [Google Scholar]
- Tewari, R. The origins of iron working in India: New evidence from the Central Ganga Plain and the Eastern Vindhyas. Antiquity 2003, 77, 536–544. [Google Scholar] [CrossRef]
- Zuo, K.J.; Olson, J.L. The evolution of functional hand replacement: From iron prostheses to hand transplantation. Plast. Surg. 2014, 22, 44–51. [Google Scholar] [CrossRef]
- Neuhaus, V. Götz von Berlichingen; Handbuch, G., Metzler, J.B., Eds.; Springer: Stuttgart, Germany, 1996; pp. 78–99. [Google Scholar]
- Cohn, H.J. Götz von Berlichingen and the Art of Military Autobiography. In War, Literature and the Arts in Sixteenth-Century Europe; Palgrave Macmillan: London, UK, 1989; pp. 22–40. [Google Scholar]
- Putti, V. Historical prostheses. 1925. J. Hand Surg. Br. 2005, 30, 310–325. [Google Scholar] [CrossRef]
- Sellegren, K.R. An Early History of Lower Limb Amputations and Prostheses. The Iowa 1982, 2, 13–27. [Google Scholar]
- Duffy Hancock, J. The Evolution of Artificial Limbs; University of Louisville School of Medicine: Louisville, KY, USA, 1929. [Google Scholar]
- An Account of a New Method of Treating Diseases of the Joints of the Knee and Elbow: In a Letter to Mr. Percival Pott. 1783. Available online: https://wellcomecollection.org/works/k8prmsey (accessed on 12 February 2025).
- Bican, O.; Kahl, L.K. History of hip surgery. In Essentials in Total Hip Arthroplasty; CRC Press: Boca Raton, FL, USA, 2024; pp. 95–101. [Google Scholar]
- Di Matteo, B.; Tarabella, V.; Filardo, G.; Viganò, A.; Tomba, P.; Marcacci, M. John Rhea Barton: The birth of osteotomy. Knee Surg. Sports Traumatol. Arthrosc. 2013, 21, 1957–1962. [Google Scholar] [CrossRef] [PubMed]
- Cumston, C.G. Spontaneous dislocation of the hip-joint following acute infectious diseases. Boston Med. Surg. J. 1910, 163, 684–688. [Google Scholar] [CrossRef]
- Myers, K.R.; Sgaglione, N.A.; Grande, D.A. Trends in biological joint resurfacing. Bone Jt. Res. 2013, 2, 193–199. [Google Scholar] [CrossRef]
- Marshall, D.A.; Pykerman, K.; Werle, J.; Lorenzetti, D.; Wasylak, T.; Noseworthy, T.; Dick, D.A.; O’Connor, G.; Sundaram, A.; Heintzbergen, S.; et al. Hip resurfacing versus total hip arthroplasty: A systematic review comparing standardized outcomes. Clin. Orthop. Relat. Res. 2014, 472, 2217–2230. [Google Scholar] [CrossRef]
- Levert, H.S. Experiments on the use of Metallic Ligatures, as applied to Arteries. Am. J. Med. Sci. 1829, 7, 17–22. [Google Scholar] [CrossRef]
- Lister, J. An address on the treatment of fracture of the patella. BMJ 1883, 2, 855–860. [Google Scholar] [CrossRef] [PubMed]
- Guyuron, B.; Vasconez, H.C. Basic Principles of Bone Fixation. In Fundamentals of Maxillofacial Surgery; Springer: New York, NY, USA, 1997; pp. 169–185. [Google Scholar]
- Poon, V.K.M.; Burd, A. In vitro cytotoxity of silver: Implication for clinical wound care. Burns 2004, 30, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Coogan, T.P.; Latta, D.M.; Snow, E.T.; Costa, M. Toxicity and carcinogenicity of nickel compounds. Crit. Rev. Toxicol. 1989, 19, 341–384. [Google Scholar] [CrossRef]
- Affatato, S. Perspectives in Total Hip Arthroplasty: Advances in Biomaterials and Their Tribological Interactions; Woodhead Publishing: New Delhi, India, 2014. [Google Scholar]
- Eynon-Lewis, N.J.; Ferry, D.; Pearse, M.F. Themistocles Gluck: An unrecognised genius. BMJ 1992, 305, 1534–1536. [Google Scholar] [CrossRef]
- Greenhagen, R.M.; Johnson, A.R.; Joseph, A. Internal fixation: A historical review. Clin. Podiatr. Med. Surg. 2011, 28, 607–618. [Google Scholar] [CrossRef]
- Lecture, W.-J. Some landmarks in the surgery of the rheumatic diseases. Ann. R. Coll. Surg. Engl. 1978, 61, 29. [Google Scholar]
- Jones, A.R. Sir William arbuthnot Lane. J. Bone Jt. Surg. Br. 1952, 34-B, 478–482. [Google Scholar] [CrossRef]
- Lane, W.A. Some remarks on the treatment of fractures. Br. Med. J. 1895, 1, 861–863. [Google Scholar] [CrossRef]
- Afshar, A.; Steensma, D.P.; Kyle, R.A. Albin lambotte: Pioneer of osteosynthesis (bone fixation). Mayo Clin. Proc. 2021, 96, 2012–2013. [Google Scholar] [CrossRef]
- Groves, E.W.H. An experimental study of the operative treatment of fractures. Br. J. Surg. 1913, 1, 438–501. [Google Scholar] [CrossRef]
- Key, J.A. Stainless steel and vitallium in internal fixation of bone. Arch. Surg. 1941, 43, 615. [Google Scholar] [CrossRef]
- Laing, P.G. Problems in the use of metals as surgical implants. J. Dent. Res. 1966, 45, 1660–1661. [Google Scholar] [CrossRef]
- Menegau, G.; Odiette, D. Growth of Connective Tissues of Bone in Tissue Cultures in the Presence of Certain Metals; Kanavel, A.B., Moynthan, L., Duval, P., Eds.; International Abstract of Surgery (supplementary to Surgery, Gynecology and Obstetrics): Chicago, IL, USA, 1936; p. 362. [Google Scholar]
- Jones, L. Interaction of bone and various metals. Arch. Surg. 1936, 32, 990. [Google Scholar] [CrossRef]
- Haas, W. “Rustfree” steel in surgery. Arch. f Orthop. u Unfall-Chir. 1936, 37, 606. [Google Scholar]
- Orsos, E. The current generated by bone sutures. Zentralbl Chir. 1925, 56, 1014. [Google Scholar]
- Perves, G.D.J. The importance of the electrolytic factor in osteosynthesis. Mém Acad. Chir. 1938, 64, 650. [Google Scholar]
- Masmonteil, F. The tolerance of bone for metallc foreign bodies. Presse Med. 1935, 43, 1935. [Google Scholar]
- Sherman, W.O. Treatment of compound fractures. Arch. Surg. 1940, 40, 838. [Google Scholar] [CrossRef]
- CS37-31; Steel Bone Plates and Screws. United States Department of Commerce, Bureau of Standards: Washington, DC, USA, 1930.
- Hudack, S. High chromium, low nickel steel in the operative fixation of fractures. Arch. Surg. 1940, 40, 867. [Google Scholar] [CrossRef]
- Lange, M. Krupp Steel Wire as Bone Suture Material. Ztschr f Orthop. Chir. 1926, 58, 1–9. [Google Scholar]
- Desch, C.H. Mr. H. brearley. Nature 1948, 162, 288. [Google Scholar] [CrossRef]
- Spires, W.P., Jr.; Kelman, D.C.; Pafford, J.A. Mechanical Evaluation of ASTM F75 Alloy in Various Metallurgical Conditions. In Quantitative Characterization and Performance of Porous Implants for Hard Tissue Applications; 100 Barr Harbor Drive, PO Box C700, Conshohocken, PA 19428-295; ASTM International: West Conshohocken, PA, USA, 1987; pp. 47–59. [Google Scholar]
- Hernigou, P.; Pariat, J. History of internal fixation (part 1): Early developments with wires and plates before World War II. Int. Orthop. 2017, 41, 1273–1283. [Google Scholar] [CrossRef] [PubMed]
- Witte, F. The history of biodegradable magnesium implants: A review. Acta Biomater. 2010, 6, 1680–1692. [Google Scholar] [CrossRef] [PubMed]
- Festas, A.J.; Ramos, A.; Davim, J.P. Medical devices biomaterials—A review. Proc. Inst. Mech. Eng. L J. Mater. Des. Appl. 2020, 234, 218–228. [Google Scholar] [CrossRef]
- Hernigou, P. Smith-Petersen and early development of hip arthroplasty. Int. Orthop. 2014, 38, 193–198. [Google Scholar] [CrossRef]
- Venable, C.S.; Stuck, W.G.; Beach, A. The effects on bone of the presence of metals: Based upon electrolysis. Ann. Surg. 1937, 105, 917–938. [Google Scholar] [CrossRef]
- Stuck, W.G. Electrolytic destruction of bone caused by metal fixation devices. J. Bone Jt. Surg. 1937, 19, 1077–1080. [Google Scholar]
- Stuck, C.V.W. Fractures: Recent advances in treatment with non-electrolytic metal appliances. J. Indiana M. A 1938, 31, 335. [Google Scholar]
- Venable, C.S. Application of neutral metal in fractures. South. Surg. 1939, 8, 456. [Google Scholar]
- Roberts, P.; Grigoris, P. Metal on Metal Articulation in Total Hip Replacement. In Interfaces in Total Hip Arthroplasty; Springer: London, UK, 2000; pp. 121–133. [Google Scholar]
- Beksaç, B.; Bek, D.; Miller, A.N.; Salvati, E.A. Thompson hip hemiarthroplasty: Asymptomatic after 44 years a case report. Inj. Extra 2008, 39, 264–266. [Google Scholar] [CrossRef]
- Shen, G. Femoral stem fixation. J. Bone Jt. Surg. Br. 1998, 80, 754–756. [Google Scholar] [CrossRef]
- Dall, D.M.; Learmonth, I.D.; Solomon, M.I.; Miles, A.W.; Davenport, J.M. Fracture and loosening of Charnley femoral stems. Comparison between first-generation and subsequent designs. J. Bone Jt. Surg. Br. 1993, 75, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Ebied, A.; Hoad-Reddick, D.A.; Raut, V. Medium-term results of the Charnley low-offset femoral stem. J. Bone Jt. Surg. Br. 2005, 87, 916–920. [Google Scholar] [CrossRef] [PubMed]
- Munemoto, M.; Grammatopoulos, G.; Tanaka, Y.; Gibbons, M.; Athanasou, N.A. The pathology of failed McKee-Farrar implants: Correlation with modern metal-on-metal-implant failure. J. Mater. Sci. Mater. Med. 2017, 28, 66. [Google Scholar] [CrossRef]
- Brown, S.R.; Davies, W.A.; DeHeer, D.H.; Swanson, A.B. Long-term survival of McKee-Farrar total hip prostheses. Clin. Orthop. Relat. Res. 2002, 402, 157–163. [Google Scholar] [CrossRef]
- Williams, D.F. The Biological Applications of Titanium and Titanium Alloys. In Materials Sciences and Implant Orthopedic Surgery; Springer: Dordrecht, The Netherlands, 1986; pp. 107–116. [Google Scholar]
- Kumar, G.; Narayan, B. Osseointegrated Titanium Implants: Requirements for Ensuring a Long-Lasting, Direct Bone-to-Implant Anchorage in Man. In Classic Papers in Orthopaedics; Springer: London, UK, 2014; pp. 507–509. [Google Scholar]
- Roos, J.; Sennerby, L.; Lekholm, U.; Jemt, T.; Gröndahl, K.; Albrektsson, T. A qualitative and quantitative method for evaluating implant success: A 5-year retrospective analysis of the Brånemark implant. Int. J. Oral. Maxillofac. Implants 1997, 12, 504–514. [Google Scholar] [PubMed]
- Marin, E.; Lanzutti, A. Biomedical applications of titanium alloys: A comprehensive review. Materials 2023, 17, 114. [Google Scholar] [CrossRef]
- Oldani, C.; Dominguez, A. Titanium as a Biomaterial for Implants. In Recent Advances in Arthroplasty; InTech: Rijeka, Croatia, 2012. [Google Scholar]
- Niinomi, M. Mechanical biocompatibilities of titanium alloys for biomedical applications. J. Mech. Behav. Biomed. Mater. 2008, 1, 30–42. [Google Scholar] [CrossRef]
- Bartlo, L.J. Effect of Microstructure on the Fatigue Properties of Ti-6Al-4V bar. In Fatigue at High Temperature; 100 Barr Harbor Drive, PO Box C700, Conshohocken, PA 19428-2959; ASTM International: Conshohocken, PA, USA, 1969; pp. 144–154. [Google Scholar]
- Jackson, J.D.; Boyd, W.K. Crevice Corrosion of Titanium. In Applications Related Phenomena in Titanium Alloys; 100 Barr Harbor Drive, PO Box C700, Conshohocken, PA 19428-2959; ASTM International: Conshohocken, PA, USA, 1968; pp. 218–226. [Google Scholar]
- Bass, C.D.; Harmsworth, C.L. Fatigue Behavior of Titanium Castings. In Proceedings of the ASME 1969 Gas Turbine Conference and Products Show, Cleveland, OH, USA, 9–13 March 1969. [Google Scholar] [CrossRef]
- El Sawy, A.A.; Shaarawy, M.A. Evaluation of metal ion release from Ti6Al4V and Co-Cr-Mo casting alloys: In vivo and in vitro study. J. Prosthodont. 2014, 23, 89–97. [Google Scholar] [CrossRef]
- Richter, M.; Matusiewicz, H. Metals and metal ions release from metallic implants in the animal body models: A review of experimental investigations. World J. Adv. Res. Rev. 2022, 16, 653–676. [Google Scholar] [CrossRef]
- Engelhart, S.; Segal, R.J. Allergic reaction to vanadium causes a diffuse eczematous eruption and titanium alloy orthopedic implant failure. Cutis 2017, 99, 245–249. [Google Scholar] [PubMed]
- Tsaryk, R.; Peters, K.; Barth, S.; Unger, R.E.; Scharnweber, D.; Kirkpatrick, C.J. The role of oxidative stress in pro-inflammatory activation of human endothelial cells on Ti6Al4V alloy. Biomaterials 2013, 34, 8075–8085. [Google Scholar] [CrossRef] [PubMed]
- Hamman, G.; Bardos, D.I. Metallographic Quality Control of Orthopaedic Implants. In Metallography as a Quality Control Tool; Springer: Boston, MA, USA, 1980; pp. 221–245. [Google Scholar]
- Venugopalan, R.; Lucas, L.C. Evaluation of restorative and implant alloys galvanically coupled to titanium. Dent. Mater. 1998, 14, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Semlitsch, M. Titanium alloys for hip joint replacements. Clin. Mater. 1987, 2, 1–13. [Google Scholar] [CrossRef]
- Henriques, V.A.R.; Silva, C.R.M. Production of titanium alloys for medical implants by powder metallurgy. Key Eng. Mater. 2001, 189, 443–448. [Google Scholar] [CrossRef]
- Jablokov, V.R.; Nutt, M.J.; Richelsoph, M.E.; Freese, H.L. The Application of Ti-15Mo Beta Titanium Alloy in High Strength Structural Orthopaedic Applications. In Titanium, Niobium, Zirconium, and Tantalum for Medical and Surgical Applications; 100 Barr Harbor Drive, PO Box C700, Conshohocken, PA 19428-2959; ASTM International: Conshohocken, PA, USA, 2006; pp. 83–100. [Google Scholar]
- Niinomi, M. Low-Modulus Ti Alloys Suitable for Rods in Spinal Fixation Devices. In Interface Oral Health Science 2016; Springer: Singapore, 2017; pp. 3–21. [Google Scholar]
- Qiu, C.; Liu, Q.; Ding, R. Significant enhancement in yield strength for a metastable beta titanium alloy by selective laser melting. Mater. Sci. Eng. A Struct. Mater. 2021, 816, 141291. [Google Scholar] [CrossRef]
- Nastac, L.; Gungor, M.N.; Ucok, I.; Klug, K.L.; Tack, W.T. Advances in investment casting of Ti–6Al–4V alloy: A review. Int. J. Cast. Met. Res. 2006, 19, 73–93. [Google Scholar] [CrossRef]
- McNaney, J.M.; Imbeni, V.; Jung, Y.; Papadopoulos, P.; Ritchie, R.O. An experimental study of the superelastic effect in a shape-memory Nitinol alloy under biaxial loading. Mech. Mater. 2003, 35, 969–986. [Google Scholar] [CrossRef]
- Chaudhari, R.; Vora, J.J.; Parikh, D.M. A Review on Applications of Nitinol Shape Memory Alloy. In Recent Advances in Mechanical Infrastructure; Springer: Singapore, 2021; pp. 123–132. [Google Scholar]
- Alipour, S.; Taromian, F.; Ghomi, E.R.; Zare, M.; Singh, S.; Ramakrishna, S. Nitinol: From historical milestones to functional properties and biomedical applications. Proc. Inst. Mech. Eng. H 2022, 236, 1595–1612. [Google Scholar] [CrossRef]
- Tarniţă, D.; Tarniţă, D.N.; Hacman, L.; Copiluş, C.; Berceanu, C. In vitro experiment of the modular orthopedic plate based on Nitinol, used for human radius bone fractures. Rom. J. Morphol. Embryol. 2010, 51, 315–320. [Google Scholar] [PubMed]
- Tarnita, D.; Bolcu, D.; Berceanu, C.; Cismaru, F.L. Theoretical and experimental studies for an orthopedic staple made up Nitinol. J. Optoelectron. Adv. Mater. 2010, 12, 2323–2332. [Google Scholar]
- Safranski, D.; Dupont, K.; Gall, K. Pseudoelastic NiTiNOL in orthopaedic applications. Shape Mem. Superelasticity 2020, 6, 332–341. [Google Scholar] [CrossRef]
- Fu, Q.G.; Liu, X.W.; Xu, S.G.; Li, M.; Zhang, C.C. Stress-shielding effect of nitinol swan-like memory compressive connector on fracture healing of upper limb. J. Mater. Eng. Perform. 2009, 18, 797–804. [Google Scholar] [CrossRef]
- Shabalovskaya, S.A.; Tian, H.; Anderegg, J.W.; Schryvers, D.U.; Carroll, W.U.; Van Humbeeck, J. The influence of surface oxides on the distribution and release of nickel from Nitinol wires. Biomaterials 2009, 30, 468–477. [Google Scholar] [CrossRef]
- Srivastava, A.; Xu, R.; Escoto, A.; Ward, C.; Patel, R.V. Design of an Ultra Thin Strain Sensor Using Superelastic Nitinol for Applications in Minimally Invasive Surgery. In Proceedings of the 2016 IEEE International Conference on Advanced Intelligent Mechatronics (AIM), Banf, AB, Canada, 12–15 July 2016; IEEE: Piscataway, NJ, USA, 2016. [Google Scholar] [CrossRef]
- Singh, D.; Sinha, S.; Singh, H.; Jagetia, A.; Gupta, S.; Gangoo, P.; Tandon, M. Use of nitinol shape memory alloy staples (NiTi clips) after cervical discoidectomy: Minimally invasive instrumentation and long-term results. Minim. Invasive Neurosurg. 2011, 54, 172–178. [Google Scholar] [CrossRef]
- Mehjabeen, A.; Song, T.; Xu, W.; Tang, H.P.; Qian, M. Zirconium alloys for orthopaedic and dental applications. Adv. Eng. Mater. 2018, 20, 1800207. [Google Scholar] [CrossRef]
- O’Brien, B. Niobium Biomaterials. In Springer Series in Biomaterials Science and Engineering; Springer: Berlin/Heidelberg, Germany, 2015; pp. 245–272. [Google Scholar]
- Mani, G.; Porter, D.; Grove, K.; Collins, S.; Ornberg, A.; Shulfer, R. A comprehensive review of biological and materials properties of Tantalum and its alloys. J. Biomed. Mater. Res. A 2022, 110, 1291–1306. [Google Scholar] [CrossRef]
- King, D.J.M.; Knowles, A.J.; Bowden, D.; Wenman, M.R.; Capp, S.; Gorley, M.; Shimwell, J.; Packer, L.; Gilbert, M.R.; Harte, A. High temperature zirconium alloys for fusion energy. J. Nucl. Mater. 2022, 559, 153431. [Google Scholar] [CrossRef]
- Jiang, N.; Bian, H.; Song, X.; Lei, Y.; Song, Y.; Lin, D.; Chen, X.; Long, W. Recent advances in joining of zirconium and zirconium alloy for nuclear industry. Met. Mater. Int. 2024, 30, 2625–2654. [Google Scholar] [CrossRef]
- Renganathan, G.; Tanneru, N.; Madurai, S.L. Orthopedical and Biomedical Applications of Titanium and Zirconium Metals. In Fundamental Biomaterials: Metals; Elsevier: Amsterdam, The Netherlands, 2018; pp. 211–241. [Google Scholar]
- Andreeva, V.V.; Glukhova, A.I. Corrosion and electrochemical properties of zirconium, titanium and titanium-zirconium alloys in solutions of hydrochloric acid and of hydrochloric acid with oxidising agents. J. Appl. Chem. 2007, 11, 390–397. [Google Scholar] [CrossRef]
- Andreeva, V.V.; Glukhova, A.I. Corrosion and electrochemical properties of titanium, zirconium and titanium-zirconium alloys in acid solutions. II. J. Appl. Chem. 1962, 12, 457–468. [Google Scholar] [CrossRef]
- Northwood, D.O.; London, I.M.; Bähen, L.E. Elastic constants of zirconium alloys. J. Nucl. Mater. 1975, 55, 299–310. [Google Scholar] [CrossRef]
- Helmi Attia, M. On the fretting wear mechanism of Zr-alloys. Tribol. Int. 2006, 39, 1320–1326. [Google Scholar] [CrossRef]
- Nomura, N. Zirconium Alloys for Orthopedic Applications. In Springer Series in Biomaterials Science and Engineering; Springer: Berlin/Heidelberg, Germany, 2015; pp. 215–221. [Google Scholar]
- Zhou, F.Y.; Wang, B.L.; Qiu, K.J.; Li, L.; Lin, J.P.; Li, H.F.; Zheng, Y.F. Microstructure, mechanical property, corrosion behavior, and in vitro biocompatibility of Zr-Mo alloys. J. Biomed. Mater. Res. B Appl. Biomater. 2013, 101, 237–246. [Google Scholar] [CrossRef]
- Rosalbino, F.; Macciò, D.; Scavino, G.; Saccone, A. Corrosion behavior of new ternary zirconium alloys as alternative materials for biomedical applications. Mater. Corros. 2015, 66, 1125–1132. [Google Scholar] [CrossRef]
- Xu, L.; Wei, C.; Deng, L.; Wang, P.; Zhong, W.; Huang, W. A review of non-biodegradable alloys implantation induced inflammatory and immune cell responses. J. Alloys Compd. 2024, 977, 173086. [Google Scholar] [CrossRef]
- Li, J.; Ai, H.-J. The responses of endothelial cells to Zr61Ti2Cu25Al12 metallic glass in vitro and in vivo. Mater. Sci. Eng. C Mater. Biol. Appl. 2014, 40, 189–196. [Google Scholar] [CrossRef]
- Leite, K.L.; Dias, M.D.; Tavares, F.O.; Silva, K.S.; Chevitarese, A.B.; Martins, M.L.; Masterson, D.; Menezes, L.R.; Gonçalves, A.F.; Maia, L.C. A Data mining analysis on niobium in dentistry: Promising alloys for dental materials. Pesqui. Bras. Odontopediatria Clín. Integr. 2024, 24, e230072. [Google Scholar] [CrossRef]
- Ali, A.M.; Thair, L.; Intisar, J. Studying Biomimetic Coated Niobium as an Alternative Dental Implant Material to Titanium (in vitro and in vivo study). Baghdad Sci. J. 2018, 15, 253–261. [Google Scholar]
- Olivares-Navarrete, R.; Olaya, J.J.; Ramírez, C.; Rodil, S.E. Biocompatibility of niobium coatings. Coatings 2011, 1, 72–87. [Google Scholar] [CrossRef]
- Ak, A. Fibroblast cell responses to vanadium and niobium Titanium alloys: A biocompatibility study. ACS Omega 2023, 8, 33802–33808. [Google Scholar] [CrossRef]
- Matsuno, H.; Yokoyama, A.; Watari, F.; Uo, M.; Kawasaki, T. Biocompatibility and osteogenesis of refractory metal implants, titanium, hafnium, niobium, tantalum and rhenium. Biomaterials 2001, 22, 1253–1262. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.L.; Li, L.; Zheng, Y.F. In vitro cytotoxicity and hemocompatibility studies of Ti-Nb, Ti-Nb-Zr and Ti-Nb-Hf biomedical shape memory alloys. Biomed. Mater. 2010, 5, 044102. [Google Scholar] [CrossRef] [PubMed]
- Yolun, A.; Şimşek, M.; Kaya, M.; Annaç, E.E.; Köm, M.; Çakmak, Ö. Fabrication, characterization, and in vivo biocompatibility evaluation of titanium-niobium implants. Proc. Inst. Mech. Eng. H 2021, 235, 99–108. [Google Scholar] [CrossRef]
- Eisenbarth, E.; Velten, D.; Müller, M.; Thull, R.; Breme, J. Biocompatibility of beta-stabilizing elements of titanium alloys. Biomaterials 2004, 25, 5705–5713. [Google Scholar] [CrossRef]
- Shi, K.; Zhang, Y.; Zhang, J.; Xie, Z. Electrochemical properties of niobium coating for biomedical application. Coatings 2019, 9, 546. [Google Scholar] [CrossRef]
- Bobyn, J.D.; Poggie, R.A.; Krygier, J.J.; Lewallen, D.G.; Hanssen, A.D.; Lewis, R.J.; Unger, A.S.; O’Keefe, T.J.; Christie, M.J.; Nasser, S.; et al. Clinical validation of a structural porous tantalum biomaterial for adult reconstruction. J. Bone Jt. Surg. Am. 2004, 86 (Suppl. S2), 123–129. [Google Scholar] [CrossRef] [PubMed]
- Levine, B.R.; Sporer, S.; Poggie, R.A.; Della Valle, C.J.; Jacobs, J.J. Experimental and clinical performance of porous tantalum in orthopedic surgery. Biomaterials 2006, 27, 4671–4681. [Google Scholar] [CrossRef]
- Dunbar, M.J.; Wilson, D.A.J.; Hennigar, A.W.; Amirault, J.D.; Gross, M.; Reardon, G.P. Fixation of a trabecular metal knee arthroplasty component: A prospective randomized study. J. Bone Jt. Surg. Am. 2009, 91, 1578–1586. [Google Scholar] [CrossRef]
- Carraro, F.; Bagno, A. Tantalum as trabecular metal for endosseous implantable applications. Biomimetics 2023, 8, 49. [Google Scholar] [CrossRef] [PubMed]
- Christie, M.J. Clinical applications of trabecular Metal. Am. J. Orthop. 2002, 31, 219–220. [Google Scholar]
- Stiehl, J.B. Trabecular metal in hip reconstructive surgery. Orthopedics 2005, 28, 662–670. [Google Scholar] [CrossRef] [PubMed]
- Schildhauer, T.A.; Peter, E.; Muhr, G.; Köller, M. Activation of human leukocytes on tantalum trabecular metal in comparison to commonly used orthopedic metal implant materials. J. Biomed. Mater. Res. A 2009, 88, 332–341. [Google Scholar] [CrossRef]
- Taylor, D.F. Acid corrosion resistance of tantalum, columbium, zirconium, and titanium. Ind. Eng. Chem. 1950, 42, 639. [Google Scholar] [CrossRef]
- Tang, Z.; Xie, Y.; Yang, F.; Huang, Y.; Wang, C.; Dai, K.; Zheng, X.; Zhang, X. Porous tantalum coatings prepared by vacuum plasma spraying enhance bmscs osteogenic differentiation and bone regeneration in vitro and in vivo. PLoS ONE 2013, 8, e66263. [Google Scholar] [CrossRef]
- Li, X.; Wang, L.; Yu, X.; Feng, Y.; Wang, C.; Yang, K.; Su, D. Tantalum coating on porous Ti6Al4V scaffold using chemical vapor deposition and preliminary biological evaluation. Mater. Sci. Eng. C Mater. Biol. Appl. 2013, 33, 2987–2994. [Google Scholar] [CrossRef]
- Kuo, T.-Y.; Chin, W.-H.; Chien, C.-S.; Hsieh, Y.-H. Mechanical and biological properties of graded porous tantalum coatings deposited on titanium alloy implants by vacuum plasma spraying. Surf. Coat. Technol. 2019, 372, 399–409. [Google Scholar] [CrossRef]
- Talha, M.; Ma, Y.; Lin, Y.; Pan, Y.; Kong, X.; Sinha, O.P.; Behera, C.K. Corrosion performance of cold deformed austenitic stainless steels for biomedical applications. Corros. Rev. 2019, 37, 283–306. [Google Scholar] [CrossRef]
- Seah, K.H.W.; Chen, X. A comparison between the corrosion characteristics of 316 stainless steel, solid titanium and porous titanium. Corros. Sci. 1993, 34, 1841–1851. [Google Scholar] [CrossRef]
- Lodhi, M.J.K.; Deen, K.M.; Greenlee-Wacker, M.C.; Haider, W. Additively manufactured 316L stainless steel with improved corrosion resistance and biological response for biomedical applications. Addit. Manuf. 2019, 27, 8–19. [Google Scholar] [CrossRef]
- Roed-Petersen, B.; Roed-Petersen, J.; Jørgensen, K.D. Nickel allergy and osteomyelitis in a patient with metal osteosynthesis of a jaw fracture. Contact Dermat. 1979, 5, 108–112. [Google Scholar] [CrossRef] [PubMed]
- Randin, J.P. Corrosion behavior of nickel-containing alloys in artificial sweat. J. Biomed. Mater. Res. 1988, 22, 649–666. [Google Scholar] [CrossRef]
- Zhang, M.; Yao, K.; Wei, M.; Zhu, P.; Wang, R.; Zhang, S.; Jin, X.; Tian, T.; Zhang, Y.; Long, K. Dermatitis due to orthopaedic implants. J. Biomater. Tissue Eng. 2019, 9, 1–9. [Google Scholar] [CrossRef]
- Imam, M.A.; Fraker, A.C.; Gilmore, C.M. Corrosion Fatigue of 316L Stainless Steel, Co-Cr-Mo Alloy, and ELI Ti-6Al-4V. Corrosion and Degradation of Implant Materials; 100 Barr Harbor Drive, PO Box C700, Conshohocken, PA 19428-2959; ASTM International: Conshohocken, PA, USA, 1979; pp. 128–143. [Google Scholar]
- Beach, J.E.; Marchica, N.V.; Taylor, D.W.; Ichter, L.L. A fatigue comparison of high strength steel, stainless steel, and titanium in asimulated ocean environment. In Proceedings of the OTC Offshore Technology Conference, Houston, TX, USA, 7 May 1978. [Google Scholar] [CrossRef]
- Lane, I.R.; Golden, L.B.; Acherman, W.L. Corrosion resistance of titanium, zirconium, and stainless steel in organic compounds. Ind. Eng. Chem. 1953, 45, 1067–1070. [Google Scholar] [CrossRef]
- Golden, L.B.; Lane, I.R.; Acherman, W.L. Corrosion resistance of titanium, zirconium, and stainless steel. Ind. Eng. Chem. 1952, 44, 1930–1939. [Google Scholar] [CrossRef]
- Sundgren, J.-E.; Bodö, P.; Lundström, I. Auger electron spectroscopic studies of the interface between human tissue and implants of titanium and stainless steel. J. Colloid. Interface Sci. 1986, 110, 9–20. [Google Scholar] [CrossRef]
- Mirvis, S.E.; Geisler, F.; Joslyn, J.N.; Zrebeet, H. Use of titanium wire in cervical spine fixation as a means to reduce MR artifacts. AJNR Am. J. Neuroradiol. 1988, 9, 1229–1231. [Google Scholar]
- Geisler, F.H.; Sutton, L.N.; Mirvis, S.E.; Zrebeet, H.; Joslyn, J.N. Titanium wire internal fixation for stabilization of injury of the cervical spine: Clinical results and postoperative magnetic resonance imaging of the spinal cord. Neurosurgery 1989, 25, 356–362. [Google Scholar] [CrossRef]
- Daga, B.; Rivera, G.; Boeri, R. Review of the regulations for the use of stainless steels for orthopedic implants in Argentina. J. Phys. Conf. Ser. 2007, 90, 012045. [Google Scholar] [CrossRef]
- Baharuddin, M.Y.; Salleh, S.-H.; Suhasril, A.A.; Zulkifly, A.H.; Lee, M.H.; Omar, M.A.; Kader, A.S.A.; Noor, A.M.; Harris, A.R.A.; Majid, N.A. Fabrication of low-cost, cementless femoral stem 316L stainless steel using investment casting technique. Artif. Organs 2014, 38, 603–608. [Google Scholar] [CrossRef] [PubMed]
- Sankaran, B. Experiences with Implantable Devices—Problems Faced by Developing Countries. In Materials Sciences and Implant Orthopedic Surgery; Springer: Dordrecht, The Netherlands, 1986. [Google Scholar]
- Azevedo, C.R.F.; Hippert, E., Jr. Failure analysis of surgical implants in Brazil. Eng. Fail. Anal. 2002, 9, 621–633. [Google Scholar] [CrossRef]
- Bakwatanisa, B.; Enywaku, A.; Kiwanuka, M.; Lamunu, C.; Mbowa, N.; Mukiibi, D.; Namayega, C.N.; Ngabirano, B.; Ntambi, H.; Reichert, W. Biomaterials use in Mulago National Referral Hospital in Kampala, Uganda: Access and affordability. J. Biomed. Mater. Res. A 2016, 104, 104–112. [Google Scholar] [CrossRef] [PubMed]
- Heiden, M.; Walker, E.; Stanciu, L. Magnesium, iron and zinc alloys, the trifecta of bioresorbable orthopaedic and vascular implantation—A review. J. Biotechnol. Biomater. 2015, 5, 1. [Google Scholar] [CrossRef]
- Pina, S.; Ferreira, J.M.F. Bioresorbable plates and screws for clinical applications: A review. J. Healthc. Eng. 2012, 3, 243–260. [Google Scholar] [CrossRef]
- Pietrzak, W.S.; Sarver, D.; Verstynen, M. Bioresorbable implants—Practical considerations. Bone 1996, 19, S109–S119. [Google Scholar] [CrossRef]
- Farraro, K.F.; Kim, K.E.; Woo, S.L.-Y.; Flowers, J.R.; McCullough, M.B. Revolutionizing orthopaedic biomaterials: The potential of biodegradable and bioresorbable magnesium-based materials for functional tissue engineering. J. Biomech. 2014, 47, 1979–1986. [Google Scholar] [CrossRef] [PubMed]
- Salama, M.; Vaz, M.F.; Colaço, R.; Santos, C.; Carmezim, M. Biodegradable iron and porous iron: Mechanical properties, degradation behaviour, manufacturing routes and biomedical applications. J. Funct. Biomater. 2022, 13, 72. [Google Scholar] [CrossRef]
- Li, P.; Dai, J.; Li, Y.; Alexander, D.; Čapek, J.; Geis-Gerstorfer, J.; Wang, G.; Han, J.; Yu, Z.; Li, A. Zinc based biodegradable metals for bone repair and regeneration: Bioactivity and molecular mechanisms. Mater. Today Bio 2024, 25, 100932. [Google Scholar] [CrossRef]
- Aljihmani, L.; Alic, L.; Boudjemline, Y.; Hijazi, Z.M.; Mansoor, B.; Serpedin, E.; Qaraqe, K. Magnesium-based bioresorbable Stent materials: Review of reviews. J. Bio Tribo-Corros. 2019, 5, 26. [Google Scholar] [CrossRef]
- Gu, X.-N.; Zheng, Y.-F. A review on magnesium alloys as biodegradable materials. Front. Mater. Sci. China 2010, 4, 111–115. [Google Scholar] [CrossRef]
- Chen, Y.; Xu, Z.; Smith, C.; Sankar, J. Recent advances on the development of magnesium alloys for biodegradable implants. Acta Biomater. 2014, 10, 4561–4573. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Liu, X.; Wu, S.; Yeung, K.W.K.; Zheng, Y.; Chu, P.K. Design of magnesium alloys with controllable degradation for biomedical implants: From bulk to surface. Acta Biomater. 2016, 45, 2–30. [Google Scholar] [CrossRef] [PubMed]
- Moravej, M.; Purnama, A.; Fiset, M.; Couet, J.; Mantovani, D. Electroformed pure iron as a new biomaterial for degradable stents: In vitro degradation and preliminary cell viability studies. Acta Biomater. 2010, 6, 1843–1851. [Google Scholar] [CrossRef] [PubMed]
- Frattolin, J.; Roy, R.; Rajagopalan, S.; Walsh, M.; Yue, S.; Bertrand, O.F.; Mongrain, R. A manufacturing and annealing protocol to develop a cold-sprayed Fe-316L stainless steel biodegradable stenting material. Acta Biomater. 2019, 99, 479–494. [Google Scholar] [CrossRef]
- Sharma, P.; Pandey, P.M. Corrosion behaviour of the porous iron scaffold in simulated body fluid for biodegradable implant application. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 99, 838–852. [Google Scholar] [CrossRef] [PubMed]
- Lu, S.; Wang, P.; Wang, Q.; Deng, P.; Yuan, Y.; Fu, X.; Yang, Y.; Tan, L.; Yang, K.; Qi, X. Biodegradable high-nitrogen iron alloy anastomotic staples: In vitro and in vivo studies. Bioact. Mater. 2024, 40, 34–46. [Google Scholar] [CrossRef]
- Hermawan, H.; Dubé, D.; Mantovani, D. Degradable metallic biomaterials: Design and development of Fe-Mn alloys for stents. J. Biomed. Mater. Res. A 2010, 93, 1–11. [Google Scholar] [CrossRef]
- Schinhammer, M.; Steiger, P.; Moszner, F.; Löffler, J.F.; Uggowitzer, P.J. Degradation performance of biodegradable Fe-Mn-C(-Pd) alloys. Mater. Sci. Eng. C Mater. Biol. Appl. 2013, 33, 1882–1893. [Google Scholar] [CrossRef]
- Dargusch, M.S.; Venezuela, J.; Dehghan-Manshadi, A.; Johnston, S.; Yang, N.; Mardon, K.; Lau, C.; Allavena, R. In vivo evaluation of bioabsorbable Fe-35Mn-1Ag: First reports on in vivo hydrogen gas evolution in Fe-based implants. Adv. Healthc. Mater. 2021, 10, e2000667. [Google Scholar] [CrossRef]
- Kafri, A.; Ovadia, S.; Yosafovich-Doitch, G.; Aghion, E. In vivo performances of pure Zn and Zn-Fe alloy as biodegradable implants. J. Mater. Sci. Mater. Med. 2018, 29, 94. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Yang, H.; Liu, Y.; Xiong, P.; Guo, H.; Huang, H.-H.; Zheng, Y. Comparative studies on degradation behavior of pure zinc in various simulated body fluids. JOM 2019, 71, 1414–1425. [Google Scholar] [CrossRef]
- SathishKumar, G.; Parameswaran, P.; Vijayan, V.; Yokeswaran, R. Effects of Ca, Cu concentration on degradation behavior of Zn alloys in Hank’s solution. Met. Powder Rep. 2020, 76, 10. [Google Scholar] [CrossRef]
- Guo, P.; Bagheri, R.; Ren, T.; Yang, L.; Xu, C.; Lin, J.; Liu, H.; Sun, W.; Song, Z. Corrosion characteristics of zinc–zirconium alloy in c-SBF and its biocompatibility in vitro/in vivo. Mater. Corros. 2020, 71, 196–208. [Google Scholar] [CrossRef]
- Huang, J.; Lai, Y.; Jin, H.; Guo, H.; Ai, F.; Xing, Q.; Yang, X.; Ross, D.J. Preparation and properties of Zn-cu alloy for potential Stent material. J. Mater. Eng. Perform. 2020, 29, 6484–6493. [Google Scholar] [CrossRef]
- Eddy Jai Poinern, G.; Brundavanam, S.; Fawcett, D. Biomedical magnesium alloys: A review of material properties, surface modifications and potential as a biodegradable orthopaedic implant. Am. J. Biomed. Eng. 2013, 2, 218–240. [Google Scholar] [CrossRef]
- Chaya, A.; Yoshizawa, S.; Verdelis, K.; Myers, N.; Costello, B.J.; Chou, D.-T.; Pal, S.; Maiti, S.; Kumta, P.N.; Sfeir, C. In vivo study of magnesium plate and screw degradation and bone fracture healing. Acta Biomater. 2015, 18, 262–269. [Google Scholar] [CrossRef] [PubMed]
- Koo, Y.; Lee, H.-B.; Dong, Z.; Kotoka, R.; Sankar, J.; Huang, N.; Yun, Y. The effects of static and dynamic loading on biodegradable magnesium pins in vitro and in vivo. Sci. Rep. 2017, 7, 14710. [Google Scholar] [CrossRef]
- Song, G. Control of biodegradation of biocompatable magnesium alloys. Corros. Sci. 2007, 49, 1696–1701. [Google Scholar] [CrossRef]
- Iulian, A.; Aurora, A.; Ana-Iulia, B.; Florin, M. In vitro biodegradation behavior of some magnesium alloys without aluminium potentially used for trauma implants. Front. Bioeng. Biotechnol. 2016, 4, 203–209. [Google Scholar] [CrossRef]
- He, L.Y.; Zhang, X.M.; Liu, B.; Tian, Y.; Ma, W.H. Effect of magnesium ion on human osteoblast activity. Braz. J. Med. Biol. Res. 2016, 49, e5257. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Ma, X.-Y.; Feng, Y.-F.; Ma, Z.-S.; Ma, T.-C.; Zhang, Y.; Zhang, Y.; Li, X.; Wang, L.; Lei, W. Magnesium ions promote the biological behaviour of rat calvarial osteoblasts by activating the PI3K/akt signalling pathway. Biol. Trace Elem. Res. 2017, 179, 284–293. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.; Kim, K.-J.; Cheon, S.; Kim, E.-M.; Kim, Y.-A.; Park, C.; Park, C.; Kim, K.K. Biochemical activity of magnesium ions on human osteoblast migration. Biochem. Biophys. Res. Commun. 2020, 531, 588–594. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Xie, B.; Wang, L.; Qin, Y.; Henneman, Z.J.; Nancollas, G.H. Influence of magnesium ions and amino acids on the nucleation and growth of hydroxyapatite. CrystEngComm 2011, 13, 1153–1158. [Google Scholar] [CrossRef]
- Shimaya, M.; Muneta, T.; Ichinose, S.; Tsuji, K.; Sekiya, I. Magnesium enhances adherence and cartilage formation of synovial mesenchymal stem cells through integrins. Osteoarthr. Cartil. 2010, 18, 1300–1309. [Google Scholar] [CrossRef]
- Wu, L.; Luthringer, B.J.C.; Feyerabend, F.; Schilling, A.F.; Willumeit, R. Effects of extracellular magnesium on the differentiation and function of human osteoclasts. Acta Biomater. 2014, 10, 2843–2854. [Google Scholar] [CrossRef]
- Brown, E.M.; Chen, C.J. Calcium, magnesium and the control of PTH secretion. Bone Miner. 1989, 5, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Sahota, O.; Mundey, M.K.; San, P.; Godber, I.M.; Hosking, D.J. Vitamin D insufficiency and the blunted PTH response in established osteoporosis: The role of magnesium deficiency. Osteoporos. Int. 2006, 17, 1013–1021. [Google Scholar] [CrossRef]
- Santos, G.G.; Nunes, V.L.C.; Marinho, S.M.O.C.; Santos, S.R.A.; Rossi, A.M.; Miguel, F.B. Biological behavior of magnesium-substituted hydroxyapatite during bone repair. Braz. J. Biol. 2021, 81, 53–61. [Google Scholar] [CrossRef]
- Havalda, R.; Pilli, S.C.; Putti, B.B. Effects of magnesium on mechanical properties of human bone. IOSR J. Pharm. Biol. Sci. 2013, 7, 8–14. [Google Scholar] [CrossRef]
- Nielsen, F.H. Magnesium deficiency and increased inflammation: Current perspectives. J. Inflamm. Res. 2018, 11, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Maier, J.A.; Castiglioni, S.; Locatelli, L.; Zocchi, M.; Mazur, A. Magnesium and inflammation: Advances and perspectives. Semin. Cell Dev. Biol. 2021, 115, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Kirkland, N.T.; Lespagnol, J.; Birbilis, N.; Staiger, M.P. A survey of bio-corrosion rates of magnesium alloys. Corros. Sci. 2010, 52, 287–291. [Google Scholar] [CrossRef]
- Wan, Y.; Xiong, G.; Luo, H.; He, F.; Huang, Y.; Zhou, X. Preparation and characterization of a new biomedical magnesium–calcium alloy. Mater. Eng. 2008, 29, 2034–2037. [Google Scholar] [CrossRef]
- Xu, Z.; Smith, C.; Chen, S.; Sankar, J. Development and microstructural characterizations of Mg–Zn–Ca alloys for biomedical applications. Mater. Sci. Eng. B Solid. State Mater. Adv. Technol. 2011, 176, 1660–1665. [Google Scholar] [CrossRef]
- Weng, W.; Biesiekierski, A.; Li, Y.; Dargusch, M.; Wen, C. A review of the physiological impact of rare earth elements and their uses in biomedical Mg alloys. Acta Biomater. 2021, 130, 80–97. [Google Scholar] [CrossRef] [PubMed]
- Rola, P.; Włodarczak, S.; Doroszko, A.; Lesiak, M.; Włodarczak, A. The bioresorbable magnesium scaffold (Magmaris)-State of the art: From basic concept to clinical application. Catheter. Cardiovasc. Interv. 2022, 100, 1051–1058. [Google Scholar] [CrossRef]
- Zhang, A.-M.; Lenin, P.; Zeng, R.-C.; Kannan, M.B. Advances in hydroxyapatite coatings on biodegradable magnesium and its alloys. J. Magnes. Alloy 2022, 10, 1154–1170. [Google Scholar] [CrossRef]
- Witte, F.; Feyerabend, F.; Maier, P.; Fischer, J.; Störmer, M.; Blawert, C.; Dietzel, W.; Hort, N. Biodegradable magnesium-hydroxyapatite metal matrix composites. Biomaterials 2007, 28, 2163–2174. [Google Scholar] [CrossRef]
- Amukarimi, S.; Mozafari, M. Biodegradable magnesium-based biomaterials: An overview of challenges and opportunities. MedComm 2021, 2, 123–144. [Google Scholar] [CrossRef]
- Gąsior, G.; Szczepański, J.; Radtke, A. Biodegradable iron-based materials-what was done and what more can be done? Materials 2021, 14, 3381. [Google Scholar] [CrossRef] [PubMed]
- Lin, W.; Zhang, H.; Zhang, W.; Qi, H.; Zhang, G.; Qian, J.; Li, X.; Qin, L.; Li, H.; Wang, X.; et al. In vivo degradation and endothelialization of an iron bioresorbable scaffold. Bioact. Mater. 2021, 6, 1028–1039. [Google Scholar] [CrossRef] [PubMed]
- Putra, N.E.; Leeflang, M.A.; Taheri, P.; Fratila-Apachitei, L.E.; Mol, J.M.C.; Zhou, J.; Zhou, J.; Zadpoor, A.A. Extrusion-based 3D printing of ex situ-alloyed highly biodegradable MRI-friendly porous iron-manganese scaffolds. Acta Biomater. 2021, 134, 774–790. [Google Scholar] [CrossRef]
- Hrubovčáková, M.; Džupon, M.; Kupková, M.; Oriňáková, R. Biodegradable iron-based foams for potential bone replacement material. Defect. Diffus. For. 2020, 405, 151–156. [Google Scholar] [CrossRef]
- Bondareva, J.V.; Dubinin, O.N.; Kuzminova, Y.O.; Shpichka, A.I.; Kosheleva, N.V.; Lychagin, A.V.; Shibalova, A.A.; Pozdnyalov, A.A.; Akhatov, I.S.; Timashev, P.S. Biodegradable iron-silicon implants produced by additive manufacturing. Biomed. Mater. 2022, 17, 035005. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Ulloa, A.; Nauman, E.; Stanciu, L. Collagen coating effects on Fe-Mn bioresorbable alloys. J. Orthop. Res. 2020, 38, 523–535. [Google Scholar] [CrossRef]
- Ravanbakhsh, S.; Paternoster, C.; Barucca, G.; Mengucci, P.; Gambaro, S.; Lescot, T.; Chevallier, T.; Fortin, M.-A.; Mantovani, D. Improving the radiopacity of Fe-Mn biodegradable metals by magnetron-sputtered W-Fe-Mn-C coatings: Application for thinner stents. Bioact. Mater. 2022, 12, 64–70. [Google Scholar] [CrossRef] [PubMed]
- Petráková, M.; Gorejová, R.; Shepa, J.; Macko, J.; Kupková, M.; Petruš, O.; Baláž, M.; Sopčák, T.; Mičušík, M.; Kožár, M.; et al. Effect of Gentamicin-Loaded Calcium Phosphate Coating and Polymeric Coating on the Degradation Properties of Biodegradable Iron-Based Biomaterials. ACS Omega 2024, 9, 48299–48314. [Google Scholar] [CrossRef] [PubMed]
- Tai, C.-C.; Huang, Y.-M.; Liaw, C.-K.; Yang, K.-Y.; Ma, C.-H.; Huang, S.-I.; Huang, C.-C.; Tsia, P.-I.; Shen, H.-H.; Sun, J.-S.; et al. Biocompatibility and biological performance of additive-manufactured bioabsorbable iron-based porous interference screws in a rabbit model: A 1-year observational study. Int. J. Mol. Sci. 2022, 23, 14626. [Google Scholar] [CrossRef]
- Edwards, D.F., 3rd; Miller, C.J.; Quintana-Martinez, A.; Wright, C.S.; Prideaux, M.; Atkins, G.J.; Thompson, W.R.; Clinkenbeard, E.L. Differential iron requirements for osteoblast and adipocyte differentiation. JBMR Plus 2021, 5, e10529. [Google Scholar] [CrossRef]
- Yang, J.; Dong, D.; Luo, X.; Zhou, J.; Shang, P.; Zhang, H. Iron overload-induced osteocyte apoptosis stimulates osteoclast differentiation through increasing osteocytic RANKL production in vitro. Calcif. Tissue Int. 2020, 107, 499–509. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Wang, H.; Qi, G.; Jiang, C.; Chen, K.; Yan, Z. Iron overload-induced ferroptosis of osteoblasts inhibits osteogenesis and promotes osteoporosis: An in vitro and in vivo study. IUBMB Life 2022, 74, 1052–1069. [Google Scholar] [CrossRef] [PubMed]
- von Brackel, F.N.; Oheim, R. Iron and bones: Effects of iron overload, deficiency and anemia treatments on bone. JBMR Plus 2024, 8, ziae064. [Google Scholar] [CrossRef] [PubMed]
- Marques, O.; Weiss, G.; Muckenthaler, M.U. The role of iron in chronic inflammatory diseases: From mechanisms to treatment options in anemia of inflammation. Blood 2022, 140, 2011–2023. [Google Scholar] [CrossRef] [PubMed]
- Wieczorek, M.; Schwarz, F.; Sadlon, A.; Abderhalden, L.A.; de Godoi Rezende Costa Molino, C.; Spahn, D.R.; Schaer, D.J.; Orav, E.J.; Egli, A.; Bischoff-Ferrai, H.A.; et al. Iron deficiency and biomarkers of inflammation: A 3-year prospective analysis of the DO-HEALTH trial. Aging Clin. Exp. Res. 2022, 34, 515–525. [Google Scholar] [CrossRef]
- Das, P.; Pathak, D.K.; Sharma, P.; Pandey, P.M. A review on the mechanical and biocorrosion behaviour of iron and zinc-based biodegradable materials fabricated using powder metallurgy routes. Corros. Rev. 2024. [Google Scholar] [CrossRef]
- Putra, N.E.; Moosabeiki, V.; Leeflang, M.A.; Zhou, J.; Zadpoor, A.A. Biodegradation-affected fatigue behavior of extrusion-based additively manufactured porous iron-manganese scaffolds. Acta Biomater. 2024, 178, 340–351. [Google Scholar] [CrossRef]
- Su, Y.; Fu, J.; Zhou, J.; Georgas, E.; Du, S.; Qin, Y.-X.; Wang, Y.; Zheng, Y.; Zhu, D. Blending with transition metals improves bioresorbable zinc as better medical implants. Bioact. Mater. 2023, 20, 243–258. [Google Scholar] [CrossRef]
- Lin, W.; Xu, L.; Li, G. Molecular insights into lysyl oxidases in cartilage regeneration and rejuvenation. Front. Bioeng. Biotechnol. 2020, 8, 359, Erratum in Front. Bioeng. Biotechnol. 2020, 8, 598323. [Google Scholar] [CrossRef]
- Kong, W.; Lyu, C.; Liao, H.; Du, Y. Collagen crosslinking: Effect on structure, mechanics and fibrosis progression. Biomed. Mater. 2021, 16, 062005. [Google Scholar] [CrossRef]
- Matthiesen, C.L.; Hu, L.; Torslev, A.S.; Poulsen, E.T.; Larsen, U.G.; Kjaer-Sorensen, K.; Thomsen, J.S.; Bruel, A.; Engild, J.J.; Oxvig, C.; et al. Superoxide dismutase 3 is expressed in bone tissue and required for normal bone homeostasis and mineralization. Free Radic. Biol. Med. 2021, 164, 399–409. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, R.; Ferns, G.A.; Sahebkar, A.; Mirshekar, M.A.; Jalali, M. Zinc supplementation is associated with a reduction in serum markers of inflammation and oxidative stress in adults: A systematic review and meta-analysis of randomized controlled trials. Cytokine 2021, 138, 155396. [Google Scholar] [CrossRef]
- Su, Y.; Fu, J.; Lee, W.; Du, S.; Qin, Y.-X.; Zheng, Y.; Wang, Y.; Zhu, D. Improved mechanical, degradation, and biological performances of Zn-Fe alloys as bioresorbable implants. Bioact. Mater. 2022, 17, 334–343. [Google Scholar] [CrossRef] [PubMed]
- Farabi, E.; Sharp, J.A.; Vahid, A.; Fabijanic, D.M.; Barnett, M.R.; Gallo, S.C. Development of high strength and ductile Zn-Al-Li alloys for potential use in bioresorbable medical devices. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 122, 111897. [Google Scholar] [CrossRef]
- Parilla, F.W.; Youngman, T.R.; Layon, D.R.; Ince, D.C.; Pashos, G.E.; Maloney, W.J.; Clohisy, J.C. Excellent 20-year results of total hip arthroplasty with highly cross-linked polyethylene on cobalt-chromium femoral heads in patients ≤50 years. J. Arthroplasty 2024, 39, 409–415. [Google Scholar] [CrossRef] [PubMed]
- Lanzutti, A.; Andreatta, F.; Vaglio, E.; Sortino, M.; Totis, G.; Fedrizzi, L. Effects of post-printing heat treatment on microstructure, corrosion and wet wear behavior of CoCrW alloy produced by L-PBF process. Prog. Addit. Manuf. 2023, 8, 1473–1487. [Google Scholar] [CrossRef]
- Mertz, K.C.; Yang, J.; Chung, B.C.; Chen, X.; Mayfield, C.K.; Heckmann, N.D. Ceramic femoral heads exhibit lower wear rates compared to cobalt chrome: A meta-analysis. J. Arthroplasty 2023, 38, 397–405. [Google Scholar] [CrossRef]
- Feng, S.; Zhang, Y.; Bao, Y.-H.; Yang, Z.; Zha, G.-C.; Chen, X.-Y. Comparison of modular and nonmodular tapered fluted titanium stems in femoral revision hip arthroplasty: A minimum 6-year follow-up study. Sci. Rep. 2020, 10, 13692. [Google Scholar] [CrossRef] [PubMed]
- Hussain, M.; Khan, S.M.; Al-Khaled, K.; Ayadi, M.; Abbas, N.; Chammam, W. Performance analysis of biodegradable materials for orthopedic applications. Mater. Today Commun. 2022, 31, 103167. [Google Scholar] [CrossRef]
- Jorgensen, D.J.; Dunand, D.C. Structure and mechanical properties of Ti–6Al–4V with a replicated network of elongated pores. Acta Mater. 2011, 59, 640–650. [Google Scholar] [CrossRef]
- Bridgeport, D.A.; Brantley, W.A.; Herman, P.F. Cobalt-chromium and nickel-chromium alloys for removable prosthodontics, Part 1: Mechanical properties. J. Prosthodont. 1993, 2, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Xu, C.; Hu, X.; Gan, W.; Wu, K.; Wang, X. Improving the Young’s modulus of Mg via alloying and compositing—A short review. J. Magnes. Alloy 2022, 10, 2009–2024. [Google Scholar] [CrossRef]
- Grünewald, T.A.; Rennhofer, H.; Hesse, B.; Burghammer, M.; Stanzl-Tschegg, S.E.; Cotte, M.; Löffler, J.F.; Weinberg, A.-M.; Lichtenegger, H.C. Magnesium from bioresorbable implants: Distribution and impact on the nano-and mineral structure of bone. Biomaterials 2016, 76, 250–260. [Google Scholar] [CrossRef] [PubMed]
- He, L.H.; Fujisawa, N.; Swain, M.V. Elastic modulus and stress-strain response of human enamel by nano-indentation. Biomaterials 2006, 27, 4388–4398. [Google Scholar] [CrossRef] [PubMed]
- Sumner, D.R. Long-term implant fixation and stress-shielding in total hip replacement. J. Biomech. 2015, 48, 797–800. [Google Scholar] [CrossRef]
- Savio, D.; Bagno, A. When the total hip replacement fails: A review on the stress-shielding effect. Processes 2022, 10, 612. [Google Scholar] [CrossRef]
- Marin, E.; Pressacco, M.; Fusi, S.; Lanzutti, A.; Turchet, S.; Fedrizzi, L. Characterization of grade 2 commercially pure Trabecular Titanium structures. Mater. Sci. Eng. C Mater. Biol. Appl. 2013, 33, 2648–2656. [Google Scholar] [CrossRef]
- Zhu, X.-K.; Joyce, J.A. Review of fracture toughness (G, K, J, CTOD, CTOA) testing and standardization. Eng. Fract. Mech. 2012, 85, 1–46. [Google Scholar] [CrossRef]
- Fujii, T.; Nose, T. Evaluation of fracture toughness for ceramic materials. ISIJ Int. 1989, 29, 717–725. [Google Scholar] [CrossRef]
- Richards, N.L. Quantitative evaluation of fracture toughness-microstructural relationships in alpha-beta titanium alloys. J. Mater. Eng. Perform. 2004, 13, 218–225. [Google Scholar] [CrossRef]
- Hendricks, P. Effects of Heat Treatment and Microstructure on Tensile and Fracturetoughness Properties of Titanium 6Al-4V Alloy Plate. In Proceedings of the 15th Structural Dynamics and Materials Conference, Reston, VA, USA, 17–19 April 1974; American Institute of Aeronautics and Astronautics: Reston, VA, USA, 1974. [Google Scholar] [CrossRef]
- Shukla, K.S.P. Nitriding of Medical Grade CoCrMo Alloys Using HIPIMS Discharge. Ph.D Thesis, Sheffield Hallam University, Sheffield, UK, 2021. [Google Scholar] [CrossRef]
- Naghib, S.M.; Mojtaba Ansari, M.; Pedram, A.; Moztarzadeh, F.; Mozafari, M. Bioactivation of 304 stainless steel surface through 45S5 bioglass coating for biomedical applications. Int. J. Electrochem. Sci. 2012, 7, 2890–2903. [Google Scholar] [CrossRef]
- Podzorova, L.I.; Il’icheva, A.A.; Pen’kova, O.I.; Alad’ev, N.A.; Volchenkova, V.A.; Kutsev, S.V.; Shvorneva, L.I. Modified composites of Al2O3–(Ce-TZP) system as materials for medical use. Inorg. Mater. Appl. Res. 2016, 7, 724–729. [Google Scholar] [CrossRef]
- Palmero, P. Zirconia-Based Composites for Biomedical Applications. In Bioceramics and Biocomposites; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2019; pp. 57–85. [Google Scholar]
- Ramesh, S.; Tolouei, R.; Tan, C.Y.; Amiriyan, M.; Yap, B.K.; Purbolaksono, J.; Hamdi, M. Influence of Magnesium Doping in Hydroxyapatite Bioceramics Sintered by Short Holding Time, Proceedings of the 5th Kuala Lumpur International Conference on Biomedical Engineering 2011 (BIOMED 2011), Kuala Lumpur, Malaysia, 20–23 June 2011; Springer: Berlin/Heidelberg, Germany, 2011; pp. 80–83. [Google Scholar]
- Hasoon Al-Moameri, H.; Majid Nahi, Z.; Raheem Rzaij, D.; TAl-Sharify, N. A review on the biomedical applications of alumina. J. Eng. Sustain. Dev. 2022, 24, 28–36. [Google Scholar] [CrossRef]
- Wu, H.; Chen, X.; Kong, L.; Liu, P. Mechanical and biological properties of titanium and its alloys for oral implant with preparation techniques: A review. Materials 2023, 16, 6860. [Google Scholar] [CrossRef] [PubMed]
- Lanzutti, A.; Magnan, M.; Vaglio, E.; Totis, G.; Sortino, M.; Fedrizzi, L. Study of the effect of L-PBF technique temporal evolution on microstructure, surface texture, and fatigue performance of Ti gr. 23 alloy. Metals 2023, 13, 1247. [Google Scholar] [CrossRef]
- Lanzutti, A.; Andreatta, F.; Rossi, L.; Di Benedetto, P.; Causero, A.; Magnan, M.; Fedrizzi, L. Corrosion fatigue failure of a high carbon CoCrMo modular hip prosthesis: Failure analysis and electrochemical study. Eng. Fail. Anal. 2019, 105, 856–868. [Google Scholar] [CrossRef]
- Huang, J.-Y.; Yeh, J.-J.; Jeng, S.-L.; Chen, C.-Y.; Kuo, R.-C. High-cycle fatigue behavior of type 316L stainless steel. Mater. Trans. 2006, 47, 409–417. [Google Scholar] [CrossRef]
- Guerchais, R.; Morel, F.; Saintier, N.; Robert, C. Influence of the microstructure and voids on the high-cycle fatigue strength of 316L stainless steel under multiaxial loading. Fatigue Fract. Eng. Mater. Struct. 2015, 38, 1087–1104. [Google Scholar] [CrossRef]
- Schopf, T.; Weihe, S.; Daniel, T.; Smaga, M.; Beck, T. Fatigue behavior and lifetime assessment of an austenitic stainless steel in the VHCF regime at ambient and elevated temperatures. Fatigue Fract. Eng. Mater. Struct. 2023, 46, 1763–1774. [Google Scholar] [CrossRef]
- Rao, J.H.; Stanford, N. A survey of fatigue properties from wrought and additively manufactured Ti-6Al-4V. Mater. Lett. 2021, 283, 128800. [Google Scholar] [CrossRef]
- Bayrak, Ö.; Yetim, A.F.; Alsaran, A.; Çelik, A. Fatigue life determination of plasma nitrided medical grade CoCrMo alloy. Fatigue Fract. Eng. Mater. Struct. 2010, 33, 303–309. [Google Scholar] [CrossRef]
- Okazaki, Y. Comparison of fatigue properties and fatigue crack growth rates of various implantable metals. Materials 2012, 5, 2981–3005. [Google Scholar] [CrossRef]
- Tsakiris, V.; Tardei, C.; Clicinschi, F.M. Biodegradable Mg alloys for orthopedic implants—A review. J. Magnes. Alloy 2021, 9, 1884–1905. [Google Scholar] [CrossRef]
- Liu, D.; Yang, D.; Li, X.; Hu, S. Mechanical properties, corrosion resistance and biocompatibilities of degradable Mg-RE alloys: A review. J. Mater. Res. Technol. 2019, 8, 1538–1549. [Google Scholar] [CrossRef]
- Kiani, F.; Wen, C.; Li, Y. Prospects and strategies for magnesium alloys as biodegradable implants from crystalline to bulk metallic glasses and composites-A review. Acta Biomater. 2020, 103, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Potzies, C.; Kainer, K.U. Fatigue of magnesium alloys. Adv. Eng. Mater. 2004, 6, 281–289. [Google Scholar] [CrossRef]
- Reza Kashyzadeh, K.; Amiri, N.; Maleki, E.; Unal, O. A critical review on improving the fatigue life and corrosion properties of magnesium alloys via the technique of adding different elements. J. Mar. Sci. Eng. 2023, 11, 527. [Google Scholar] [CrossRef]
- Cao, W.; Hench, L.L. Bioactive materials. Ceram. Int. 1996, 22, 493–507. [Google Scholar] [CrossRef]
- Zhao, X. Introduction to Bioactive Materials in Medicine. In Bioactive Materials in Medicine; Elsevier: Amsterdam, The Netherlands, 2011; pp. 1–13. [Google Scholar]
- Posada, O.; Tate, R.; Meek, R.M.; Grant, M. In vitro analyses of the toxicity, immunological, and gene expression effects of cobalt-chromium alloy wear debris and co ions derived from metal-on-metal hip implants. Lubricants 2015, 3, 539–568. [Google Scholar] [CrossRef]
- Xia, Z.; Kwon, Y.-M.; Mehmood, S.; Downing, C.; Jurkschat, K.; Murray, D.W. Characterization of metal-wear nanoparticles in pseudotumor following metal-on-metal hip resurfacing. Nanomedicine 2011, 7, 674–681. [Google Scholar] [CrossRef]
- Ras Sørensen, S.-A.L.; Jørgensen, H.L.; Sporing, S.L.; Lauritzen, J.B. Revision rates for metal-on-metal hip resurfacing and metal-on-metal total hip arthroplasty—A systematic review. Hip Int. 2016, 26, 515–521. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Lee, G.; Li, S.; Hu, Z.; Zhao, K.; Rogers, J.A. Advances in bioresorbable materials and electronics. Chem. Rev. 2023, 123, 11722–11773. [Google Scholar] [CrossRef] [PubMed]
- Waelti, S.L.; Markart, S.; Willems, E.P.; Fischer, T.; Dietrich, T.J.; Ditchfield, M.; Mattissek, C.; Krebs, T. Radiographic features of magnesium-based bioabsorbable screw resorption in paediatric fractures. Pediatr. Radiol. 2022, 52, 2368–2376. [Google Scholar] [CrossRef] [PubMed]
- Byun, S.-H.; Lim, H.-K.; Kim, S.-M.; Lee, S.-M.; Kim, H.-E.; Lee, J.-H. The bioresorption and guided bone regeneration of absorbable hydroxyapatite-coated magnesium mesh. J. Craniofac. Surg. 2017, 28, 518–523. [Google Scholar] [CrossRef]
- Kuhlmann, J.; Bartsch, I.; Willbold, E.; Schuchardt, S.; Holz, O.; Hort, N.; Hoche, D.; Heineman, W.R.; Witte, F. Fast escape of hydrogen from gas cavities around corroding magnesium implants. Acta Biomater. 2013, 9, 8714–8721. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.-K.; Lee, K.-B.; Kim, S.-Y.; Bode, K.; Jang, Y.-S.; Kwon, T.-Y.; Jeon, M.H.; Lee, M.-H. Gas formation and biological effects of biodegradable magnesium in a preclinical and clinical observation. Sci. Technol. Adv. Mater. 2018, 19, 324–335. [Google Scholar] [CrossRef] [PubMed]
- Jassim, S.S.; Patel, S.; Wardle, N.; Tahmassebi, J.; Middleton, R.; Shardlow, D.L.; Stephen, A.; Hutchinson, J.; Haddad, F.S. Five-year comparison of wear using oxidised zirconium and cobalt-chrome femoral heads in total hip arthroplasty: A multicentre randomised controlled trial. Bone Jt. J. 2015, 97-B, 883–889. [Google Scholar] [CrossRef]
- Jasty, M.; Bragdon, C.R.; Lee, K.; Hanson, A.; Harris, W.H. Surface damage to cobalt-chrome femoral head prostheses. J. Bone Jt. Surg. Br. 1994, 76-B, 73–77. [Google Scholar] [CrossRef]
- Viceconti, M.; Baleani, M.; Squarzoni, S.; Toni, A. Fretting wear in a modular neck hip prosthesis. J. Biomed. Mater. Res. 1997, 35, 207–216. [Google Scholar] [CrossRef]
- Elkins, J.M.; Callaghan, J.J.; Brown, T.D. Stability and trunnion wear potential in large-diameter metal-on-metal total hips: A finite element analysis. Clin. Orthop. Relat. Res. 2014, 472, 529–542. [Google Scholar] [CrossRef]
- Collier, J.P.; Mayor, M.B.; Jensen, R.E.; Surprenant, V.A.; Surprenant, H.P.; McNamar, J.L.; Belec, L. Mechanisms of failure of modular prostheses. Clin. Orthop. Relat. Res. 1992, 285, 129–139. [Google Scholar] [CrossRef]
- Konttinen, Y.T.; Pajarinen, J.; Takakubo, Y.; Gallo, J.; Nich, C.; Takagi, M.; Goodman, S.B. Macrophage polarization and activation in response to implant debris: Influence by “particle disease” and “ion disease”. J. Long. Term. Eff. Med. Implants 2014, 24, 267–281. [Google Scholar] [CrossRef] [PubMed]
- Landgraeber, S.; Jäger, M.; Jacobs, J.J.; Hallab, N.J. The pathology of orthopedic implant failure is mediated by innate immune system cytokines. Mediators Inflamm. 2014, 2014, 185150. [Google Scholar] [CrossRef] [PubMed]
- Hallab, N.J.; Jacobs, J.J. Chemokines associated with pathologic responses to orthopedic implant debris. Front. Endocrinol. 2017, 8, 5. [Google Scholar] [CrossRef] [PubMed]
- Anderson, J.M.; Rodriguez, A.; Chang, D.T. Foreign body reaction to biomaterials. Semin. Immunol. 2008, 20, 86–100. [Google Scholar] [CrossRef]
- Mariani, E.; Lisignoli, G.; Borzì, R.M.; Pulsatelli, L. Biomaterials: Foreign bodies or tuners for the immune response? Int. J. Mol. Sci. 2019, 20, 636. [Google Scholar] [CrossRef]
- Anderson, J.M. Multinucleated giant cells. Curr. Opin. Hematol. 2000, 7, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Chrcanovic, B.R.; Gomes, C.C.; Gomez, R.S. Peripheral giant cell granuloma associated with dental implants: A systematic review. J. Stomatol. Oral. Maxillofac. Surg. 2019, 120, 456–461. [Google Scholar] [CrossRef]
- Molina-Ruiz, A.M.; Requena, L. Foreign body granulomas. Dermatol. Clin. 2015, 33, 497–523. [Google Scholar] [CrossRef]
- Zhang, X.; Morham, S.G.; Langenbach, R.; Young, D.A.; Xing, L.; Boyce, B.F.; Puzas, E.J.; Rosier, R.N.; O’Keefe, R.J.; Schwarz, E.M. Evidence for a direct role of cyclo-oxygenase 2 in implant wear debris-induced osteolysis. J. Bone Miner. Res. 2001, 16, 660–670. [Google Scholar] [CrossRef]
- de Souza, W.; Gemini-Piperni, S.; Ruivo, C.; Bastos, N.; Almeida, S.; Lopes, D.; Cordoso, P.; Oliveira, M.J.; Sumner, D.R.; Ross, R.d; et al. Osteoblasts-derived exosomes as potential novel communicators in particle-induced periprosthetic osteolysis. Mater. Today Bio 2024, 28, 101189. [Google Scholar] [CrossRef] [PubMed]
- Dong, J.; Zhang, L.; Ruan, B.; Lv, Z.; Wang, H.; Wang, Y.; Jiang, Q.; Cao, W. NRF2 is a critical regulator and therapeutic target of metal implant particle-incurred bone damage. Biomaterials 2022, 288, 121742. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Li, J.; Wu, S.; Wang, J.; Tang, Q. Accumulation of blood chromium and cobalt in the participants with metal objects: Findings from the 2015 to 2018 National Health and Nutrition Examination Survey (NHANES). BMC Geriatr. 2023, 23, 72. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Q.; Pan, X.; Chen, Y.; Lian, Q.; Gao, J.; Xu, Y.; Wang, J.; Shi, Z.; Cheng, H. Prosthetic metals: Release, metabolism and toxicity. Int. J. Nanomed. 2024, 19, 5245–5267. [Google Scholar] [CrossRef]
- Scharf, B.; Clement, C.C.; Zolla, V.; Perino, G.; Yan, B.; Elci, S.G.; Purdue, E.; Goldring, S.; Macaluso, F.; Cobelli, N.; et al. Molecular analysis of chromium and cobalt-related toxicity. Sci. Rep. 2014, 4, 5729. [Google Scholar] [CrossRef]
- Thyssen, J.P. Nickel and cobalt allergy before and after nickel regulation—Evaluation of a public health intervention. Contact Dermat. 2011, 65 (Suppl. S1), 1–68. [Google Scholar] [CrossRef]
- Pacheco, K.A. Allergy to surgical implants. Clin. Rev. Allergy Immunol. 2019, 56, 72–85. [Google Scholar] [CrossRef]
- Thomas, P.; Summer, B. Diagnosis and management of patients with allergy to metal implants. Expert. Rev. Clin. Immunol. 2015, 11, 501–509. [Google Scholar] [CrossRef] [PubMed]
- Thyssen, J.P.; Menné, T.; Lidén, C.; Julander, A.; Jensen, P.; Jakobsen, S.S.; Søballe, K.; Gotfredsen, K.; Jellesen, M.S.; Johansen, J.D. Cobalt release from implants and consumer items and characteristics of cobalt sensitized patients with dermatitis. Contact Dermat. 2012, 66, 113–122. [Google Scholar] [CrossRef]
- Balogh, E.; Paragh, G.; Jeney, V. Influence of iron on bone homeostasis. Pharmaceuticals 2018, 11, 107. [Google Scholar] [CrossRef]
- Meng, G.; Wu, X.; Yao, R.; He, J.; Yao, W.; Wu, F. Effect of zinc substitution in hydroxyapatite coating on osteoblast and osteoclast differentiation under osteoblast/osteoclast co-culture. Regen. Biomater. 2019, 6, 349–359. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Feyerabend, F.; Schilling, A.F.; Willumeit-Römer, R.; Luthringer, B.J.C. Effects of extracellular magnesium extract on the proliferation and differentiation of human osteoblasts and osteoclasts in coculture. Acta Biomater. 2015, 27, 294–304. [Google Scholar] [CrossRef] [PubMed]
- Yamasaki, K.; Hagiwara, H. Excess iron inhibits osteoblast metabolism. Toxicol. Lett. 2009, 191, 211–215. [Google Scholar] [CrossRef]
- Ying Kei, C.L. Iron-Substituted Hydroxyapatite. In Handbook of Ionic Substituted Hydroxyapatites; Elsevier: Amsterdam, The Netherlands, 2020; pp. 259–282. [Google Scholar]
- Ratnayake, J.T.B.; Mucalo, M.; Dias, G.J. Substituted hydroxyapatites for bone regeneration: A review of current trends. J. Biomed. Mater. Res. B Appl. Biomater. 2017, 105, 1285–1299. [Google Scholar] [CrossRef] [PubMed]
- Thian, E.S.; Konishi, T.; Kawanobe, Y.; Lim, P.N.; Choong, C.; Ho, B.; Aizawa, M. Zinc-substituted hydroxyapatite: A biomaterial with enhanced bioactivity and antibacterial properties. J. Mater. Sci. Mater. Med. 2013, 24, 437–445. [Google Scholar] [CrossRef] [PubMed]
- Vasto, S.; Mocchegiani, E.; Malavolta, M.; Cuppari, I.; Listì, F.; Nuzzo, D.; Ditta, V.; Candore, G.; Caruso, C. Zinc and inflammatory/immune response in aging. Ann. N. Y. Acad. Sci. 2007, 1100, 111–122. [Google Scholar] [CrossRef] [PubMed]
- Martins, A.C.; Almeida, J.I.; Lima, I.S.; Kapitão, A.S.; Gozzelino, R. Iron metabolism and the inflammatory response. IUBMB Life 2017, 69, 442–450. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Berndt, C.C.; Gross, K.A.; Kucuk, A. Material fundamentals and clinical performance of plasma-sprayed hydroxyapatite coatings: A review. J. Biomed. Mater. Res. 2001, 58, 570–592. [Google Scholar] [CrossRef]
- Diamanti, M.V.; Sebastiani, M.; Mangione, V.; Del Curto, B.; Pedeferri, M.P.; Bemporad, E.; Cigada, A.; Carassiti, F. Multi-step anodizing on Ti6Al4V components to improve tribomechanical performances. Surf. Coat. Technol. 2013, 227, 19–27. [Google Scholar] [CrossRef]
- Marin, E.; Offoiach, R.; Regis, M.; Fusi, S.; Lanzutti, A.; Fedrizzi, L. Diffusive thermal treatments combined with PVD coatings for tribological protection of titanium alloys. Mater. Des. 2016, 89, 314–322. [Google Scholar] [CrossRef]
- Lanzutti, A.; Raffaelli, A.; Magnan, M.; Fedrizzi, L.; Regis, M.; Marin, E. Microstructural and mechanical study of an induction nitrided Ti gr.5 hip prosthesis component. Surf. Coat. Technol. 2019, 377, 124895. [Google Scholar] [CrossRef]
- Mändl, S.; Rauschenbach, B. Improving the biocompatibility of medical implants with plasma immersion ion implantation. Surf. Coat. Technol. 2002, 156, 276–283. [Google Scholar] [CrossRef]
- Pelletier, J.; Anders, A. Plasma-based ion implantation and deposition: A review of physics, technology, and applications. IEEE Trans. Plasma Sci. IEEE Nucl. Plasma Sci. Soc. 2005, 33, 1944–1959. [Google Scholar] [CrossRef]
- Marin, E.; Lanzutti, A.; Nakamura, M.; Zanocco, M.; Zhu, W.; Pezzotti, G.; Andreatta, F. Corrosion and scratch resistance of DLC coatings applied on chromium molybdenum steel. Surf. Coat. Technol. 2019, 378, 124944. [Google Scholar] [CrossRef]
- Safin Kaosar Saad, K.; Saba, T.; Bin Rashid, A. Application of PVD coatings in medical implantology for enhanced performance, biocompatibility, and quality of life. Heliyon 2024, 10, e35541. [Google Scholar] [CrossRef]
- Domínguez-Trujillo, C.; Peón, E.; Chicardi, E.; Pérez, H.; Rodríguez-Ortiz, J.A.; Pavón, J.J.; García-Couce, J.; Galván, J.C.; García-Moreno, F.; Torres, Y. Sol-gel deposition of hydroxyapatite coatings on porous titanium for biomedical applications. Surf. Coat. Technol. 2018, 333, 158–162. [Google Scholar] [CrossRef]
- Owens, G.J.; Singh, R.K.; Foroutan, F.; Alqaysi, M.; Han, C.-M.; Mahapatra, C.; Kim, H.-W.; Knowles, J.C. Sol–gel based materials for biomedical applications. Prog. Mater. Sci. 2016, 77, 1–79. [Google Scholar] [CrossRef]
- Boccaccini, A.R.; Keim, S.; Ma, R.; Li, Y.; Zhitomirsky, I. Electrophoretic deposition of biomaterials. J. R. Soc. Interface 2010, 7 (Suppl. S5), S581–S613. [Google Scholar] [CrossRef]
- Makurat-Kasprolewicz, B.; Ossowska, A. Electrophoretically deposited titanium and its alloys in biomedical engineering: Recent progress and remaining challenges. J. Biomed. Mater. Res. B Appl. Biomater. 2024, 112, e35342. [Google Scholar] [CrossRef]
- Liu, F.; Wang, H.; Zhao, B.; Zhang, W.; Wang, Q.; Niu, Y.; Wang, Y.; Yu, H.; Wang, Q.; Yang, K. Study on deposition of biomedical Ta coating on Ti6Al4V alloy substrate by CVD and its properties. Surf. Coat. Technol. 2025, 495, 131593. [Google Scholar] [CrossRef]
- Grant, D.M.; McColl, I.R.; Golozar, M.A.; Wood, J.V.; Braithwaite, N.S.J. Plasma assisted CVD for biomedical applications. Diam. Relat. Mater. 1992, 1, 727–730. [Google Scholar] [CrossRef]
- Zhang, C.; Duan, G.; Li, J.; Xiao, D.; Shi, F.; Duan, K.; Guo, T.; Fan, X.; Weng, J. Hydrothermal growth of biomimetic calcium phosphate network structure on titanium surface for biomedical application. Ceram. Int. 2023, 49, 16652–16660. [Google Scholar] [CrossRef]
- Wen, S.; Liu, X.; Ding, J.; Liu, Y.; Lan, Z.; Zhang, Z.; Chen, G. Hydrothermal synthesis of hydroxyapatite coating on the surface of medical magnesium alloy and its corrosion resistance. Prog. Nat. Sci. 2021, 31, 324–333. [Google Scholar] [CrossRef]
- Asmawi, R.; Ibrahim, M.H.I.; Amin, A.M.; Mustafa, N.; Noranai, Z. Development of bioactive ceramic coating on titanium alloy substrate for biomedical application using dip coating method. IOP Conf. Ser. Mater. Sci. Eng. 2017, 226, 012179. [Google Scholar] [CrossRef]
- Aksakal, B.; Hanyaloglu, C. Bioceramic dip-coating on Ti-6Al-4V and 316L SS implant materials. J. Mater. Sci. Mater. Med. 2008, 19, 2097–2104. [Google Scholar] [CrossRef]
- Zhang, X.; Pfeiffer, S.; Rutkowski, P.; Makowska, M.; Kata, D.; Yang, J.; Graule, T. Laser cladding of manganese oxide doped aluminum oxide granules on titanium alloy for biomedical applications. Appl. Surf. Sci. 2020, 520, 146304. [Google Scholar] [CrossRef]
- Weng, F.; Chen, C.; Yu, H. Research status of laser cladding on titanium and its alloys: A review. Mater. Eng. 2014, 58, 412–425. [Google Scholar] [CrossRef]
- Huang, W.; Zalnezhad, E.; Musharavati, F.; Jahanshahi, P. Investigation of the tribological and biomechanical properties of CrAlTiN and CrN/NbN coatings on SST 304. Ceram. Int. 2017, 43, 7992–8003. [Google Scholar] [CrossRef]
- Dolatabadi, A.; Mosatghimi, J.; Pershin, V. A New Design for Coating Thin Film Alumina on Stainless Steel for Biomedical Applications. In Proceedings of the International Conference on MEMS, NANO and Smart Systems, Banff, AB, Canada, 23 July 2003; IEEE: Piscataway, NJ, USA, 2003. [Google Scholar] [CrossRef]
- Snyders, R.; Bousser, E.; Amireault, P.; Klemberg-Sapieha, J.E.; Park, E.; Taylor, K.; Taylor, K.; Casey, K.; Martinu, L. Tribo-mechanical properties of DLC coatings deposited on nitrided biomedical stainless steel. Plasma Process Polym. 2007, 4, S640–S646. [Google Scholar] [CrossRef]
- Liu, B.; Zhang, X.; Xiao, G.-Y.; Lu, Y.-P. Phosphate chemical conversion coatings on metallic substrates for biomedical application: A review. Mater. Sci. Eng. C Mater. Biol. Appl. 2015, 47, 97–104. [Google Scholar] [CrossRef]
- Oliver, J.-A.N.; Su, Y.; Lu, X.; Kuo, P.-H.; Du, J.; Zhu, D. Bioactive glass coatings on metallic implants for biomedical applications. Bioact. Mater. 2019, 4, 261–270. [Google Scholar] [CrossRef]
- Muthulakshmi, L.; Anand Kumar, B.; Rajasekar, A.; Annaraj, J.; Pruncu, C.I. The benefits of k-Carrageenan-gelatin hybrid composite coating on the medical grade stainless steel (SS304) used as anticorrosive barrier. Mater. Chem. Phys. 2021, 258, 123909. [Google Scholar] [CrossRef]
- Trzaskowska, P.A.; Kuźmińska, A.; Butruk-Raszeja, B.; Rybak, E.; Ciach, T. Electropolymerized hydrophilic coating on stainless steel for biomedical applications. Colloids Surf. B Biointerfaces 2018, 167, 499–508. [Google Scholar] [CrossRef] [PubMed]
- Noori, M.; Atapour, M.; Ashrafizadeh, F.; Elmkhah, H.; di Confiengo, G.G.; Ferraris, S.; Perero, S.; Cardu, M.; Spriano, S. Nanostructured multilayer CAE-PVD coatings based on transition metal nitrides on Ti6Al4V alloy for biomedical applications. Ceram. Int. 2023, 49, 23367–23382. [Google Scholar] [CrossRef]
- Jiang, J.Y.; Xu, J.L.; Liu, Z.H.; Deng, L.; Sun, B.; Liu, S.D.; Wang, L.; Liu, H.Y. Preparation, corrosion resistance and hemocompatibility of the superhydrophobic TiO2 coatings on biomedical Ti-6Al-4V alloys. Appl. Surf. Sci. 2015, 347, 591–595. [Google Scholar] [CrossRef]
- Hatem, A.; Lin, J.; Wei, R.; Torres, R.D.; Laurindo, C.; Soares, P. Tribocorrosion behavior of DLC-coated Ti-6Al-4V alloy deposited by PIID and PEMS + PIID techniques for biomedical applications. Surf. Coat. Technol. 2017, 332, 223–232. [Google Scholar] [CrossRef]
- Narayanan, R.; Seshadri, S.K.; Kwon, T.Y.; Kim, K.H. Calcium phosphate-based coatings on titanium and its alloys. J. Biomed. Mater. Res. B Appl. Biomater. 2008, 85, 279–299. [Google Scholar] [CrossRef]
- Li, S.J.; Niinomi, M.; Akahori, T.; Kasuga, T.; Yang, R.; Hao, Y.L. Fatigue characteristics of bioactive glass-ceramic-coated Ti-29Nb-13Ta-4.6 Zr for biomedical application. Biomaterials 2004, 25, 3369–3378. [Google Scholar] [CrossRef]
- Wang, L.; Shang, X.; Hao, Y.; Wan, G.; Dong, L.; Huang, D.; Yang, X.; Sun, J.; Wang, Q.; Zha, G.; et al. Bi-functional titanium-polydopamine-zinc coatings for infection inhibition and enhanced osseointegration. RSC Adv. 2019, 9, 2892–2905. [Google Scholar] [CrossRef]
- Szaraniec, B.; Pielichowska, K.; Pac, E.; Menaszek, E. Multifunctional polymer coatings for titanium implants. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 93, 950–957. [Google Scholar] [CrossRef]
- Tsai, C.-E.; Hung, J.; Hu, Y.; Wang, D.-Y.; Pilliar, R.M.; Wang, R. Improving fretting corrosion resistance of CoCrMo alloy with TiSiN and ZrN coatings for orthopedic applications. J. Mech. Behav. Biomed. Mater. 2021, 114, 104233. [Google Scholar] [CrossRef] [PubMed]
- Falub, C.V.; Müller, U.; Thorwarth, G.; Parlinska-Wojtan, M.; Voisard, C.; Hauert, R. In vitro studies of the adhesion of diamond-like carbon thin films on CoCrMo biomedical implant alloy. Acta Mater. 2011, 59, 4678–4689. [Google Scholar] [CrossRef]
- Coşkun, M.I.; Karahan, İ.H.; Yücel, Y. Optimized Electrodeposition Concentrations for Hydroxyapatite Coatings on CoCrMo biomedical alloys by computational techniques. Electrochim. Acta 2014, 150, 46–54. [Google Scholar] [CrossRef]
- Qin, L.; Sun, H.; Hafezi, M.; Zhang, Y. Polydopamine-assisted immobilization of chitosan brushes on a textured CoCrMo alloy to improve its tribology and biocompatibility. Materials 2019, 12, 3014. [Google Scholar] [CrossRef]
- Garbuz, D.S.; Hu, Y.; Kim, W.Y.; Duan, K.; Masri, B.A.; Oxland, T.R.; Burt, H.; Wang, R.; Duncan, C.P. Enhanced gap filling and osteoconduction associated with alendronate-calcium phosphate-coated porous tantalum. J. Bone Jt. Surg. Am. 2008, 90, 1090–1100. [Google Scholar] [CrossRef]
- Cheng, S.; Ke, J.; Yao, M.; Shao, H.; Zhou, J.; Wang, M.; Ji, X.; Zhong, G.; Peng, F.; Ma, L.; et al. Improved osteointegration and angiogenesis of strontium-incorporated 3D-printed tantalum scaffold via bioinspired polydopamine coating. J. Mater. Sci. Technol. 2021, 69, 106–118. [Google Scholar] [CrossRef]
- Fontaine, A.B.; Koelling, K.; Passos, S.D.; Cearlock, J.; Hoffman, R.; Spigos, D.G. Polymeric surface modifications of tantalum stents. J. Endovasc. Ther. 1996, 3, 276–283. [Google Scholar] [CrossRef]
- Li, Z.-Y.; Cai, Z.-B.; Ding, Y.; Cui, X.-J.; Yang, Z.-B.; Zhu, M.-H. Characterization of graphene oxide/ZrO2 composite coatings deposited on zirconium alloy by micro-arc oxidation. Appl. Surf. Sci. 2020, 506, 144928. [Google Scholar] [CrossRef]
- Ionita, D.; Vardaki, M.; Stan, M.S.; Dinischiotu, A.; Demetrescu, I. Enhance stability and in vitro cell response to a bioinspired coating on zr alloy with increasing chitosan content. J. Bionic Eng. 2017, 14, 459–467. [Google Scholar] [CrossRef]
- Hornberger, H.; Virtanen, S.; Boccaccini, A.R. Biomedical coatings on magnesium alloys—A review. Acta Biomater. 2012, 8, 2442–2455. [Google Scholar] [CrossRef]
- Shadanbaz, S.; Dias, G.J. Calcium phosphate coatings on magnesium alloys for biomedical applications: A review. Acta Biomater. 2012, 8, 20–30. [Google Scholar] [CrossRef] [PubMed]
- Zhen, Z.; Xi, T.F.; Zheng, Y.F. Surface Modification by Natural Biopolymer Coatings on Magnesium Alloys for Biomedical Applications. In Surface Modification of Magnesium and Its Alloys for Biomedical Applications; Elsevier: Amsterdam, The Netherlands, 2015; pp. 301–333. [Google Scholar]
- Fu, J.; Zhu, Q.; Chen, Z.; Zhao, J.; Wu, S.; Zhao, M.; Xu, S.; Lai, D.; Fu, G.; Zhang, W. Polydopamine (PDA) coatings with endothelial vascular growth factor (VEGF) immobilization inhibiting neointimal formation post zinc (zn) wire implantation in rat aortas. Biomater. Res. 2023, 27, 84. [Google Scholar] [CrossRef]
- Velásquez-García, L.F.; Kornbluth, Y. Biomedical applications of metal 3D printing. Annu. Rev. Biomed. Eng. 2021, 23, 307–338. [Google Scholar] [CrossRef] [PubMed]
- Zadpoor, A.A. Additively manufactured porous metallic biomaterials. J. Mater. Chem. B Mater. Biol. Med. 2019, 7, 4088–4117. [Google Scholar] [CrossRef]
- Guoqing, Z.; Junxin, L.; Chengguang, Z.; Juanjuan, X.; Xiaoyu, Z.; Anmin, W. Design Optimization and Manufacturing of Bio-fixed tibial implants using 3D printing technology. J. Mech. Behav. Biomed. Mater. 2021, 117, 104415. [Google Scholar] [CrossRef] [PubMed]
- Sterk, S.; Silva, M.E.T.; Fernandes, A.A.; Huß, A.; Wittek, A. Development of new surgical mesh geometries with different mechanical properties using the design freedom of 3D printing. J. Appl. Polym. Sci. 2023, 140, e54687. [Google Scholar] [CrossRef]
- Chen, H.; Wang, C.; Zhu, X.; Zhang, K.; Fan, Y.; Zhang, X. Fabrication of porous titanium scaffolds by stack sintering of microporous titanium spheres produced with centrifugal granulation technology. Mater. Sci. Eng. C Mater. Biol. Appl. 2014, 43, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Lee, P.D.; Dashwood, R.J.; Lindley, T.C. Titanium foams for biomedical applications: A review. Mater. Technol. 2010, 25, 127–136. [Google Scholar] [CrossRef]
- Papazoglou, D.P. Additively Manufactured Ti-6Al-4V Biomimetic Lattice Structures for Patient-Specific Orthopedic Implants: The Effect of Unit Cell Geometry, Pore Size, and Pulsed Electromagnetic Field Stimulation on the Osseointegration of Mg-63 Cells in Vitro, Mechanical Properties, and Surface Characterization. Master’s Thesis, University of Dayton, Dayton, OH, USA, 2023. [Google Scholar]
- Dong, Z.; Zhao, X. Application of TPMS structure in bone regeneration. Eng. Regen. 2021, 2, 154–162. [Google Scholar] [CrossRef]
- Pugliese, R.; Graziosi, S. Biomimetic scaffolds using triply periodic minimal surface-based porous structures for biomedical applications. SLAS Technol. 2023, 28, 165–182. [Google Scholar] [CrossRef]
- Li, S.; Lee, W.-T.; Yeom, J.-T.; Kim, J.G.; Oh, J.S.; Lee, T.; Liu, Y.; Nam, T.-H. Towards bone-like elastic modulus in Ti Nb Sn alloys with large recovery strain for biomedical applications. J. Alloys Compd. 2022, 925, 166724. [Google Scholar] [CrossRef]
- Wang, R.; Wang, R.; Chen, D.; Qin, G.; Zhang, E. Novel CoCrWNi alloys with Cu addition: Microstructure, mechanical properties, corrosion properties and biocompatibility. J. Alloys Compd. 2020, 824, 153924. [Google Scholar] [CrossRef]
- Jiang, F.; Zhu, W.; Zhao, C.; Li, Y.; Wei, P.; Wan, T.; Ye, H.; Pan, S.; Ren, F. A strong, wear- and corrosion-resistant, and antibacterial Co–30 at.% Cr–5 at.% Ag ternary alloy for medical implants. Mater. Des. 2019, 184, 108190. [Google Scholar] [CrossRef]
- Brar, H.S.; Berglund, I.S.; Allen, J.B.; Manuel, M.V. The role of surface oxidation on the degradation behavior of biodegradable Mg-RE (Gd, Y, Sc) alloys for resorbable implants. Mater. Sci. Eng. C Mater. Biol. Appl. 2014, 40, 407–417. [Google Scholar] [CrossRef]
- Szyba, D.; Bajorek, A.; Babilas, D.; Temleitner, L.; Łukowiec, D.; Babilas, R. New resorbafble Ca-Mg-Zn-Yb-B-Au alloys: Structural and corrosion resistance characterization. Mater. Des. 2022, 213, 110327. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).