Abstract
The application of wide-bandgap semiconductors in next-generation power modules requires cost-effective Cu particles and a reduced bonding time in the die attachment process to enable efficient industrial-scale manufacturing. Therefore, this study aimed to analyze the effect of Cu particle size variation on pressure-assisted sinter-bondability and bond line shear strength. Cu particles were synthesized through a simple wet-chemical process, in which pH variation was employed to obtain submicrometer-sized Cu particles with average diameters of 500, 300, and 150 nm. The synthesized particles exhibited pure Cu composition, forming only a native oxide layer on their surfaces. In pastes containing these Cu particles, smaller particle sizes led to the delayed evaporation of the reducing solvent, which in turn delayed the exothermic reactions associated with particle sintering and oxidation. However, the sintering-induced exothermic peak became more pronounced as the particle size decreased, confirming that smaller particles improved sinterability. Pressure-assisted sinter bonding performed in air at 300 °C indicated that a decreased particle size contributed to the densification of the bond line structure and an increase in shear strength. Specifically, the paste containing 150 nm Cu particles achieved a highly dense microstructure and an exceptional shear strength of 36.7 MPa within just 30 s of sinter bonding. These findings demonstrate that reducing the particle size is essential for enhancing the sinter-bondability of cost-effective Cu particle-based sinter-bonding pastes.
1. Introduction
As the transition from combustion-engine vehicles to electric vehicles accelerates, the application of wide-bandgap (WBG) semiconductors to improve power module efficiency is expected to gain momentum [1,2,3,4,5]. Unlike Si-based power devices, which typically operate in the mid-100 °C range, WBG semiconductors can operate at much higher temperatures, often exceeding 250 °C [6]. However, this approach poses a reliability challenge at the chip level [7,8]. Specifically, the solder used for die attachment in power devices may undergo remelting or experience a significant reduction in mechanical strength at high temperatures. Consequently, solder joints must be replaced with sintered bonding materials.
With advancements in power module technology, Ag sinter bonding has evolved in recent decades and is now entering industrial applications in SiC power modules [9,10,11]. Bonding materials utilizing Ag nanoparticles exhibit exceptional reliability even in high-temperature environments and boast a thermal conductivity of up to 300 W/m·K [12,13], which is significantly superior to that of conventional solder joints. Furthermore, Ag joints have a much higher initial bond strength than traditional solder joints [14]. However, because sinter bonding relies on the solid-state sintering mechanism of Ag particles, the bonding process takes longer than reflow soldering [9,10,11,12,13].
Recent research on sinter bonding has focused on reducing material costs, leading to the active exploration of Cu-based sinter bonding [15,16,17,18,19,20,21,22,23,24]. Compared with Ag, the primary challenge with Cu particles is their susceptibility to oxidation [25], which inhibits solid-state sintering by forming an oxide layer on the surface. To mitigate this, reducing atmospheres are used during the bonding process, or reducing agents are incorporated into the paste formulation [26,27,28,29]. In the latter case, the characteristics of the reducing agent directly influence the sintering behavior of the Cu particles [16,19,26,27,28,29,30,31]. Therefore, the development of an optimal reducing agent is the key to achieving high-performance Cu sinter bonding. Consequently, paste manufacturers consider their proprietary reducing agents as essential intellectual property.
Another critical factor in sinter-bonding pastes is the size and morphology of the filler particles. However, particle size has been prioritized over shape. Therefore, it is essential to quantitatively assess how changes in Cu particle size influence sinter-bondability under controlled conditions, where all other material and process variables remain constant. Moreover, the paste must exhibit excellent reduction properties to enable rapid sinter bonding. Zuo et al. analyzed the effect of Cu particle size on pressure-assisted sinter bonding at 250 °C [30]. They investigated Cu particles of 20 and 100 nm and a 10 wt% mixture of 20 and 100 nm particles. Their results showed that 20 nm particles exhibited higher shear strength than 100 nm particles, whereas the mixture of 20 and 100 nm particles achieved the highest bond line density and the strongest shear strength. However, the paste formulation and organic solvent used as the reducing agent were not optimized. Consequently, despite using sub-100 nm nanoparticles and applying the optimal process conditions, the shear strength did not reach 17 MPa. Therefore, to better understand the effect of the Cu particle size variation, it is necessary to use at least three different particle sizes and conduct a comparative analysis of the shear strength values obtained under the optimal reduction formulation of the paste. Subsequently, Liu et al. synthesized Cu particles with four different average sizes (57.5, 144.5, 104.5, and 193.4 nm) and formulated a paste using polyethylene glycol [31]. They conducted sinter bonding at 300 °C for 1 h under a nitrogen flow without applying external pressure. Surprisingly, the 144.5 nm particles exhibited the highest bond line shear strength. This contradicts the theoretical expectations, as the highest packing density does not necessarily occur at intermediate particle sizes. The authors attributed this high shear strength to the lower void content of the sintered microstructure. However, further investigation under pressure-assisted sintering conditions is necessary to clarify the relationship between Cu particle size and shear strength.
In this study, we leveraged our expertise in reducing agent technology to formulate a Cu paste and systematically analyzed the effect of Cu particle size on the pressure-assisted sinter bonding characteristics. Cu particles were synthesized using an identical method, except for variations in the pH, ensuring minimal changes in particle properties other than size. The synthesized Cu particles had average sizes of 500, 300, and 150 nm.
2. Materials and Methods
2.1. Synthesis of Cu Particles
Submicron-sized Cu particles were synthesized using an aqueous reduction method [31]. Deionized (DI) water (50 mL) was placed in a 500 mL vial, followed by the addition of 1.26 M copper(II) oxide (CuO, Samchun Pure Chemical Co., Ltd., Gunpo, Republic of Korea) and 1.27 M oxalic acid (H2C2O4, Daejung Chemicals & Metals Co., Ltd., Siheung, Republic of Korea). The mixture was stirred at 300 rpm and allowed to react for 20 h. A reducing agent solution was prepared by dissolving 1.5 M L-ascorbic acid (C6H8O6, 99%, Daejung Chemicals & Metals Co., Ltd., Siheung, Republic of Korea) in 100 mL DI water. After the Cu precursor solution reacted for 20 h, the reducing agent solution was added, and the mixture was stirred at 300 rpm for 5 min. To adjust the pH, a 5 M sodium hydroxide (NaOH, 98%, Samchun Pure Chemical Co., Ltd., Gunpo, Republic of Korea) solution was prepared and added dropwise until the pH reached 9, 10, or 11. Finally, the mixed solution was placed in a 90 °C water bath and stirred at 300 rpm for 30 min to synthesize the Cu particles. The resulting suspension was stirred continuously and air-cooled to room temperature.
The Cu particles were collected by centrifugation. The supernatant was removed and the residual organic substances in the suspension were diluted and eliminated by sequentially replacing the liquid with DI water or ethanol. Two consecutive DI water rinses were performed, followed by a single ethanol rinse to facilitate the drying process. The washed Cu particles were then dried in a vacuum chamber at 5 × 10−2 torr for 10 h at room temperature before collection.
2.2. Preparation of Cu Pastes
For paste preparation, a reducing formulation was prepared by mixing EW-10 solvent (Epsilon Epowder, Seongnam, Republic of Korea), which has been verified for its excellent reduction capability on Cu particle surface oxide layers, with glycerol (C3H8O3, Sigma-Aldrich Co. LLC, Saint Louis, MO, USA) at a weight ratio of 4:6. The synthesized Cu particles were uniformly mixed with the reducing formulation using a vortex mixer to produce a Cu paste.
2.3. Sinter Bonding
For sinter bonding, a stencil mask was used to print Cu paste onto a dummy Cu substrate in a 3 × 3 × 0.1 mm3 patterned area. A dummy Cu chip measuring 3 × 3 × 1 mm3 was aligned and placed on top of the printed pattern. To minimize bonding interference caused by the vaporization of the paste components, the sandwich-structured specimen was pre-heated at 150 °C for 30 s on a hot plate to dry the paste. Finally, the sandwich-structured specimen was rapidly heated to 300 °C in ambient air while applying a pressure of 10 MPa to perform die bonding via sinter bonding. The thermocompression bonding equipment was custom-built, and the heating rate to 300 °C was approximately 30 °C/s.
2.4. Characterization
The morphology of the synthesized Cu particles was observed using high-resolution field-emission scanning electron microscopy (HR-FE-SEM; SU8010, Hitachi High-Technologies Corp., Tokyo, Japan) at an accelerating voltage of 10 kV. The phase analysis of the synthesized Cu particles was performed using X-ray diffraction (XRD; DE/D8 Advance, Bruker, Billerica, MA, USA). In addition, for a precise surface phase analysis, X-ray photoelectron spectroscopy (XPS; K-Alpha+, Thermo Fisher Scientific, Waltham, MA, USA) was performed.
The thermal behavior of the Cu pastes was analyzed by thermogravimetry–differential thermal analysis (TG-DTA; DTG-60, Shimadzu, Kyoto, Japan) at a heating rate of 20 °C/min in ambient air.
To observe the microstructure of the bond line after sinter bonding, the sandwich-structured specimen was mounted in a vertical position, and its cross-section was prepared through grinding and polishing. The bond line microstructure was examined using scanning electron microscopy (SEM; SU8010, Hitachi High-Technologies Corp., Tokyo, Japan) in back-scattered electron (BSE) mode.
The strength of the formed bond line was evaluated by shear testing using a microshear tester (Hawk 8200S, Kovis Technology, Seoul, Republic of Korea). The shear test was conducted by positioning the shear tip 150 μm above the substrate surface and moving it at a speed of 200 μm/s to shear the dummy chip. The maximum stress recorded before bond line failure was measured and determined as the shear strength value. For each bonding condition, the shear strength was measured for five specimens and the average value reported. After shear testing, the fracture surface microstructure was observed at low and high magnification using SEM.
3. Results and Discussion
3.1. Characteristics of Synthesized Cu Particles
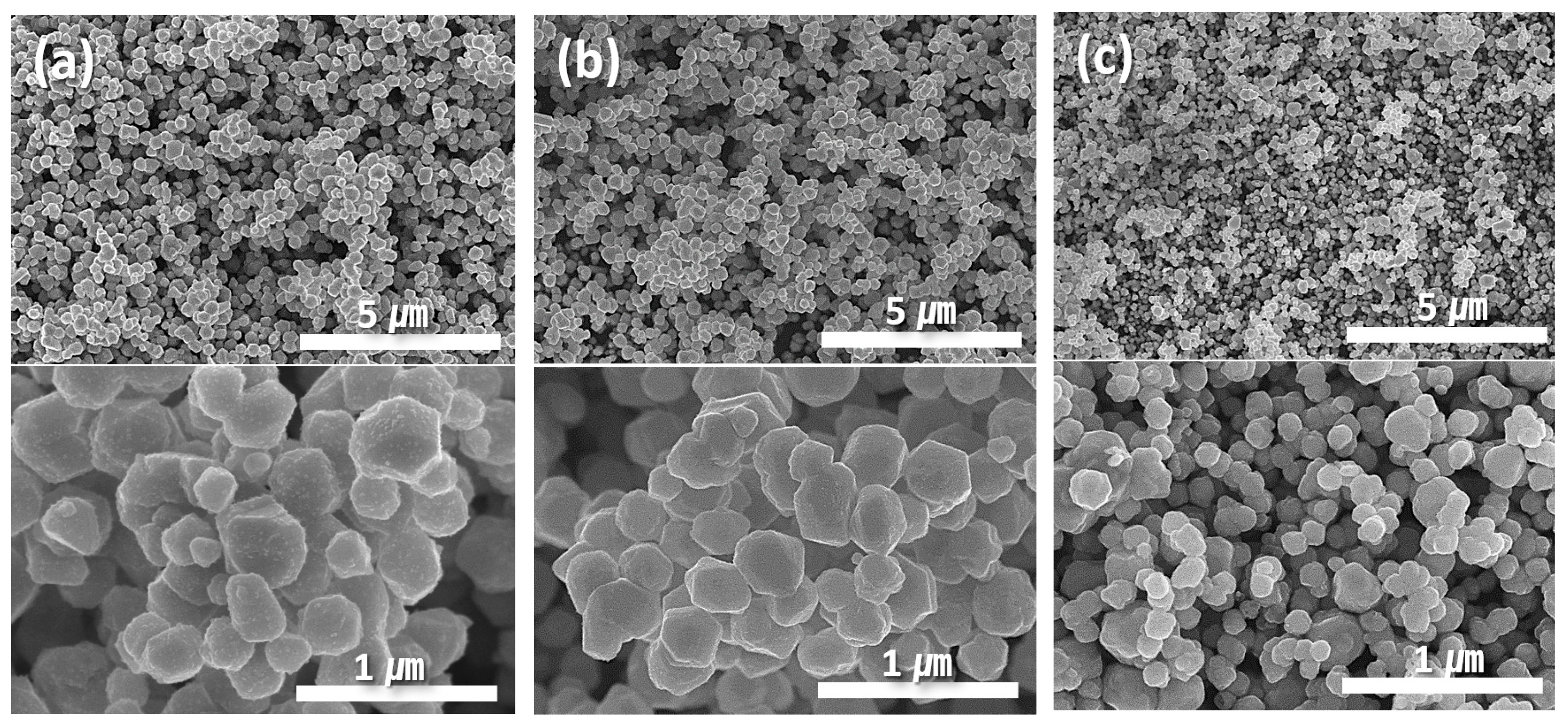
Figure 1 illustrates the morphological changes in the synthesized particles as a function of pH. Regardless of the pH, all synthesized particles exhibited a pseudo-spherical shape with a polyhedral morphology. An increase in pH resulted in a decrease in the average particle size. Image analysis determined that the average particle sizes at pH 9, 10, and 11 were 500 (±85), 300 (±70), and 150 (±30) nm, respectively. The reduction in the particle size was accompanied by a decrease in the standard deviation, resulting in a more uniform size distribution.
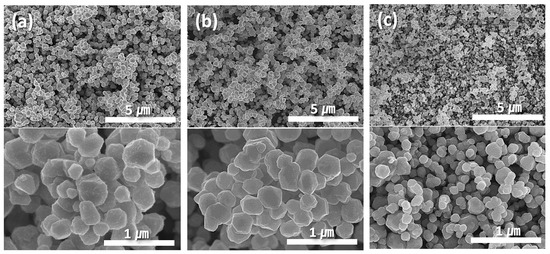
Figure 1.
SEM images showing morphologies of (a) 500, (b) 300, and (c) 150 nm Cu particles synthesized at (a) pH 9, (b) 10, and (c) 11.
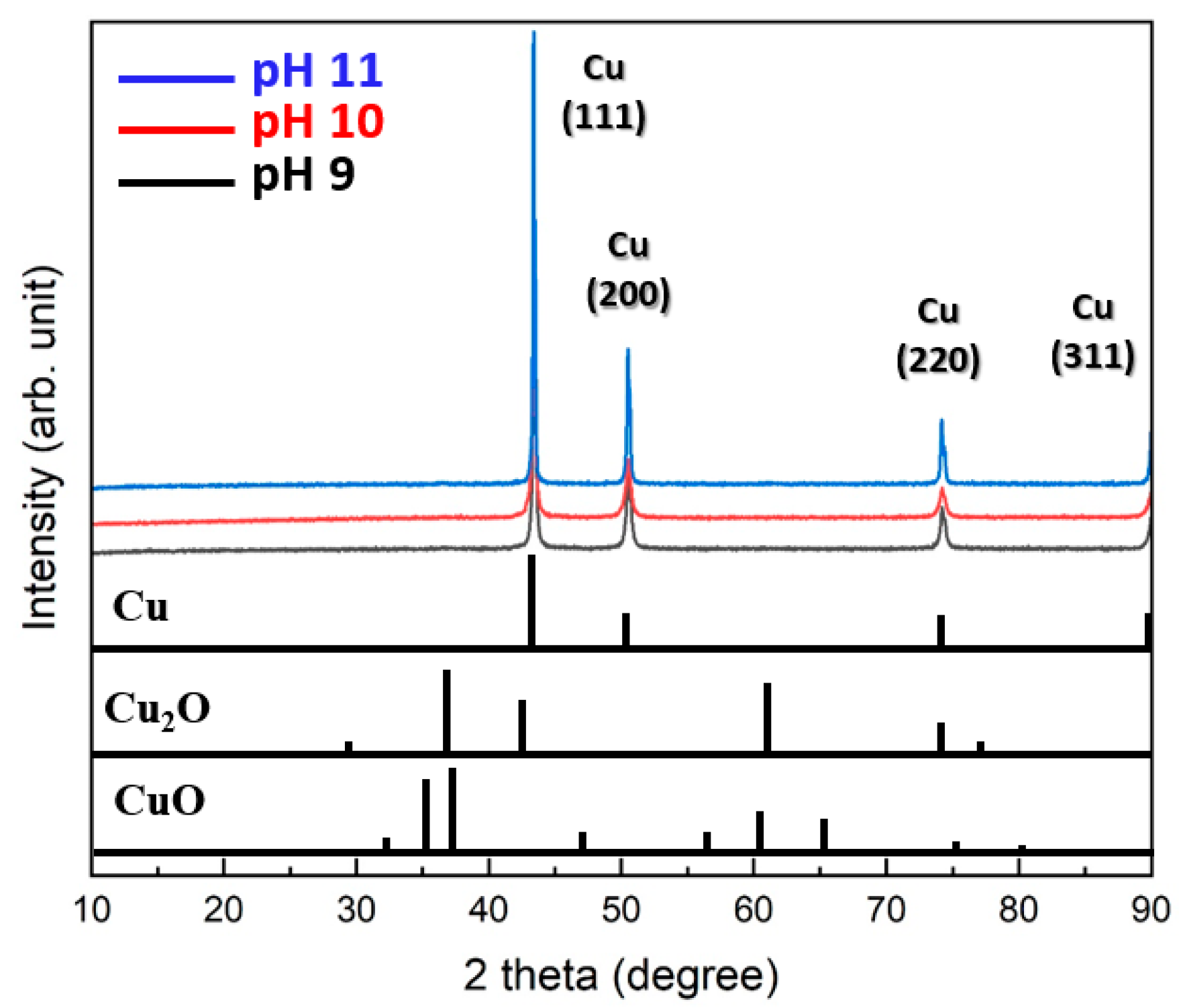
Figure 2 summarizes the XRD patterns of the particles synthesized at different pH levels. All particles synthesized at pH 9, 10, and 11 exhibited only pure Cu phases without any detectable oxides such as Cu2O or CuO. Specifically, the (111) plane exhibited the most prominent growth, followed by clear detection of the (200) and (220) planes. These results indicate that the synthesis method is highly effective for producing pure Cu particles.
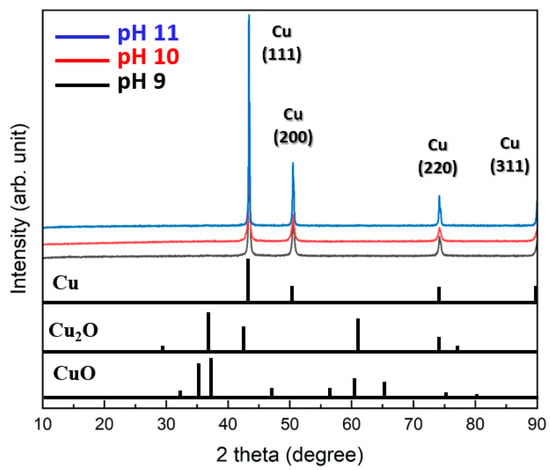
Figure 2.
Cu 2p XPS spectra of 150 nm Cu particles synthesized at a pH of 11.
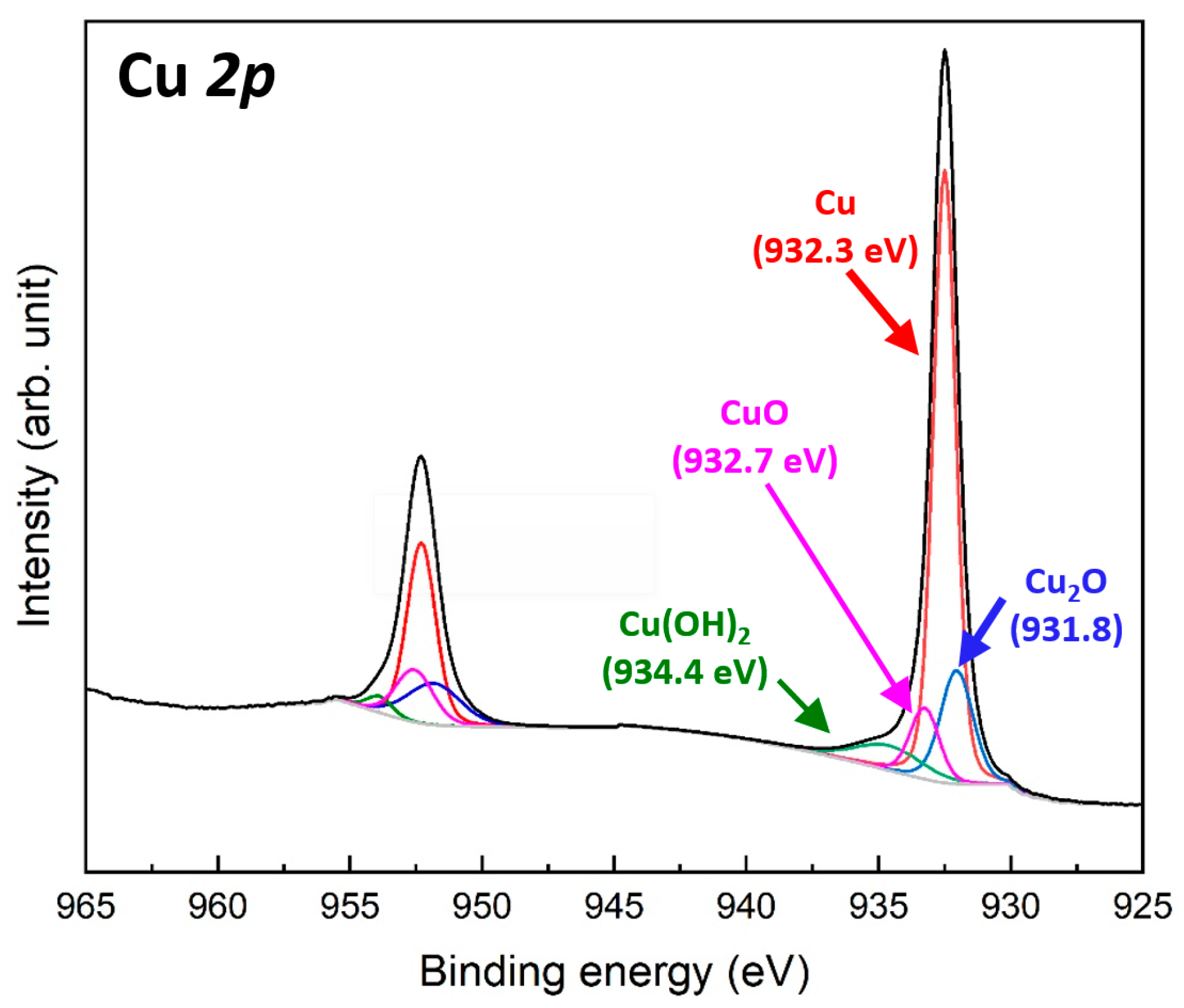
Figure 3 shows the Cu 2p XPS spectra of the Cu particles synthesized at pH 11, which exhibited the smallest particle size among the synthesis conditions. The two spectra measured at 932.3 and 952.1 eV correspond to the Cu 2p3/2 and Cu 2p1/2 orbitals, respectively [32]. The energy separation of approximately 19.8 eV confirms the presence of elemental Cu [33]. The Cu 2p3/2 spectrum can be deconvoluted into three components: the main Cu0 peak at 932.3 eV, a minor Cu+ peak at 931.8 eV associated with Cu2O formation, and a weak Cu2+ peak at 932.7 eV related to CuO formation [34]. These results indicate the formation of an extremely thin oxide layer on the surface of the synthesized Cu particles. In addition, a weak Cu2+ peak was detected at 934.4 eV, corresponding to Cu(OH)2 formation [35], which likely resulted from residual moisture or water adsorption. A similar trend was observed in the Cu 2p1/2 spectrum. These results confirm that the Cu particles synthesized using the aqueous reduction method possess a thin native oxide layer on their surfaces, along with minor moisture adsorption.
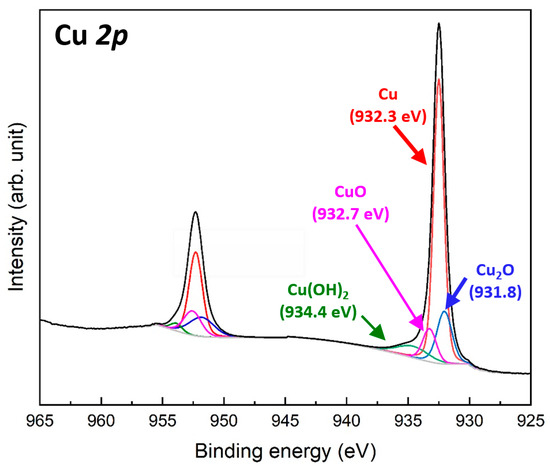
Figure 3.
Cu 2p XPS spectra of 150 nm Cu particles synthesized at pH 11.
3.2. Properties of Prepared Cu Pastes
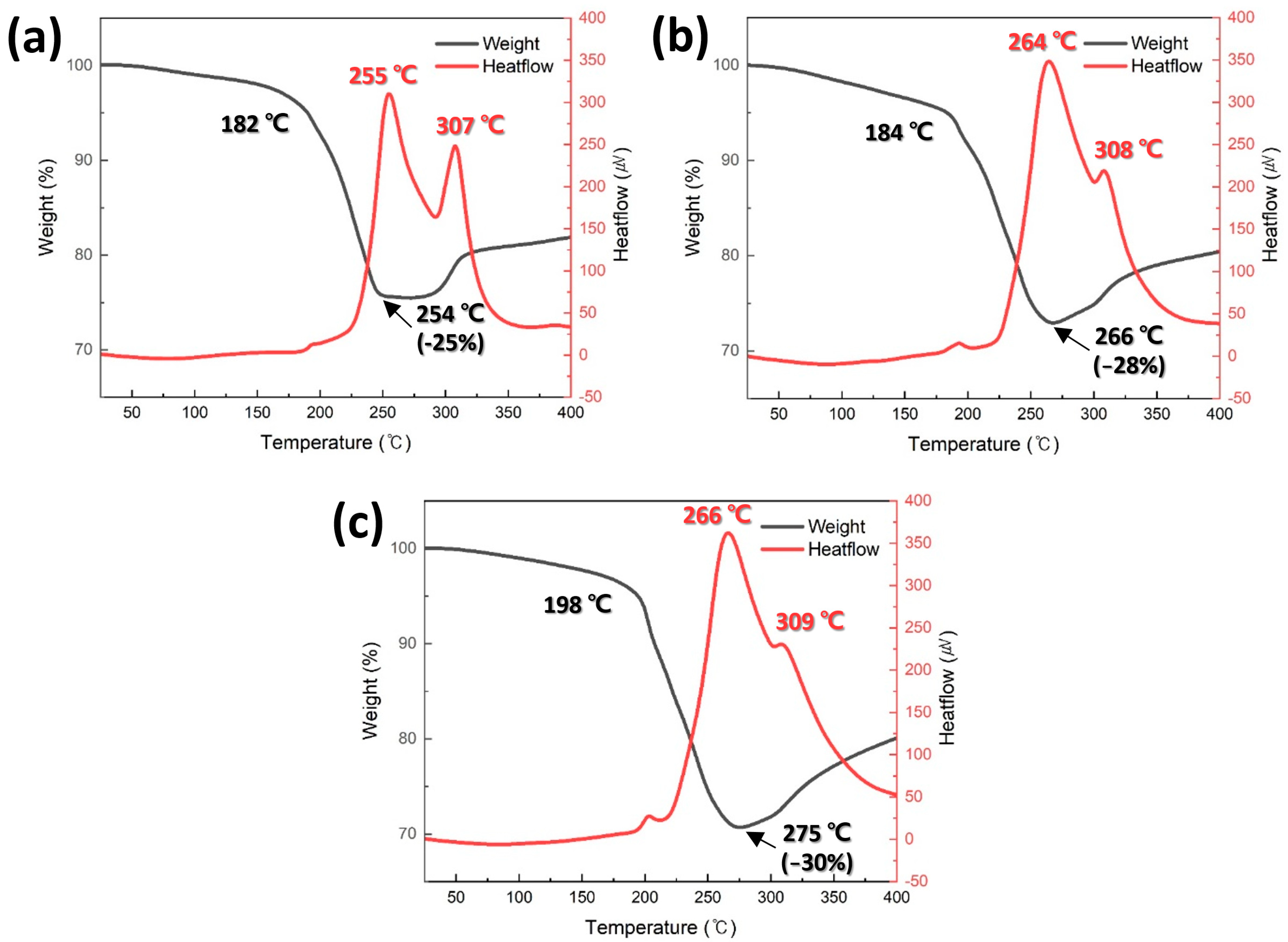
Figure 4 shows the TG-DTA thermograms for the pastes containing Cu particles synthesized at different pH levels. In all pastes, a rapid weight loss by the evaporation of the reducing solvent began in the temperature range of 182–198 °C. Notably, as the average Cu particle size decreased, the onset temperature of the rapid weight loss increased. This trend can be attributed to the increased total surface area of the smaller particles, which leads to a higher amount of reducing solvent wetting the surface, thereby delaying solvent evaporation. Subsequently, a weight increase owing to the oxidation of Cu particles was observed in the temperature range of 254–275 °C. Similarly to the onset temperature of the weight loss, the onset temperature of the weight increase increased with decreasing particle size. This can also be explained by the increased amount of reducing solvent wetting the Cu particles, which delays oxidation. When the initial weight of each paste was set to 100%, the minimum weight recorded for each paste was measured as −25%, −28%, and −30%, shown in Figure 4a–c, respectively, indicating that the maximum weight loss increased as the average particle size decreased. Although the evaporation of the reducing solvent was delayed for smaller particles, the oxidation onset temperature was further delayed, leading to an equivalent higher weight reduction.
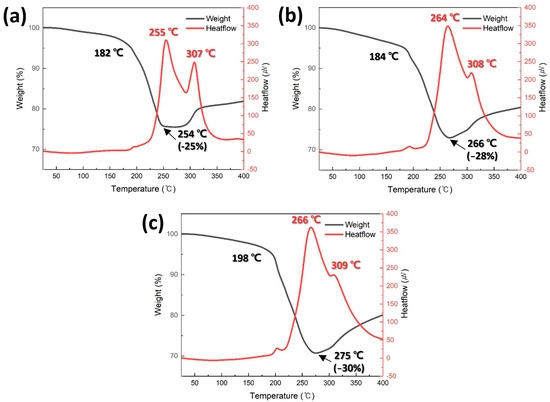
Figure 4.
TG-DTA results of pastes containing (a) 500, (b) 300, and (c) 150 nm Cu particles synthesized at (a) pH 9, (b) 10, and (c) 11.
Two distinct exothermic peaks were observed in the DTA curves of all the pastes, and the intensity of these peaks increased as the average Cu particle size decreased. The lower-temperature peak is attributed to the sintering of Cu particles, whereas the higher-temperature peak is associated with Cu particle oxidation. The relatively high sintering peak temperature observed in Figure 4c appears to contradict previous reports, suggesting that smaller particle sizes reduce the sintering temperature. However, this is likely due to the increased residual solvent content, which delays the sintering process. Nevertheless, the peak intensity increases with decreasing particle size, strongly supporting the established theory that smaller particle sizes improve sinterability [36]. It is important to note that these sintering-induced exothermic peaks were measured under nonpressurized conditions. Under pressure-assisted conditions, the exothermic peaks are expected to be much more pronounced owing to the increased contact area between the particles. Furthermore, the order of the oxidation-related peak temperatures at higher temperatures further confirms the previously mentioned oxidation-delay effect.
3.3. Sinter Bonding Properties
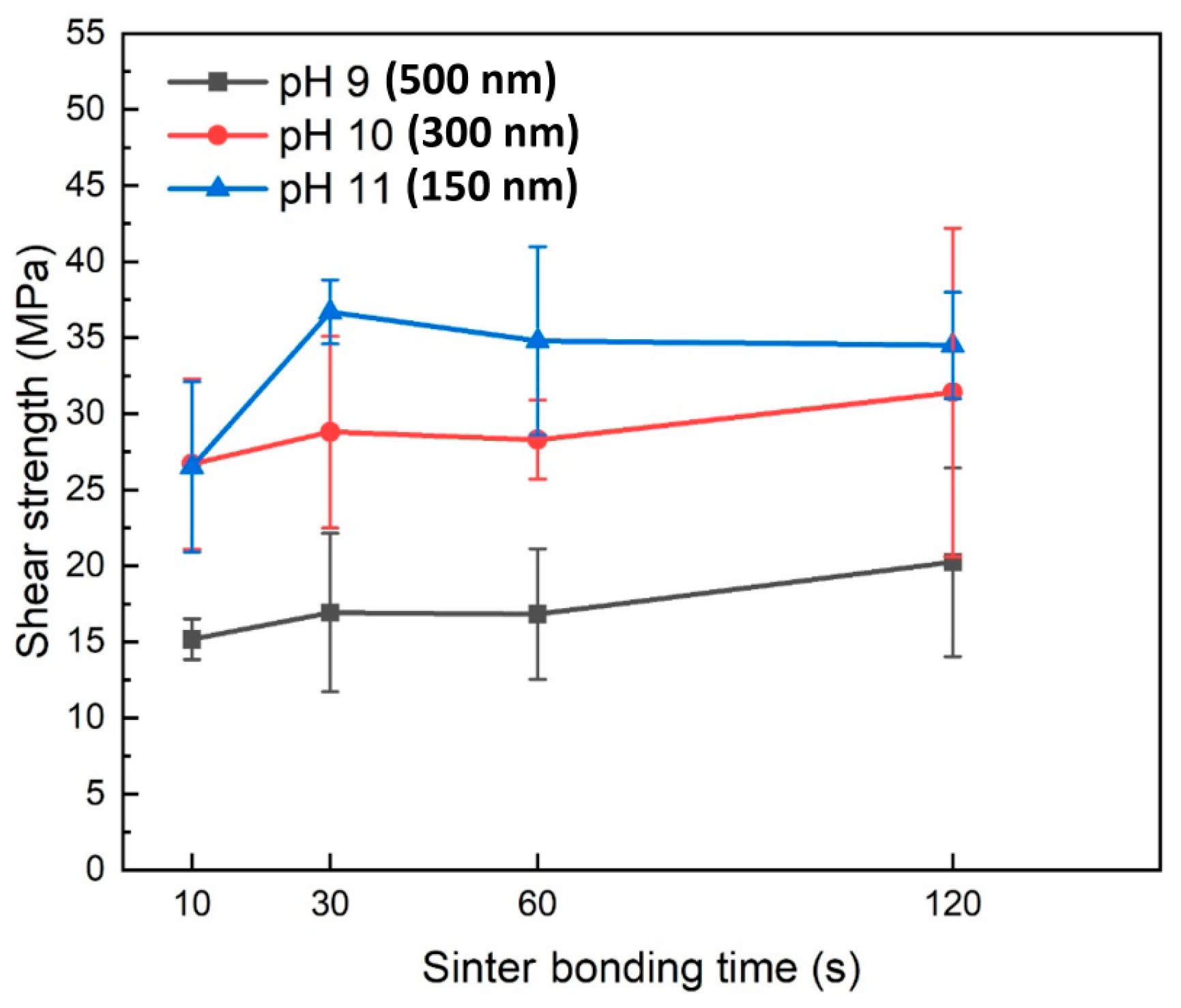
Figure 5 shows the shear strength values of the formed joints as a function of bonding time when pressure-assisted sinter bonding was performed in air at 300 °C using the pastes containing Cu particles synthesized at different pH levels. The shear strength results indicated that a decrease in Cu particle size led to a significant increase in the bonding strength. Specifically, in the bonding time range of 10–120 s, the paste containing 500 nm Cu nanoparticles synthesized at pH 9 exhibited a shear strength of 15.2–19.8 MPa. In contrast, the paste with 300 nm Cu nanoparticles synthesized at pH 10 showed a significantly higher shear strength of 26.7–31.4 MPa, with a general trend of increasing shear strength as bonding time increased. The paste containing 150 nm Cu nanoparticles synthesized at pH 11 initially displayed a shear strength similar to that of the 300 nm Cu nanoparticles at a bonding time of 10 s. However, at 30 s, it exhibited the highest shear strength of 34.5 MPa. Achieving an exceptional shear strength of approximately 35 MPa with only 30 s of bonding in air is an outstanding sinter bonding result that has rarely been reported [15,16,17,18,19,20,22,23,26,27,28,29,30,31]. However, as the bonding time increased to 60 and 120 s, the shear strength no longer increased and instead showed a gradual decline.
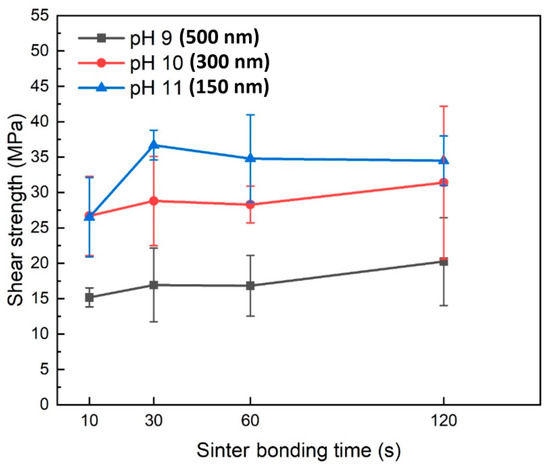
Figure 5.
Shear strengths of bond lines sintered between Cu finishes under 10 MPa at 300 °C in air using pastes containing Cu particles synthesized at different pH levels.
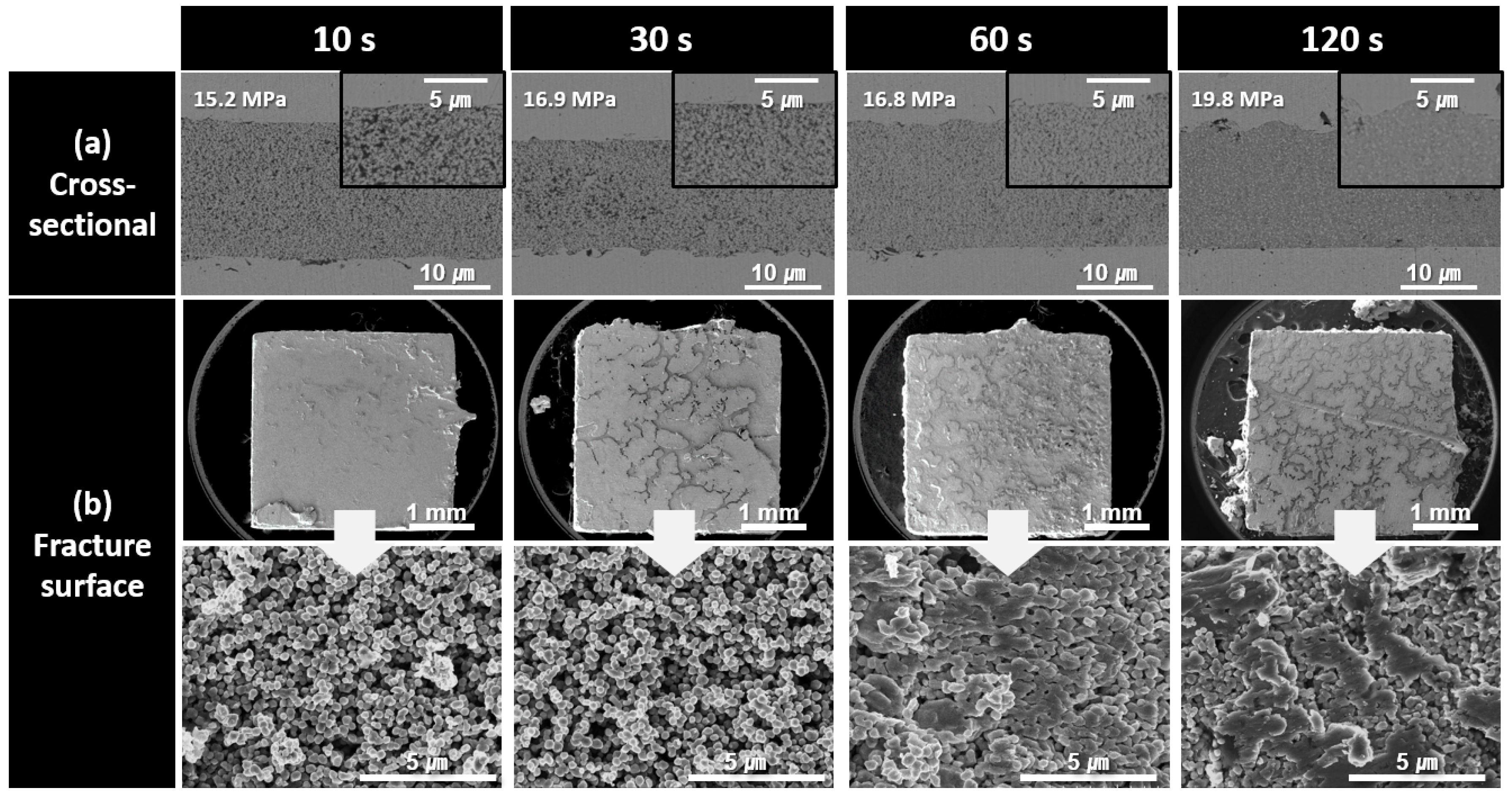
To investigate the causes of the observed shear strength trends, the cross-sectional and fracture microstructures of the bond lines were analyzed as a function of the bonding time for each paste type. Figure 6 shows the cross-sectional and fracture microstructures of the bond lines formed using the paste containing 500 nm Cu nanoparticles synthesized at pH 9 for different bonding times. In the cross-sectional microstructures of the bond lines, bonding for an extremely short duration of 10 s resulted in a highly porous and loose structure. As the bonding time increased to 120 s, the microstructural density gradually improved. However, even after 120 s of bonding, porous structures were still observed in the high-magnification cross-sectional images, indicating that bonding at 300 °C under 10 MPa pressure was insufficient to achieve a fully dense bond line. The low density of the bond line was further confirmed by the fracture images obtained after shear testing. For up to 30 s of bonding, the Cu particle morphology remained intact, suggesting that sintering progressed primarily to the extent of neck formation between the particles. At 60 s, some particle morphologies began to disappear, and after 120 s, coarse structures induced by sintering began to appear. However, the presence of visible particle shapes even at 120 s indicated that the sintering process was still incomplete. Consequently, all the fractured samples bonded for up to 120 s exhibited an interfacial failure mode, with fractures occurring along the upper and lower interfaces of the bond line. The low microstructural densities and interfacial failure modes observed in the fracture surfaces are consistent with the low strength values measured in Figure 5.
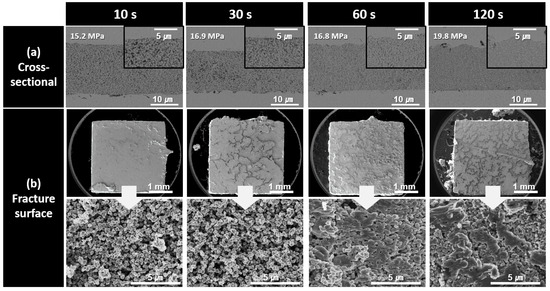
Figure 6.
SEM images of bond lines sintered between Cu finishes under 10 MPa at 300 °C in air using pastes containing 500 nm Cu particles synthesized at pH 9. (a) Cross-sectional microstructures and (b) fracture surfaces of bond lines for different bonding times.
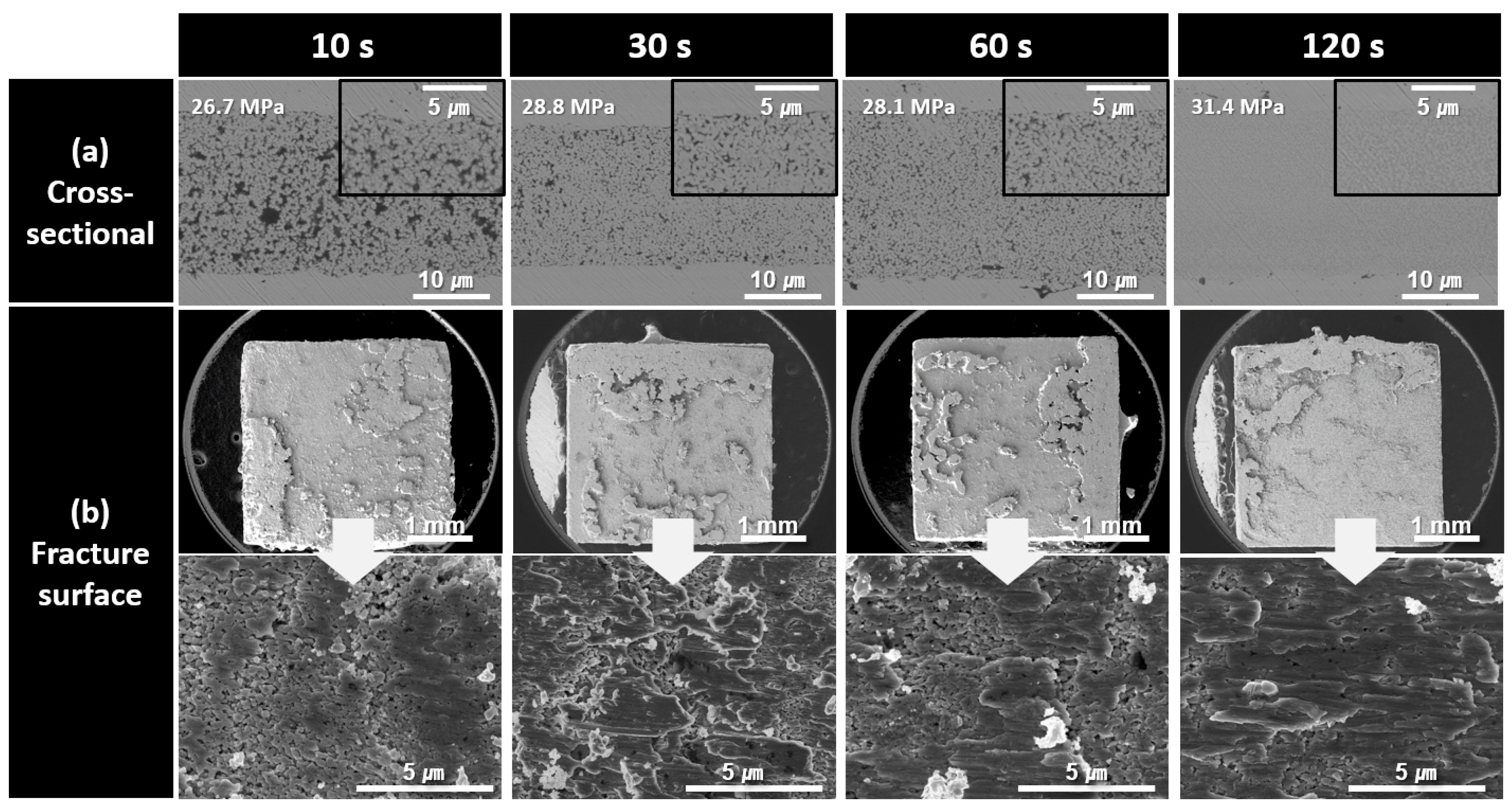
The cross-sectional and fracture microstructures of the bond lines formed using the paste containing 300 nm Cu nanoparticles synthesized at pH 10 for different bonding times are shown in Figure 7. Similarly to the previous observations, the density of the bond lines gradually increased as the bonding time increased from 10 to 120 s. The improved sinterability of the 300 nm Cu nanoparticles is clearly evident in the fracture images. After 10 s of bonding, the fracture image showed a structure in which some particle shapes remained; however, large coarse structures had already formed and were sheared under stress. This corresponds well with the high shear strength of 26.7 MPa observed in Figure 5. The similar microstructures and failure modes observed in the fracture surfaces are consistent with the strength results shown in Figure 5, which were similar despite the increase in the bonding time.
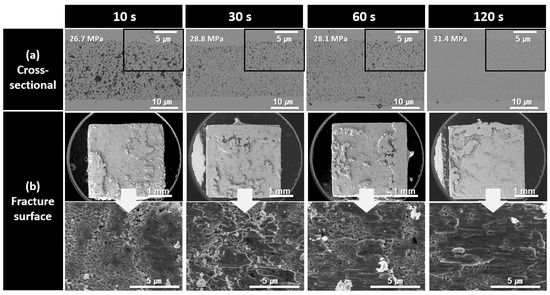
Figure 7.
SEM images of bond lines sintered between Cu finishes under 10 MPa at 300 °C in air using pastes containing 300 nm Cu particles synthesized at pH 10. (a) Cross-sectional microstructures and (b) fracture surfaces of bond lines for different bonding times.
As the bonding time increased to 30, 60, and 120 s, the extent of the coarsened structures on the fracture surfaces gradually expanded, although no significant structural changes were observed. For all samples bonded for up to 120 s, fractures predominantly propagated within the bond line, exhibiting a cohesive failure mode.
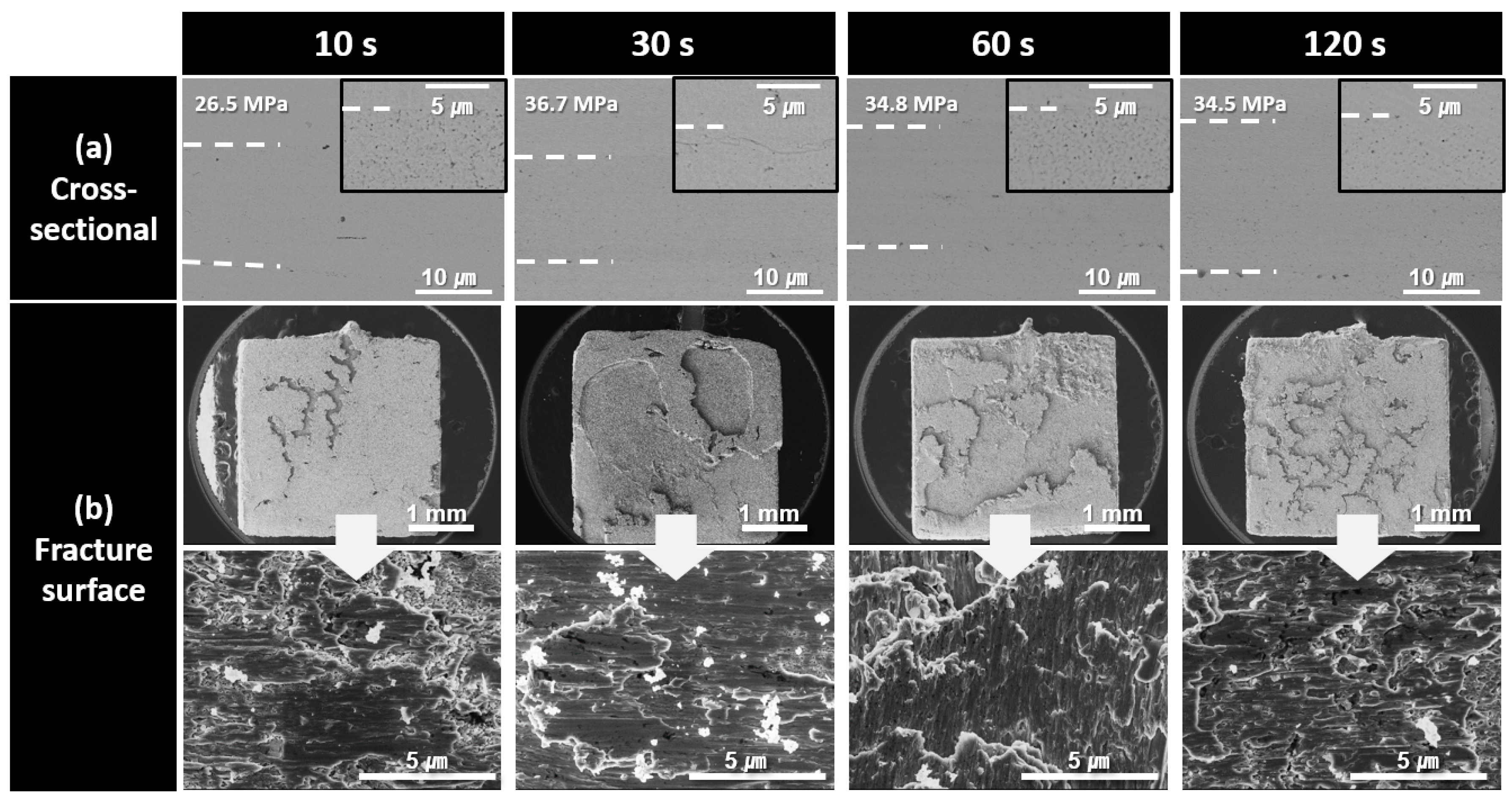
Figure 8 shows the cross-sectional and fracture microstructures of the bond lines formed using the paste containing 150 nm Cu nanoparticles synthesized at pH 11 for different bonding times. A highly porous bond line was clearly observed after 10 s of bonding, whereas a significantly denser bond line microstructure was achieved after 30 s of bonding. For samples bonded for 30 s or longer, the fracture surfaces exhibited a structure resembling the bulk metal torn apart by a shear force without any remaining particle-shaped structures. The fracture surfaces exhibited elongated metallic structures with minimal void formation. However, in the fracture surface of the sample bonded for 120 s, the elongated patterns were reduced, and a somewhat brittle microstructure was observed. This brittleness is attributed to the increased oxygen content in the bond line owing to prolonged bonding in high-temperature air. The increased oxygen level likely led to the formation of fine oxide phases, such as Cu2O [24], which contributed to the increased brittleness of the bond line. As a result, the decrease in shear strength after reaching its peak at 30 s, as was observed in Figure 5, can be explained by the oxidation occurring in the bond line during prolonged bonding at 60 and 120 s. In addition, the noticeably lower shear strength observed at 10 s of bonding, despite the relatively good bond line microstructure, was attributed to insufficient sinter bonding at the interface because of the excessively short bonding time. Consequently, a significant number of fractures occurred near the bond line/substrate interface. Nevertheless, the fracture surfaces of all the samples bonded for up to 120 s were primarily formed within the bond line itself, classifying the failure mode as a cohesive failure.
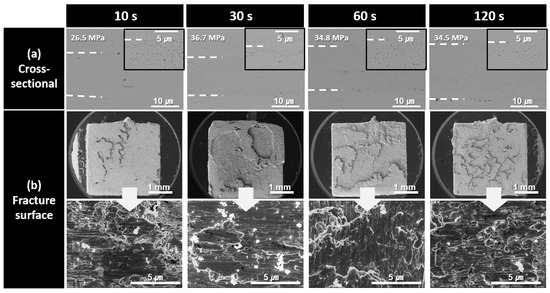
Figure 8.
SEM images of bond lines sintered between Cu finishes under 10 MPa at 300 °C in air using pastes containing 150 nm Cu particles synthesized at pH 11. (a) Cross-sectional microstructures and (b) fracture surfaces of bond lines for different bonding times.
The fundamental driving force of sintering is the reduction in the overall free energy of a system. On a macroscopic scale, this process is characterized by the growth of sintering necks at the contacts between the particles. Microscopically, it involves atomic diffusion processes such as surface diffusion during the initial sintering stage. The presence of smaller particles, with their high specific surface area and pronounced surface curvature, leads to a significant increase in surface energy. This excess energy is the primary reason why smaller particles demonstrate enhanced sinterability at the same sintering temperature.
4. Conclusions
Submicrometer-sized Cu particles with average diameters of 500, 300, and 150 nm were successfully synthesized by adjusting the pH to 9, 10, and 11, respectively. The XRD analysis of the synthesized particles did not detect any oxide phases such as Cu2O or CuO. However, the XPS analysis confirmed the presence of a very thin oxide layer on the particle surfaces. Considering that this oxide layer is a native oxide that is difficult to suppress, the synthesized particles can be regarded to be pure Cu.
Thermal analysis revealed that smaller Cu particles exhibited a delayed evaporation of the reducing solvent owing to capillary effects. This resulted in the delayed onset of both sintering- and oxidation-induced exothermic reactions. However, under nonpressurized conditions, the sintering-induced exothermic peak became more pronounced as the particle size decreased, indirectly confirming that a smaller particle size improves sinterability. In pressure-assisted sinter bonding performed in air at 300 °C, the decrease in particle size contributed to the densification of the bond line structure and an increase in shear strength, particularly after 30 s of bonding. For instance, using a paste containing Cu particles with an average diameter of 150 nm, an excellent shear strength of 36.7 MPa was achieved after 30 s of solid-state sinter bonding. The bond line formed under these conditions exhibited such a high density that voids were barely visible in the low-magnification images. However, as the bonding time increased further, the densification effect became negligible, whereas the oxygen content in the bond line increased, leading to the formation of brittle oxides that suppressed additional shear strength improvements. These findings clearly indicate that in the development of cost-effective sinter-bonding pastes utilizing Cu particles, reducing the particle size is essential for enhancing sinter bondability, particularly if an appropriate reducing agent is developed and uniform particle dispersion in the paste is ensured. In addition, smaller particle sizes contributed to shorter bonding times, further improving the efficiency of the sinter bonding process.
Author Contributions
Conceptualization, J.-H.L.; methodology, H.K. and J.-H.L.; validation, H.K.; formal analysis, H.K. and J.-H.L.; investigation, H.K.; resources, H.K. and J.-H.L.; data curation, H.K.; writing—original draft preparation, J.-H.L.; writing—review and editing, J.-H.L.; visualization, H.K.; supervision, J.-H.L.; project administration, J.-H.L.; funding acquisition, J.-H.L. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by a National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (No.: 2021R1A2C1007400).
Data Availability Statement
The original contributions presented in the study are included in the article, and further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Robles, E.; Matallana, A.; Aretxabalets, I.; Andreu, J.; Fernández, M.; Martin, J.L. The role of power device technology in the electric vehicle powertrain. Int. J. Energy Res. 2021, 46, 22222–22265. [Google Scholar]
- Kumar, A.; Moradpour, M.; Losito, M.; Franke, W.-T.; Ramasamy, S.; Baccoli, R.; Gatto, G. Wide band gap devices and their application in power electronics. Energies 2022, 15, 9172. [Google Scholar] [CrossRef]
- Yadlapalli, R.T.; Kotapati, A.; Kandipati, R.; Koritala, C.S. A review on energy efficient technologies for electric vehicle applications. J. Energy Storage 2022, 50, 104214. [Google Scholar]
- Chaudhary, O.S.; Denaï, M.; Refaat, S.S.; Pissanidis, G. Technology and applications of wide bandgap semiconductor materials: Current state and future trends. Energies 2023, 16, 6689. [Google Scholar] [CrossRef]
- Via, F.L.; Alquiwe, D.; Giannazzo, F.; Kimoto, T.; Neudeck, P.; Ou, H.; Roncaglia, A.; Saddow, S.H.; Tudisco, S. Emerging SiC applications beyond power electronic devices. Micromachines 2023, 14, 1200. [Google Scholar] [CrossRef] [PubMed]
- Seal, S.; Mantooth, H.A. High performance silicon carbidepower packaging–past trends, present practices, and future directions. Energy 2017, 10, 341. [Google Scholar]
- Sakairi, H.; Yanagi, T.; Otake, H.; Kuroda, N.; Tanigawa, H. Measurement methodology for accurate modeling of SiC MOSFET switching behavior over wide voltage and current ranges. IEEE Trans. Power Electron. 2018, 33, 7314–7325. [Google Scholar]
- Zhang, L.; Yuan, X.; Wu, X.; Shi, C.; Zhang, J.; Zhang, Y. Performance evaluation of high power SiC MOSFET modules in comparison to Si IGBT modules. IEEE J. Emerg. Slected Topics Power Electron. 2024. Early Acess. [Google Scholar]
- Sahuri, A.; Billaud, Y.; Signor, L.; Saury, D.; Milhet, X. Experimental investigation of thermal conductivity during aging of nonporous sintered silver. Acta Mater. 2023, 257, 119109. [Google Scholar]
- Wakamoto, K.; Namazu, T. Mechanical characterization of sintered silver materials for power device packaging: A review. Energies 2024, 17, 4105. [Google Scholar] [CrossRef]
- Zhang, B.; Lu, X.; Ma, H.; Wang, D.; Mei, Y.-H. Development of silver paste with high sintering driving force for reliable packaging of power electronics. IEEE Trans. Compon. Packag. Manuf. Technol. 2024, 14, 10–17. [Google Scholar] [CrossRef]
- Du, C.; Zou, G.; Huo, J.; Feng, B.; Liu, L. Generative AI-enabled microstructure design of porous thermal interface materials with desired effective thermal conductivity. J. Mater. Sci. 2023, 58, 16160–16171. [Google Scholar]
- Zhang, H.O.; Bai, H.L.; Jia, Q.; Guo, W.; Liu, L.; Zou, G.S. High electrical and thermal conductivity of nano-Ag paste for power electronic applications. Acta Mater. Sin. 2020, 33, 1543–1555. [Google Scholar]
- Suganuma, K.; Kim, S.-J.; Kim, K.-S. High-temperature lead-free solders: Properties and possibilities. JOM 2009, 66, 64–71. [Google Scholar]
- Jianfeng, Y.; Guisheng, Z.; Anming, H.; Zhou, Y.N. Preparation of PVP coated Cu NPs and the application for low-temperature bonding. J. Mater. Chem. 2011, 21, 15981–15986. [Google Scholar]
- Li, J.; Yu, X.; Shi, T.; Cheng, C.; Fan, J.; Cheng, S.; Liao, G.; Tang, Z. Low-temperature and low-pressure Cu–Cu bonding by highly sinterable Cu nanoparticle paste. Nanoscale Res. Lett. 2017, 12, 255. [Google Scholar]
- Yoon, J.W.; Back, J.H. Effect of sintering conditions on the mechanical strength of Cu-sintered joints for high-power applications. Materials 2018, 11, 2105. [Google Scholar] [CrossRef]
- Li, J.; Liang, Q.; Shi, T.; Fan, J.; Gong, B.; Feng, C.; Tang, Z. Design of Cu nanoaggregates composed of ultra-small Cu nanoparticles for Cu-Cu thermocompression bonding. J. Alloys Compd. 2019, 772, 793–800. [Google Scholar]
- Peng, Y.; Mou, Y.; Liu, J.; Chen, M. Fabrication of high-strength Cu–Cu joint by low-temperature sintering micron–nano Cu composite paste. J. Mater. Sci. Mater. Electron. 2020, 31, 8456–8463. [Google Scholar]
- Chen, T.F.; Siow, K.S. Comparing the mechanical and thermal-electrical properties of sintered copper (Cu) and sintered silver (Ag) joints. J. Alloys Compd. 2021, 866, 158783. [Google Scholar]
- Choi, E.B.; Lee, J.-H. Tens-of-seconds solid-state sinter-bonding technique in air using in situ reduction of surface oxide layers on easily bendable dendritic Cu particles. Appl. Surf. Sci. 2022, 580, 152347. [Google Scholar]
- Kim, M.-S.; Kim, D.; Roh, M.-H.; Nishikawa, H. Highly reliable micro-scale Cu sintered joint by oxidation-reduction bonding process under thermal cycling. Microelectron. Reliab. 2023, 150, 115123. [Google Scholar]
- Xin, J.; Gao, Y.; Zhang, C.; Yang, L.; Liu, S.; Li, K.; Zhou, M.; Liu, Y.; Zhang, J.; Cai, W. High performance Cu sintering joint for power devices enabled by in-situ generation of Cu particles with multi-level hierarchical structures. J. Mater. Process. Technol. 2024, 329, 118435. [Google Scholar]
- Jung, S.H.; Lee, J.-H. Ultrafine dendritic Cu particles for extremely fast pressure-assisted sintering under air and pore-free bond lines. J. Mater. Res. Technol. 2025, 35, 3045–3057. [Google Scholar]
- Son, J.; Yu, D.-Y.; Kim, Y.-C.; Kim, S.-I.; Byun, D.; Bang, J. Thermal reliability of Cu sintering joints for high-temperature die attach. Microelectron. Reliab. 2023, 147, 115002. [Google Scholar]
- Liu, J.; Chen, H.; Ji, H.; Li, M. Highly conductive Cu-Cu joint formation by low-temperature sintering of formic acid-treated Cu nanoparticles. ACS Appl. Mater. Interfaces 2016, 8, 33289–33298. [Google Scholar]
- Mou, Y.; Cheng, H.; Peng, Y.; Chen, M. Fabrication of reliable Cu-Cu joints by low temperature bonding isopropanol stabilized Cu nanoparticles in air. Mater. Lett. 2018, 229, 353–356. [Google Scholar]
- Cui, Z.; Jia, Q.; Wang, Y.; Li, D.; Wang, C.-P.; Zhang, H.; Lu, Z.; Ma, L.; Zou, G.; Guo, F. Enhanced shear strength and microstructure of Cu–Cu interconnection by low-temperature sintering of Cu nanoparticles. J. Mater. Sci. Mater. Electron. 2024, 35, 743. [Google Scholar]
- Zuo, Y.; Carter-Searjeant, S.; Green, M.; Mills, L.; Mannan, S.H. High bond strength Cu joints fabricated by rapid and pressureless in situ reduction-sintering of Cu nanoparticles. Mater. Lett. 2020, 276, 128260. [Google Scholar]
- Zuo, Y.; Shen, J.; Xu, H.; Gao, R. Effect of different sizes of Cu nanoparticles on the shear strength of Cu-Cu joints. Mater. Lett. 2017, 199, 13–16. [Google Scholar]
- Liu, W.; Wang, H.; Huang, K.-S.; Wang, C.-M.; Albert, T.W. Low temperature and pressureless Cu-to-Cu direct bonding by green synthesized Cu nanoparticles. J. Taiwan Inst. Chem. Eng. 2021, 125, 394–401. [Google Scholar] [CrossRef]
- Xia, J.; Xu, Y.; Hu, B.; Lin, J. A rapid approach to urushiol–copper(I) coordination polymer under UV irradiation. Prog. Org. Coat. 2021, 65, 510–513. [Google Scholar] [CrossRef]
- Pauly, N.; Tougaard, S.; Yubero, F. Determination of the Cu 2p primary excitation spectra for Cu, Cu2O and CuO. Sur. Sci. 2014, 29, 17–22. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Z.; Zang, D.; Feng, L. Structural and opto-electrical properties of Cu–Al–O thin films prepared by magnetron sputtering method. Vacuum 2014, 99, 160–165. [Google Scholar] [CrossRef]
- Awad, N.K.; Ashour, E.A.; Allam, N.K. Unravelling the composition of the surface layers formed on Cu, Cu-Ni, Cu-Zn and Cu-Ni-Zn in clean and polluted environments. Appl. Sirf. Sci. 2015, 346, 158–164. [Google Scholar] [CrossRef]
- Mackenzie, J.K.; Shuttleworth, R. A phenomenological theory of sintering. Proc. Phys. Soc. B 1949, 62, 833–852. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).