Investigation into the Effects of Citric Acid on the Corrosion Behavior of AM 350 Stainless Steel Using Electrochemical Impedance Spectroscopy
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
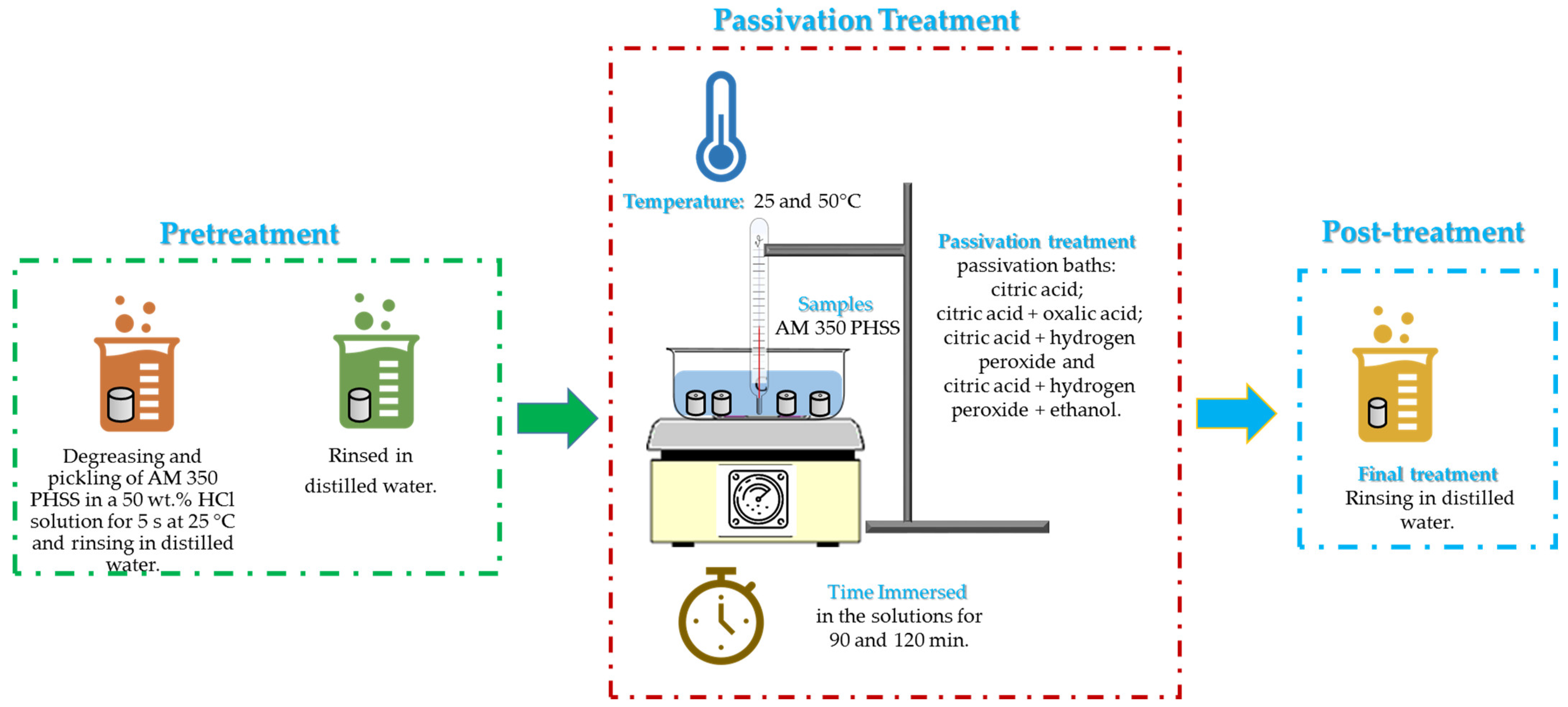
2.2. Passivation Treatment
- i.
- Passivated AM 350 steel samples were cleaned by ultrasound (10 min in ethanol) and immersed in deionized water.
- ii.
- The passivation baths used were as follows:
- a.
- citric acid (C6H8O7) 25% p/v;
- b.
- citric acid (C6H8O7) 25% p/v + oxalic acid (C2H2O4) 10% v/v;
- c.
- citric acid (C6H8O7) 25% p/v + hydrogen peroxide (H2O2) 10% v/v;
- d.
- citric acid (C6H8O7) 25% p/v + hydrogen peroxide (H2O2) 10% v/v + ethane (C2H6O) 5% v/v.
- iii.
- The bath temperatures were 25 and 50 °C, and the immersion times were set at 90 and 120 min.
- iv.
- The samples were rinsed with deionized water and air-dried to finish the treatment.
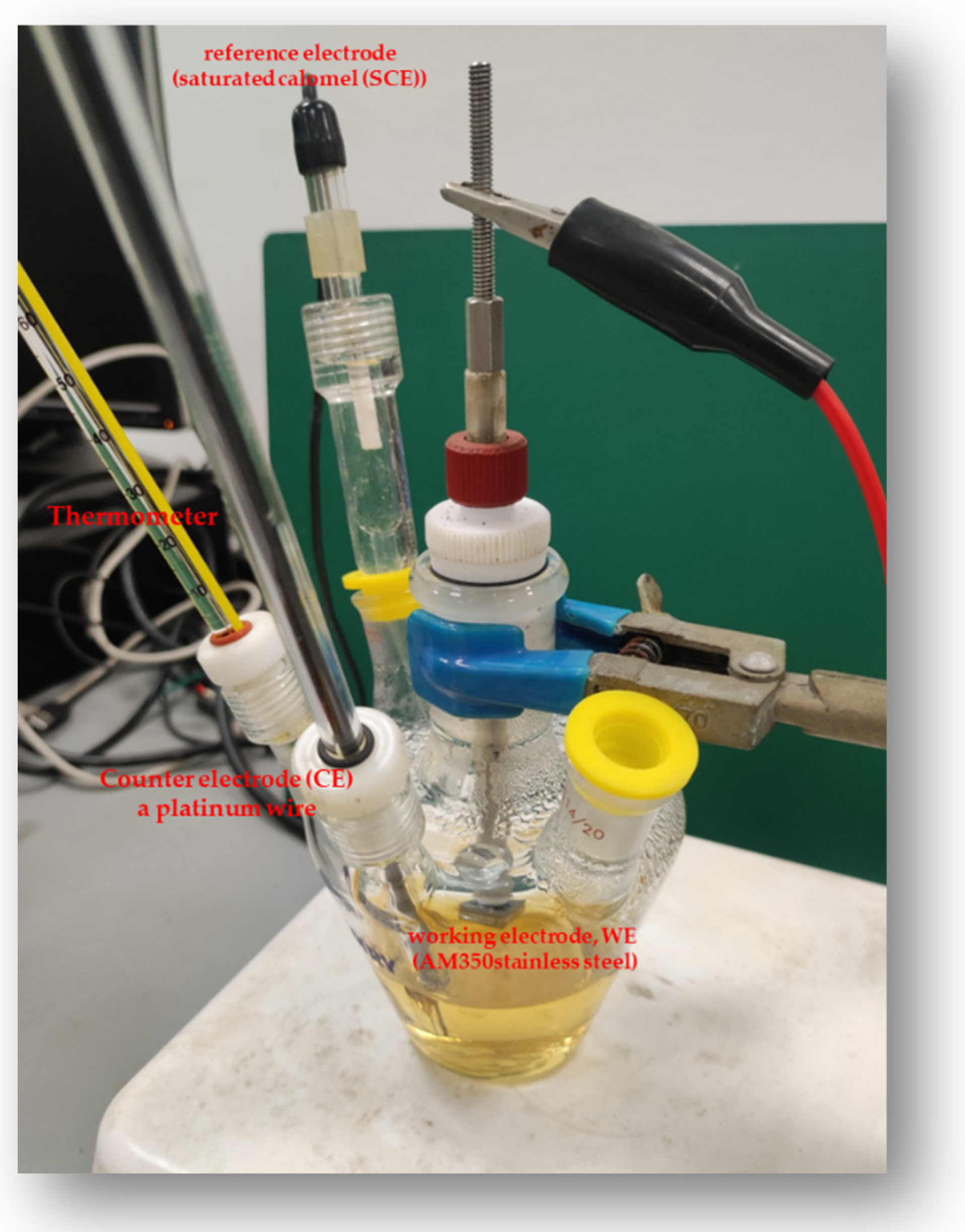
2.3. Corrosion Measurements
2.4. Microstructural Characterization
3. Results
3.1. SEM Microstructural Analysis
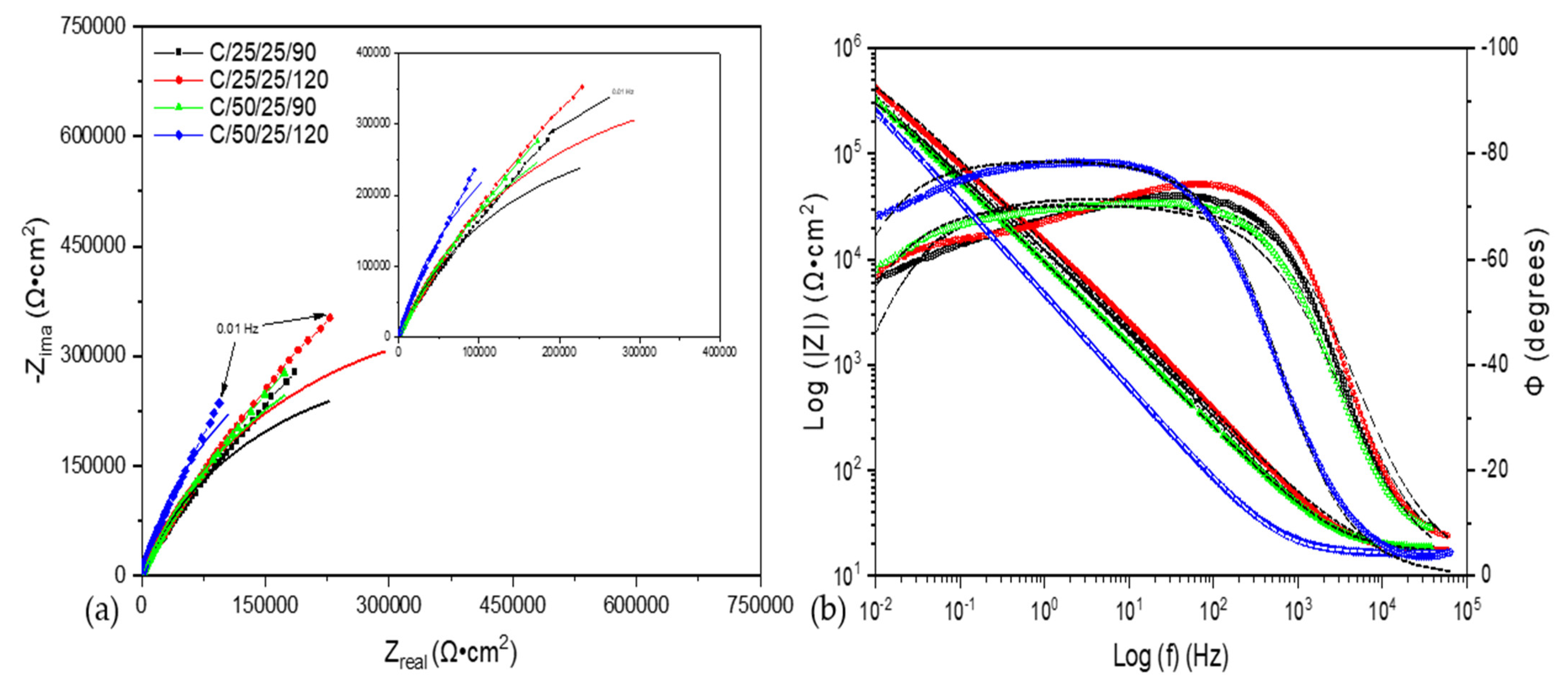
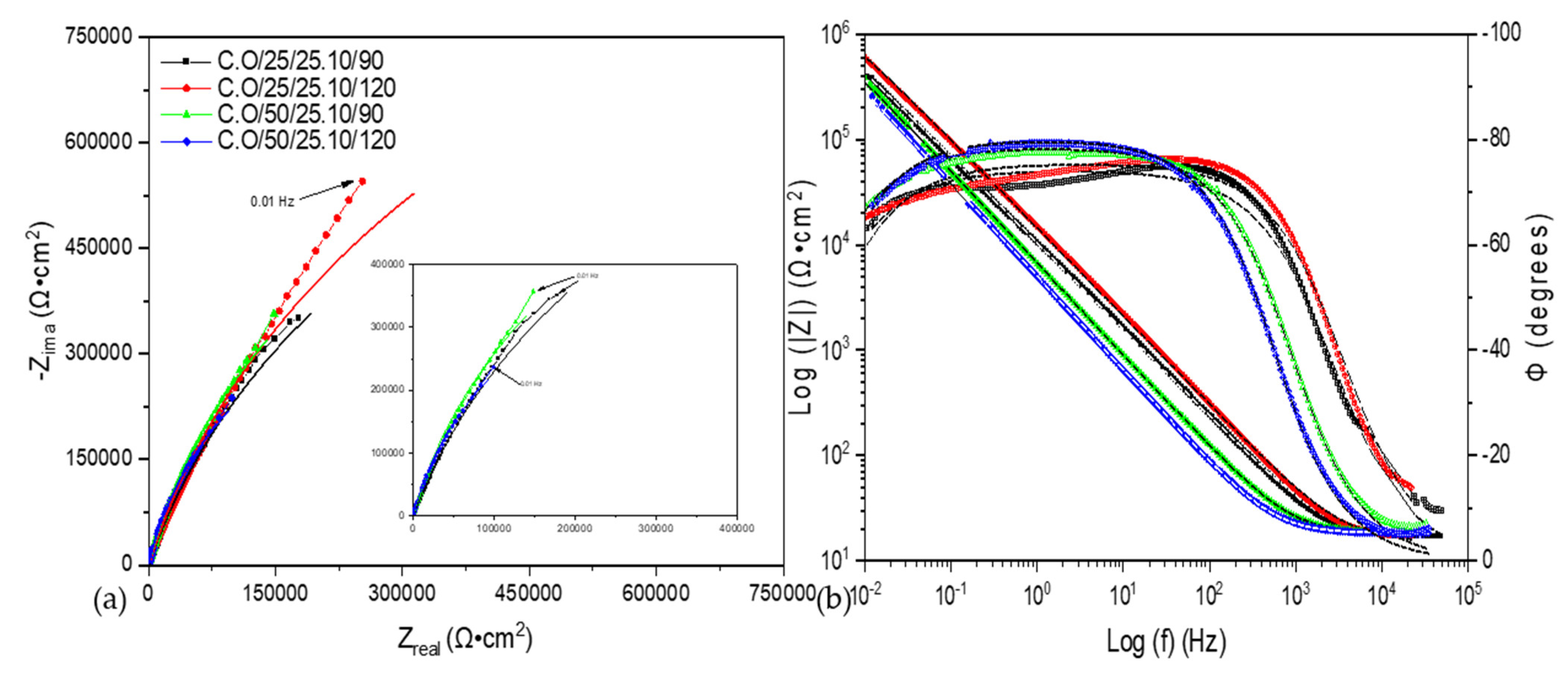
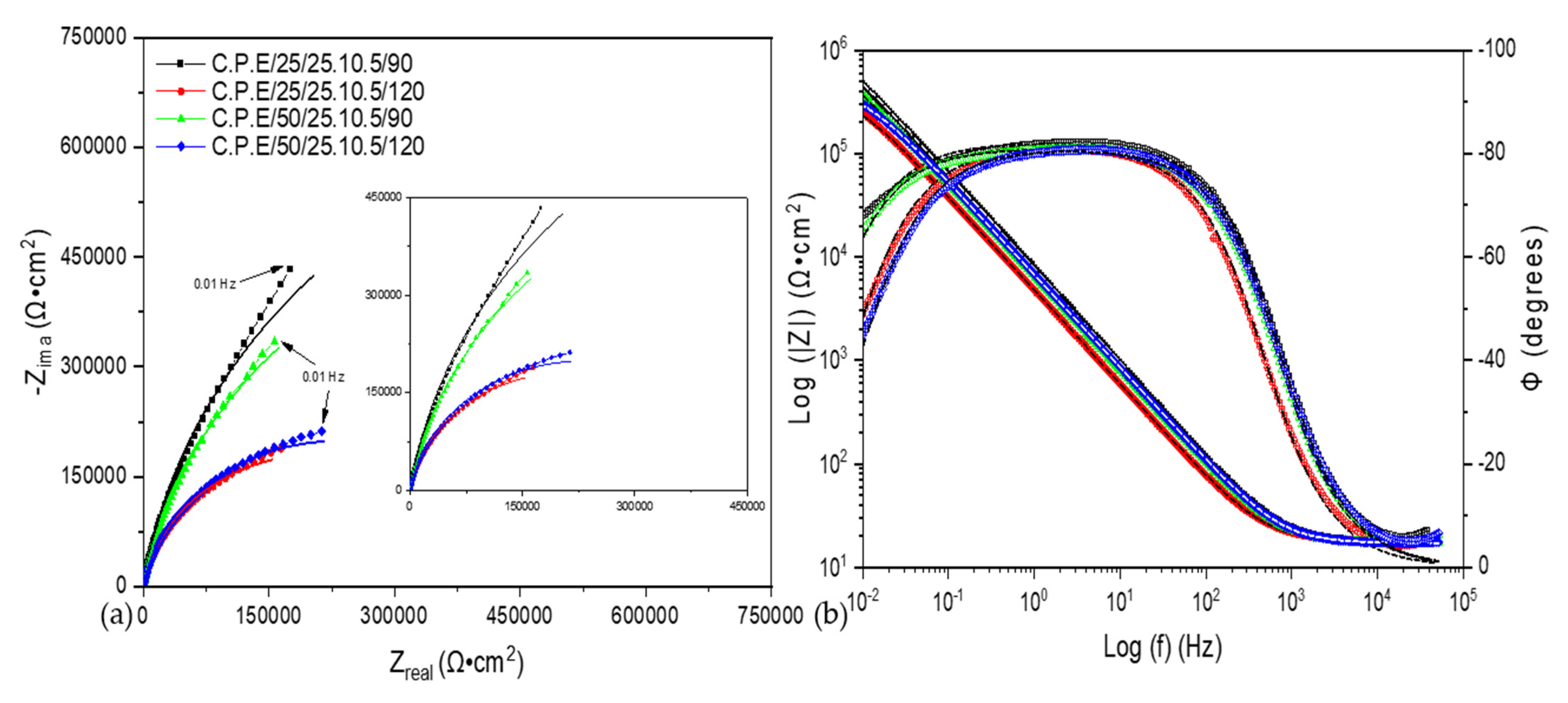
3.2. Electrochemical Impedance Spectroscopy (EIS)
4. Discussion
5. Conclusions
- The impedance parameters provided relevant information concerning the role of chlorides and corrosion resistance.
- The equivalent circuit presented a one-time-constant and two-time-constant corrosion system.
- The results indicate that a two-time-system or double-layer equivalent circuit is associated with a system that is susceptible to pitting due to the behavior of the second barrier, where the n values are 0.6, indicating that the second layer is heterogeneous due to cracks and pittings.
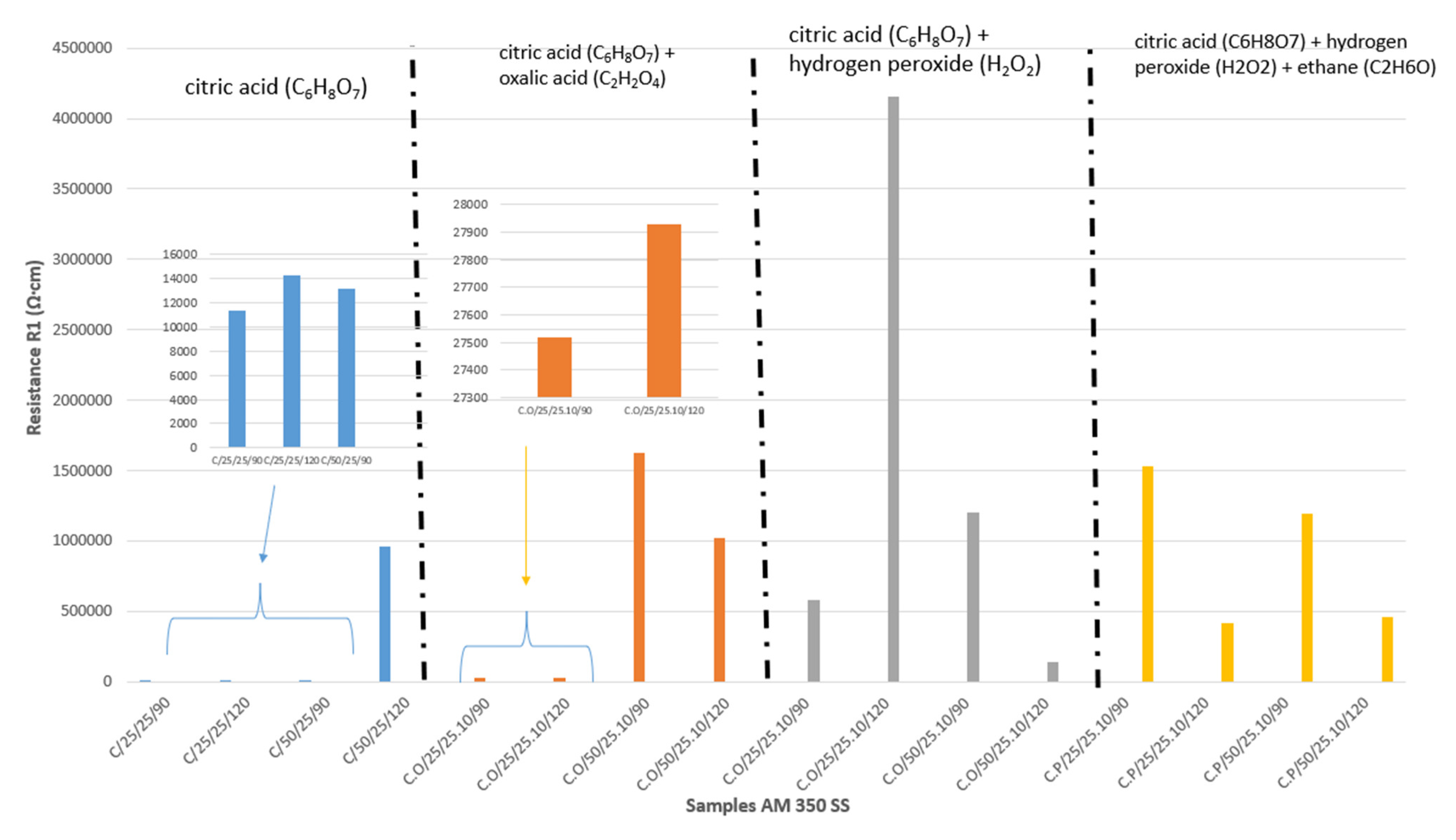
- The AM 350 stainless steels passivated in citric and oxalic acid presented the higher corrosion resistance with values of 6 × 105 Ω·cm2 for sample C.O/25/25.10/120, indicating that with an increase in time, the passivate will be generated in a better way.
- After evaluation using EIS, it was observed that adding peroxide and ethanol helped to make the passivation process more stable at 50 °C because when passivation was performed in that media, the corrosion resistance increased when the passivation temperature increased. However, when the time increased, the corrosion resistance decreased.
- The citric acid passivation process in stainless steels could represent an environmentally friendly alternative to the frequently used nitric acid passivation process.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Villegas-Tovar, J.; Gaona-Tiburcio, C.; Lara-Banda, M.; Maldonado-Bandala, E.; Baltazar-Zamora, M.A.; Cabral-Miramontes, J.; Nieves-Mendoza, D.; Olguin-Coca, J.; Estupiñan-Lopez, F.; Almeraya-Calderón, F. Electrochemical Corrosion Behavior of Passivated Precipitation Hardening Stainless Steels for Aerospace Applications. Metals 2023, 13, 835. [Google Scholar] [CrossRef]
- Gialanella, S.; Malandruccolo, A. Aerospace Alloys; Topics in Mining, Metallurgy and Materials Engineering; Bergmann, C.P., Ed.; Springer: Cham, Switzerland, 2020; ISSN 2364-3307. [Google Scholar] [CrossRef]
- Mouritz, P.A. Introduction to Aerospace Materials; Woodhead Publishing: Cambridge, UK, 2012. [Google Scholar]
- Koch, G.H.; Brongers, M.P.H.; Thompson, N.G.; Virmani, P.Y.; Payer, J.H. Chapter 1: Costs of corrosion in the United States. In Handbook of Environmental Degradation of Materials; Kutz, M., Ed.; William Andrew: New York, NY, USA, 2005; pp. 3–24. [Google Scholar]
- Chatterjee, M.; Patra, A.; Babu, R.; Narayana, R. Aerospace Materials and Technologies Volume 2: Aerospace Materials and Material Technologies, 1st ed.; Prasad Eswara, N., Wanhill, R.J.H., Eds.; Springer Science + Business Media: Singapore, 2017; pp. 3–24. [Google Scholar]
- Banda, M.L.; Gaona-Tiburcio, C.; Zambrano-Robledo, P.; Cabral, M.J.A.; Estupinan, F.; Baltazar-Zamora, M.A.; Almeraya-Calderon, F. Corrosion Behaviour of 304 Austenitic, 15-5PH and 17-4PH Passive Stainless Steels in acid solutions. Int. J. Electrochem. Sci. 2018, 13, 10314–10324. [Google Scholar] [CrossRef]
- Cobb, H.M. The History of Stainless Steel; ASM International: Novelty, OH, USA, 2010. [Google Scholar]
- Samaniego-Gámez, O.; Almeraya-Calderón, F.; Chacón-Nava, J.; Maldonado-Bandala, E.; Nieves-Mendoza, D.; Flores-De los Rios, J.P.; Jáquez-Muñoz, J.M.; Delgado, A.D.; Gaona-Tiburcio, C. Corrosion Behavior of Passivated CUSTOM450 and AM350 Stainless Steels For Aeronautical Applications. Metals 2022, 12, 666. [Google Scholar] [CrossRef]
- Almeraya-Calderon, F.; Villegas-Tovar, M.; Maldonado-Bandala, E.; Lara-Banda, M.; Baltazar-Zamora, M.A.; Santiago-Hurtado, G.; Nieves-Mendoza, D.; Lopez-Leon, L.D.; Jaquez-Muñoz, J.M.; Estupiñán-López, F.; et al. Use of Electrochemical Noise for the Study of Corrosion by Passivated CUSTOM 450 and AM 350 Stainless Steels. Metals 2024, 14, 341. [Google Scholar] [CrossRef]
- Lopes, J.C. Material selection for aeronautical structural application. Sci. Technol. Adv. Mat. 2008, 20, 78–82. [Google Scholar]
- Lo, K.H.; Shek, C.H.; Lai, J.K.L. Recent developments in stainless steels. Mat. Sci. Eng. R. 2009, 65, 39–104. [Google Scholar] [CrossRef]
- Gaona-Tiburcio, C.; Montoya, R.M.; Cabral, M.J.; Estupiñan, L.F.; Zambrano, R.P.; Orozco, C.R.; Chacon-Nava, J.; Baltazar Z, M.; Almeraya-Calderon, F. Corrosion Resistance of Multilayer Coatings Deposited by PVD on Inconel 718 Using Electrochemical Impedance Spectroscopy Technique. Coatings 2020, 10, 521. [Google Scholar] [CrossRef]
- Farrar, J. The Alloy Tree—A Guide to Low-Alloy Steels, Stainless Steels and Nickel-Base Alloys; Woodhead Publishing Limited and CRC Press: Sawston, UK, 2004. [Google Scholar]
- ASM International. Introduction to Stainless Steel. In Alloy Digest Sourcebook—Stainless Steel, 3rd ed.; David, J.R., Davis & Associates, Eds.; ASM International: Novelty, OH, USA, 2000; pp. 2–3. [Google Scholar]
- Almeraya-Calderón, F.; Samaniego-Gámez, O.; Maldonado-Bandala, E.; Nieves-Mendoza, D.; Olguín-Coca, J.; Jáquez-Muñoz, J.M.; Cabral-Miramontes, J.; Flores-De los Rios, J.P.; Bautista-Margulis, R.G.; Gaona-Tiburcio, C. Corrosion Behavior of Passivated Martensitic and Semi-Austenitic Precipitation Hardening Stainless Steel. Metals 2022, 12, 1033. [Google Scholar] [CrossRef]
- Almeraya-Calderón, F.; Villegas-Tovar, M.; Maldonado-Bandala, E.; Lara-Banda, M.; Baltazar-Zamora, M.A.; Landa-Ruiz, L.; Nieves-Mendoza, D.; Jaquez-Muñoz, J.M.; Estupiñan-Lopez, F.; Cabral-Miramontes, J.; et al. Localized Corrosion of CUSTOM 450 and AM 350 Stainless Steels in H2SO4 and NaCl Solutions. Materials 2025, 18, 988. [Google Scholar] [CrossRef]
- Gaydos, S.P. Passivation of aerospace stainless parts with citric acid solutions. Plat. Surf. Finish. 2003, 90, 20–25. [Google Scholar]
- Schweitzer, P.A. Metallic Materials: Physical, Mechanical, and Corrosion Properties; M. Dekker: New York, NY, USA, 2003. [Google Scholar]
- Benavides, S. Corrosion Control in the Aerospace Industry; Woodhead Publishing: Cambridge, UK, 2009. [Google Scholar]
- Lewis, P.; Kolody, M. Alternative to Nitric Acid Passivation of Stainless Steel Alloys. In Technology Evaluation for Environmental Risk Mitigation Compendium, Proceedings of the NASA Technology Evaluation for Environmental Risk Mitigation Principal Center (TEERM), Kennedy Space Center, FL, USA, 13 January 2008; Department of Defense (DoD) and NASA, Kennedy Space Center: Merritt Island, FL, USA, 2008. [Google Scholar]
- ASTM A967-17; Standard Specification for Chemical Passivation Treatments for Stainless Steel Parts. ASTM International: West Conshohocken, PA, USA, 1999.
- NASA. Technical Reports Sever. Available online: https://ntrs.nasa.gov/citations/19680010964 (accessed on 15 May 2024).
- Gaona Tiburcio, C.; Samaniego Gámez, O.; Jáquez Muñoz, J.M.; Baltazar Zamora, M.A.; Landa-Ruiz, L.; Lira Martínez, A.; Flores De los Rios, J.P.; Cabral Miramontes, J.; Estupinán-López, F.; Almeraya Calderon, F. Frequency-Time Domain Analysis of Electrochemical Noise of Passivated AM350 Stainless Steel for Aeronautical Applications. Int. J. Electrochem. Sci. 2022, 17, 220950. [Google Scholar] [CrossRef]
- El-Taib Heakal, F.; Ameer, M.A.; El-Aziz, A.M.; Fekry, A.M. Electrochemical behavior of Mo-containing austenitic stainless steel in buffer solutions. Mater. Werkst. 2004, 35, 407–413. [Google Scholar] [CrossRef]
- El-Taib Heakal, F.; Ghoneim, A.; Fekry, A. Stability of spontaneous passive films on high strength Mo-containing stainless steels in aqueous solutions. J. Appl. Electrochem. 2007, 37, 405–413. [Google Scholar] [CrossRef]
- Bragaglia, M.; Cherubini, V.; Cacciotti, I.; Rinaldi, M.; Mori, S.; Soltani, P.; Nanni, F.; Kaciulis, S.; Montesperelli, G. Citric Acid Aerospace Stainless Steel Passivation: A Green Approach. In Proceedings of the CEAS Aerospace Europe Conference 2015, Delft, The Netherlands, 7–11 September 2015. [Google Scholar]
- Marcelin, S.; Pébèrea, N.; Régnierb, S. Electrochemical characterisation of a martensitic stainless steel in a neutral chloride solution. Electrochim. Acta 2013, 87, 32–40. [Google Scholar] [CrossRef]
- Lara-Banda, M.; Gaona-Tiburcio, C.; Zambrano-Robledo, P.; Delgado-E., M.; Cabral-Miramontes, J.; Nieves-Mendoza, D.; Maldonado-Bandala, E.; Estupiñan-Lopez, F.; Chacón-Nava, J.; Almeraya-Calderón, F. Alternative to Nitric Acid Passivation of 15-5 and 17-4PH Stainless Steel Using Electrochemical Techniques. Materials 2020, 13, 2836. [Google Scholar] [CrossRef]
- AM 350 Technical Data. Available online: https://www.hightempmetals.com/techdata/hitempAM350data.php (accessed on 30 November 2024).
- ASTM A380-17; Standard Practice for Cleaning, Descaling and Passivation of Stainless-Steel Parts, Equipment, and Systems. ASTM International: West Conshohocken, PA, USA, 1999.
- ASTM E3-95; Standard Practice for Preparation of Metallographic Specimens. ASTM International: West Conshohocken, PA, USA, 1995.
- Gallego-Juárez, J.A.; Graff, K.F. Power Ultrasonics—Applications of High-Intensity Ultrasound; Woodhead Publishing: Cambridge, UK, 2015. [Google Scholar]
- SAE AMS 2700F; Passivation of Corrosion Resistant Steels. Aerospace Material Specification. SAE International: Warrendale, PA, USA, 2018.
- ASTM G44–99; Standard Practice for Exposure of Metals and Alloys by Alternate Immersion in Neutral 3.5% Sodium Chloride Solution. ASTM International: West Conshohocken, PA, USA, 1999.
- ASTM G106-15; Standard Practice for Verification of Algorithm and Equipment for Electrochemical Impedance Measurements. ASTM International: West Conshohocken, PA, USA, 2015.
- Macdonald, D.D. The history of the Point Defect Model for the passive state: A brief review of film growth aspects. Electrochim. Acta 2011, 56, 1761–1772. [Google Scholar]
- Duan, Z.; Man, C.; Dong, C.; Cui, Z.; Kong, D.; Wang, L.; Wang, X. Pitting behavior of SLM 316L stainless steel exposed to chloride environments with different aggressiveness: Pitting mechanism induced by gas pores. Corros. Sci. 2020, 67, 108520. [Google Scholar]
- Choudhary, S.; Qiu, Y.; Thomas, S.; Birbilis, N. Element-resolved electrochemical analysis of transpassive dissolution and repassivation behavior of the multi-principal element alloy AlTiVCr. Electrochim. Acta 2020, 362, 137104. [Google Scholar] [CrossRef]
- Tian, H.; Sun, F.; Chu, F.; Wang, L.; Wang, X.; Cui, X. Passivation behavior and surface chemistry of 316 SS in the environment containing Cl− and NH4+. J. Electroanal. Chem. 2021, 886, 115138. [Google Scholar]
- Samaniego-Gámez, P.; Almeraya-Calderón, F.; Martin, U.; Ress, R.; Gaona-Tiburcio, C.; Silva-Vidaurri, J.; Cabral-Miramontes, J.; Bastidas, J.; Chacón-Nava, J.; Bastidas, M.D. Effect of sealing treatment on the corrosion behavior of anodized AA2099 aluminum-lithium alloy. Rev. Metal. 2020, 56, e180. [Google Scholar] [CrossRef]
- Blanco, G.; Bautista, A.; Takenouti, H. EIS Study of Passivation of Austenitic and Duplex Stainless Steels Reinforcements in Simulated Pore Solutions. Cem. Concr. Compos. 2006, 28, 212–219. [Google Scholar] [CrossRef]
- Warren, J.; Wei, D.Y. The low cycle fatigue behavior of the controlled transformation stainless steel alloy AM355 at 121, 204 and 315 °C. Mater. Sci. Eng. A 2008, 475, 148–156. [Google Scholar] [CrossRef]
- Tseng, K.H.; Chen, Y.C.; Chen, Y.C. Micro Plasma Arc Welding of AM 350 Precipitation Hardening Alloys. Appl. Mech. Mater. 2012, 121, 2681–2685. [Google Scholar] [CrossRef]
- Favor, R.J.; Deel, O.L.; Achbach, W.P. Design Information on AM-350 Stainless Steel for Aircraft and Missiles; Defense Metals Information Center, Battelle Memorial Institute: Columbus, OH, USA, 1961; Volume 156. [Google Scholar]
- Grubać, Z.; Metikoš-Huković, M. EIS Study of Solid-State Transformations in the Passivation Process of Bismuth in Sulfide Solution. J. Electroanal. Chem. 2004, 565, 85–94. [Google Scholar] [CrossRef]
- Shaikh, H.; George, G.; Khatak, H.S. Failure analysis of an AM 350 steel bellows. Fail. Anal. 2001, 8, 571–576. [Google Scholar] [CrossRef]
- Lin, C.K.; Chu, C.C. Mean stress effects on low-cycle fatigue for a precipitation-hardening martensitic stainless steel in different tempers. Fatigue Fract. Eng. Mater. Struct. 2000, 23, 545–553. [Google Scholar] [CrossRef]
- Boudinar, Y.; Belmokre, K.; Touzet, M.; Devos, O.; Puiggali, M. Investigation of the Passivation Process of Plastically Deformed 316L Stainless Steel Using the High Frequency Capacitance Obtained by EIS. Mater. Corros. 2019, 70, 206–215. [Google Scholar] [CrossRef]
- Freire, L.; Carmezim, M.J.; Ferreira, M.G.S.; Montemor, M.F. The Electrochemical Behaviour of Stainless Steel AISI 304 in Alkaline Solutions with Different PH in the Presence of Chlorides. Electrochim. Acta 2011, 56, 5280–5289. [Google Scholar] [CrossRef]
- Recio-Hernandez, J.; Galicia-García, M.; Silva-Jiménez, H.; Malpica-Calderón, R.; Ordoñez-Casanova, E.G. EIS Evaluation of Corrosion Resistance of AISI 304 Stainless Steel Exposed to Pseudomonas Stutzeri. Int. J. Electrochem. Sci. 2021, 16, 21058. [Google Scholar] [CrossRef]
- Olugbade, T.O.; Omiyale, B.O. Corrosion Study and Surface Analysis of the Passivation Film on Surface-Deformed AISI 304 Stainless Steel. Chem. Afr. 2022, 5, 1663–1670. [Google Scholar] [CrossRef]
- Zhang, H.; Zhao, J.; Yang, C.; Shen, M.; Zhang, X.; Xi, T.; Yin, L.; Zhao, H.; Liu, X.; Liu, L.; et al. Corrosion Resistance of Cu-Bearing 316L Stainless Steel Tuned by Various Passivation Potentials. Surf. Interface Anal. 2021, 53, 592–602. [Google Scholar] [CrossRef]
- Chokri, A.; Ben Rhouma, A.; Sahlaoui, H. Evaluation of Passivation and Corrosion Behavior of AISI 316L SS with Low Ferrite Content Using EIS Test in Artificial Seawater Solution; Lecture Notes in Mechanical Engineering; Springer: Cham, Switzerland, 2024; pp. 19–28. [Google Scholar] [CrossRef]
- Cheng, X.; Li, X.; Yang, L.; Du, C. Corrosion Resistance of 316L Stainless Steel in Acetic Acid by EIS and Mott-Schottky. J. Wuhan Univ. Technol. Mater. Sci. Ed. 2008, 23, 574–578. [Google Scholar] [CrossRef]
- Niu, W.; Lillard, R.S.; Li, Z.; Ernst, F. Properties of the Passive Film Formed on Interstitially Hardened AISI 316L Stainless Steel. Electrochim. Acta 2015, 176, 410–419. [Google Scholar] [CrossRef]
- Kang, J.; Li, J.; Zhao, K.Y.; Bai, X.; Yong, Q.L.; Su, J. Passivation Behaviors of Super Martensitic Stainless Steel in Weak Acidic and Weak Alkaline NaCl Solutions. J. Iron Steel Res. Int. 2015, 22, 1156–1163. [Google Scholar] [CrossRef]
- Lai, W.Y.; Zhao, W.Z.; Yin, Z.F.; Zhang, J. EIS and XPS Studies on Passive Film of AISI 304 Stainless Steel in Dilute Sulfuric Acid Solution. Surf. Interface Anal. 2012, 44, 418–425. [Google Scholar] [CrossRef]
- Wallinder, D.; Pan, J.; Leygraf, C.; Delblanc-Bauer, A. Eis and XPS Study of Surface Modification of 316LVM Stainless Steel after Passivation. Corros. Sci. 1998, 41, 275–289. [Google Scholar] [CrossRef]
- Alves, V.A.; Brett, C.M.A. Characterisation of Passive Films Formed on Mild Steels in Bicarbonate Solution by EIS. Electrochim. Acta 2002, 47, 2081–2091. [Google Scholar] [CrossRef]
- Cabrera-Sierra, R.; Hallen, J.M.; Vazquez-Arenas, J.; Vázquez, G.; González, I. EIS characterization of tantalum and niobium oxide films based on a modification of the point defect model. J. Electroanal. Chem. 2010, 638, 51–58. [Google Scholar] [CrossRef]
- Chávez-Díaz, M.P.; Luna-Sánchez, R.M.; Vazquez-Arenas, J.; Lartundo-Rojas, L.; Hallen, J.M.; Cabrera-Sierra, R. XPS and EIS Studies to Account for the Passive Behavior of the Alloy Ti-6Al-4V in Hank’s Solution. J. Solid State Electrochem. 2019, 23, 3187–3196. [Google Scholar] [CrossRef]
- Magar, H.S.; Hassan, R.Y.A.; Mulchandani, A. Electrochemical Impedance Spectroscopy (EIS): Principles, Construction, and Biosensing Applications. Sensors 2021, 21, 6578. [Google Scholar] [CrossRef]
- Macdonald, J.R. Generalizations of “universal dielectric response” and a general distribution-of-activation-energies model for dielectric and conductive systems. J. App. Phys. 1985, 58, 1971–1978. [Google Scholar] [CrossRef]
- Macdonald, J.R. Frequency response of inifield dielectric and conductive systems involving an exponetial distribution of activation energy. J. Appl. Phys. 1985, 58, 1955–1970. [Google Scholar] [CrossRef]



| Element | AM 350 |
|---|---|
| Fe | Balance |
| C | 0.07–0.11 |
| Cr | 16.0–17.0 |
| Ni | 4.0–5.0 |
| Mo | 2.50–3.25 |
| Mn | 0.50–1.25 |
| N | 0.07–0.13 |
| Si | ≤0.50 |
| S | 0.030 |
| Passivation Baths | Temperature (°C) | Concentration | Time (min) |
|---|---|---|---|
| citric acid (C6H8O7) | 25 and 50 | (C6H8O7) 25% p/v | 90 and 120 |
| citric acid (C6H8O7) + oxalic acid (C2H2O4) | 25 and 50 | (C6H8O7) 25% p/v + (C2H2O4) 10% v/v | 90 and 120 |
| citric acid (C6H8O7) + hydrogen peroxide (H2O2) | 25 and 50 | (C6H8O7) 25% p/v + (H2O2) 10% v/v | 90 and 120 |
| citric acid (C6H8O7) + hydrogen peroxide (H2O2) + ethane (C2H6O) | 25 and 50 | (C6H8O7) 25% p/v + (H2O2) 10% v/v + (C2H6O) 5% v/v | 90 and 120 |
| Sample | Rsol (Ω·cm) | CPE1 (μF/cm2) | n1 | R1 (Ω·cm) | CPE2 (μF/cm2) | n2 | R2 (Ω·cm) | X2 |
|---|---|---|---|---|---|---|---|---|
| C/25/25/90 | 17 ± 1.5 | 1.45 × 10−5 ± 1.5 × 10−7 | 0.84 ± 0.1 | 11,360 ± 40 | 8.16 × 10−6 ± 2 × 10−8 | 0.59 ± 0.05 | 1,994,500 ± 58 | 5.4 × 10−4 |
| C/25/25/120 | 16 ± 2.0 | 1.01 × 10−5 ± 2.1 × 10−7 | 0.86 ± 0.1 | 14,283 ± 11 | 8.49 × 10−6 ± 1 × 10−8 | 0.62 ± 0.05 | 3,654,800 ± 63 | 5.0 × 10−4 |
| C/50/25/90 | 17 ± 1.3 | 2.06 × 10−5 ± 1.6 × 10−7 | 0.81 ± 0.1 | 13,204 ± 20 | 5.62 × 10−6 ± 1.8 × 10−8 | 0.65 ± 0.05 | 1,383,400 ± 87 | 6.4 × 10−4 |
| C/50/25/120 | 16 ± 0.9 | 4.29 × 10−5 ± 2.2 × 10−7 | 0.88 ± 0.1 | 962,470 ± 23 | - | - | - | 1.9 × 10−3 |
| Sample | Rsol (Ω·cm) | CPE1 (μF/cm2) | n1 | R1 (Ω·cm) | CPE2 (μF/cm2) | n2 | R2 (Ω·cm) | X2 |
|---|---|---|---|---|---|---|---|---|
| C.O/25/25.10/90 | 15 ± 1 | 1.65 × 10−5 ± 0.9 × 10−7 | 0.86 ± 0.02 | 27,519 ± 21 | 5.96 × 10−6 ± 1 × 10−8 | 0.76 ± 0.02 | 2,063,800 ± 120 | 1.6 × 10−3 |
| C.O/25/25.10/120 | 15.81 ± 0.5 | 1.16 × 10−5 ± 0.7 × 10−7 | 0.87 ± 0.06 | 27,929 ± 36 | 3.97 × 10−6 ± 0.6 × 10−8 | 0.65 ± 0.02 | 5,091,900 ± 98 | 5.2 × 10−4 |
| C.O/50/25.10/90 | 17.91 ± 0.8 | 3.00 × 10−5 ± 1.8 × 10−7 | 0.87 ± 0.02 | 1,624,200 ± 240 | - | - | - | 1.1 × 10−3 |
| C.O/50/25.10/120 | 18.2 ± 0.4 | 3.86 × 10−5 ± 1.3 × 10−7 | 0.89 ± 0.03 | 1,022,800 ± 603 | - | - | - | 1.3 × 10−3 |
| Sample | Rsol (Ω·cm) | CPE1 (μF/cm2) | n1 | R1 (Ω·cm) | CPE2 (μF/cm2) | n2 | R2 (Ω·cm) | X2 |
|---|---|---|---|---|---|---|---|---|
| C.P/25/25.10/90 | 16 ± 0.5 | 2.50 × 10−5 ± 1 × 10−7 | 0.91 ± 0.02 | 584,230 ± 86 | - | - | - | 1.0 × 10−3 |
| C.P/25/25.10/120 | 17 ± 0.8 | 1.65 × 10−5± 2 × 10−7 | 0.93 ± 0.02 | 4,155,700 ± 70 | - | - | - | 1.7 × 10−3 |
| C.P/50/25.10/90 | 16 ± 0.8 | 2.24 × 10−5± 1 × 10−7 | 0.91 ± 0.02 | 1,201,800 ± 63 | - | - | - | 1.2 × 10−3 |
| C.P/50/25.10/120 | 16 ± 0.9 | 4.07 × 10−5± 1 × 10−7 | 0.94 ± 0.02 | 145,210 ± 23 | 7.67 × 10−5 ± 1.6 × 10−7 | 0.92 ± 0.02 | 130,830 ± 17 | 5.5 × 10−3 |
| Sample | Rsol (Ω·cm) | CPE1 (μF/cm2) | n1 | R1 (Ω·cm) | X2 |
|---|---|---|---|---|---|
| C.P.E/25/25.10.5/90 | 17 ± 0.5 | 2.42 × 10−5 ± 6 × 10−7 | 0.91 ± 0.15 | 1,530,900 ± 130 | 1.7 × 10−3 |
| C.P.E/25/25.10.5/120 | 18 ± 0.3 | 3.99 × 10−5 ± 7 × 10−7 | 0.90 ± 0.17 | 419,510 ± 99 | 1.6 × 10−3 |
| C.P.E/50/25.10.5/90 | 17 ± 0.5 | 3.02 × 10−5 ± 3 × 10−7 | 0.90 ± 0.15 | 1,197,500 ± 178 | 1.1 × 10−3 |
| C.P.E/50/25.10.5/120 | 17 ± 0.2 | 2.83 × 10−5 ± 4 × 10−7 | 0.90 ± 0.1 | 464,940 ± 169 | 1.1 × 10−3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gaona-Tiburcio, C.; Villegas-Tovar, M.; Maldonado-Bandala, E.; Lara-Banda, M.; Baltazar-Zamora, M.A.; Méndez-Ramírez, C.T.; Nieves-Mendoza, D.; Almaguer-Cantu, V.; Jaquez-Muñoz, J.M.; Landa-Gómez, A.; et al. Investigation into the Effects of Citric Acid on the Corrosion Behavior of AM 350 Stainless Steel Using Electrochemical Impedance Spectroscopy. Metals 2025, 15, 420. https://doi.org/10.3390/met15040420
Gaona-Tiburcio C, Villegas-Tovar M, Maldonado-Bandala E, Lara-Banda M, Baltazar-Zamora MA, Méndez-Ramírez CT, Nieves-Mendoza D, Almaguer-Cantu V, Jaquez-Muñoz JM, Landa-Gómez A, et al. Investigation into the Effects of Citric Acid on the Corrosion Behavior of AM 350 Stainless Steel Using Electrochemical Impedance Spectroscopy. Metals. 2025; 15(4):420. https://doi.org/10.3390/met15040420
Chicago/Turabian StyleGaona-Tiburcio, Citlalli, Miguel Villegas-Tovar, Erick Maldonado-Bandala, María Lara-Banda, Miguel Angel Baltazar-Zamora, Ce Tochtli Méndez-Ramírez, Demetrio Nieves-Mendoza, Verónica Almaguer-Cantu, Jesus Manuel Jaquez-Muñoz, Aldo Landa-Gómez, and et al. 2025. "Investigation into the Effects of Citric Acid on the Corrosion Behavior of AM 350 Stainless Steel Using Electrochemical Impedance Spectroscopy" Metals 15, no. 4: 420. https://doi.org/10.3390/met15040420
APA StyleGaona-Tiburcio, C., Villegas-Tovar, M., Maldonado-Bandala, E., Lara-Banda, M., Baltazar-Zamora, M. A., Méndez-Ramírez, C. T., Nieves-Mendoza, D., Almaguer-Cantu, V., Jaquez-Muñoz, J. M., Landa-Gómez, A., & Almeraya-Calderón, F. (2025). Investigation into the Effects of Citric Acid on the Corrosion Behavior of AM 350 Stainless Steel Using Electrochemical Impedance Spectroscopy. Metals, 15(4), 420. https://doi.org/10.3390/met15040420










