Corrosion Behavior of Mild Steel in Various Environments Including CO2, H2S, and Their Combinations
Abstract
:1. Introduction
2. Experimental Procedures
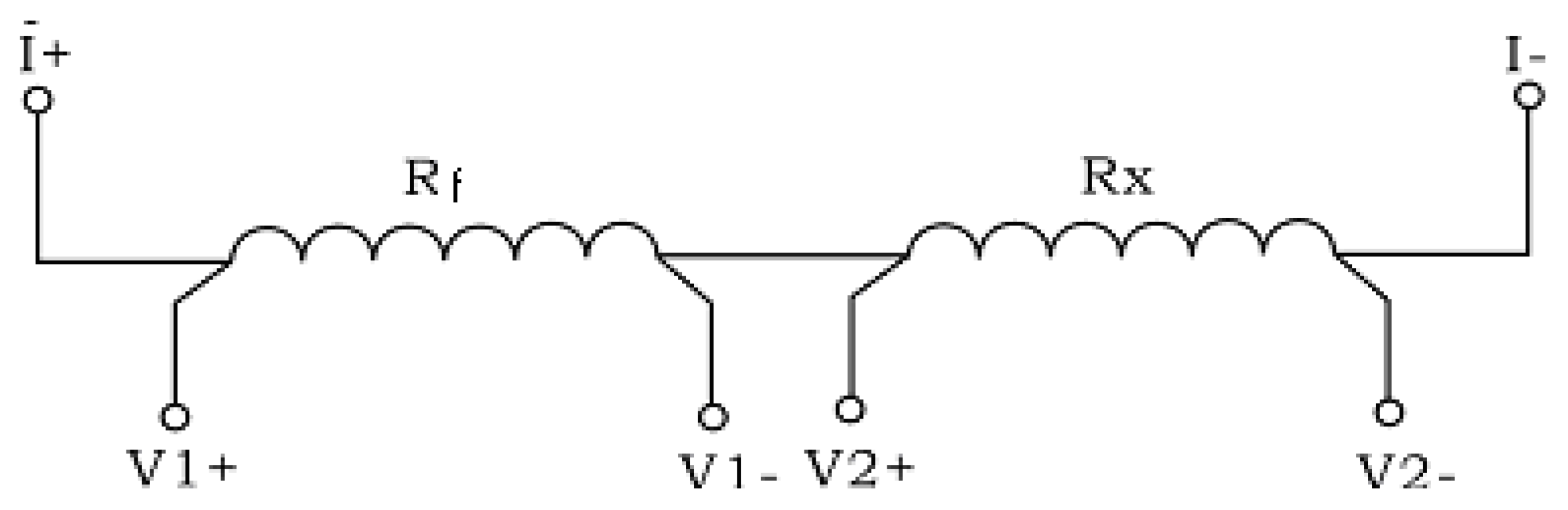
2.1. Test Materials and ER Probes
2.2. Experimental Equipment
2.3. Condition of the Test Medium
2.4. Experimental Procedure and Data Acquisition
3. Results and Discussion
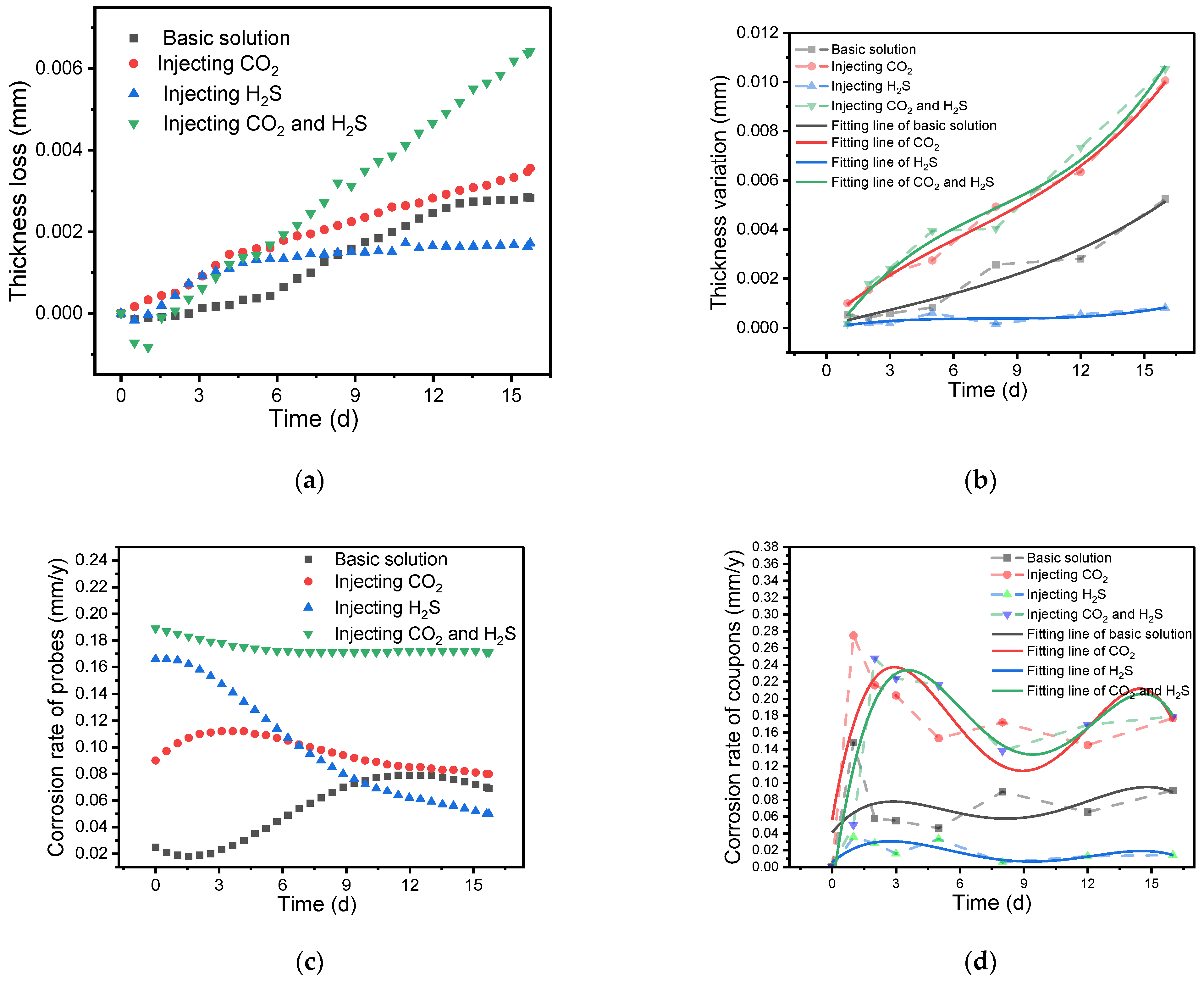
3.1. Analysis of Corrosion Rate
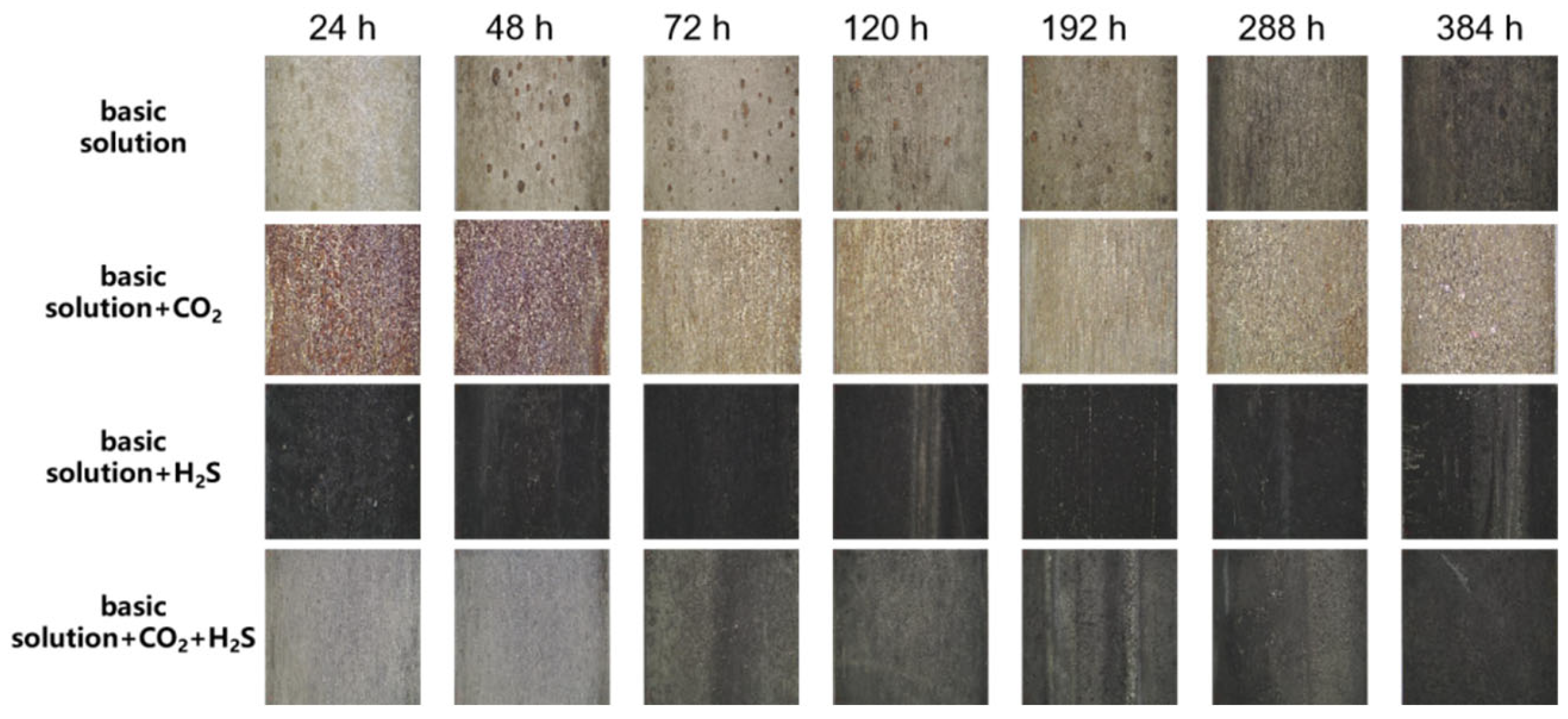
3.2. Corrosion Product Analysis and Morphology Observation
3.2.1. Color Analysis of Corrosion Precipitates
3.2.2. XRD Analysis of Corrosion Precipitation Products
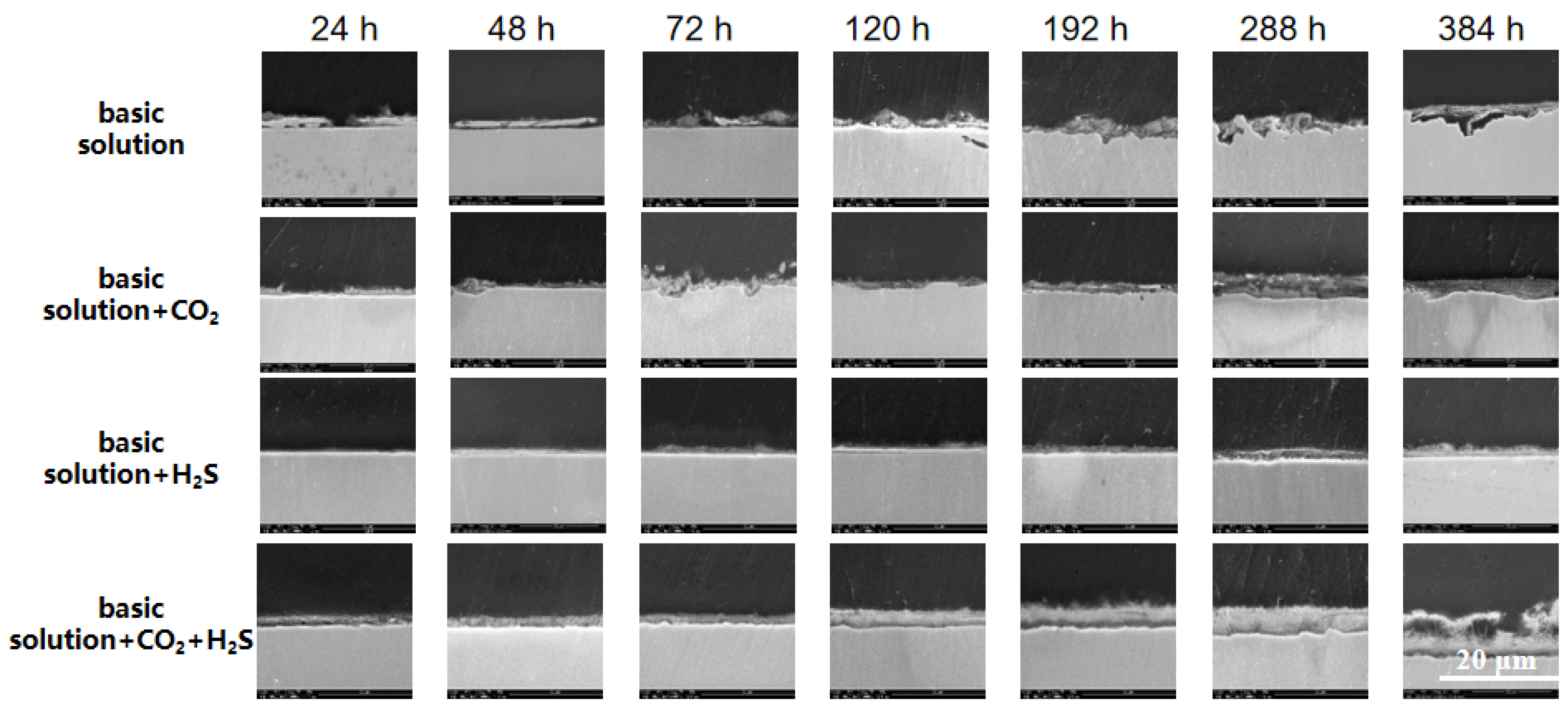
3.3. Structural Analysis of Corrosion Product Films
3.3.1. Thickness and Characterization of Corrosion Product Films
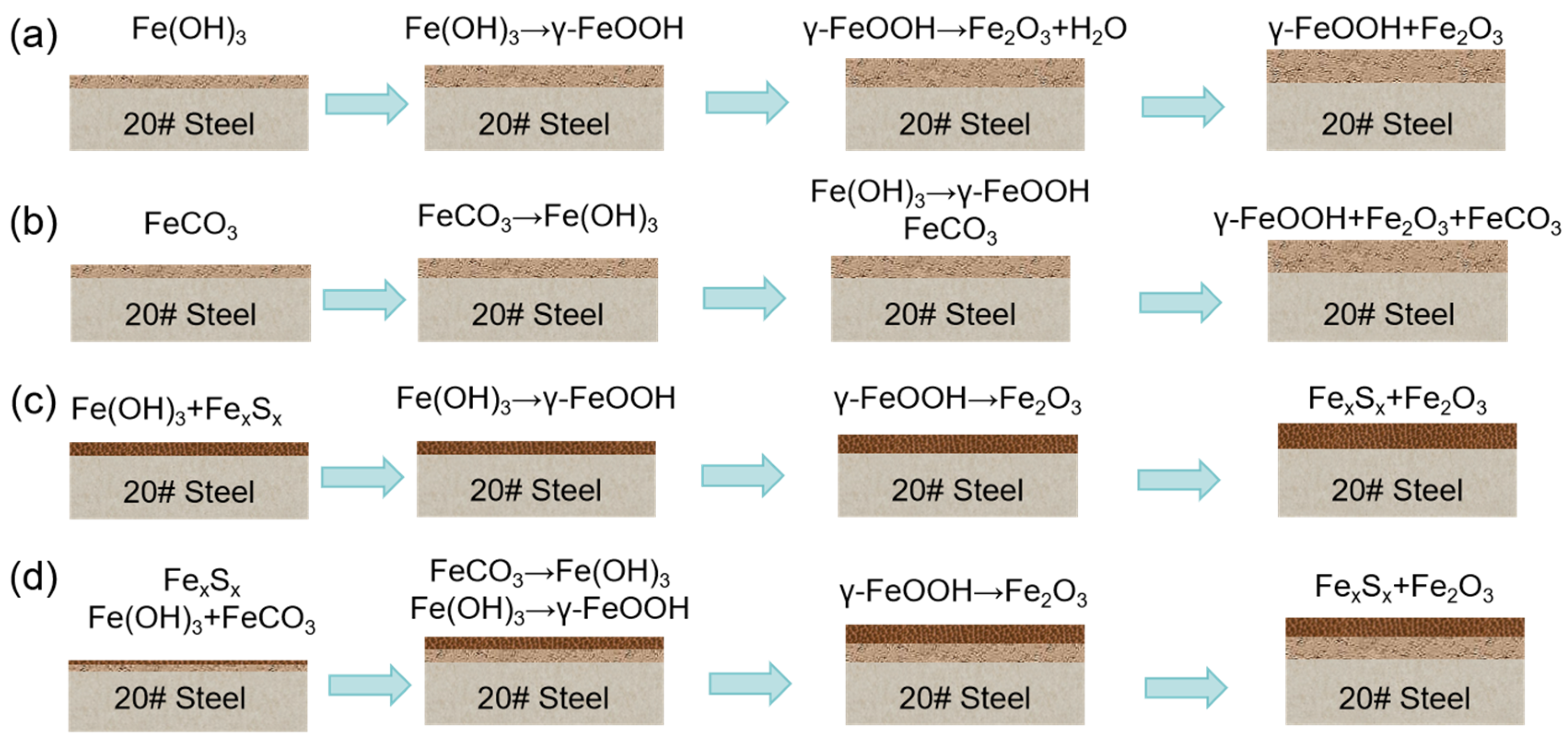
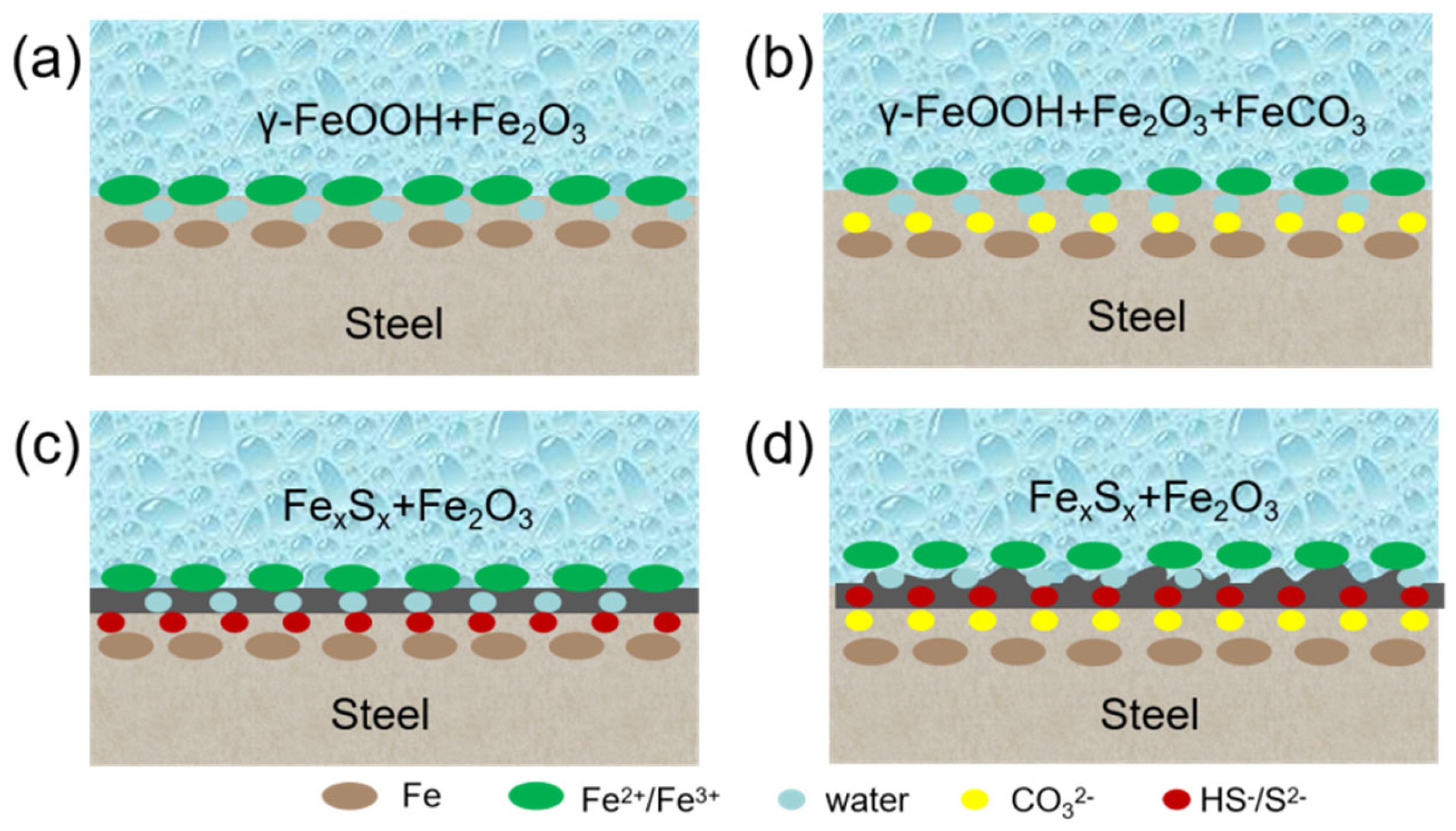
3.3.2. Modeling of Corrosion Product Film Growth and Damage
3.4. Modeling of Corrosion in CO2-H2S Environment
4. Conclusions
- This study pioneeringly employs the electrical resistance method, utilizing a large plane probe, to investigate corrosion in simulated oilfield sewage containing CO2 and H2S under laboratory conditions. The obtained corrosion process curve distinctly exhibits the stages of film formation to maturation, revealing characteristics unattainable through synchronous specimen testing. Contrary to previous beliefs regarding the unsuitability of the resistance method due to the electronic conductivity of FexSx, our experiments demonstrate its efficacy for corrosion monitoring in H2S-containing environments.
- Throughout the maturation of the corrosion product film, material conversion within the film, including transformations from iron oxide to iron sulfide, significantly influences corrosion behavior.
- In the presence of both CO2 and H2S, neither inhibits uniform corrosion nor decelerates it; instead, it facilitates the corrosion process.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gong, Q.; Wu, H.; Yang, F.; Tang, Z. Corrosion of pipeline steel weld metals containing alloying elements in CO2/H2S environment. Gas Sci. Eng. 2023, 109, 104846. [Google Scholar] [CrossRef]
- NACE TM0177-2005; Laboratory Testing of Metals for Resistance to Specific Forms of Environmental Cracking in H2S Environments. NACE International: Houston, TX, USA, 2005.
- Mansfeld, F. The influence of carbon dioxide on corrosion. Corrosion 1988, 44, 33–46. [Google Scholar]
- Ben, M.E.A.; Seghier, B.; Keshtegar, K.F.; Tee, T.; Zayed, R.; Abbassi, N.T.T. Prediction of maximum pitting corrosion depth in oil and gas pipelines. Eng. Fail. Anal. 2020, 112, 104505. [Google Scholar]
- Seghier, M.B.; Hoche, D.; Zheludkevich, M. Prediction of the internal corrosion rate for oil and gas pipeline: Implementation of ensemble learning techniques. J. Nat. Gas Sci. Eng. 2022, 99, 104425. [Google Scholar] [CrossRef]
- Nocito, F.; Dibenedetto, A. Atmospheric CO2 mitigation technologies: Carbon capture utilization and storage. Curr. Opin. Green Sustain. Chem. 2020, 21, 34–43. [Google Scholar] [CrossRef]
- Zhao, Z. Corrosion mechanism and prevention of buried gas gathering pipeline in micro H2S/CO2 environment. Oil-Gas Field Surf. Eng. 2021, 40, 73–76. [Google Scholar]
- Zheng, Y.; Jing, N.; Bruce, B. Electrochemical model of mild steel corrosion in a mixed H2S/CO2 aqueous environment in the absence of protective corrosion product layers. Corrosion 2014, 71, 316–325. [Google Scholar] [CrossRef]
- El-Kady, A.; Amin, M.T.; Khan, F.; El-Halwagi, M. Analysis of CO2 pipeline regulations from a safety perspective for offshore carbon capture, utilization, and storage (CCUS). J. Clean. Prod. 2024, 439, 140734. [Google Scholar] [CrossRef]
- Zhou, Y.; Xie, F.; Wang, D.; Wang, Y.; Wu, M. Carbon capture, utilization and storage (CCUS) pipeline steel corrosion failure analysis: A review. Eng. Fail. Anal. 2024, 155, 107745. [Google Scholar] [CrossRef]
- Chen, L.; Liu, Z.; Huang, F.; Dong, J.; Wang, C.; Yuan, J.; Fu, Q. Experimental study of corrosion resistance of 431M2 stainless steel coatings in a CO2-saturated NaCl solution deposited by extreme high-speed laser cladding. Mater. Today Commun. 2024, 38, 108202. [Google Scholar]
- Sun, W.; Nesic, S. A mechanistic model of uniform hydrogen sulfide/carbon dioxide corrosion of mild steel. Corrosion 2009, 65, 291–307. [Google Scholar] [CrossRef]
- Wang, C.; Yan, M.; Zhao, X.; Li, P.; Wang, H. Research progress of H2S/CO2 corrosion in oil and gas development. J. Xi’an Shiyou Univ. 2005, 20, 66–71. [Google Scholar]
- Li, D.P.; Zhang, L.; Yang, J.W.; Lu, M.X.; Ding, J.H.; Liu, M.L. Effect of H2S concentration on the corrosion behavior of pipeline steel under the coexistence of H2S and CO2. Int. J. Miner. Metall. Mater. 2014, 21, 388–394. [Google Scholar] [CrossRef]
- Choi, Y.S.; Nešić, S.; Ling, S. Effect of H2S on the CO2 corrosion of carbon steel in acidic solutions. Electrochim. Acta 2011, 56, 1752. [Google Scholar] [CrossRef]
- Li, W.F.; Zhou, Y.J.; Xue, Y. Corrosion behavior of 110S tube steel in environments of high H2S and CO2 content. J. Iron Steel Res. Int. 2012, 19, 59. [Google Scholar] [CrossRef]
- Ma, H.Y.; Cheng, X.L.; Li, G.Q.; Chen, S.H.; Quan, Z.L.; Zhao, S.Y.; Niu, L. The influence of hydrogen sulfide on corrosion of iron under different conditions. Corros. Sci. 2000, 42, 1669. [Google Scholar] [CrossRef]
- Tang, J.W.; Shao, Y.W.; Zhang, T.; Meng, G.Z.; Wang, F.H. Corrosion behaviour of carbon steel in different concentrations of HCl solutions containing H2S at 90 °C. Corros. Sci. 2011, 53, 1715. [Google Scholar] [CrossRef]
- Nešić, S. Key issues related to modelling of internal corrosion of oil and gas pipelines: A review. Corros. Sci. 2007, 49, 4308. [Google Scholar] [CrossRef]
- Yin, Z.F.; Zhao, W.Z.; Bai, Z.Q.; Feng, Y.R.; Zhou, W.J. Corrosion behavior of SM 80SS tube steel in stimulant solution containing H2S and CO2. Electrochim. Acta 2008, 53, 3690. [Google Scholar] [CrossRef]
- Davoodi, A.; Pakshir, M.; Babaiee, M.; Ebrahimi, G.R. A comparative H2S corrosion study of 304L and 316L stainless steels in acidic media. Corros. Sci. 2011, 53, 399. [Google Scholar] [CrossRef]
- Tang, J.W.; Shao, Y.W.; Guo, J.B.; Zhang, T.; Meng, G.Z.; Wang, F.H. The effect of H2S concentration on the corrosion behavior of carbon steel at 90 °C. Corros. Sci. 2010, 52, 2050. [Google Scholar] [CrossRef]
- Zhongwen, J.I.; Shoumin, C.H.E.N.; Lipeng, G.O.U.; Qili, M.A. Multi-media environment corrosion mechanism and protection technology of gathering and transportation pipeline in oilfield. Appl. Chem. Ind. 2024, 53, 1612–1615. [Google Scholar]
- Khalaf, A.H.; Xiao, Y.; Xu, N.; Wu, B.H.; Li, H.; Lin, B.; Nie, Z.; Tang, J.L. Emerging AI technologies for corrosion monitoring in oil and gas industry: A comprehensive review. Eng. Fail. Anal. 2024, 155, 107735. [Google Scholar] [CrossRef]
- Alamri, A.H. Localized corrosion and mitigation approach of steel materials used in oil and gas pipelines—An overview. Eng. Fail. Anal. 2020, 116, 104735. [Google Scholar] [CrossRef]
- Lin, N.; Huang, H.; Li, S.L. Study on erosion numerical simulation of elbows of pipelines in natural gas gathering stations. Sci. Technol. Eng. 2020, 20, 8543–8549. [Google Scholar]
- Obot, I.B.; Solomon, M.M.; Umoren, S.A.; Suleiman, R.; Elanany, M.; Alanazi, N.M.; Sorour, A.A. Progress in the development of sour corrosion inhibitors: Past, present, and future perspectives. J. Ind. Eng. Chem. 2019, 79, 1–18. [Google Scholar] [CrossRef]
- Yan, T.; Xu, L.-C.; Zeng, Z.-X.; Pan, W.-G. Mechanism and anti-corrosion measures of carbon dioxide corrosion in CCUS: A review. iScience 2024, 27, 108594. [Google Scholar] [CrossRef]
- Wei, L.; Pang, X.; Gao, K. Effect of small amount of H2S on the corrosion behavior of carbon steel in the dynamic supercritical CO2 environments. Corros. Sci. 2016, 103, 132–144. [Google Scholar] [CrossRef]
- Liu, Z.; Gao, X.; Du, L.; Li, J.; Li, P.; Bai, X.; Misra, R.D.K. Corrosion behavior of low-alloy pipeline steel exposed to H2S/CO2-saturated saline solution. J. Mater. Eng. Perform. 2017, 26, 1010–1017. [Google Scholar] [CrossRef]
- Srinivasan, S.; Tebbal, S. Critical factors in predicting CO2/H2S corrosion in multiphase systems. In CORROSION 98; NACE International: San Diego, CA, USA, 1998. [Google Scholar]
- NACE International. H2S-CO2 environment. In CORROSION; Paper No. 1559; NACE International: Houston, TX, USA, 2012. [Google Scholar]
- Pessu, F.; Barker, R.; Neville, A. The influence of pH on localized corrosion behavior of X65 carbon steel in CO2-saturated brines. Corrosion 2015, 71, 1452–1466. [Google Scholar] [CrossRef]
- Pessu, F.; Barker, R.; Neville, A. Pitting and uniform corrosion of X65 carbon steel in sour corrosion environments: The influence of CO2, H2S and temperature. Corrosion 2017, 73, 12454. [Google Scholar] [CrossRef] [PubMed]
- Asmara, Y.; Kurniawan, T.; Ijost, I. Corrosion prediction for corrosion rate of carbon steel in oil and gas environment: A review. Indones. J. Sci. Technol. 2018, 3, 64–74. [Google Scholar] [CrossRef]
- Reddy, M.S.B.; Ponnamma, D.; Sadasivuni, K.K.; Aich, S.; Kailasa, S.; Parangusan, H.; Ibrahim, M.; Eldeib, S.; Shehata, O.; Ismail, M.; et al. Sensors in advancing the capabilities of corrosion detection: A review. Sens. Actuators A Phys. 2021, 332 Pt 1, 113086. [Google Scholar] [CrossRef]
- Banan, A.; Asadi, S. Novel cationic and non-ionic biopolyurethanes for effective inhibition of mild steel corrosion in H2S-CO2 environment. Ind. Crops Prod. 2024, 221, 119320. [Google Scholar] [CrossRef]
- Keshk, A.A.; Elsayed, N.H.; Almutairi, F.M.; Al-Anazi, M.; Said, S.; Althurwi, H.M.; Albalawi, R.K.; El-Aassar, M.R. Effect of green and sustainable extracted fucoidan polysaccharide as a corrosion inhibitor in 3.5% NaCl. Biomass Convers. Biorefinery 2024, 14, 28219–28232. [Google Scholar] [CrossRef]
- ASTM Standard G1-03; Standard Practice for Preparing, Cleaning, and Evaluating Corrosion Test Specimens. ASTM International: West Conshohocken, PA, USA, 2017.
- Qian, Y.H.; Ma, C.H.; Niu, D.; Xu, J.J.; Li, M.S. Influence of Alloyed Chromium on the Atmospheric Corrosion Resistance of Weathering Steels. Corros. Sci. 2013, 74, 424–429. [Google Scholar] [CrossRef]
- NACE SP0775-2023; Preparation, Installation, Analysis, and Interpretation of Corrosion Coupons in Oilfield Operations. NACE International: Houston, TX, USA, 2023.
- ASTM Standard G96-90; Standard Guide for Online Monitoring of Corrosion in Plant Equipment (Electrical and Electrochemical Methods). ASTM International: West Conshohocken, PA, USA, 2018.
- ASTM B826-09(2020); Standard Test Method for Monitoring Atmospheric Corrosion Tests by Electrical Resistance Probes. ASTM: West Conshohocken, PA, USA, 2020.
- Kim, S.; McCormick, B.; Carlson, K.; Lee, S.; Kim, G.; Lee, K.; Yun, Y.-M.; Kang, W. Characterization of flowback and produced water from shale oil and gas wells fractured with different levels of recycled water. Water Pract. Technol. 2024, 19, 4022–4035. [Google Scholar] [CrossRef]
- Kamal, M.S.; Hussein, I.; Mahmoud, M.; Sultan, A.S.; Saad, M.A.S. Oilfield scale formation and chemical removal: A review. J. Pet. Sci. Eng. 2018, 171, 127–139. [Google Scholar] [CrossRef]
- Wu, Q.L.; Zhang, Z.H.; Dong, X.M.; Yang, J.Q. Corrosion Behavior of Low-Alloy Steel Containing 1% Chromium in CO2 Environments. Corros. Sci. 2013, 75, 400–408. [Google Scholar] [CrossRef]
- López, D.A.; Pérez, T.; Simison, S.N. The Influence of Microstructure and Chemical Composition of Carbon and Low Alloy Steels in CO2 Corrosion. A State-of-the-Art Appraisal. Mater. Des. 2003, 24, 561–575. [Google Scholar] [CrossRef]
- Liu, Z.; Gao, X.; Du, L.; Li, J.; Li, P.; Yu, C.; Misra, R.D.K.; Wang, Y. Comparison of corrosion behaviour of low-alloy pipeline steel exposed to H2S/CO2-saturated brine and vapour-saturated H2S/CO2 environments. Electrochim. Acta 2017, 232, 528–541. [Google Scholar] [CrossRef]
- Lee, K.-L.; Nesic, S. The Effect of Trace Amount of H2S on CO2 Corrosion Investigated by Using the EIS Technique. In CORROSION 2005; NACE International: Houston, TX, USA, 2005. [Google Scholar]
- Sun, W.; Nešić, S.; Woollam, R.C. The effect of temperature and ionic strength on iron carbonate (FeCO3) solubility limit. Corros. Sci. 2009, 51, 1273–1276. [Google Scholar] [CrossRef]
- Gao, S.; Jin, P.; Brown, B.; Young, D.; Nesic, S.; Singer, M. Corrosion behavior of mild steel in sour environments at elevated temperatures. In CORROSION; NACE International: Houston, TX, USA, 2017. [Google Scholar]
- Ning, J.; Zheng, Y.; Brown, B.; Young, D.; Nesic, S. The role of iron sulfide polymorphism in localized H2S corrosion of mild steel. Corros. Sci. 2017, 73, 155–168. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Xu, M.; Hu, T.; Xue, G.; Chen, F.; Zhao, H.; Zhou, H.; Lei, Y.; Zhu, K. Effects of H2S content on the corrosion behavior of gas storage reservoir injection and production pipeline steel in CO2-H2S environment. Mater Today Commun 2024, 41, 110364. [Google Scholar] [CrossRef]
- Zhang, G.A.; Zeng, Y.; Guo, X.P.; Jiang, F.; Shi, D.Y.; Chen, Z.Y. Electrochemical corrosion behavior of carbon steel under dynamic high pressure H2S/CO2 environment. Corros. Sci. 2012, 65, 37–47. [Google Scholar] [CrossRef]
- Xiang, Y.; Xu, M.; Choi, Y.-S. State-of-the-art overview of pipeline steel corrosion in impure dense CO2 for CCS transportation: Mechanisms and models. Corros. Eng. Sci. Technol. 2017, 52, 485–509. [Google Scholar] [CrossRef]
- Zheng, S.; Li, C.; Chen, C.; Yang, M.; Qi, Y. Stress-oriented hydrogen-induced cracking of L360QS steel. Mater. Perform. 2012, 51, 71–75. [Google Scholar] [CrossRef]
- Li, W.F.; Zhou, Y.J.; Xue, Y. Corrosion behavior of tubing steel in an environment with high H2S and CO2 content. J. Wuhan Univ. Technol. Mater. Sci. Ed. 2013, 28, 1038–1043. [Google Scholar] [CrossRef]





| Element | Si | Mn | P | S | Al | Cr | Ni | Cu | C | Fe |
|---|---|---|---|---|---|---|---|---|---|---|
| Content (wt.%) | 0.2 | 0.41 | 0.013 | 0.009 | 0.029 | 0.01 | 0.01 | 0.01 | 0.18 | balance |
| Chemical Composition | MgSO4 | CaSO4 | Na2CO3 | NaHCO3 | NaSO4 | NaCl | H2O |
|---|---|---|---|---|---|---|---|
| Mass fraction (wt.%) | 0.180 | 0.642 | 0.557 | 5.310 | 8.732 | 16.480 | balance |
| Injected Gases | Physical State | Experimental Period | Remarks | ||
|---|---|---|---|---|---|
| Group A | Basic solution | N2 (for oxygen repellent) | 60 °C, atmospheric pressure | Corrosion coupons were taken and analyzed 24 h, 48 h, 72 h, 120 h, 192 h, 288 h, and 384 h after the start of the experiment. | Dynamic stirring conditions were not performed in this experiment, and the medium dynamics were generated naturally by the energized gas. |
| Group B | H2S | ||||
| Group C | CO2 | ||||
| Group D | H2S+CO2 |
| Basic Solution | Basic Solution+CO2 | Basic Solution+H2S | Basic Solution+CO2+H2S | |
|---|---|---|---|---|
| Cumulative corrosion rate | 1. Within 288 h, the corrosion rate gradually increases | 1. Within 72 h, the corrosion rate gradually increases | 1. The initial corrosion rate is higher; it is significantly higher than the situation where only CO2 is introduced | 1. It has the highest initial corrosion rate |
| 2. The corrosion rate reaches its peak at approximately 288 h and then shows a decreasing trend | 2. The corrosion rate reaches its peak around 72 h and then shows a slow decreasing trend | 2. After the formation of the corrosion product film, the corrosion rate gradually decreases | 2. The corrosion rate gradually stabilizes after 72 h and is significantly higher than in the other three situations | |
| Thickness loss | 1. Within 144 h, there is minimal change in thickness | 1. Within 96 h, there is minimal change in thickness | 1. Within 96 h, the thickness change is similar to the situation where only CO2 is introduced | 1. Within 96 h, the thickness change is similar to the situation where only CO2 or H2S is introduced |
| 2. As corrosion progresses, the thickness loss gradually increases | 2. As corrosion progresses, the thickness loss slowly increases | 2. As corrosion progresses, the thickness loss remains almost stable and unchanged | 2. As corrosion progresses, the thickness loss gradually increases and is significantly higher than the thickness loss in the original solution |
| Data Acquisition | Similarity | The changes in the corrosion rate and thickness trends shown in the initial formation of the corrosion product film to the stabilization stage are consistent. |
| Difference | The ER probe can collect a large amount of data continuously, and the obtained data can show strong regularity without fitting. The corrosion coupon can only collect a small amount of data at intervals, and the data obtained are more dispersed and require complex post-processing such as fitting. | |
| Data Analysis | Similarity | The pattern of the endpoints obtained by the two methods is the same. |
| Difference | At low corrosion rates (in the case of the basic solution and with only H2S passed through), the pattern reflected by the ER probe and the corrosion coupon do not agree with each other. | |
| Usage Environment | Similarity | Both methods can be performed in the field and are suitable for different types of corrosive environments. |
| Difference | The data obtained from the ER probe and the corrosion coupon under the condition of CO2 alone are not identical. |
| Solution | End-of-Period Corrosion Rate | |||
|---|---|---|---|---|
| ER Probe | Corrosion Coupon | |||
| Corrosion Rate | Classification | Corrosion Rate | Classification | |
| Basic solution | 0.0069 | Moderate | 0.0913 | Moderate |
| +CO2 | 0.08 | Moderate | 0.177 | High |
| +H2S | 0.05 | Moderate | 0.0142 | Low |
| +H2S-CO2 | 0.171 | High | 0.179 | High |
| Solution | End-of-Period Corrosion Rate | |||
|---|---|---|---|---|
| ER Probe | Corrosion Coupon | |||
| Thickness Loss | Classification | Thickness Loss | Classification | |
| Basic solution | 0.04166 | Moderate | 5.24 × 10−4 | Moderate |
| +CO2 | 0.03973 | Moderate | 0.00101 | High |
| +H2S | 0.06615 | Moderate | 8.1 × 10−5 | Low |
| +H2S-CO2 | 0.05753 | High | 0.00105 | High |
| Chemical Compound | Solubility Product Constant (Ksp) |
|---|---|
| Fe(OH)2 | 8.0 × 10−16 |
| Fe(OH)3 | 4.0 × 10−38 |
| FeCO3 | 3.2 × 10−11 |
| FeS | 6.3 × 10−18 |
| FeS2 | 4.0 × 10−22 |
| Fe2O3 | 10−30~10−40 |
| Fe3O4 | 10−40~10−50 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yue, Y.; Yin, Z.; Li, S.; Zhang, Z.; Zhang, Q. Corrosion Behavior of Mild Steel in Various Environments Including CO2, H2S, and Their Combinations. Metals 2025, 15, 440. https://doi.org/10.3390/met15040440
Yue Y, Yin Z, Li S, Zhang Z, Zhang Q. Corrosion Behavior of Mild Steel in Various Environments Including CO2, H2S, and Their Combinations. Metals. 2025; 15(4):440. https://doi.org/10.3390/met15040440
Chicago/Turabian StyleYue, Yuanguang, Zhibiao Yin, Shiming Li, Ziyue Zhang, and Qifu Zhang. 2025. "Corrosion Behavior of Mild Steel in Various Environments Including CO2, H2S, and Their Combinations" Metals 15, no. 4: 440. https://doi.org/10.3390/met15040440
APA StyleYue, Y., Yin, Z., Li, S., Zhang, Z., & Zhang, Q. (2025). Corrosion Behavior of Mild Steel in Various Environments Including CO2, H2S, and Their Combinations. Metals, 15(4), 440. https://doi.org/10.3390/met15040440






