Fabrication of Corrosion Resistance Micro-Nanostructured Superhydrophobic Anodized Aluminum in a One-Step Electrodeposition Process
Abstract
:1. Introduction
2. Experimental Procedure
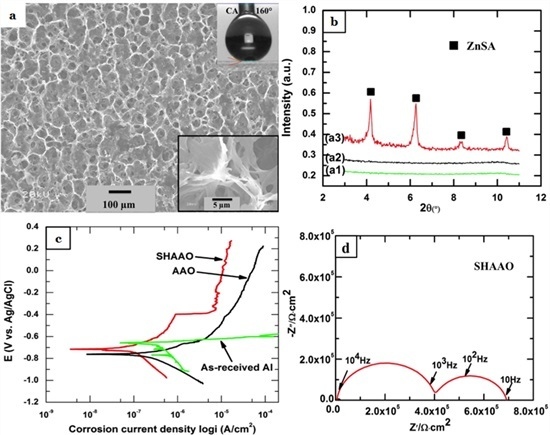
3. Results and Discussion
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| AAO | Anodized aluminum oxide |
| SHAAO | Superhydrophobic anodized aluminum oxide |
| SA | Stearic acid |
| ZnSA | Zinc stearate |
| EIS | Electrochemical impedance spectroscopy |
| OCP | Open circuit potential |
| CA | Contact angle |
| SEM | Scanning electron microscope |
| XRD | X-ray diffraction |
| FTIR | Fourier transform infrared spectroscopy |
| EDX | Energy dispersive X-ray spectrometer |
References
- Dotzauer, D.M.; Dai, J.; Sun, L.; Bruening, M.L. Catalytic membranes prepared using layer-by-layer adsorption of polyelectrolyte/metal nanoparticle films in porous supports. Nano Lett. 2006, 6, 2268–2272. [Google Scholar] [CrossRef] [PubMed]
- Simovic, S.; Losic, D.; Vasilev, K. Controlled drug release from porous materials by plasma polymer deposition. Chem. Commun. 2010, 46, 1317–1319. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Meng, G.; Huang, Q.; Xu, Q.; Chu, Z.; Zhu, C. FITC-modified PPy nanotubes embedded in nanoporous AAO membrane can detect trace PCB20 via fluorescence ratiometric measurement. Chem. Commun. 2011, 47, 3808–3810. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Goebl, J.; Yin, Y. Templated synthesis of nanostructured materials. Chem. Soc. Rev. 2013, 42, 2610–2653. [Google Scholar] [CrossRef] [PubMed]
- Jani, A.M.M.; Anglin, E.J.; McInnes, S.J.P.; Losic, D.; Shapter, J.G.; Voelcker, N.H. Nanoporous anodic aluminium oxide membranes with layered surface chemistry. Chem. Commun. 2009, 21, 3062–3064. [Google Scholar] [CrossRef] [PubMed]
- Bouchama, L.; Azzouz, N.; Boukmouche, N.; Chopart, J.P.; Daltin, A.L.; Bouznit, Y. Enhancing aluminum corrosion resistance by two-step anodizing process. Surf. Coat. Technol. 2013, 235, 676–684. [Google Scholar] [CrossRef]
- Huang, Y.; Sarkar, D.K.; Chen, X.-G. A one-step process to engineer superhydrophobic copper surfaces. Mater. Lett. 2010, 64, 2722–2724. [Google Scholar] [CrossRef]
- Ciasca, G.; Papi, M.; Chiarpotto, M.; de Ninno, A.; Giovine, E.; Campi, G.; Gerardino, A.; de Spirito, M.; Businaro, L. Controlling the cassie-to-wenzel transition: An easy route towards the realization of tridimensional arrays of biological objects. Nano-Micro Lett. 2014, 6, 280–286. [Google Scholar] [CrossRef]
- Zhang, F.; Chen, S.; Dong, L.; Lei, Y.; Liu, T.; Yin, Y. Preparation of superhydrophobic films on titanium as effective corrosion barriers. Appl. Surf. Sci. 2011, 257, 2587–2591. [Google Scholar] [CrossRef]
- Yin, B.; Fang, L.; Tang, A.-Q.; Huang, Q.-L.; Hu, J.; Mao, J.-H.; Bai, G.; Bai, H. Novel strategy in increasing stability and corrosion resistance for super-hydrophobic coating on aluminum alloy surfaces. Appl. Surf. Sci. 2011, 258, 580–585. [Google Scholar] [CrossRef]
- Huang, Y.; Sarkar, D.K.; Chen, X.-G. Superhydrophobic aluminum alloy surfaces prepared by chemical etching process and their corrosion resistance properties. Appl. Surf. Sci. 2015, 356, 1012–1024. [Google Scholar] [CrossRef]
- Huang, Y.; Sarkar, D.K.; Gallant, D.; Chen, X.-G. Corrosion resistance properties of superhydrophobic copper surfaces fabricated by one-step electrochemical modification process. Appl. Surf. Sci. 2013, 282, 689–694. [Google Scholar] [CrossRef]
- Zuo, Z.; Liao, R.; Guo, C.; Yuan, Y.; Zhao, X.; Zhuang, A.; Zhang, Y. Fabrication and anti-icing property of coral-like superhydrophobic aluminum surface. Appl. Surf. Sci. 2015, 331, 132–139. [Google Scholar] [CrossRef]
- Watson, G.S.; Green, D.W.; Schwarzkopf, L.; Li, X.; Cribb, B.W.; Myhra, S.; Watson, J.A. A gecko skin micro/nano structure—A low adhesion, superhydrophobic, anti-wetting, self-cleaning, biocompatible, antibacterial surface. Acta Biomater. 2015, 21, 109–122. [Google Scholar] [CrossRef] [PubMed]
- Vengatesh, P.; Kulandainathan, M.A. Hierarchically ordered self-lubricating superhydrophobic anodized aluminum surfaces with enhanced corrosion resistance. ACS Appl. Mater. Interfaces 2015, 7, 1516–1526. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, S.; Zhang, Y.; Yu, M.; Liu, J. Fabrication of superhydrophobic layered double hydroxides films with different metal cations on anodized aluminum 2198 alloy. Mater. Lett. 2015, 142, 137–140. [Google Scholar] [CrossRef]
- Liu, W.; Luo, Y.; Sun, L.; Wu, R.; Jiang, H.; Liu, Y. Fabrication of the superhydrophobic surface on aluminum alloy by anodizing and polymeric coating. Appl. Surf. Sci. 2013, 264, 872–878. [Google Scholar] [CrossRef]
- Fujii, T.; Aoki, Y.; Habazaki, H. Superhydrophobic hierarchical surfaces fabricated by anodizing of oblique angle deposited Al–Nb alloy columnar films. Appl. Surf. Sci. 2011, 257, 8282–8288. [Google Scholar] [CrossRef]
- Liu, Q.; Kang, Z. One-step electrodeposition process to fabricate superhydrophobic surface with improved anticorrosion property on magnesium alloy. Mater. Lett. 2014, 137, 210–213. [Google Scholar] [CrossRef]
- Huang, Y.; Sarkar, D.K.; Chen, X.-G. Superhydrophobic nanostructured zno thin films on aluminum alloy substrates by electrophoretic deposition process. Appl. Surf. Sci. 2015, 327, 327–334. [Google Scholar] [CrossRef]
- He, T.; Wang, Y.; Zhang, Y.; Lv, Q.; Xu, T.; Liu, T. Super-hydrophobic surface treatment as corrosion protection for aluminum in seawater. Corros. Sci. 2009, 51, 1757–1761. [Google Scholar] [CrossRef]




© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, Y.; Sarkar, D.K.; Chen, X.-G. Fabrication of Corrosion Resistance Micro-Nanostructured Superhydrophobic Anodized Aluminum in a One-Step Electrodeposition Process. Metals 2016, 6, 47. https://doi.org/10.3390/met6030047
Huang Y, Sarkar DK, Chen X-G. Fabrication of Corrosion Resistance Micro-Nanostructured Superhydrophobic Anodized Aluminum in a One-Step Electrodeposition Process. Metals. 2016; 6(3):47. https://doi.org/10.3390/met6030047
Chicago/Turabian StyleHuang, Ying, Dilip K. Sarkar, and X.-Grant Chen. 2016. "Fabrication of Corrosion Resistance Micro-Nanostructured Superhydrophobic Anodized Aluminum in a One-Step Electrodeposition Process" Metals 6, no. 3: 47. https://doi.org/10.3390/met6030047
APA StyleHuang, Y., Sarkar, D. K., & Chen, X.-G. (2016). Fabrication of Corrosion Resistance Micro-Nanostructured Superhydrophobic Anodized Aluminum in a One-Step Electrodeposition Process. Metals, 6(3), 47. https://doi.org/10.3390/met6030047