Kinetic Study of Ag Leaching from Arsenic Sulfosalts in the S2O32?-O2-NaOH System
Abstract
:1. Introduction
2. Materials and Methods
3. Results
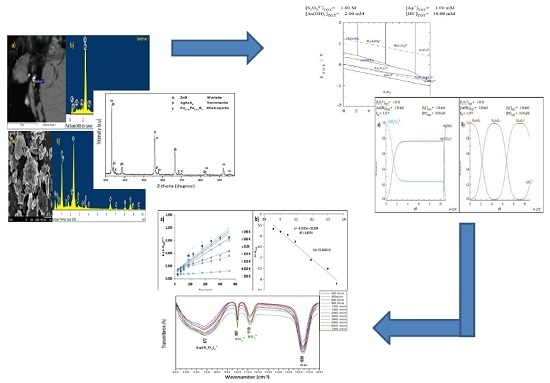
3.1. Characterization
Mineralogical Analysis by X-Ray Diffraction (XRD)
3.2. Simulation of Speciation of Ag-S2O32−-O2 System
3.3. Kinetic Study of Silver Leaching in Thiosulfate System in NaOH Medium of Zn Concentrate
3.3.1. Particle Size Effect
3.3.2. Concentration Effect
3.3.3. Temperature Effect
3.3.4. Stirring Rate Effect
3.3.5. Solid-Liquid Ratio Effect
4. Discussion
5. Conclusions
- (1)
- The presence of sulfides of the base metal, such as chalcopyrite (Cu0.25Fe0.75S2) and wurtzite (ZnS), was identified through chemical and mineralogical characterization of the powder samples of the Zn concentrate. These species, which are typical of the mining district of Zimapan, Mexico, correspond to the mineral matrix of the concentrate. Chemical analysis yielded 258.40 mg·Ag·kg−1. The presence of Ag is related to the formation of As sulfosalts, such as trechmannite (AgAsS2), which was identified in the XDR and SEM–EDS results.
- (2)
- Thermodynamically speaking, silver-thiosulfate complexes Ag(S2O3)35− and Ag(S2O3)23− form under the same pH conditions and oxidizing potentials. However, the latter is more abundant. Under the conditions used for the experiments, the formation of AsO42− and of SO42− ions was identified in the Pourbaix diagrams. The presence of the latter was confirmed through vibrations obtained by FTIR.
- (3)
- A reduction in particle size has a significant effect on the dissolution rate of Ag, since a larger surface area is in contact with the leaching solution, which facilitates the formation of Ag complexes.
- (4)
- A [S2O32−] ≥ 0.75 mol·L−1 is suggested in order to secure a sufficient number of ions to allow for the formation of silver–thiosulfate complexes. The influence of the leaching agent, where there are two reaction orders (n = 0.08 and n = 0), can be attributed to the fact that dilute thiosulfate solutions (<0.01 mol·L−1) decompose more rapidly than concentrated solutions (>0.1 mol·L−1). The reaction order corresponding to [OH−] (n = 0.13) has a greater influence than that of the complexing agent on the reaction rate.
- (5)
- The activation energy value obtained in the system was 47.16 kJ·mol−1, which indicates that the chemical stages of the process occur in time intervals longer than the matter transport stages. This was confirmed by the adjustment conducted for the shrinking core model (kexpt = 1 − (1 − X)1/3). On the other hand, the stirring rate did not have a considerable effect on the leaching rate of Ag.
- (6)
- We obtained the kinetic equation of the dissolution of Ag contained in the Zn concentrate from Zimapan, Mexico, which corresponds to the chemical control mechanism. The equation is valid in the temperature range of 298–338 K and in the concentration range of 0.05–0.75 mol·L−1 S2O32− and 0.3–0.6 mol·L−1 OH−.
- (7)
- The experimental conditions at which the best recovery (98.9%) was achieved are as follows: [OH−] = 0.1 mol·L−1, [S2O32−] = 0.5 mol·L−1, d = 74 µm, 338 K, pH = 9.0, RPM = 670 min−1, PO2 = 1 atm, and tr = 360 min.
Author Contributions
Conflicts of Interest
References
- López-Méndez, T.A.; Rivas-Ríos, V.C. Anuario Estadístico de la Minería Mexicana, 2014. Servicio Geológico Mexicano (SGM), 2015. Available online: www.sgm.gob.mx/productos/pdf/Anuario_2015_Edicion_2016.pdf (accessed on 24 June 2016).
- González-Partida, E.; Carrillo-Chávez, A.; Levresse, G.; Tritlla, J.; Camprub, A. Genetic implications of fluid inclusions in skarn chimney ore, Las Animas Zn-Pb-Ag- (F) deposit, Zimapán, Mexico. Ore Geol. Rev. 2003, 23, 91–96. [Google Scholar] [CrossRef]
- Frontino-Paulino, J.; Afonso, J.C.; Mantovano, J.L.; Vianna, C.A.; Silva-Dias da Cunha, J.W. Recovery of tungsten by liquid-liquid extraction from a wolframite concentrate after fusion with sodium hydroxide. Hydrometallurgy 2012, 127–128, 121–124. [Google Scholar] [CrossRef]
- Martins, J.I.; Lima, J.L.; Moreira, A.; Costa, S.C. Tungsten recovery from alkaline leach solutions as synthetic scheelite. Hydrometallurgy 2007, 85, 110–115. [Google Scholar] [CrossRef]
- Luna, R.M.; Lapidus, G.T. Cyanidation Kinetics of Silver Sulfide. Hidrometallurgy 2000, 56, 171–188. [Google Scholar] [CrossRef]
- Zhongwei, Z.; Jiangtao, L.; Shibo, W.; Honggui, L.; Maosheng, L.; Peimei, S.; Yunjiao, L. Extracting tungsten from scheelite concentrate with caustic soda by autoclaving process. Hydrometallurgy 2011, 108, 152–156. [Google Scholar] [CrossRef]
- Alp, I.; Celep, O.; Paktunç, D.; Thibault, Y. Influence of potassium hydroxide pretreatment on the extraction of gold and silver from a refractory ore. Hydrometallurgy 2014, 146, 64–71. [Google Scholar] [CrossRef]
- Nogarte, H.; Haque, N. Energy and greenhouse gas impacts of mining and mineral processing operations. J. Clean. Prod. 2010, 18, 266–274. [Google Scholar] [CrossRef]
- Senanayake, G. A surface adsorption/reaction mechanism for gold oxidation by copper(II) in ammoniacal thiosulfate solutions. J. Colloid Interface Sci. 2005, 286, 253–257. [Google Scholar] [CrossRef] [PubMed]
- Kholmogorov, A.; Kononova, O.; Danilenko, N.; Goryaeva, N.; Shatnykh, K.; Kachin, S. Recovery of silver from thiosulfate and thiocyanate leach solutions by adsorption on anion exchange resins and activated carbon. Hydrometallurgy 2007, 88, 189–195. [Google Scholar] [CrossRef]
- Hancock, G.F. Energy requirements for manufacture of some non-ferrous metals. Met. Technol. 1984, 11, 290–299. [Google Scholar] [CrossRef]
- Hernández, J.; Patino, F.; Rivera, I.; Reyes, I.A.; Flores, M.U.; Juárez, J.C.; Reyes, M. Leaching kinetics in cyanide media of Ag contained in the industrial mining-metallurgical wastes in the state of Hidalgo, Mexico. Int. J. Min. Sci. Technol. 2014, 24, 689–694. [Google Scholar] [CrossRef]
- Deschenes, G.; Rousseau, M.; Tardif, J. Effect of the composition of some sulphide minerals on cyanidation and use of lead nitrate and oxygen to alleviate their impact. Hidrometallurgy 1998, 11, 123–142. [Google Scholar] [CrossRef]
- Xie, F.; Dreisinger, D.B. Use of ferricyanide for gold and silver cyanidation. Trans. Nonferr. Met. Soc. China 2009, 19, 176–189. [Google Scholar] [CrossRef]
- Briones, R.; Lapidus, G.T. The leaching of silver sulfide with the thiosulfate-ammonia-cupric ion system. Hydrometallurgy 1998, 50, 243–260. [Google Scholar] [CrossRef]
- Alvarado-Macías, G.; Fuentes-Aceituno, J.C.; Nava-Alonso, F. Study of silver leaching with the thiosulfate-nitrite-copper alternative system: Effect of thiosulfate concentration and leaching temperature. Miner. Eng. 2016, 86, 140–148. [Google Scholar] [CrossRef]
- Alonso, A.R.; Lapidus, G.T.; González, I. Selective silver electroseparation from ammoniacal thiosulfate leaching solutions using a rotating cylinder electrode reactor (RCE). Hydrometallurgy 2008, 92, 115–123. [Google Scholar] [CrossRef]
- Alonso, A.R.; Lapidus, G.T.; González, I. A strategy to determine the potential interval for selective silver electrodeposition from ammoniacal thiosulfate solutions. Hydrometallurgy 2007, 85, 144–153. [Google Scholar] [CrossRef]
- Rivera, I.; Patiño, F.; Roca, A.; Cruells, M. Kinetics of metallic silver leaching in the O2-thiosulfate system. Hydrometallurgy 2015, 156, 63–70. [Google Scholar] [CrossRef]
- Lapidus, G.T. Mathematical modelling of metal leaching in nonporous minerals. Chem. Eng. Sci. 1992, 47, 1933–1941. [Google Scholar] [CrossRef]
- Grosse, A.C.; Dicinoski, G.W.; Shaw, M.J.; Haddad, P.R. Leaching and recovery of gold using ammoniacal thiosulfate leach liquors (a review). Hydrometallurgy 2003, 69, 1–21. [Google Scholar] [CrossRef]
- Jeffrey, M.I. Kinetic aspects of gold and silver leaching in ammonia-thiosulfate solutions. Hydrometallurgy 2001, 60, 7–16. [Google Scholar] [CrossRef]
- Muir, D.M.; Aylmore, M.G. Thiosulfate as an alternative to cyanide for gold processing-Issues and impediments. Miner. Process. Extr. Metall. 2005, 15, 113–116. [Google Scholar] [CrossRef]
- Alonso-Gómez, A.R.; Lapidus, G.T. Inhibition of lead solubilization during the leaching of gold and silver in ammoniacal thiosulfate solutions (effect of phosphate addition). Hydrometallurgy 2009, 99, 89–96. [Google Scholar] [CrossRef]
- Feng, D.; Van Deventer, J.S.J. Ammoniacal thiosulphate leaching of gold in the presence of pyrite. Hydrometallurgy 2006, 82, 126–132. [Google Scholar] [CrossRef]
- Solís-Marcial, O.J.; Lapidus, G.T. Study of the dissolution of chalcopyrite in sulfuric acid solutions containing alcohols and organic acid. Electrochim. Acta 2014, 140, 434–437. [Google Scholar] [CrossRef]
- Aguirre, C.L.; Toro, N.; Carvajal, N.; Watling, H.; Aguirre, C. Leaching of chalcopyrite (CuFeS2) with an imidazolium-based ionic liquid in the presence of chloride. Miner. Eng. 2016, 99, 60–66. [Google Scholar] [CrossRef]
- Mohammadi, E.; Pourabdoli, M.; Ghobeiti-Hasab, M.; Heidarpour, A. Ammoniacal thiosulfate leaching of refractory oxide gold ore. Int. J. Miner. Process. 2017, 164, 6–10. [Google Scholar] [CrossRef]
- Heath, F.A.; Jeffrey, M.I.; Zhang, H.G.; Rumball, J.A. Anaerobic thiosulfate leaching: Development of in situ gold leaching systems. Miner. Eng. 2008, 21, 424–433. [Google Scholar] [CrossRef]
- Zhang, S. Oxidation of Refractory Gold Concentrates and Simultaneous Dissolution of Gold in Aerated Alkaline Solutions. Ph.D. Thesis, Murdoch University Western Australia, Murdoch, Australia, March 2004. Available online: http://researchrepository.murdoch.edu.au/id/eprint/422/1/01Front.pdf (accessed on 9 October 2015).
- Aylmore, M.G.; Muir, D.M. Thiosulfate leaching of gold. A review. Miner. Eng. 2001, 14, 135–174. [Google Scholar] [CrossRef]
- Zipperian, D.; Raghavan, S.; Wilson, J.P. Gold and silver extraction by amoniacal thiosulfate leaching from a rhyolite ore. Hydrometallurgy 1988, 19, 361–375. [Google Scholar] [CrossRef]
- Villaseñor, C.G.; Peterson, E.; Avedaño, S.; Gómez-Caballero, A.; Sousa, J.; Reyes-Salas, M. Minerales del grupo de la tetrahedrita en las minas lomo de toro y las ánimas, Zimapán Hidalgo. Actas INAGEQ 1996, 2, 124–129. Available online: http://biblat.unam.mx/es/revista/actas-inageq/articulo/minerales-del-grupo-de-la-tetrahedrita-en-las-minas-lomo-del-toro-y-las-animas-zimapan-hidalgo (accessed on 14 September 2016).
- Rodríguez-Rodríguez, C. Estudio de la Oxidación de Sulfuros Con Ozono Para la Recuperación de Metales Preciosos Contenidos en Minerales Refractarios. Ph.D. Thesis, Centro de Investigación y Estudios Avanzados del Instituto Politécnico Nacional, Coahuila, México, 2014; pp. 21–26. [Google Scholar]
- Parga, J.R.; Carrillo, F.R. Avances en los métodos de recuperación de oro y plata de minerales refractarios. Rev. Met. Madr. 1996, 32, 4. Available online: http://revistademetalurgia.revistas.csic.es/index.php/revistademetalurgia/article/viewFile/907/920 (accessed on 12 July 2016). [CrossRef]
- Bundschuh, J.; Bhattacharya, P.; Nath, B.; Naidu, R.; Ng, J.; Guilherme, L.R.G.; Ma, L.Q.; Kim, K.W.; Jean, J.S. Arsenic ecotoxicology: The interface between geosphere, hydrosphere andbiosphere. J. Hazard. Mater. 2013, 262, 883–886. [Google Scholar] [CrossRef] [PubMed]
- Levenspiel, O. Ingeniería de las Reacciones Químicas, 2nd ed.; Reverté: Barcelona, España, 2002; pp. 397–441. [Google Scholar]
- Ongley, L.K.; Sherman, L.; Armienta, A.; Concilio, A.; Salinas, C.F. Arsenic in the soils of Zimapán, México. Environ. Pollut. 2007, 145, 793–799. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Tovar, R.; Téllez-Hernández, J.; Monroy-Fernández, M.G. Influence of minerals from the tailings in the bioaccessibility of arsenic, lead, zinc and cadmium, in the mining district Zimapán, México. Rev. Int. Contam. Ambient. 2012, 28, 3, ISSN 0188-4999. [Google Scholar]
- Martínez-Frías, J.; Marín-Ramos, J.D. Sulfuros y Sulfonales de Metales Nobles, 1st ed.; Textos Universitarios: Madrid, España, 1995; pp. 44–48. [Google Scholar]
- Kametani, H.; Aoki, A. Potential-pH diagrams of the SO2-H2O and S2O3-H2O Systems at 90 degrees. Trans. Natl. Inst. Met. 1976, 18, 20–30. [Google Scholar]
- Osseo-Asare, K. A numerical method for computing hydrometallurgical activity-activity diagrams. Hydrometallurgy 1979, 4, 217–232. [Google Scholar] [CrossRef]
- Dhawale, S.W. Thiosulfate. J. Chem. Educ. 1993, 70, 12–14, ISSN 0021-9584. [Google Scholar] [CrossRef]
- Aylmore, M.G. Treatment of a refractory gold-copper sulfide concentrate by copper ammoniacal thiosulfate leaching. Miner. Eng. 2001, 14, 615–637. [Google Scholar] [CrossRef]
- Qian, G.; Jiexue, H.; Cao, C. Kinetics of gold leaching from sulfide gold concentrates with thiosulfate solution. Trans. Nonferr. Met. Soc. China 1993, 3, 30–36. Available online: http://www.ysxbcn.com/down/upfile/soft/2010626/1993-4-5.pdf (accessed on 3 April 2016).
- Abbruzzese, C.; Fornyari, P.; Massidda, R.; Veglio, F.; Ubaldini, S. Thiosulfate leaching for gold hydrometallurgy. Hydrometallurgy 1995, 39, 265–276. [Google Scholar] [CrossRef]
- Ballester, A.; Verdeja, L.; Sancho, J. Metalurgia Extractiva Vol. II Procesos de Obtención, 2nd ed.; Síntesis: Madrid, España, 2013; pp. 42–49. [Google Scholar]
- Aazami, M.; Lapidus, G.T.; Azadeh, A. The effect of solution parameters on the thiosulfate leaching of Zarshouran refractory gold ore. Int. J. Miner. Process. 2014, 131, 43–50. [Google Scholar] [CrossRef]
- Habashi, F. Texbook of Hydrometallurgy, 2nd ed.; Metallurgie Extractive: Québec City, QC, Canada, 1999; pp. 287–306. [Google Scholar]
- Teja-Ruiz, A.M.; Juárez-Tapia, J.C.; Hernández-Cruz, L.E.; Reyes-Pérez, M.; Patiño-Cardona, F.; Reyes-Dominguez, I.A.; Flores-Guerrero, M.U.; Palacios-Beas, E.G. Influence of Temperature on the Formation of Ag Complexed in a S2O32−-O2 System. Minerals 2017, 7, 16. [Google Scholar] [CrossRef]













| Parameter | Experimental Conditions of Ag Leaching | |||||
|---|---|---|---|---|---|---|
| Particle Size Effect | [S2O32−] Effect | [OH−] Effect | Temperature Effect | Stirring Rate Effect | Effect of Solid/Liquid Ratio | |
| Temperature (K) | 298 | 298 | 298 | 298, 303, 313, 323, 328, 333 and 338 | 298 | 298 |
| Particle diameter (μm) | 105, 74, 53, 44, 37 and <37 | 74 | 74 | 74 | 74 | 74 |
| Stirring rate (min−1) | 670 | 670 | 670 | 670 | 300, 500, 670 and 800 | 670 |
| [S2O32−] (mol·L−1) | 0.5 | 1.5, 1.25, 1, 0.75, 0.5, 0.1 and 0.05 | 0.5 | 0.5 | 0.5 | 0.5 |
| [OH−] (mol·L−1) | 0.1 | 0.1 | 0.6, 0.4, 0.3, 0.2, 0.1, 0.033 and 0.01 | 0.1 | 0.1 | 0.1 |
| Solid/liquid ratio | 1:25 | 1:25 | 1:25 | 1:25 | 1:25 | 1:50, 1:25, 1:15, 1:10 and 1:5. |
| Element | As | Cd | Co | Cu | Fe | Mg | Pb | Ag | Zn | Sb | Sn | S |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Weight (%) | 0.11 | 0.33 | 0.01 | 2.94 | 5.74 | 0.03 | 0.21 | 0.03 | 46.82 | 0.07 | 0.02 | 36.44 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Teja Ruiz, A.M.; Juárez Tapia, J.C.; Reyes Domínguez, I.A.; Hernández Cruz, L.E.; Pérez, M.R.; Patiño Cardona, F.; Flores Guerrero, M.U. Kinetic Study of Ag Leaching from Arsenic Sulfosalts in the S2O32?-O2-NaOH System. Metals 2017, 7, 411. https://doi.org/10.3390/met7100411
Teja Ruiz AM, Juárez Tapia JC, Reyes Domínguez IA, Hernández Cruz LE, Pérez MR, Patiño Cardona F, Flores Guerrero MU. Kinetic Study of Ag Leaching from Arsenic Sulfosalts in the S2O32?-O2-NaOH System. Metals. 2017; 7(10):411. https://doi.org/10.3390/met7100411
Chicago/Turabian StyleTeja Ruiz, Aislinn M., Julio C. Juárez Tapia, Iván A. Reyes Domínguez, Leticia E. Hernández Cruz, Martín Reyes Pérez, Francisco Patiño Cardona, and Mizraim U. Flores Guerrero. 2017. "Kinetic Study of Ag Leaching from Arsenic Sulfosalts in the S2O32?-O2-NaOH System" Metals 7, no. 10: 411. https://doi.org/10.3390/met7100411
APA StyleTeja Ruiz, A. M., Juárez Tapia, J. C., Reyes Domínguez, I. A., Hernández Cruz, L. E., Pérez, M. R., Patiño Cardona, F., & Flores Guerrero, M. U. (2017). Kinetic Study of Ag Leaching from Arsenic Sulfosalts in the S2O32?-O2-NaOH System. Metals, 7(10), 411. https://doi.org/10.3390/met7100411