On the Microstructures and Fatigue Behaviors of 316L Stainless Steel Metal Injection Molded with Gas- and Water-Atomized Powders
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Sintering Behaviors
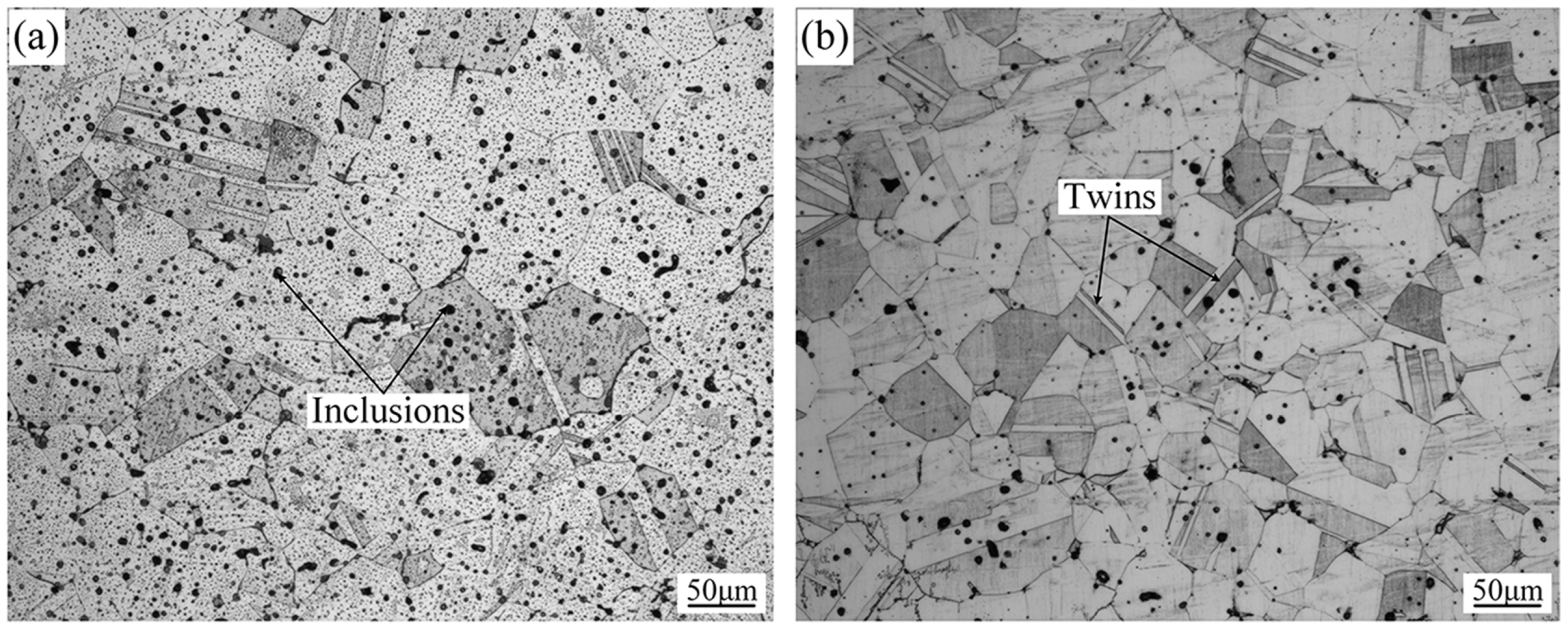
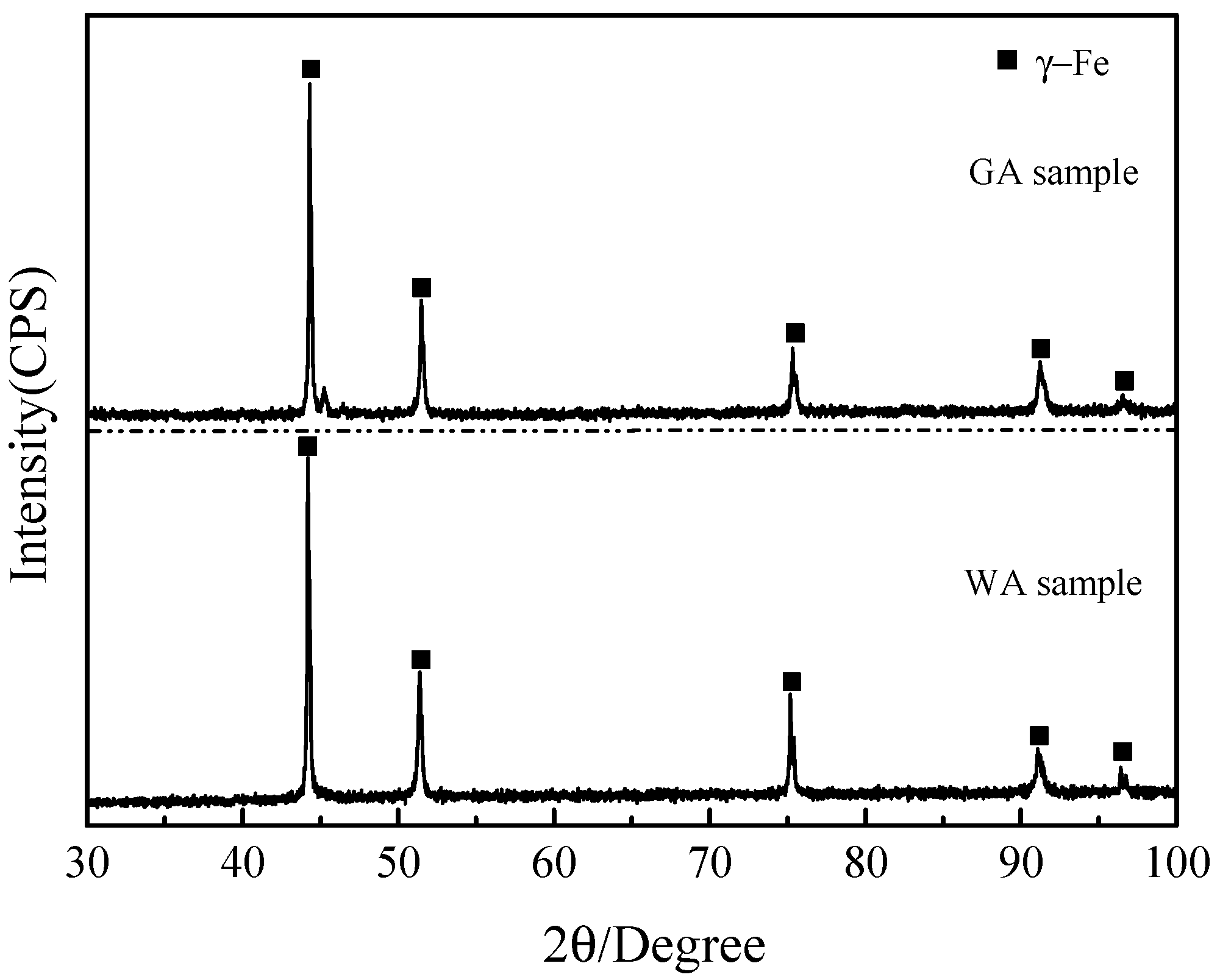
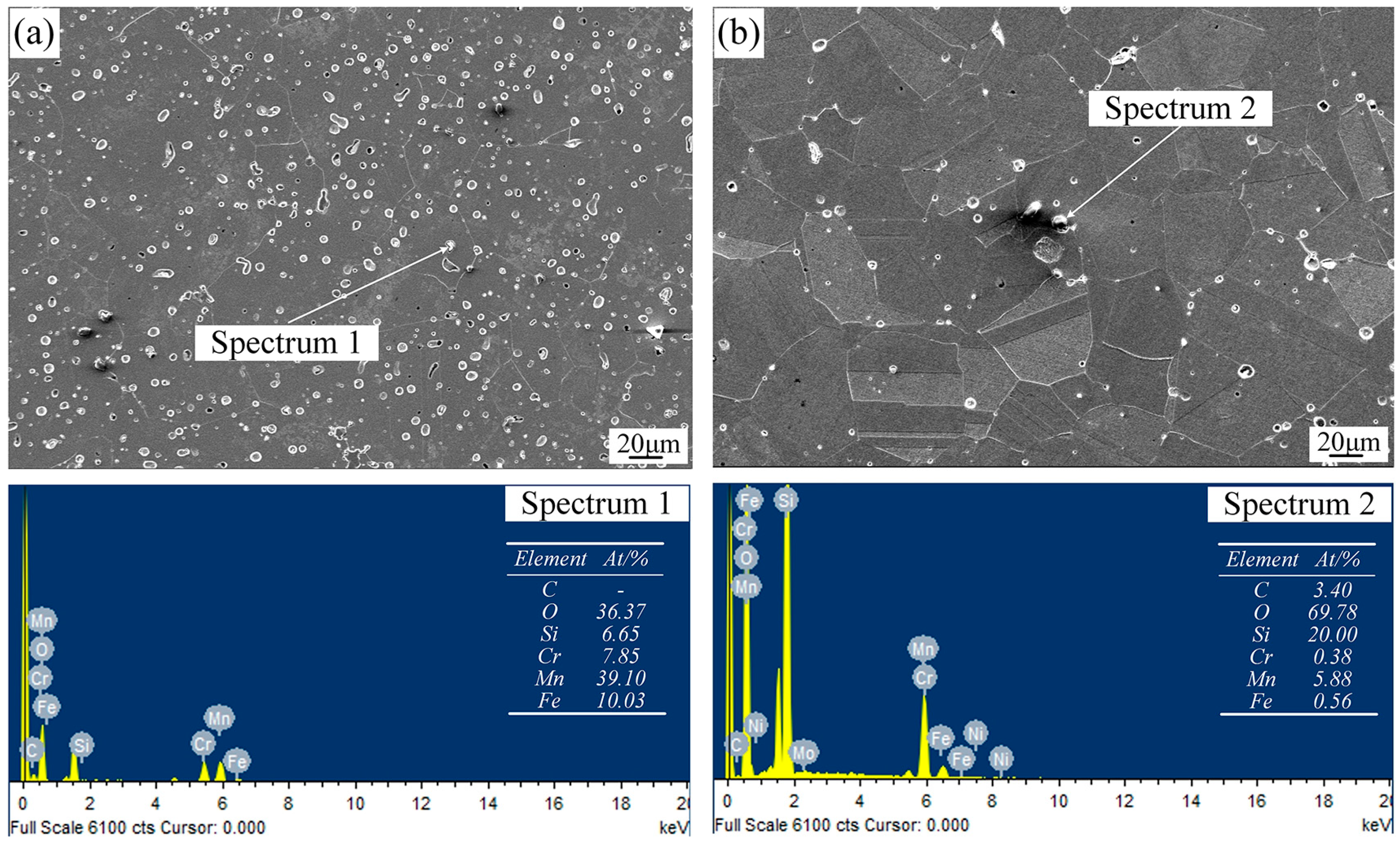
3.2. Microstructures and Phase Identification
3.3. Tensile Properties
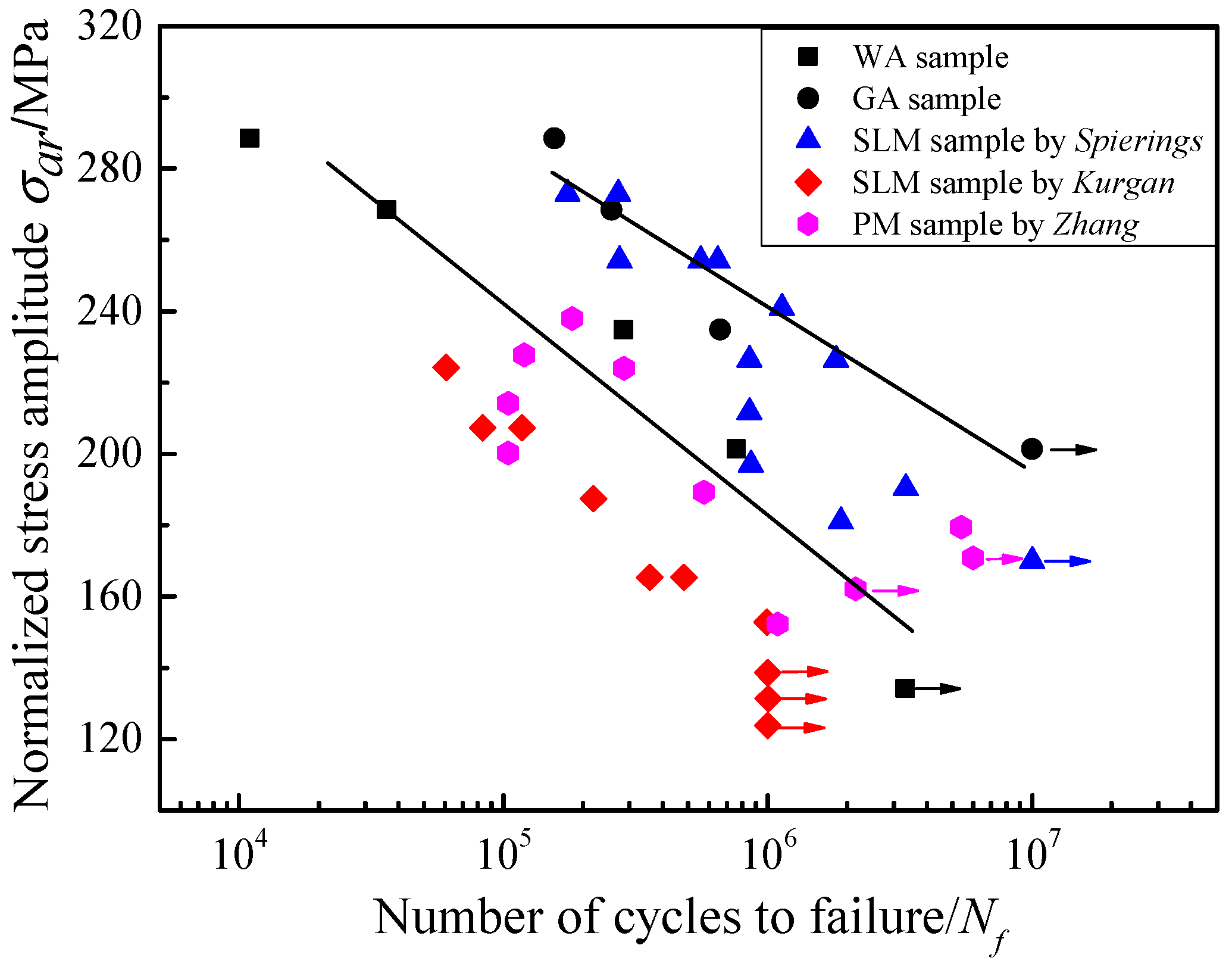
3.4. Fatigue Tests on Different Samples
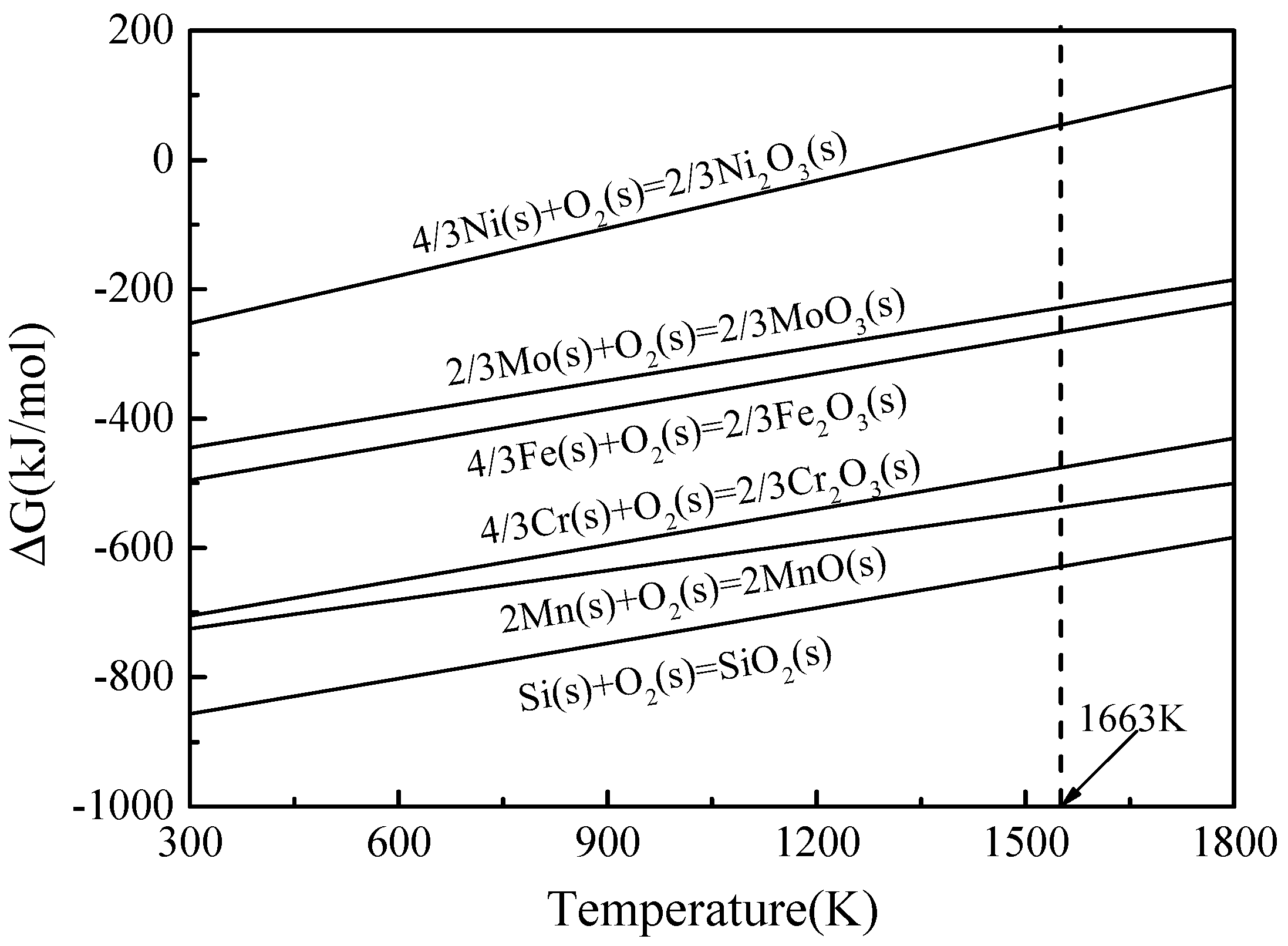
4. Discussion
5. Conclusions
- (1)
- Due to the different oxygen contents in the WA and GA powders, the oxygen contents in the sintered WA and GA samples are 2000 ppm and 600 ppm, respectively. The presence of oxygen has a negative influence on the sintering process. GA sample starts to sinter at a lower temperature than the WA sample and exhibits smaller shrinkage in the dilatometry test. The sintered densities of the GA and WA samples are 7.88 g/cm3 than 7.65 g/cm3, respectively.
- (2)
- The oxygen tends to react with Si and Mn to produce oxide particles during sintering. Due to the high oxygen content, more SiO2 and MnO particles are formed in the WA samples than in the GA samples, which in turn decreases the amount of Si and Mn dissolving into the base metal of the WA samples. The different existing status of Mn and Si in the sintered samples have significant influences on their mechanical properties.
- (3)
- The oxides not only result in low sintered density and lead to stress concentration but also compromise the solution strengthening the effect of the Mn and Si in the base metal. As a result, the tensile strength, yield strength and the elongation of the GA samples are 560 MPa, 205 MPa, and 58%, respectively, which are much higher than those of the WA samples (i.e., 450 MPa, 170 MPa, and 29%, respectively).
- (4)
- In the fatigue tests, the oxides become the initiation sites for fatigue cracks and interact with cracks during their propagation. Therefore, the fatigue lives of the GA samples are about one order of magnitude longer than the WA samples. The fatigue behaviors of the sintered GA samples are also superior to those fabricated by powder metallurgy and selective laser melting, which were reported in other researches.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cherry, J.A.; Davies, H.M.; Mehmood, S.; Lavery, N.P.; Brown, S.G.R.; Sienz, J. Investigation into the effect of process parameters on microstructural and physical properties of 316L stainless steel parts by selective laser melting. Int. J. Adv. Manuf. Technol. 2015, 76, 869–879. [Google Scholar] [CrossRef]
- Aslam, M.; Ahmad, F.; Yusoff, P.S.M.B.M.; Altaf, K.; Omar, M.A.; German, R.M. Powder injection molding of biocompatible stainless steel biodevices. Powder Technol. 2016, 295, 84–95. [Google Scholar] [CrossRef]
- Fredriksson, W.; Petrini, D.; Edström, K.; Björefors, F.; Nyholm, L. Corrosion resistances and passivation of powder metallurgical and conventionally cast 316L and 2205 stainless steels. Corros. Sci. 2013, 67, 268–280. [Google Scholar] [CrossRef]
- Kamath, C.; El-Dasher, B.; Gallegos, G.F.; King, W.E.; Sisto, A. Density of additively-manufactured, 316L SS parts using laser powder-bed fusion at powers up to 400 W. Int. J. Adv. Manuf. Technol. 2014, 74, 65–78. [Google Scholar] [CrossRef]
- Manchoul, S.; Seddik, R.; Grissa, R.; Sghaier, R.B.; Fathallah, R. A predictive approach to investigate the effect of ultrasonic shot peening on a high-cycle fatigue performance of an AISI 316L target. Int. J. Adv. Manuf. Technol. 2018, 95, 3437–3451. [Google Scholar] [CrossRef]
- Zhang, M.; Sun, C.N.; Zhang, X.; Wei, J.; Hardacre, D.; Li, H. Predictive models for fatigue property of laser powder bed fusion stainless steel 316L. Mater. Des. 2018, 145, 42–54. [Google Scholar] [CrossRef]
- Sang, P.; Kim, D.; Lin, D.; Park, S.; Ahn, S. Rheological Characterization of Powder Mixture Including a Space Holder and Its Application to Metal Injection Molding. Metals 2017, 7, 120. [Google Scholar] [CrossRef]
- Berginc, B.; Kampus, Z.; Sustarsic, B. Influence of feedstock characteristics and process parameters on properties of MIM parts made of 316L. Powder Metall. 2013, 50, 172–183. [Google Scholar] [CrossRef]
- Ji, C.H.; Loh, N.H.; Khor, K.A.; Tor, S.B. Sintering study of 316L stainless steel metal injection molding parts using Taguchi method: Final density. Mater. Sci. Eng. A 2001, 311, 74–82. [Google Scholar] [CrossRef]
- Amin, A.M.; Ibrahim, M.H.I.; Asmawi, R.; Mustaffa, N.; Hashim, M.Y. Thermal Debinding and Sintering of water atomised SS316L Metal Injection Moulding Process. IOP Conf. Ser. Mater. Sci. Eng. 2017, 226, 12155. [Google Scholar] [CrossRef]
- Manonukul, A.; Muenya, N.; Léaux, F.; Amaranan, S. Effects of replacing metal powder with powder space holder on metal foam produced by metal injection moulding. J. Mater. Process. Technol. 2010, 210, 529–535. [Google Scholar] [CrossRef]
- Setasuwon, P.; Bunchavimonchet, A.; Danchaivijit, S. The effects of binder components in wax/oil systems for metal injection molding. J. Mater. Process. Technol. 2008, 196, 94–100. [Google Scholar] [CrossRef]
- Raza, M.R.; Ahmad, F.; Omar, M.A.; German, R.M. Effects of cooling rate on mechanical properties and corrosion resistance of vacuum sintered powder injection molded 316L stainless steel. J. Mater. Process. Technol. 2012, 212, 164–170. [Google Scholar] [CrossRef]
- Karatas, C.; Saritas, S. Rheological properties of mixed gas and water atomized stainless steel powder MIM feedstock. Int. J. Powder Metall. 2001, 37, 39–44. [Google Scholar]
- Cai, L.; German, R.M. Powder injection molding using water atomized 316l stainless steel. Int. J. Powder Metall. 1995, 31, 257–264. [Google Scholar]
- Nylund, A.; Tunberg, T.; Bertilsson, H.; Carlstrom, E.; Olefjord, I. Injection molding of gas and water-atomized stainless steel powders. Int. J. Powder Metall. 1995, 31, 365–373. [Google Scholar]
- Suri, P.; Koseski, R.P.; German, R.M. Erratum: Microstructural evolution of injection molded gas- and water-atomized 316L stainless steel powder during sintering. Mater. Sci. Eng. A 2005, 402, 341–348. [Google Scholar] [CrossRef]
- Yu, P.; Qian, M.; Li, L.; Schaffer, G.B. On the infiltration mode during fabrication of aluminium composite. Acta Mater. 2010, 58, 3790–3797. [Google Scholar] [CrossRef]
- Sercombe, T.B.; Schaffer, G.B. On the role of tin in the infiltration of aluminium by aluminium for rapid prototyping applications. Scr. Mater. 2004, 51, 905–908. [Google Scholar] [CrossRef]
- Homeny, J.; Buckley, M.M. Transmission electron microscopy study of an aluminum oxide fiber/aluminum-magnesium alloy metal matrix composite interface. Mater. Lett. 1991, 10, 421–424. [Google Scholar] [CrossRef]
- Mahmoud, M.M.; Link, G.; Thumm, M. The role of the native oxide shell on the microwave sintering of copper metal powder compacts. J. Alloy. Compd. 2015, 627, 231–237. [Google Scholar] [CrossRef]
- Hao, H.; Ye, S.; Yu, K.; Chen, P.; Gu, R.; Yu, P. The role of alloying elements on the sintering of Cu. J. Alloy. Compd. 2016, 684, 91–97. [Google Scholar] [CrossRef]
- Klar, E.; Samal, P.K. Powder Metallurgy Stainless Steels: Processing, Microstructures, and Properties; ASM International: Materials Park Campus, OH, USA, 2007; pp. 59–96. [Google Scholar]
- Sayman, O.; Sen, F.; Celik, E.; Arman, Y. Thermal stress analysis of Wc–Co/Cr–Ni multilayer coatings on 316L steel substrate during cooling process. Mater. Des. 2009, 30, 770–774. [Google Scholar] [CrossRef]
- Wu, Y.; German, R.M.; Blaine, D.; Marx, B.; Schlaefer, C. Effects of residual carbon content on sintering shrinkage, microstructure and mechanical properties of injection molded 17-4 PH stainless steel. J. Mater. Sci. 2002, 37, 3573–3583. [Google Scholar] [CrossRef]
- Lide, D.R.; Baysinger, G.; Berger, L.I.; Goldberg, R.N.; Kehiaian, H.V.; Kuchitsu, K.; Rosenblatt, G.; Roth, D.L.; Zwillinger, D. CRC Handbook of Chemistry and Physics; CRC Press: Boca Raton, FL, USA, 2005; pp. 6–187. [Google Scholar]
- Wang, T.; Li, X.; Zhang, Y.; Li, H.; Zhang, B.; Feng, J. Regulating the interfacial morphology of electron beam welded pure Ti/2024Al dissimilar joint. J. Mater. Process. Technol. 2017, 245, 227–231. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, T.; Zhang, B.; Wang, Y.; Feng, J. Microstructure Evolution and Embrittlement of Electron Beam Welded TZM Alloy Joint. Mater. Sci. Eng. A 2017, 700, 512–518. [Google Scholar] [CrossRef]
- Ramirez, A.J.; Lippold, J.C. High temperature behavior of Ni-base weld metal: Part II—Insight into the mechanism for ductility dip cracking. Mater. Sci. Eng. A 2004, 380, 245–258. [Google Scholar] [CrossRef]
- Misra, S.; Mandal, N.; Dhar, R.; Chakraborty, C. Mechanisms of deformation localization at the tips of shear fractures: Findings from analogue experiments and field evidence. J. Geophys. Res. Solid Earth 2009, 114. [Google Scholar] [CrossRef]
- Qin, E.W.; Lu, L.; Tao, N.R.; Tan, J.; Lu, K. Enhanced fracture toughness and strength in bulk nanocrystalline Cu with nanoscale twin bundles. Acta Mater. 2009, 57, 6215–6225. [Google Scholar] [CrossRef]
- Li, W.P.; Wang, X.G.; Liu, B.; Fang, Q.H.; Jiang, C. Fracture mechanisms of a Mo alloyed CoCrFeNi high entropy alloy: In-situ SEM investigation. Mater. Sci. Eng. A 2018, 723, 79–88. [Google Scholar] [CrossRef]
- Zhang, C.; Song, X.; Lu, P.; Hu, X. Effect of microstructure on mechanical properties in weld-repaired high strength low alloy steel. Mater. Des. 2012, 36, 233–242. [Google Scholar] [CrossRef]
- Kurgan, N.; Varol, R. Mechanical properties of P/M 316L stainless steel materials. Powder Technol. 2010, 201, 242–247. [Google Scholar] [CrossRef]
- Spierings, A.B.; Starr, T.L.; Wegener, K. Fatigue performance of additive manufactured metallic parts. Rapid Prototyp. J. 2013, 19, 88–94. [Google Scholar] [CrossRef]
- Dowling, N.E.; Calhoun, C.A.; Arcari, A. Mean stress effects in stress-life fatigue and the Walker equation. Fatigue Fract. Eng. Mater. Struct. 2009, 32, 163–179. [Google Scholar] [CrossRef]
- Manson, S.S.; Muralidharan, U. Fatigue Life Prediction in Bending From Axial Fatigue Information. Fatigue Fract. Eng. Mater. Struct. 1987, 9, 357–372. [Google Scholar] [CrossRef]







| Powders | Si | Mn | Ni | Cr | Mo | C | O | Fe |
|---|---|---|---|---|---|---|---|---|
| WA powder | 0.5 | 1.5 | 11.0 | 17.0 | 2.0 | - | 0.30 | Bal. |
| GA powder | 0.16 |
| Parameters | Injection | De-Binding | Sintering | |
|---|---|---|---|---|
| Catalytic | Thermal | |||
| Temperature/°C | 195 | 120 | 300/450/600 | 1390 |
| Time/min | - | 240 | 60 | 180 |
| Samples | O Contents (wt%) | Densities (g·cm−3) | Yield Strength (MPa) | Tensile Strength (MPa) | Elongation (%) |
|---|---|---|---|---|---|
| WA sample | 0.20 | 7.65 | 170 ± 5 | 460 ± 15 | 29 ± 3 |
| GA sample | 0.06 | 7.88 | 205 ± 8 | 560 ± 5 | 58 ± 2 |
| Stress Amplitude σa/MPa | Normalized Stress Amplitude σar/MPa | Number of Cycles to Failure/Nf | |
|---|---|---|---|
| WA Samples | GA Samples | ||
| 193.5 | 288.5 | 1.10 × 104 | 1.56 × 105 |
| 180 | 268.3 | 3.61 × 104 | 2.56 × 105 |
| 157.5 | 234.8 | 2.85 × 105 | 6.63 × 105 |
| 135 | 201.2 | 7.61 × 105 | ≥1.00 × 107 |
| 90 | 134.2 | ≥3.32 × 106 | - |
| Reaction | ∆H (kJ/mol) | ∆S (kJ/mol K) | ∆G = ∆H − T∆S (kJ/mol) |
|---|---|---|---|
| −911 | −0.182 | ∆G = 0.182T − 911 | |
| −770 | −0.150 | ∆G = 0.150T − 770 | |
| −760 | −0.183 | ∆G = 0.183T − 760 | |
| −550 | −0.183 | ∆G = 0.183T − 550 | |
| −497 | −0.173 | ∆G = 0.173T − 497 | |
| −326 | −0.245 | ∆G = 0.245T − 326 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Feng, E.; Mo, W.; Lv, Y.; Ma, R.; Ye, S.; Wang, X.; Yu, P. On the Microstructures and Fatigue Behaviors of 316L Stainless Steel Metal Injection Molded with Gas- and Water-Atomized Powders. Metals 2018, 8, 893. https://doi.org/10.3390/met8110893
Zhang Y, Feng E, Mo W, Lv Y, Ma R, Ye S, Wang X, Yu P. On the Microstructures and Fatigue Behaviors of 316L Stainless Steel Metal Injection Molded with Gas- and Water-Atomized Powders. Metals. 2018; 8(11):893. https://doi.org/10.3390/met8110893
Chicago/Turabian StyleZhang, Yongyun, Ensheng Feng, Wei Mo, Yonghu Lv, Rui Ma, Shulong Ye, Xiaogang Wang, and Peng Yu. 2018. "On the Microstructures and Fatigue Behaviors of 316L Stainless Steel Metal Injection Molded with Gas- and Water-Atomized Powders" Metals 8, no. 11: 893. https://doi.org/10.3390/met8110893
APA StyleZhang, Y., Feng, E., Mo, W., Lv, Y., Ma, R., Ye, S., Wang, X., & Yu, P. (2018). On the Microstructures and Fatigue Behaviors of 316L Stainless Steel Metal Injection Molded with Gas- and Water-Atomized Powders. Metals, 8(11), 893. https://doi.org/10.3390/met8110893





