Sliding Wear Behavior of Friction Couples Primarily Selected for Corrosion Resistance: Iron Boride/Iron Boride and Iron Boride/Yttria-Stabilized Zirconia
Abstract
:1. Introduction
2. Materials and Methods
3. Results
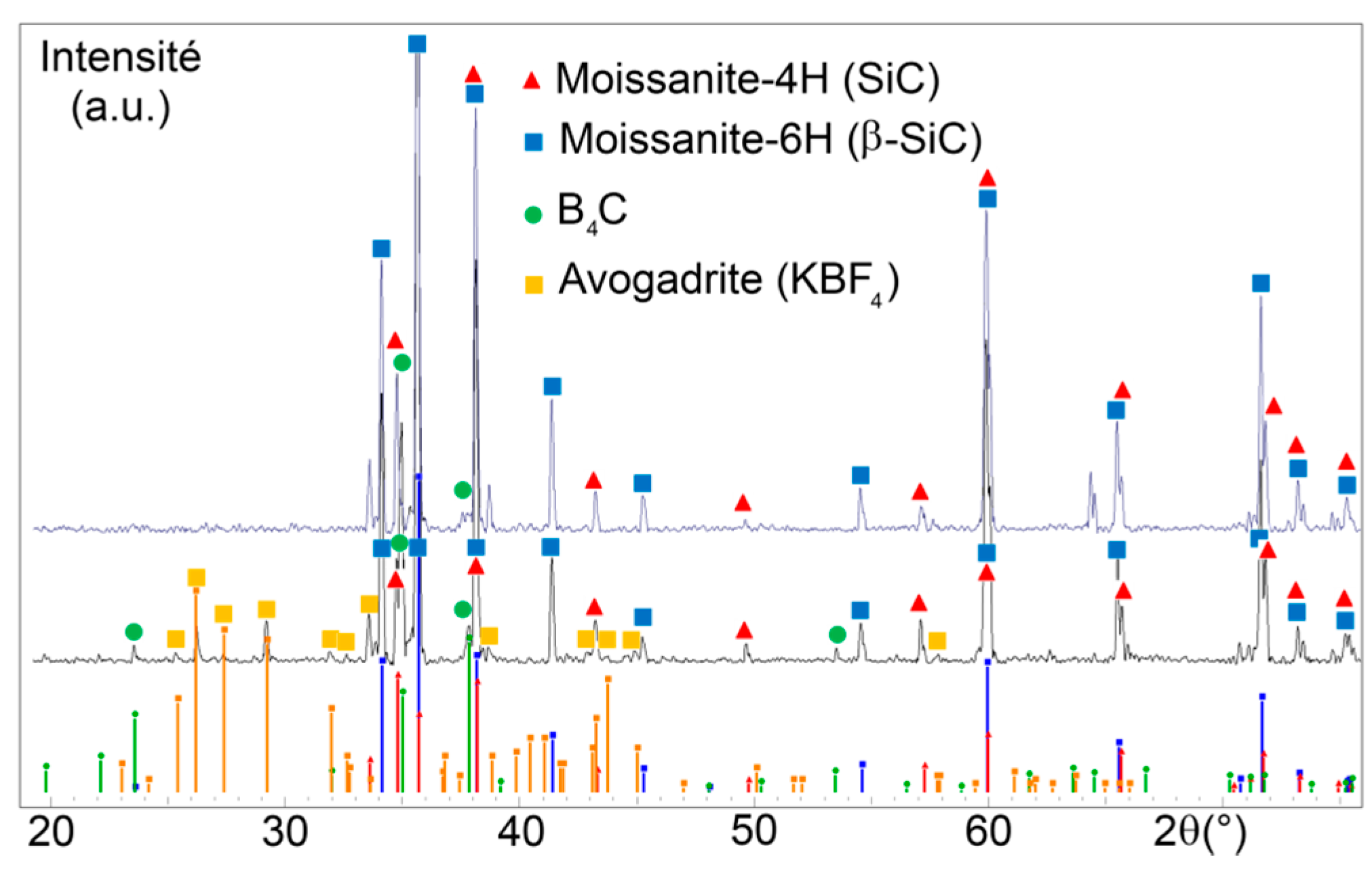
3.1. Phase Identification in the Powder after Boriding
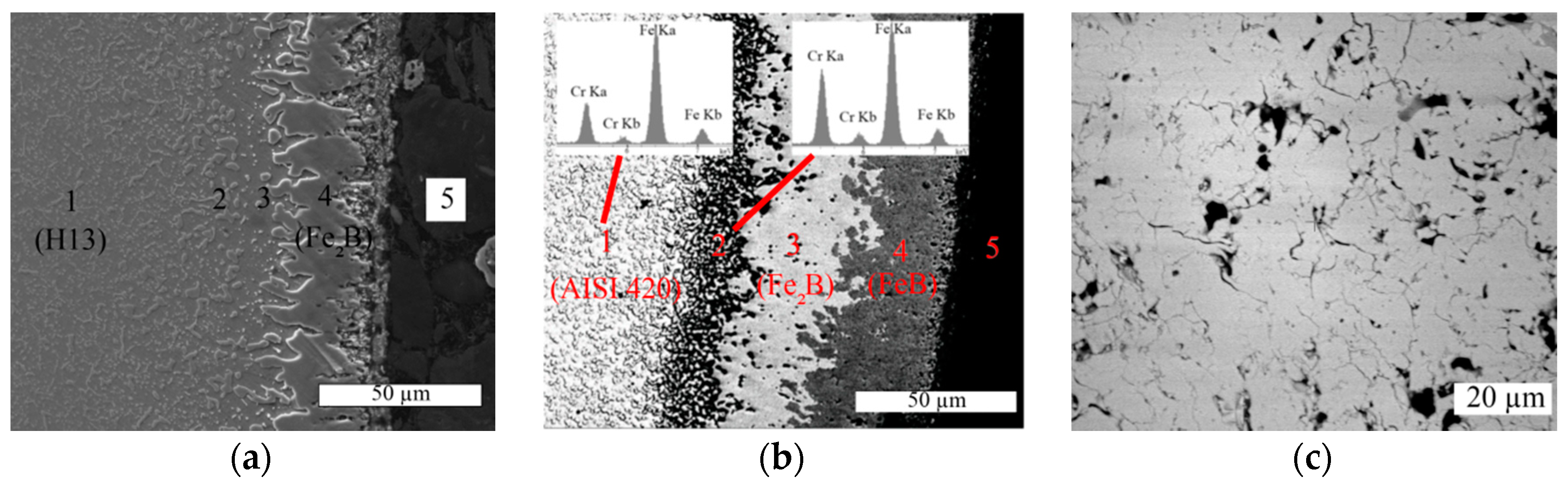
3.2. Characterisation of Borided H13 Steel
3.3. Characterisation of Borided AISI 420 Steel
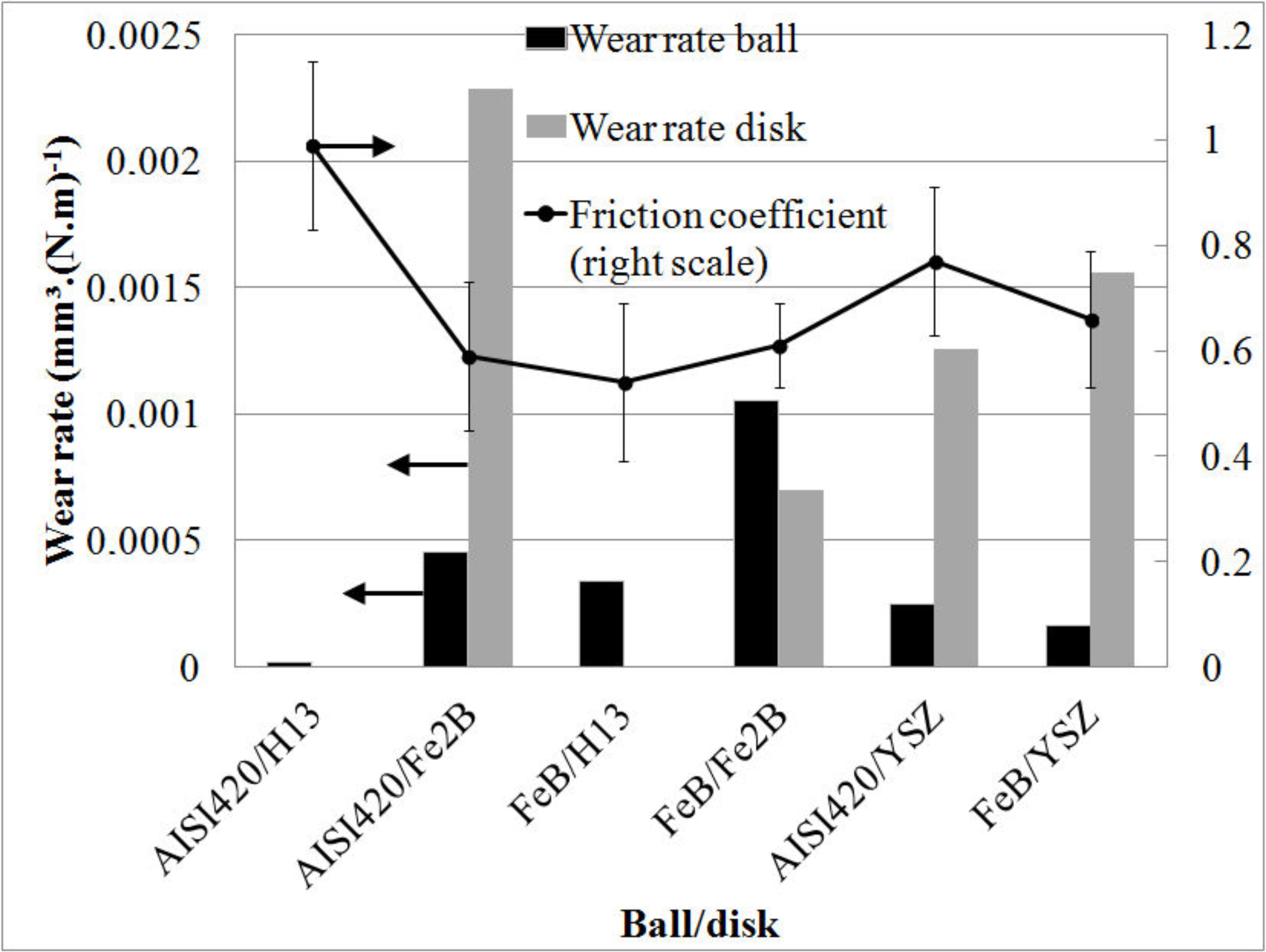
3.4. Pin-on-Disk Testing
4. Discussion
5. Conclusions
- The boriding layer consists of solely irregular Fe2B on H13 tool steel and consists of both FeB and Fe2B on AISI 420 steel.
- The friction coefficients of FeB/Fe2B and FeB/YSZ couples are significantly lower than the steel/steel couple. In the first case, wear rates are ~10−3 mm³·(N·m)−1. In all the studied couples, dynamic friction coefficient is not inferior to 0.5, except possibly for the FeB/H13 couple when FeB remains nearly intact.
- Strong vibrations occur, due to three-body abrasion. The third bodies may be oxides due to the local heating of the steel, as well as Fe2B from treated H13 steel or porous YSZ from plasma sprayed disks.
- Even if both surfaces may be sufficiently corrosion resistant for foundry applications, the measured friction forces still suggest detrimental affinity between YSZ and iron borides. This couple may be recommended if the YSZ is the replaceable counter part of the slide. This conclusion is valid only in the case of a punctual exposure to molten metal. Since the corrosion by molten metal is only a selection criterion in the test, and not part of it, present work does not replace field tests. The tribological resistance studied here is a necessary condition to the resistance in real conditions, not a sufficient condition.
Author Contributions
Funding
Conflicts of Interest
References
- D’Ans, P.; Degrez, M. A strategy for the selection of multiple materials and processes fulfilling inherently incompatible functions: The case of successive surface treatments. Surf. Coat. Technol. 2015, 276, 349–359. [Google Scholar] [CrossRef]
- Von Matushka, A.G. Boronizing; Carl Hansen Verlag, Heyden & Son: München, Germany; Wien, Austria; Philadelphia, PA, USA, 1980. [Google Scholar]
- Audisio, S.; Caillet, M.; Galerie, A.; Mazille, H. Revêtements et Traitements de Surface—Fonctionnalités, Durabilité, Procédés; Presses Polytechniques et Universitaires Romandes: Lausanne, Switzerland, 1999. [Google Scholar]
- Spence, T.W.; Makhlouf, M.M. Characterization of the operative mechanism in potassium fluoborate activated pack boriding of steels. J. Mater. Process. Technol. 2005, 168, 127–136. [Google Scholar] [CrossRef]
- Keddam, M.; Kulka, M.; Makuch, N.; Pertek, A.; Małdzinski, L. A kinetic model for estimating the boron activation energies in the FeB and Fe2B layers during the gas-boriding of Armco iron: Effect of boride incubation times. Appl. Surf. Sci. 2014, 298, 155–163. [Google Scholar] [CrossRef]
- Kulka, M.; Makuch, N.; Pertek, A.; Maldzinski, L. Simulation of the growth kinetics of boride layers formed on Fe during gas boriding in H2-BCl3 atmosphere. J. Solid State Chem. 2013, 199, 196–203. [Google Scholar] [CrossRef]
- Flores-Rentería, M.A.; Ortiz-Domínguez, M.; Keddam, M.; Damián-Mejía, O.; Elias-Espinosa, M.; Flores-González, M.A.; Medina-Moreno, S.A.; Cruz-Avilés, A.; Villanueva-Ibañez, M. A Simple Kinetic Model for the Growth of Fe2B Layers on AISI 1026 Steel During the Powder-pack Boriding. High Temp. Mater. Proc. 2015, 34, 1–11. [Google Scholar] [CrossRef]
- Ortiz-Domínguez, M.; Keddam, M.; Elias-Espinosa, M.; Damián-Mejía, O.; Flores-Rentería, M.A.; Arenas-Flores, A.; Hernández-Ávila, J. Investigation of boriding kinetics of AISI D2 steel. Surf. Eng. 2014, 30, 490–497. [Google Scholar] [CrossRef]
- Genel, K. Boriding kinetics of H13 steel. Vacuum 2006, 80, 451–457. [Google Scholar] [CrossRef]
- Keddam, M.; Ortiz-Domínguez, M.; Elias-Espinosa, M.; Damián-Mejía, O.; Arenas-Flores, A.; Gómez-Vargas, O.A.; Abreu-Quijano, M.; Aldana-González, J.I.; Zuno-Silva, J. Growth Kinetics of the Fe2B Coating on AISI H13 Steel. Trans. Indian Inst. Met. 2015, 68, 433–442. [Google Scholar] [CrossRef]
- Dybkov, V.I. Growth of boride layers on the 13% Cr steel surface in a mixture of amorphous boron and KBF4. J. Mater. Sci. 2007, 42, 6614–6627. [Google Scholar] [CrossRef]
- Xie, F.; Chen, J.; Wang, S. Effects and mechanisms of an alternating current field on pack boriding. Vacuum 2018, 148, 41–47. [Google Scholar] [CrossRef]
- Türkmen, I.; Yalamaç, E. Growth of the Fe2B layer on SAE 1020 steel employed a boron source of H3BO3 during the powder-pack boriding method. J. Alloys Compd. 2018, 744, 658–666. [Google Scholar] [CrossRef]
- Kulka, M.; Makuch, N.; Piasecki, A. Nanomechanical characterization and fracture toughness of FeB and Fe2B iron borides produced by gas boriding of Armco iron. Surf. Coat. Technol. 2017, 325, 515–532. [Google Scholar] [CrossRef]
- Tabur, M.; Izciler, M.; Gul, F.; Karacan, I. Abrasive wear behavior of boronized AISI 8620 steel. Wear 2009, 266, 1106–1112. [Google Scholar] [CrossRef]
- Ulutan, M.; Celik, O.; Gasan, H.; Er, U. Effect of Different Surface Treatment Methods on the Friction and Wear Behavior of AISI 4140 Steel. J. Mater. Sci. Technol. 2010, 26, 251–257. [Google Scholar] [CrossRef]
- Gunes, I. Wear Behavior of Plasma Paste Boronized of AISI 8620 Steel with Borax and B2O3 Paste Mixtures. J. Mater. Sci. Technol. 2013, 29, 662–668. [Google Scholar] [CrossRef]
- Stewart, K. Boronizing protects metals against wear. Adv. Mater. Proc. 1997, 151, 23–25. [Google Scholar]
- Gök, M.; Küçük, Y.; Erdoğan, A.; Öge, M.; Kanca, E.; Günen, A. Dry sliding wear behavior of borided hot-work tool steel at elevated temperatures. Surf. Coat. Technol. 2017, 328, 54–62. [Google Scholar] [CrossRef]
- Soydan, Y.; Köksal, S.; Demirer, A.; Çelik, V. Sliding Friction and Wear Behavior of Pack-Boronized AISI 1050, 4140, and 8620 Steels. Tribol. Trans. 2008, 51, 74–81. [Google Scholar] [CrossRef]
- Düzcükoğlu, H.; Çalik, A.; İmrek, H.; Karakaş, M. Examination of Pitting and Wear in Borided, Carburized, and Borocarburized AISI 8620 Gears. Tribol. Trans. 2010, 53, 485–490. [Google Scholar] [CrossRef]
- Kayali, Y.; Anaturk, B. Investigation of electrochemical corrosion behavior in a 3.5 wt.% NaCl solution of boronized dual-phase steel. Mater. Des. 2013, 46, 776–783. [Google Scholar] [CrossRef]
- Cartier, M. Guide D’emploi des Traitements de Surfaces Appliqués aux Problèmes de Frottement; Tec&Doc: Paris, France, 2000. [Google Scholar]
- Ma, S.; Xing, J.; Fu, H.; He, Y.; Bai, Y.; Li, Y.; Bai, Y. Interface characteristics and corrosion behaviour of oriented bulk Fe2B alloy in liquid zinc. Corros. Sci. 2014, 78, 71–80. [Google Scholar] [CrossRef]
- Biddulph, R.H. Boronizing for erosion resistance. Thin Solid Films 1977, 45, 341–347. [Google Scholar] [CrossRef]
- Dybkov, V.I.; Goncharuk, L.V.; Khoruzha, V.G.; Samelyuk, A.V.; Sidorko, V.R. Growth kinetics and abrasive wear resistance of boride layers on Fe-15Cr alloy. Mater. Sci. Technol. 2011, 27, 1502–1512. [Google Scholar] [CrossRef]
- Dokumaci, E.; Özkan, I.; Önay, B. Effect of boronizing on the cyclic oxidation of stainless steel. Surf. Coat. Technol. 2013, 232, 22–25. [Google Scholar] [CrossRef]
- Tsipas, D.N.; Triantafyllidis, G.K.; Kipkemoi Kiplagat, J.; Psillaki, P. Degradation behaviour of boronized carbon and high alloy steels in molten aluminium and zinc. Mater. Lett. 1998, 37, 128–131. [Google Scholar] [CrossRef]
- Hairy, P.; Dussaussois, R. Performance comparison of twelve new anti-soldering surface treatments for high pressure die casting. Fonderie Fondeur d’Aujourd’hui 2003, 227, 30–41. [Google Scholar]
- Yang, H.P.; Wu, X.C.; Min, Y.A.; Wu, T.R.; Gui, J.Z. Plasma boriding of high strength alloy steel with nanostructured surface layer at low temperature assisted by air blast shot peening. Surf. Coat. Technol. 2013, 228, 229–233. [Google Scholar] [CrossRef]
- Lou, D.C.; Akselsen, O.M.; Onsøien, M.I.; Solberg, J.K.; Berget, J. Surface modification of steel and cast iron to improve corrosion resistance in molten aluminium. Surf. Coat. Technol. 2006, 200, 5282–5288. [Google Scholar] [CrossRef]
- D’Ans, P.; Bondoux, C.; Degrandcourt, C.; Bakrim, M.; Dille, J.; Segers, L.; Degrez, M. Thermal fatigue of anticorrosive coatings and multilayer coatings: A performance index approach. Mater. Sci. Forum 2008, 595–598, 941–950. [Google Scholar] [CrossRef]
- Habig, K.H. Wear protection of steels by boriding, vanadizing, nitriding and hardening. Mater. Eng. 1980, 2, 83–92. [Google Scholar] [CrossRef]
- Habig, K.H. Adhesive wear of boride and nitride layers on steel. Inst. Phys. Conf. Ser. 1986, 75, (Sci. Hard Mater.). 963–971. [Google Scholar]
- Garcia-Bustos, E.; Figueroa-Guadarrama, M.A.; Rodríguez-Castro, G.A.; Gómez-Vargas, O.A.; Gallardo-Hernandez, E.A.; Campos-Silva, I. The wear resistance of boride layers measured by the four-ball test. Surf. Coat. Technol. 2013, 215, 241–246. [Google Scholar] [CrossRef]
- Bourithis, L.; Papaefthymiou, S.; Papadimitriou, G.D. Plasma transferred arc boriding of a low carbon steel: Microstructure and wear properties. Appl. Surf. Sci. 2002, 200, 203–218. [Google Scholar] [CrossRef]
- Cimenoglu, H.; Atar, E.; Motallebzadeh, A. High temperature tribological behaviour of borided surfaces based on the phase structure of the boride layer. Wear 2014, 309, 152–158. [Google Scholar] [CrossRef]
- Taktak, S. Tribological behaviour of borided bearing steels at elevated temperatures. Surf. Coat. Technol. 2006, 201, 2230–2239. [Google Scholar] [CrossRef]
- Joshi, A.; Hosmani, S.; Dumbre, J. Tribological Performance of Boronized, Nitrided, and Normalized AISI 4140 Steel against Hydrogenated Diamond-Like Carbon-Coated AISI D2 Steel. Tribol. Trans. 2015, 58, 500–510. [Google Scholar] [CrossRef]
- Yasuda, T.; Banno, A.; Ito, T.; Kiyoshi, K.; Ishibayashi, K. Thermal Spraying Composite Material Containing Molybdenum Boride and a Coat Formed by Thermal Spraying. U.S. Patent US6238807, 29 May 2001. [Google Scholar]
- Mizuno, H.; Kitamura, J.; Osawa, S.; Itsukaichi, T. Development of durable spray coatings in molten aluminum alloy. In Proceedings of the ITSC 2005 Conference, Bâle, Switzerland, 2–4 May 2005; ASM Thermal Spray Society: Materials Park, OH, USA, 2005; pp. 80–85. [Google Scholar]
- Medvedovski, E. Wear-resistant engineering ceramics. Wear 2001, 249, 821–828. [Google Scholar] [CrossRef]
- Ramalingam, S.; Zheng, L. Film-substrate interface stresses and their role in the tribological performance. Tribol. Int. 1995, 28, 145–161. [Google Scholar] [CrossRef]
- Kameo, K.; Friedrich, K.; Bartolomé, J.F.; Díaz, M.; López-Esteban, S.; Moya, J.S. Sliding wear of ceramics and cermets against steel. J. Eur. Ceram. Soc. 2003, 23, 2867–2877. [Google Scholar] [CrossRef]
- Suh, M.-S.; Chae, Y.-H.; Kim, S.-S. Friction and wear behavior of structural ceramics sliding against zirconia. Wear 2008, 264, 800–806. [Google Scholar] [CrossRef]
- Banchet, V.; Fridrici, V.; Abry, J.C.; Kapsa, P. Wear and friction characterization of materials for hip prosthesis. Wear 2007, 263, 1066–1071. [Google Scholar] [CrossRef]
- Basu, B.; Vitchev, R.G.; Vleugels, J.; Celis, J.P.; Van der Biest, O. Influence of humidity on the fretting wear of self-mated tetragonal zirconia ceramics. Acta Mater. 2000, 48, 2461–2471. [Google Scholar] [CrossRef]
- dos Santos de Almeida, E.; Milan, J.; Costa, H.; Krelling, A.; da Costa, C. Sliding wear of borided sintered AISI M2 steel coated with AlTiN/CrN multilayer. Wear 2018, 410–411, 11–24. [Google Scholar] [CrossRef]
- Standard Test Method for Wear Testing with a Pin-on-Disk Apparatus; G99-05; ASTM International: West Conshohocken, PA, USA, 2010.
- Holmberg, K.; Matthews, A. Coatings Tribology; Elsevier: Amsterdam, The Netherlands, 1994. [Google Scholar]
- Zambelli, G.; Vincent, L. Matériaux et Contacts—Une Approche Tribologique; Presses Polytechniques et Universitaires Romandes: Lausanne, Switzerland, 1998. [Google Scholar]
- D’Ans, P.; Dille, J.; Degrez, M. Thermal fatigue resistance of plasma sprayed yttria-stabilised zirconia onto borided hot work tool steel, bonded with a NiCrAlY coating: Experiments and modelling. Surf. Coat. Technol. 2011, 205, 3378–3386. [Google Scholar] [CrossRef] [Green Version]
- Habraken, L.; de Brouwer, J.-L. De Ferri Metallographia; Presses Académiques Européennes: Bruxelles, Belgium, 1966. [Google Scholar]



| Shape | Layers (From Outside to the Substrate) | Studied Material in Wear Test |
|---|---|---|
| Disk | H13 | H13 |
| Disk | Fe2B; H13 | Fe2B |
| Disk | YSZ; NiCrAlY; Fe2B | YSZ |
| Ball | AISI 420 | AISI 420 |
| Ball | FeB; Fe2B; AISI 420 | FeB |
| Material | AISI 420 | Fe2B | FeB | H13 | Fe2B | NiCrAlY | YSZ |
|---|---|---|---|---|---|---|---|
| Object | Ball | Ball | Ball | Disk | Disk | Disk | Disk |
| HV | 220 | 1600 | 2000 | 220 | 1300 | 320 | 1000 |
| Load (N) | 1.962 | 1.962 | 1.962 | 1.962 | 0.981 | 0.981 | 1.962 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
D’Ans, P.; Degrez, M. Sliding Wear Behavior of Friction Couples Primarily Selected for Corrosion Resistance: Iron Boride/Iron Boride and Iron Boride/Yttria-Stabilized Zirconia. Metals 2018, 8, 1071. https://doi.org/10.3390/met8121071
D’Ans P, Degrez M. Sliding Wear Behavior of Friction Couples Primarily Selected for Corrosion Resistance: Iron Boride/Iron Boride and Iron Boride/Yttria-Stabilized Zirconia. Metals. 2018; 8(12):1071. https://doi.org/10.3390/met8121071
Chicago/Turabian StyleD’Ans, Pierre, and Marc Degrez. 2018. "Sliding Wear Behavior of Friction Couples Primarily Selected for Corrosion Resistance: Iron Boride/Iron Boride and Iron Boride/Yttria-Stabilized Zirconia" Metals 8, no. 12: 1071. https://doi.org/10.3390/met8121071
APA StyleD’Ans, P., & Degrez, M. (2018). Sliding Wear Behavior of Friction Couples Primarily Selected for Corrosion Resistance: Iron Boride/Iron Boride and Iron Boride/Yttria-Stabilized Zirconia. Metals, 8(12), 1071. https://doi.org/10.3390/met8121071





