Deposition of Al Thin Film on Steel Substrate: The Role of Thickness on Crystallization and Grain Growth
Abstract
:1. Introduction
2. Experimental Details
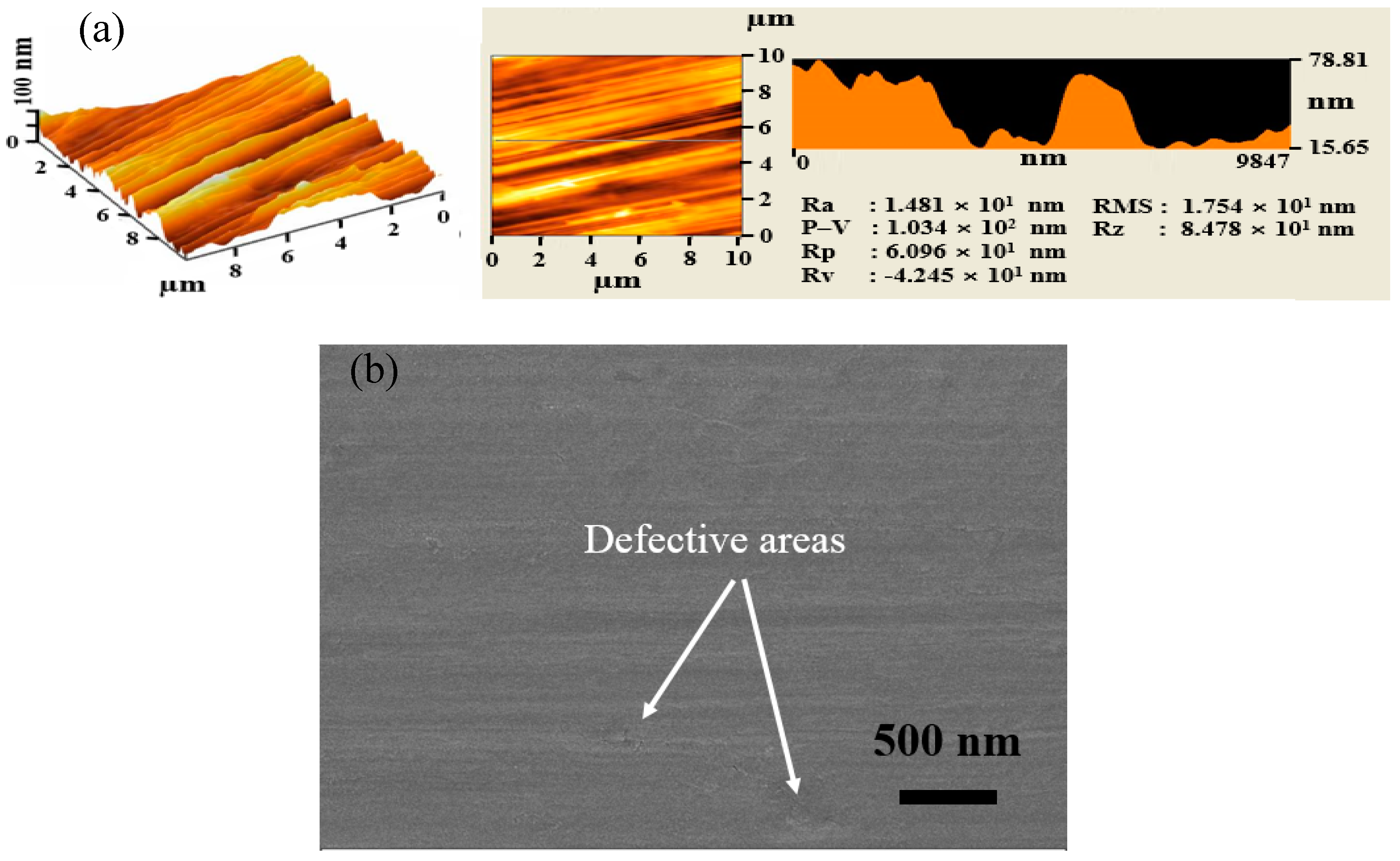
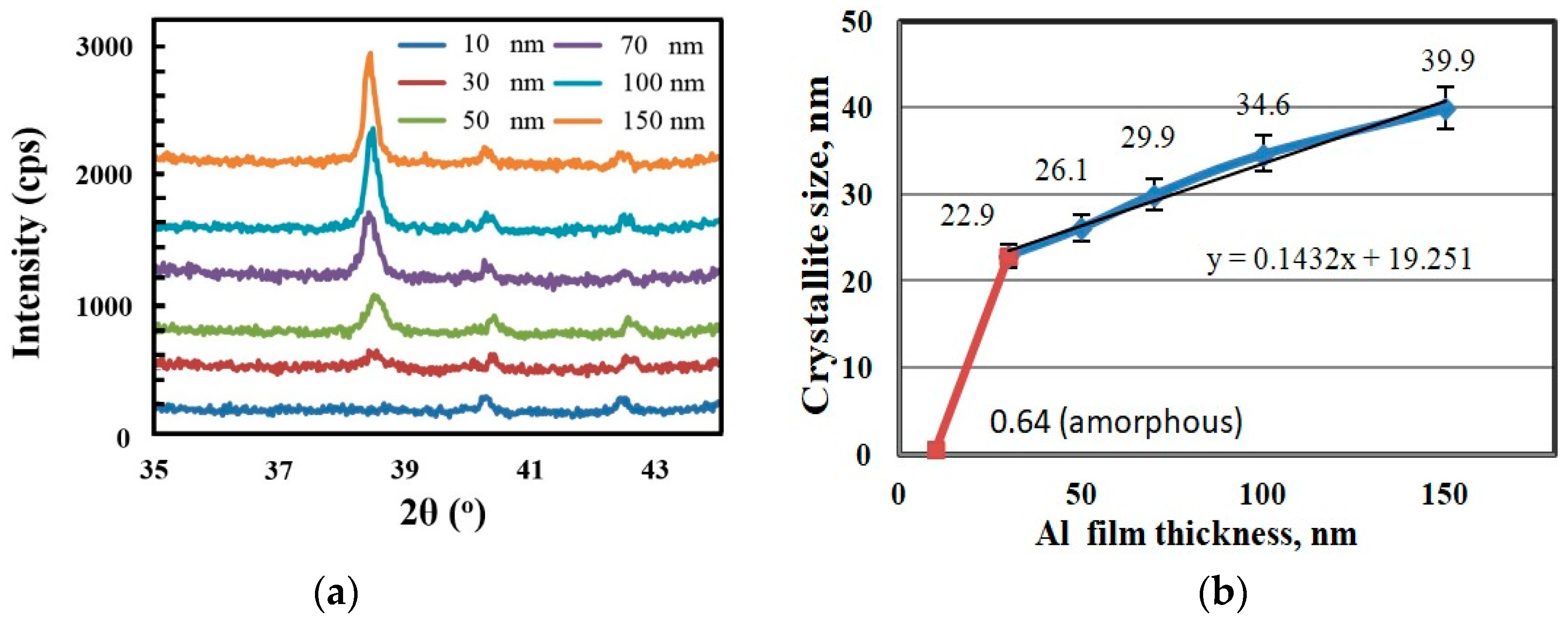
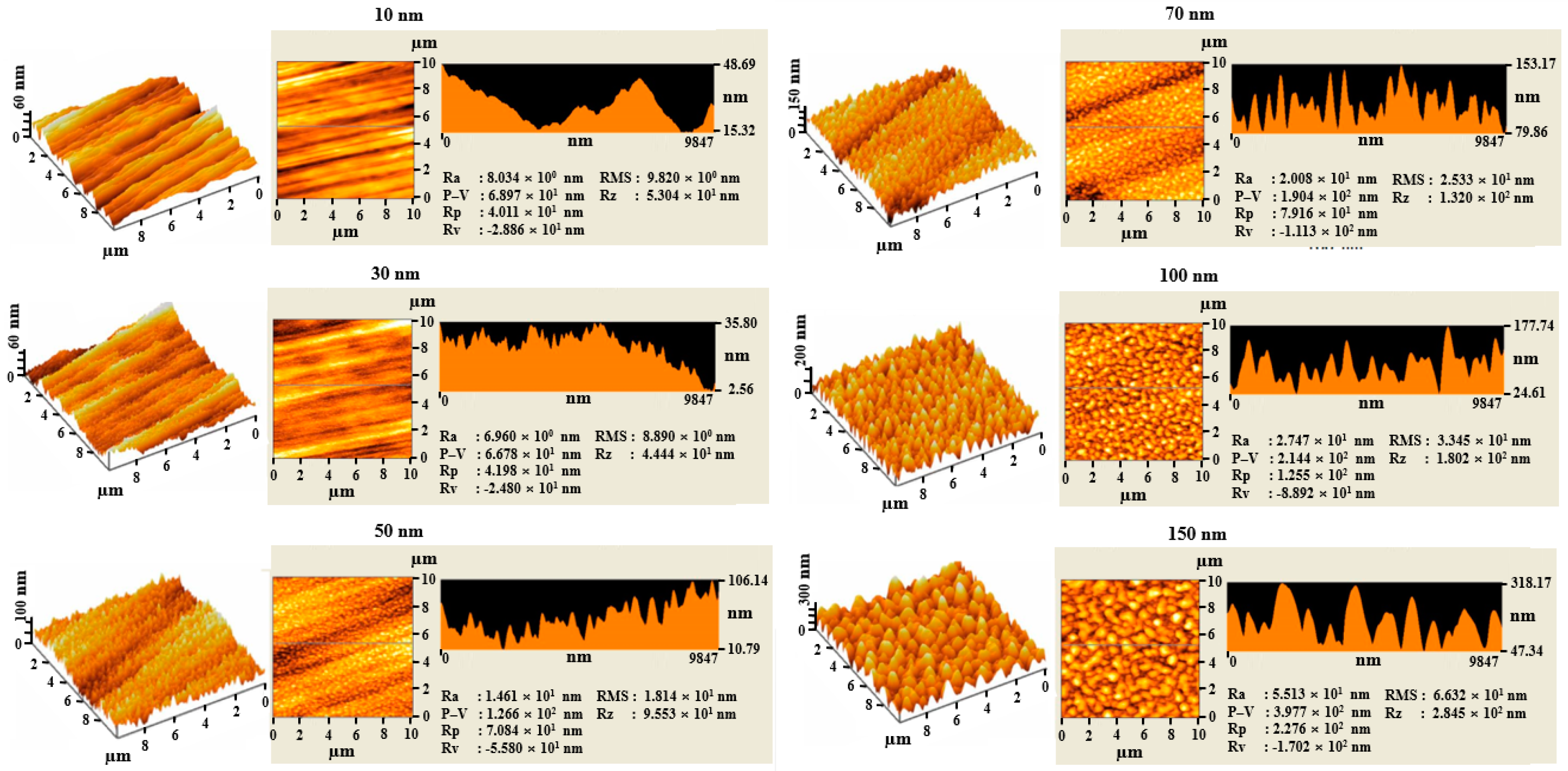
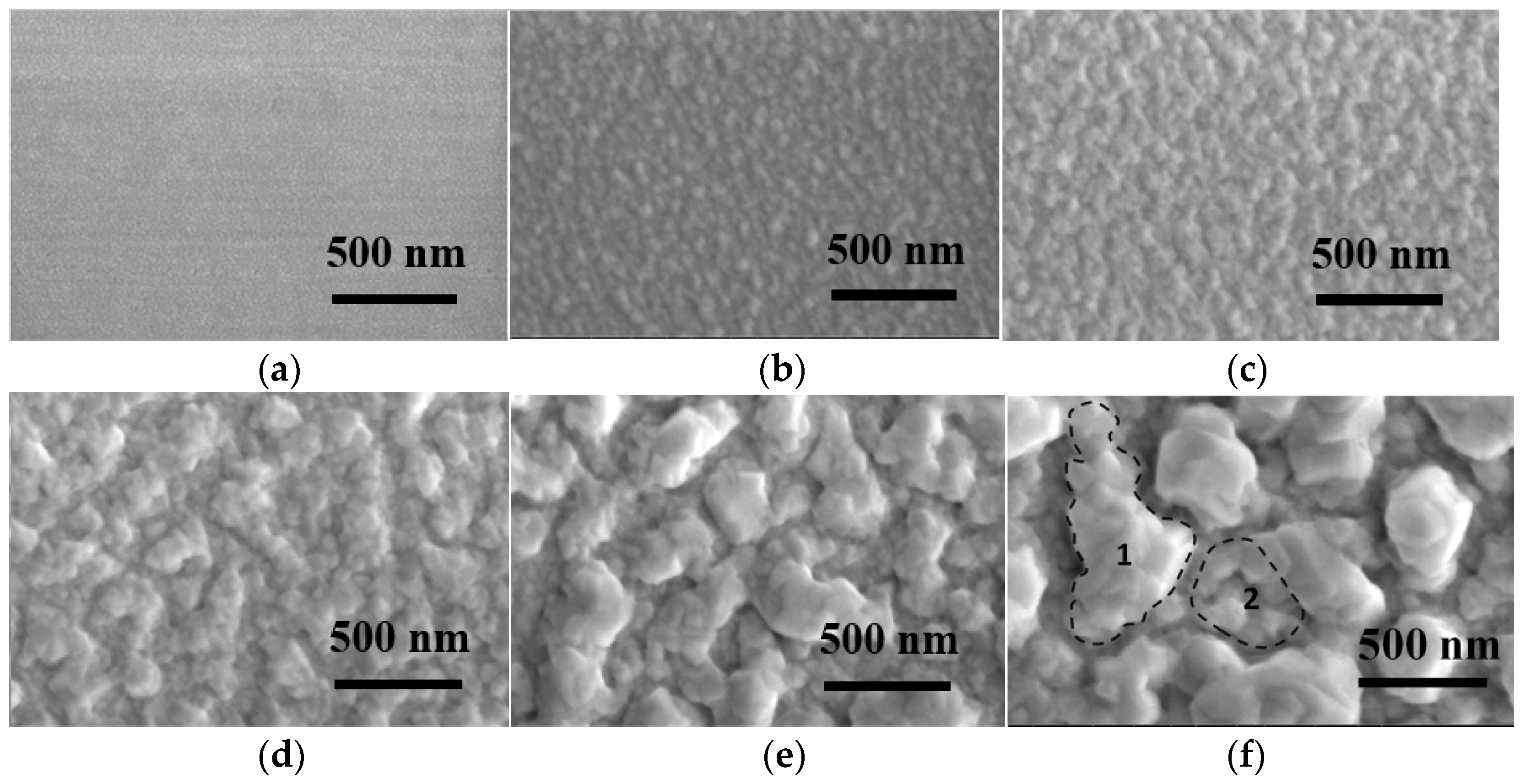
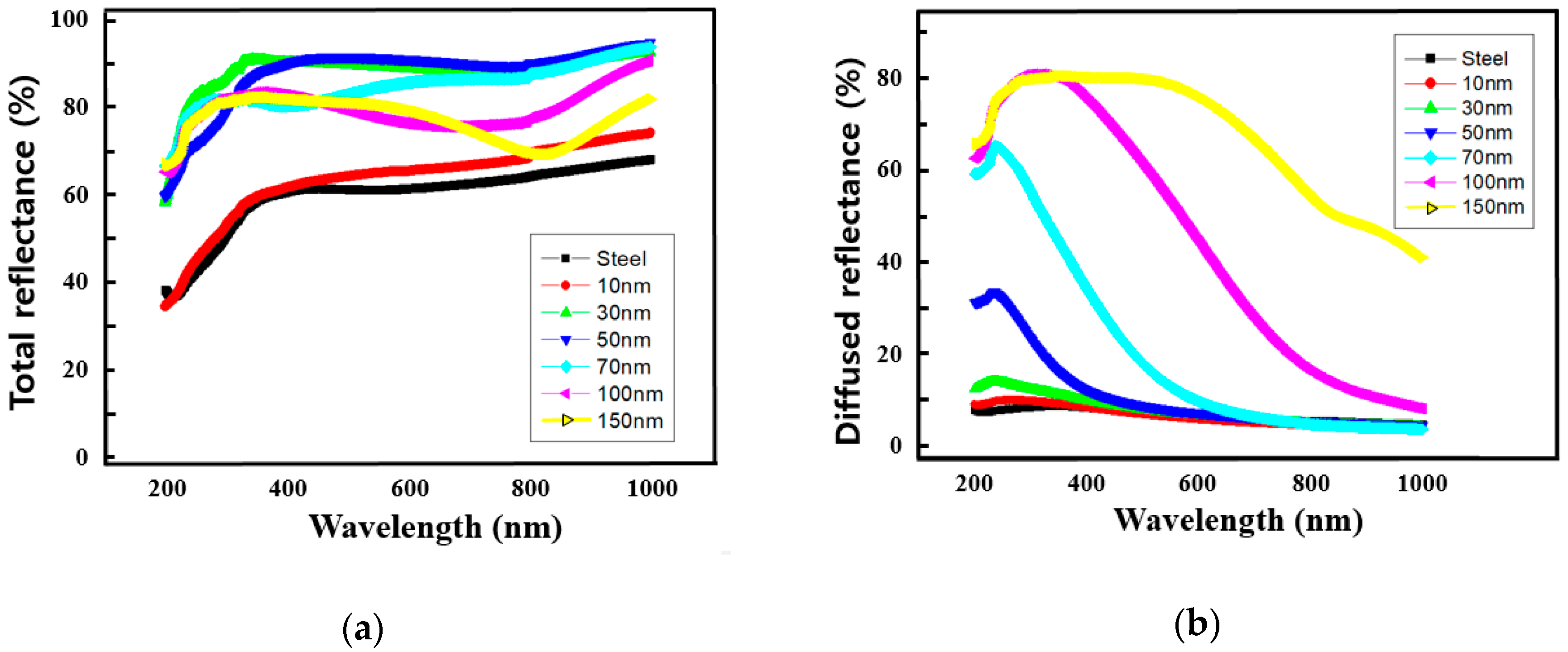
3. Results and Discussion
4. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Mansoor, S.B.; Yilbas, B.S. Phonon transport in a curved aluminum thin film due to laser short pulse irradiation. Opt. Laser Technol. 2018, 101, 107–115. [Google Scholar] [CrossRef]
- Beck, G.; Funk, S. Correlation between optical appearance and orientation of aluminum. Surf. Coat. Technol. 2012, 206, 2371–2379. [Google Scholar] [CrossRef]
- Green, M.L.; Levy, R.A.; Nuzzo, R.G.; Coleman, E. Aluminum films prepared by metal-organic low pressure chemical vapor deposition. Thin Solid Films 1984, 114, 367–377. [Google Scholar] [CrossRef]
- Vandembroucq, D.; Tarrats, A.; Greffet, J.-J.; Roux, S.; Plouraboué, F. Light scattering from cold rolled aluminum surfaces. Opt. Commun. 2001, 187, 289–294. [Google Scholar] [CrossRef] [Green Version]
- Cheung, C.H.; Djurišić, A.B.; Kwong, C.Y.; Tam, H.L.; Cheah, K.W.; Liu, Z.T.; Chan, W.K.; Chui, P.C.; Chan, J.; Rakić, A.D. Reduced angular dependence of the emission from tris(8-hydroxyquinoline) aluminum based microcavity. Opt. Commun. 2005, 248, 287–293. [Google Scholar] [CrossRef] [Green Version]
- Jung, K.H.; Yun, S.J.; Lee, S.H.; Lee, Y.J.; Lee, K.S.; Lim, J.W.; Kim, K.-B.; Kim, M.J.; Schropp, R.E.I. Double-layered Ag–Al back reflector on stainless steel substrate for a-Si:H thin film solar cells. Sol. Energy Mater. Sol. Cells 2016, 145, 368–374. [Google Scholar] [CrossRef]
- Nieto, D.; Cambronero, F.; Arias, M.T.F.; Farid, N.; O’Connor, G.M. Aluminum thin film enhanced IR nanosecond laser-induced frontside etching of transparent materials. Opt. Lasers Eng. 2017, 88, 233–242. [Google Scholar] [CrossRef]
- Rohrer, G.S.; Liu, X.; Liu, J.; Darbal, A.; Kelly, M.N.; Chen, X.; Berkson, M.A.; Nuhfer, N.T.; Coffey, K.R.; Barmak, K. The grain boundary character distribution of highly twinned nanocrystalline thin film aluminum compared to bulk microcrystalline aluminum. J. Mater. Sci. 2017, 52, 9819–9833. [Google Scholar] [CrossRef]
- Oh, C.S.; Bae, J.S.; Choa, S.H.; Lee, H.J. High Temperature Behavior of Al Thin Film. In Experimental Analysis of Nano and Engineering Materials and Structures, Proceedings of the 13th International Conference on Experimental Mechanics, Alexandroupolis, Greece, 1–6 July 2007; Springer: Berlin, Germany, 2007. [Google Scholar]
- Kaune, G.; Metwalli, E.; Meier, R.; Körstgens, V.; Schlage, K.; Couet, S.; Röhlsberger, R.; Roth, S.V.; Müller-Buschbaum, P. Growth and Morphology of Sputtered Aluminum Thin Films on P3HT Surfaces. ACS Appl. Mater. Interfaces 2011, 3, 1055–1062. [Google Scholar] [CrossRef]
- Yu-Qing, X.; Xing-Cun, L.; Qiang, C.; Wen-Wen, L.; Qiao, Z.; Li-Jun, S.; Zhong-Wei, L.; Zheng-Duo, W.; Li-Zhen, Y. Characteristics and properties of metal aluminum thin films prepared by electron cyclotron resonance plasma-assisted atomic layer deposition technology. Chin. Phys. B 2012, 21, 078105. [Google Scholar]
- Kim, K.-B.; Kim, M.J.; Lee, H.C.; Park, S.W.; Jeon, C.W. Copper indium gallium selenide (CIGS) solar cell devices on steel substrates coated with thick SiO2-based insulating material. Mater. Res. Bull. 2017, 85, 168–175. [Google Scholar] [CrossRef]
- Kim, M.J.; Kim, K.-B.; Jeon, C.W.; Lee, D.Y.; Lee, S.N.; Lee, J.M.; Lee, H.C. CIGS solar cell devices on steel substrates coated with Na containing AlPO4. J. Phys. Chem. Solids 2015, 86, 223–228. [Google Scholar] [CrossRef]
- Mahmoudabadi, F.; Ma, X.; Hatalis, M.K.; Shah, K.N.; Levendusky, T.L. Amorphous IGZO TFTs and circuits on conformable aluminum substrates. Solid State Electron. 2014, 101, 57–62. [Google Scholar] [CrossRef]
- Cheon, J.H.; Bae, J.H.; Jang, J. Mechanical stability of poly-Si TFT on metal foil. Solid State Electron. 2008, 52, 473–477. [Google Scholar] [CrossRef]
- Garbacz, H.; Wiecinski, P.; Adamczyk-Cieslak, B.; Mizera, J.; Kurzydlowski, K.J. Studies of Aluminium Coatings Deposited by Vacuum Evaporation and Magnetron Sputtering. J. Microsc. 2009, 237, 475–480. [Google Scholar] [CrossRef]
- Mwema, F.M.; Oladijo, O.P.; Akinlabi, S.A.; Akinlabi, E.T. Properties of physically deposited thin aluminium film coatings: A review. J. Alloys Compd. 2018, 747, 306–323. [Google Scholar] [CrossRef]
- Ivkov, J.; Salamon, K.; Radic, N.; Soric, M. Thermal Stability of Al-Mo Thin Film Alloys. J. Alloys Compd. 2015, 646, 1109–1115. [Google Scholar] [CrossRef]
- Dietrich, S.; Schneegans, M.; Moske, M.; Samwer, K. Investigation on Metallurgical Properties and Electromigration in AlCu Metallizations for VLSI Applications. Thin Solid Film. 1996, 275, 159–163. [Google Scholar] [CrossRef]
- Bordo, K.; Rubahn, H. Effect of Deposition Rate on Structure and Surface Morphology of Thin Evaporated Al Films on Dielectrics and Semiconductors. Mater. Sci. 2012, 18, 313–317. [Google Scholar] [CrossRef]
- Khachatryan, H.; Lee, S.N.; Kim, K.B.; Kim, H.K.; Kim, M.J. Al thin film: The effect of substrate type on Al film formation and morphology. J. Phys. Chem. Solids 2018, 122, 109–117. [Google Scholar] [CrossRef]
- Lee, H.; Singh, J.K.; Ismail, M.A.; Bhattacharya, C. Corrosion Resistance Properties of Aluminium Coating Applied by Arc Thermal Spray in SAE J2334 Solution with exposure Periods. Metals 2016, 6, 55. [Google Scholar]
- Shibata, H.; Murota, M.; Hashimoto, K. The Effects of Al (111) Crystal Orientation on Electromigration in Half-Micron Layered Al Interconnects. Jpn. J. Appl. Phys. 1993, 32, 4479–4484. [Google Scholar] [CrossRef]
- Liu, Z.J.; Shen, Y.G.; He, L.P.; Fu, T. Surface Evolution and Dynamic Scaling of Sputter-Deposited thin Al Films on Ti(100) Substrates. Appl. Surf. Sci. 2004, 226, 371–377. [Google Scholar] [CrossRef]
- Lugolole, R.; Obwoya, S.K. The Effect of Thickness of Aluminium Films on Optical Reflectance. J. Ceram. 2015, 213635, 1–6. [Google Scholar] [CrossRef]
- Kim, S.D.; Rhee, J.K.; Hwang, I.S.; Park, H.M.; Park, H.C. Surface Condition Effects of the Inter-Metal Dielectrics on Interconnect Aluminum Film Properties. Thin Solid Film. 2001, 401, 273–278. [Google Scholar] [CrossRef]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khachatryan, H.; Lee, S.-N.; Kim, K.-B.; Kim, M. Deposition of Al Thin Film on Steel Substrate: The Role of Thickness on Crystallization and Grain Growth. Metals 2019, 9, 12. https://doi.org/10.3390/met9010012
Khachatryan H, Lee S-N, Kim K-B, Kim M. Deposition of Al Thin Film on Steel Substrate: The Role of Thickness on Crystallization and Grain Growth. Metals. 2019; 9(1):12. https://doi.org/10.3390/met9010012
Chicago/Turabian StyleKhachatryan, Hayk, Sung-Nam Lee, Kyoung-Bo Kim, and Moojin Kim. 2019. "Deposition of Al Thin Film on Steel Substrate: The Role of Thickness on Crystallization and Grain Growth" Metals 9, no. 1: 12. https://doi.org/10.3390/met9010012



