Vacuum Brazing Ti–15–3 with a TiNiNb Braze Alloy
Abstract
:1. Introduction
2. Materials and Methods
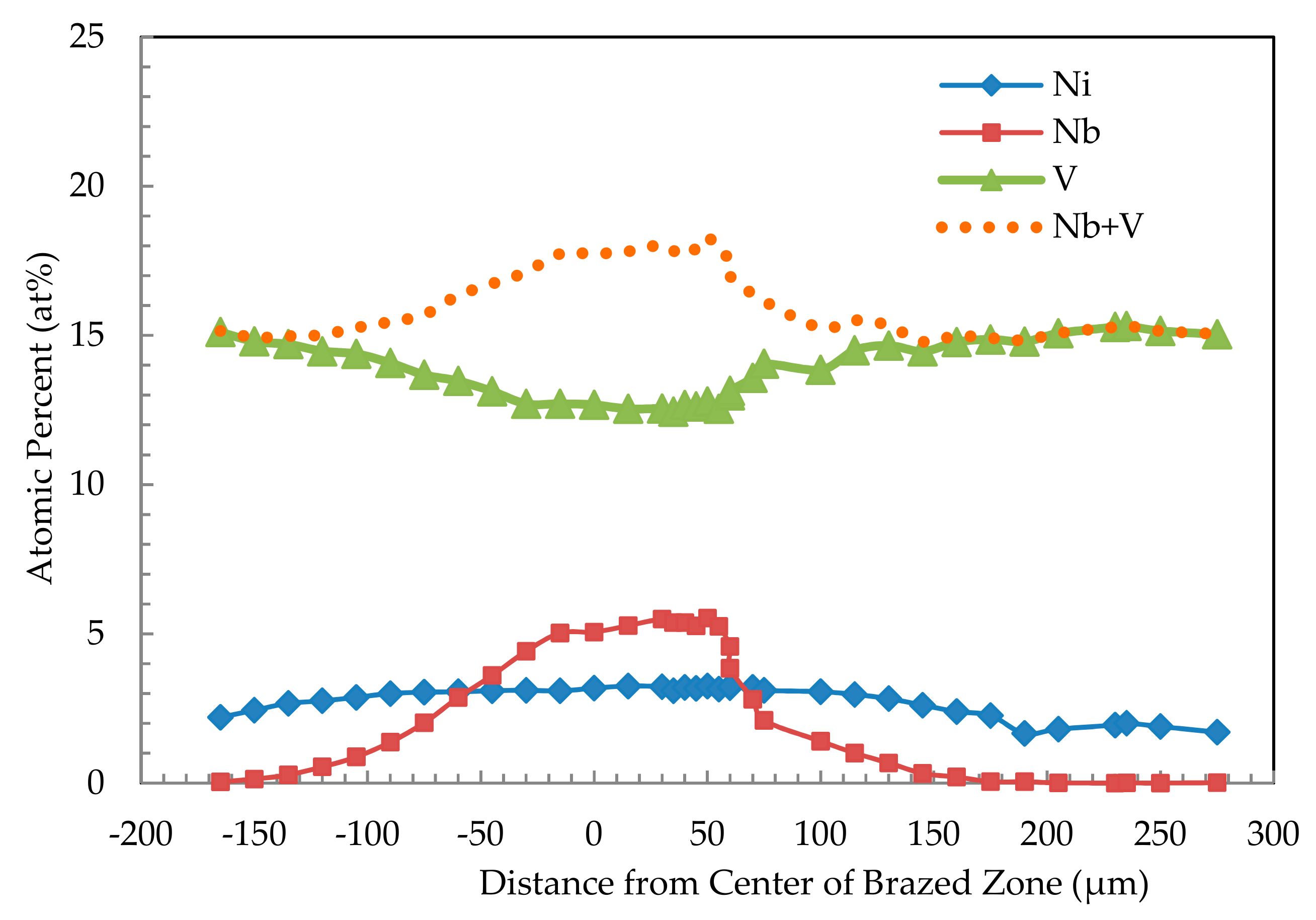
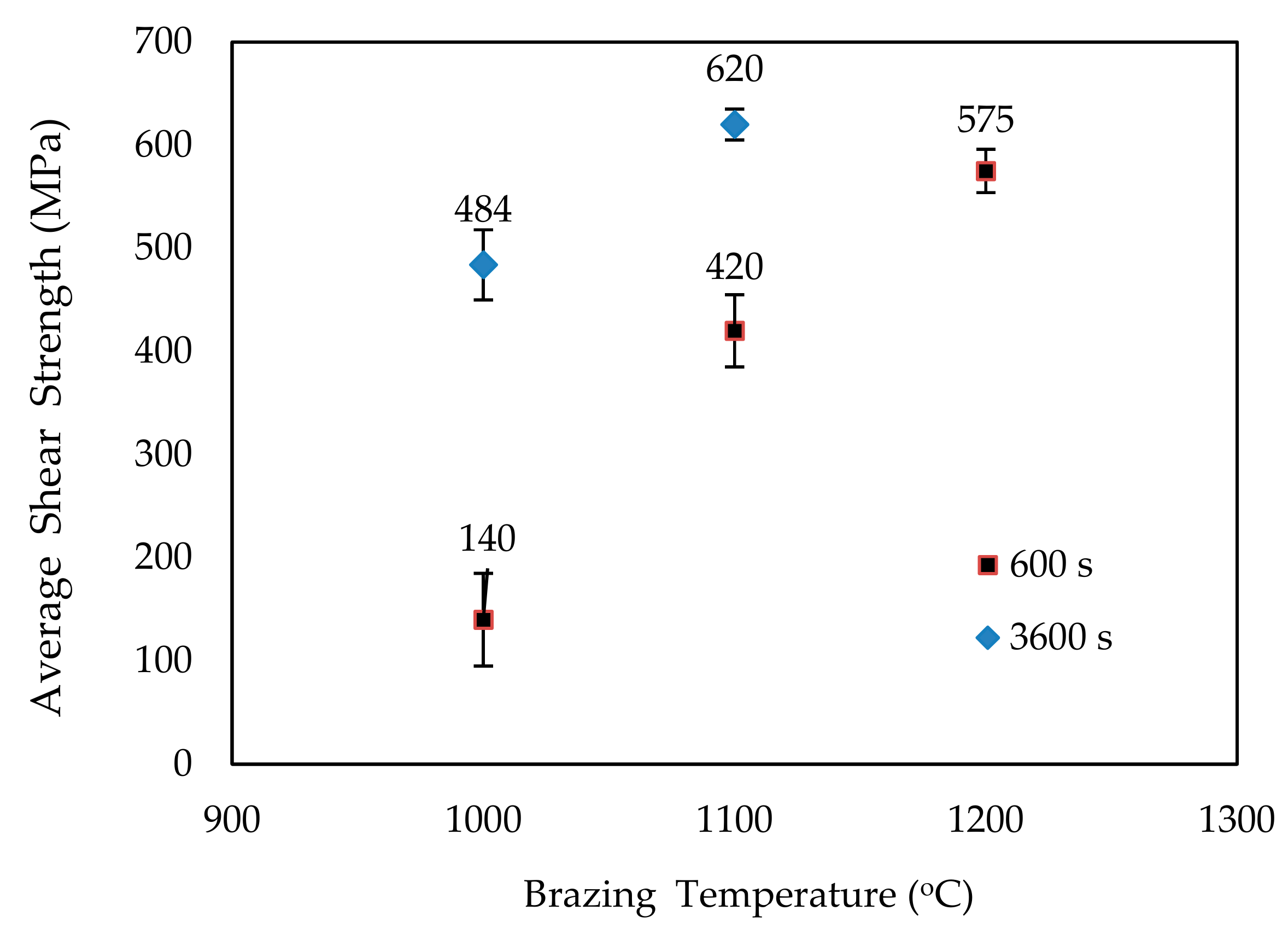
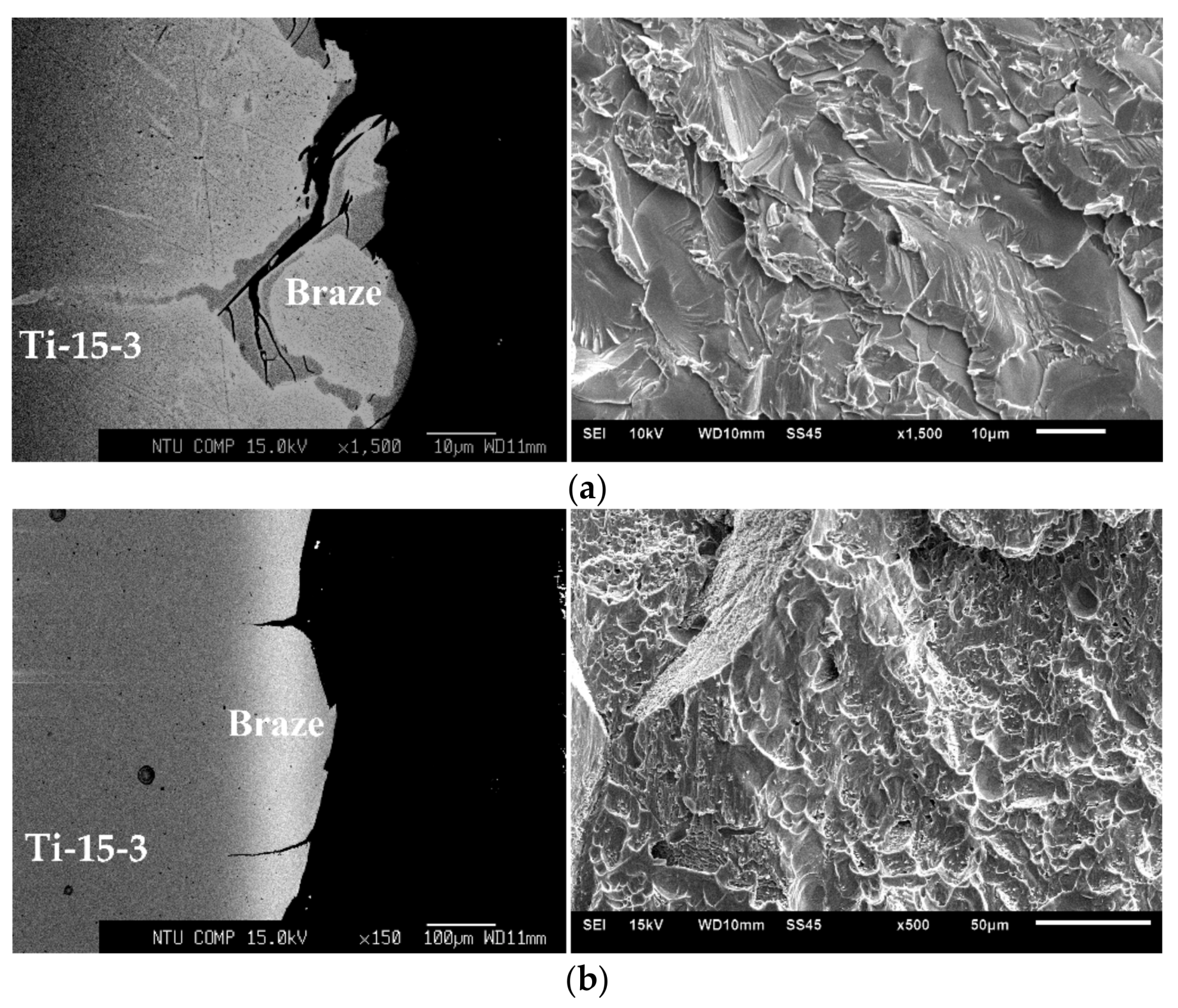
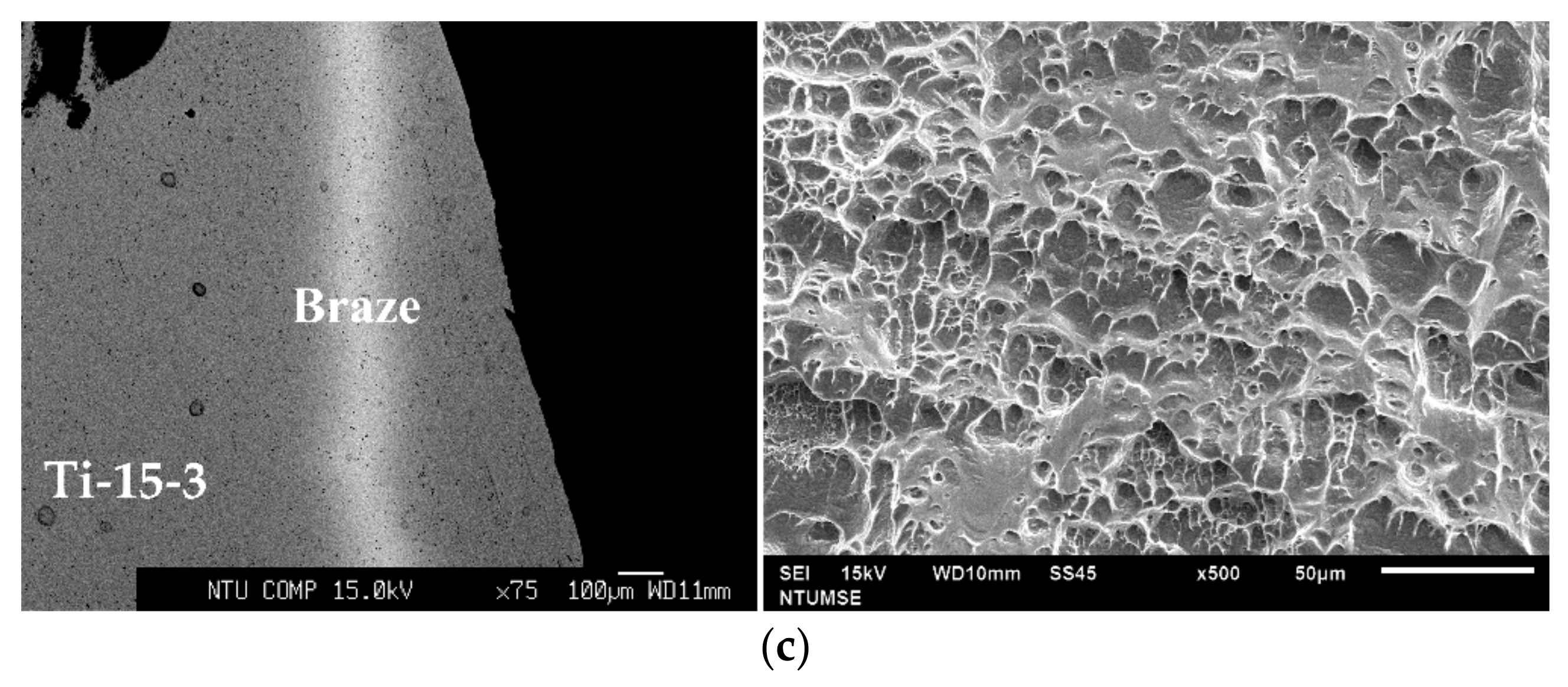
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Olson, D.L. Metals Handbook: Volume 6, Welding Brazing and Soldering; ASM International: Materials Park, OH, USA, 1990. [Google Scholar]
- Humpston, G.; Jacobson, D.M. Principles of Soldering and Brazing; ASM International: Materials Park, OH, USA, 1993. [Google Scholar]
- Yao, Q.; Cheng, H.C.; Fan, J.L.; Yan, H.X.; Zhang, C.G. High strength Mo/Ti6Al4V diffusion bonding joints: Interfacial microstructure and mechanical properties. Inter. J. Refrac. Met. Hard Mater. 2019, 82, 159–166. [Google Scholar] [CrossRef]
- Oliveira, J.P.; Panton, B.; Zeng, Z.; Andrei, C.M.; Zhou, Y.; Miranda, R.M.; Fernandes, F.M.B. Laser joining of NiTi to Ti6Al4V using a Niobium interlayer. Acta Mater. 2016, 105, 9–15. [Google Scholar] [CrossRef]
- Velmurugan, C.; Senthilkumar, V.; Sarala, S.; Arivarasan, J. Low temperature diffusion bonding of Ti-6Al-4V and duplex stainless steel. J. Mater. Proc. Tech. 2016, 234, 273–279. [Google Scholar] [CrossRef]
- Zhou, X.F.; Duan, J.A.; Zhang, F.; Zhong, S.S. The study on mechanical strength of titanium-aluminum dissimilar butt joints by laser welding-brazing process. Materials 2019, 12, 712. [Google Scholar] [CrossRef] [PubMed]
- Ogura, T.; Miyoshi, K.; Matsumura, T.; Imai, T.; Saida, K. Improvement of joint strength in dissimilar friction welding of Ti-6Al-4V alloy to type-718 nickel-based alloy using the Au-Ni interlayer. Sci. Tech. Weld. Join. 2019, 24, 327–333. [Google Scholar] [CrossRef]
- Xia, Y.Q.; Dong, H.G.; Hao, X.H.; Li, P.; Li, S. Vacuum brazing of Ti6Al4V alloy to 316L stainless steel using a Ti-Cu-based amorphous filler metal. J. Mater. Process. Tech. 2019, 269, 35–44. [Google Scholar] [CrossRef]
- Yeh, T.Y.; Shiue, R.K.; Chang, C.S. Microstructural observation of brazed Ti-15-3 alloy using the clad Ti-20Zr-20Cu-20Ni foil. ISIJ Inter. 2013, 53, 726–728. [Google Scholar] [CrossRef]
- Simoes, S. Recent progress in the joining of titanium alloys to ceramics. Metals 2018, 8, 876. [Google Scholar] [CrossRef]
- Ganjeh, E.; Sarkhosh, H.; Bajgholi, M.E.; Khorsand, H.; Ghaffari, M. Increasing Ti–6Al–4V brazed joint strength equal to the base metal by Ti and Zr amorphous filler alloys. Mater. Charac. 2012, 71, 31–40. [Google Scholar] [CrossRef]
- Chen, W.S.; Wang, C.Y.; Shiue, R.K. Brazing Inconel 625 using the copper foil. Metall. Mater. Trans. A 2013, 44A, 5724–5731. [Google Scholar] [CrossRef]
- Chang, C.T.; Wu, Z.Y.; Shiue, R.K.; Chang, C.S. Infrared brazing Ti-6Al-4V and SP-700 alloys using the Ti-20Zr-20Cu-20Ni braze alloy. Mater. Lett. 2007, 61, 842–845. [Google Scholar] [CrossRef]
- Guedes, A.; Pinto, A.M.P.; Vieira, M.F.; Viana, F. Joining Ti-47Al-2Cr-2Nb with a Ti/(Cu,Ni)/Ti clad-laminated braze alloy. J. Mater. Sci. 2003, 38, 2409–2414. [Google Scholar] [CrossRef] [Green Version]
- Zou, Z.H.; Zeng, F.H.; Wu, H.B.; Liu, J.; Li, Y.; Gu, Y.; Yuan, T.C.; Zhang, F.Q. The joint strength and fracture mechanisms of TC4/TC4 and TA0/TA0 brazed with Ti-25Cu-15Ni braze alloy. J. Mater. Eng. Perf. 2017, 26, 2079–2085. [Google Scholar] [CrossRef]
- Ren, H.S.; Xiong, H.P.; Chen, B.; Pang, S.J.; Chen, B.Q.; Ye, L. Microstructures and mechanical properties of vacuum brazed Ti3Al/TiAl joints using two Ti-based filler metals. J. Mater. Sci. Tech. 2016, 32, 372–380. [Google Scholar] [CrossRef]
- Chang, C.T.; Du, Y.C.; Shiue, R.K.; Chang, C.S. Infrared brazing of high-strength titanium alloys by Ti-15Cu-15Ni and Ti-15Cu-25Ni filler foils. Mater. Sci. Eng. 2006, A420, 155–164. [Google Scholar] [CrossRef]
- Pang, S.; Sun, L.; Xiong, H.; Chen, C.; Liu, Y.; Li, H.; Zhang, T. A multi component TiZr-based amorphous brazing filler metal for high-strength joining of titanium alloy. Scripta Mater. 2016, 117, 55–59. [Google Scholar] [CrossRef]
- Wang, Y.; Cai, X.Q.; Yang, Z.W.; Wang, D.P.; Liu, X.G.; Liu, Y.C. Effects of Nb content in Ti–Ni–Nb brazing alloys on the microstructure and mechanical properties of Ti–22Al–25Nb alloy brazed joints. J. Mater. Sci. Tech. 2017, 33, 682–689. [Google Scholar] [CrossRef]
- Wang, S.B.; Kao, C.S.; Tsay, L.W.; Shiue, R.K. The application of 40Ti-35Ni-25Nb filler foil in brazing commercially pure titanium. Metals 2018, 8, 154. [Google Scholar] [CrossRef]
- Walter, J.L.; Jackson, M.R.; Sims, C.T. Titanium and its Alloys: Principles of Alloying Titanium; ASM International: Materials Park, OH, USA, 1993. [Google Scholar]
- Zhang, W.J.; Liu, H.H.; Ding, H.; Fujii, H. Grain refinement and superplastic flow in friction stir processed Ti-15V-3Cr-3Sn-3Al alloy. J. Alloys Comp. 2019, 803, 901–911. [Google Scholar] [CrossRef]
- Zou, C.H.; Zhou, Q.; Wang, L. A step deformation method for superplasticity improvement of coarse-grained Ti-15V-3Cr-3Sn-3Al. Chin. J. Aeronaut. 2018, 31, 1619–1624. [Google Scholar] [CrossRef]
- Lin, C.; Shiue, R.K.; Wu, S.K.; Yang, T.E. Infrared brazed joints of Ti50Ni50 shape memory alloy and Ti-15-3 alloy using two Ag-based fillers. Materials 2019, 12, 1603. [Google Scholar] [CrossRef] [PubMed]
- Sandpaper. Wikipedia. Available online: https://en.wikipedia.org/wiki/Sandpaper (accessed on 4 October 2019).
- Massalski, T.B. Binary Alloy Phase Diagrams; ASM international: Materials Park, OH, USA, 1990. [Google Scholar]
- Smith, W.F. Structure and Properties of Engineering Alloys; McGraw-Hill Inc.: New York, NY, USA, 1993. [Google Scholar]


| Material/wt% | Ti | Ni | Nb | V | Al | Cr | Sn |
|---|---|---|---|---|---|---|---|
| Ti–15–3 | 76 | - | - | 15 | 3 | 3 | 3 |
| Ti–35Ni–25Nb | 40 | 35 | 25 | - | - | - | - |
| Position/at% | Ti | Ni | Nb | V | Al | Cr | Sn | Phase |
|---|---|---|---|---|---|---|---|---|
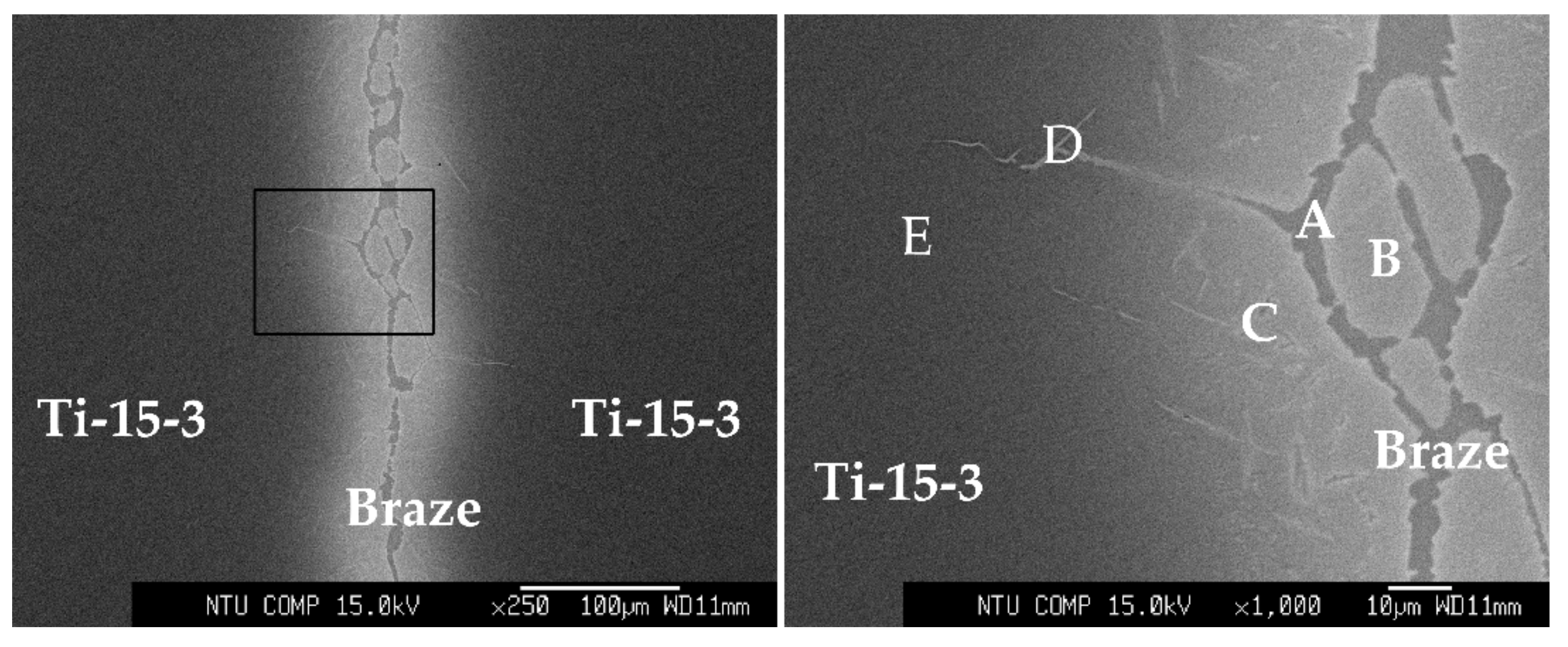
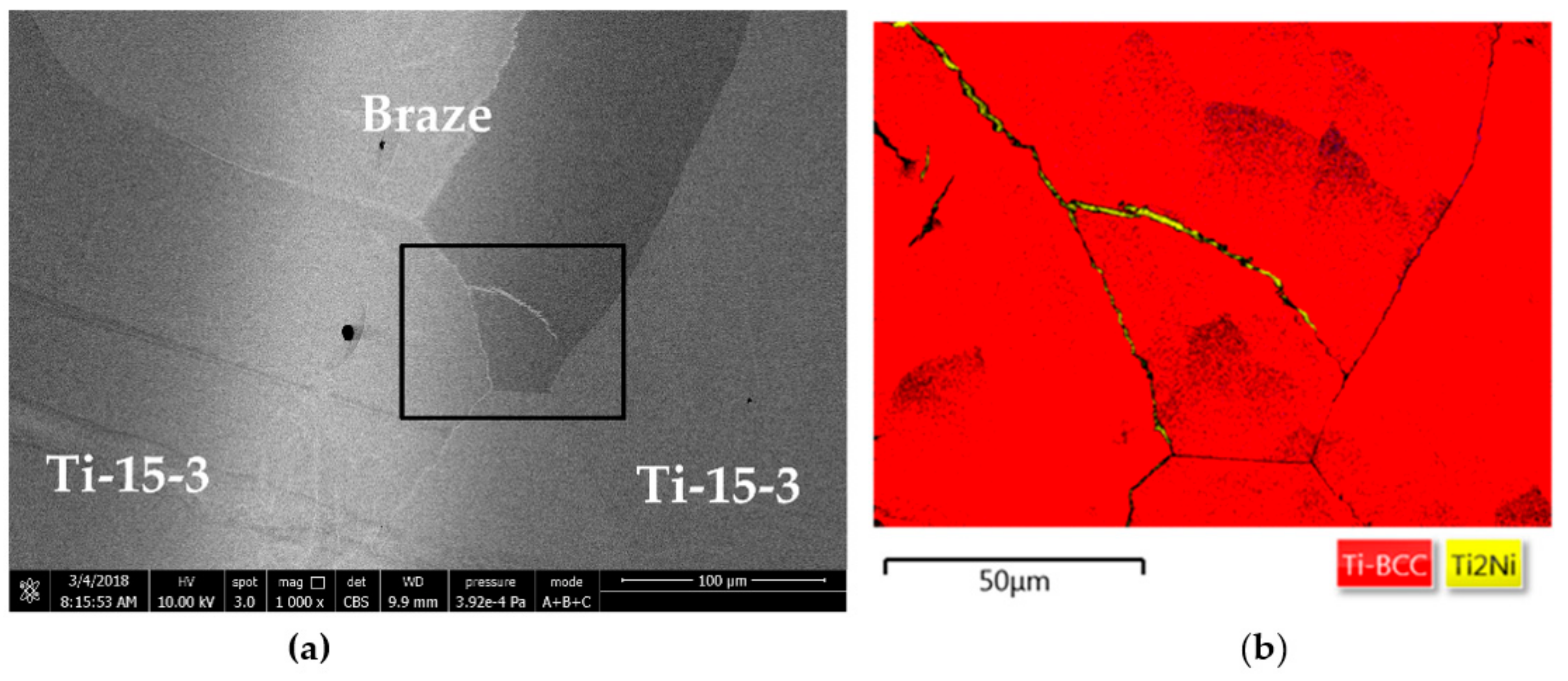
| A | 60.3 | 34.1 | 1.8 | 1.8 | 1.2 | 0.6 | 0.1 | Ti2Ni |
| B | 64.5 | 5.5 | 15.4 | 8.9 | 2.5 | 1.6 | 1.4 | (β-Ti,Nb)-rich |
| C | 61.4 | 24.0 | 3.8 | 6.1 | 1.9 | 1.5 | 1.2 | close to Ti2Ni |
| D | 61.4 | 32.1 | 0.7 | 3.0 | 1.1 | 1.2 | 0.4 | Ti2Ni |
| E | 67.3 | 4.4 | 6.2 | 11.6 | 6.0 | 2.1 | 2.3 | close to Ti–15–3 |
| Position/at% | Ti | Ni | Nb | V | Al | Cr | Sn | Phase |
|---|---|---|---|---|---|---|---|---|
| F | 67.8 | 13.9 | 4.7 | 7.3 | 4.1 | 1.6 | 0.5 | close to Ti2Ni |
| G | 72.4 | 3.9 | 3.7 | 11.3 | 5.5 | 2.1 | 1.0 | (β-Ti,Nb)-rich |
| Position/at% | Ti | Ni | Nb | V | Al | Cr | Sn | Phase |
|---|---|---|---|---|---|---|---|---|
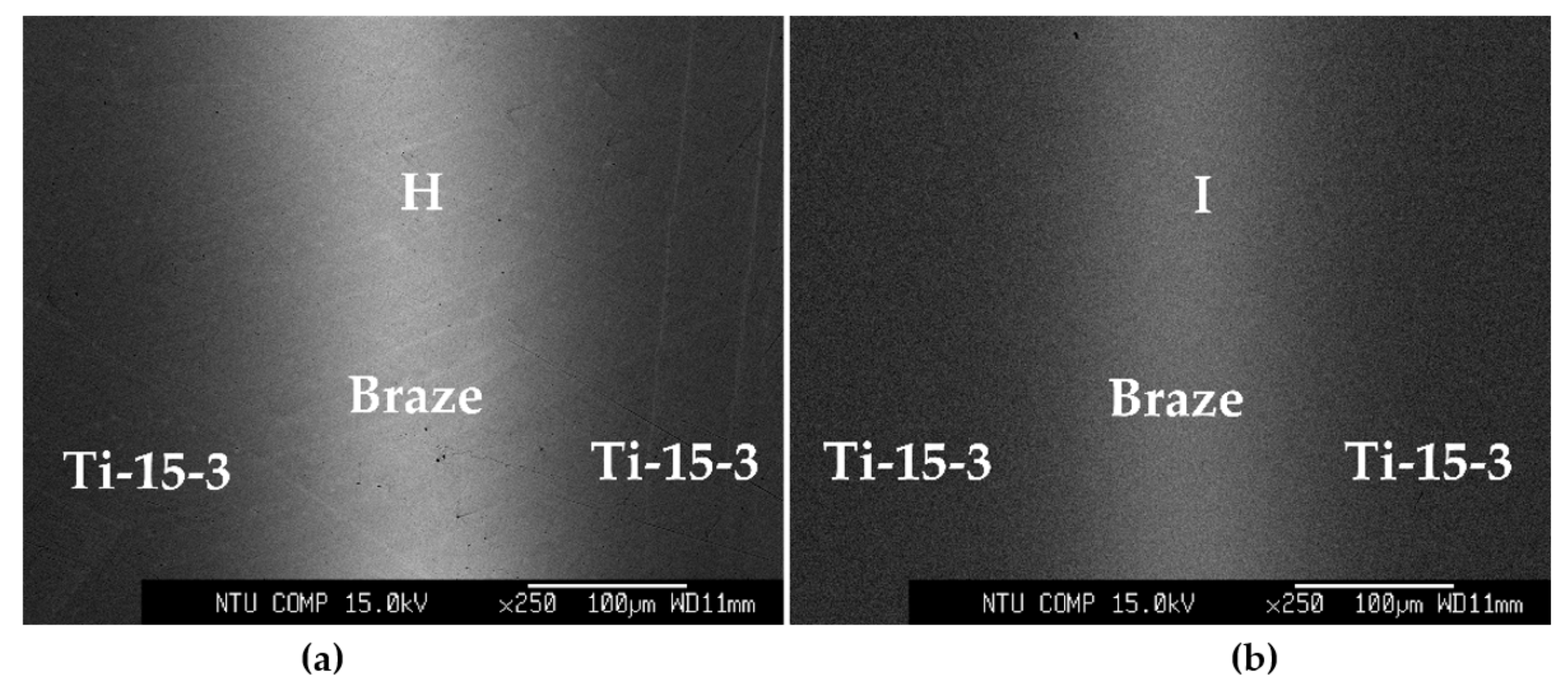
| H | 71.4 | 2.4 | 5.6 | 12.5 | 4.5 | 2.6 | 1.0 | (β-Ti,Nb)-rich |
| I | 71.2 | 3.2 | 6.0 | 10.9 | 4.7 | 2.9 | 1.0 | (β-Ti,Nb)-rich |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kao, C.-S.; Tsay, L.-W.; Wang, S.-B.; Shiue, R.-K. Vacuum Brazing Ti–15–3 with a TiNiNb Braze Alloy. Metals 2019, 9, 1085. https://doi.org/10.3390/met9101085
Kao C-S, Tsay L-W, Wang S-B, Shiue R-K. Vacuum Brazing Ti–15–3 with a TiNiNb Braze Alloy. Metals. 2019; 9(10):1085. https://doi.org/10.3390/met9101085
Chicago/Turabian StyleKao, Chuan-Sheng, Leu-Wen Tsay, Shan-Bo Wang, and Ren-Kae Shiue. 2019. "Vacuum Brazing Ti–15–3 with a TiNiNb Braze Alloy" Metals 9, no. 10: 1085. https://doi.org/10.3390/met9101085
APA StyleKao, C.-S., Tsay, L.-W., Wang, S.-B., & Shiue, R.-K. (2019). Vacuum Brazing Ti–15–3 with a TiNiNb Braze Alloy. Metals, 9(10), 1085. https://doi.org/10.3390/met9101085






