Influence of the Compaction Pressure and Sintering Temperature on the Mechanical Properties of Porous Titanium for Biomedical Applications
Abstract
:1. Introduction
2. Experimental Methods
2.1. Sample Processing
2.2. Porosity Characterisation
2.3. Mechanical Testing
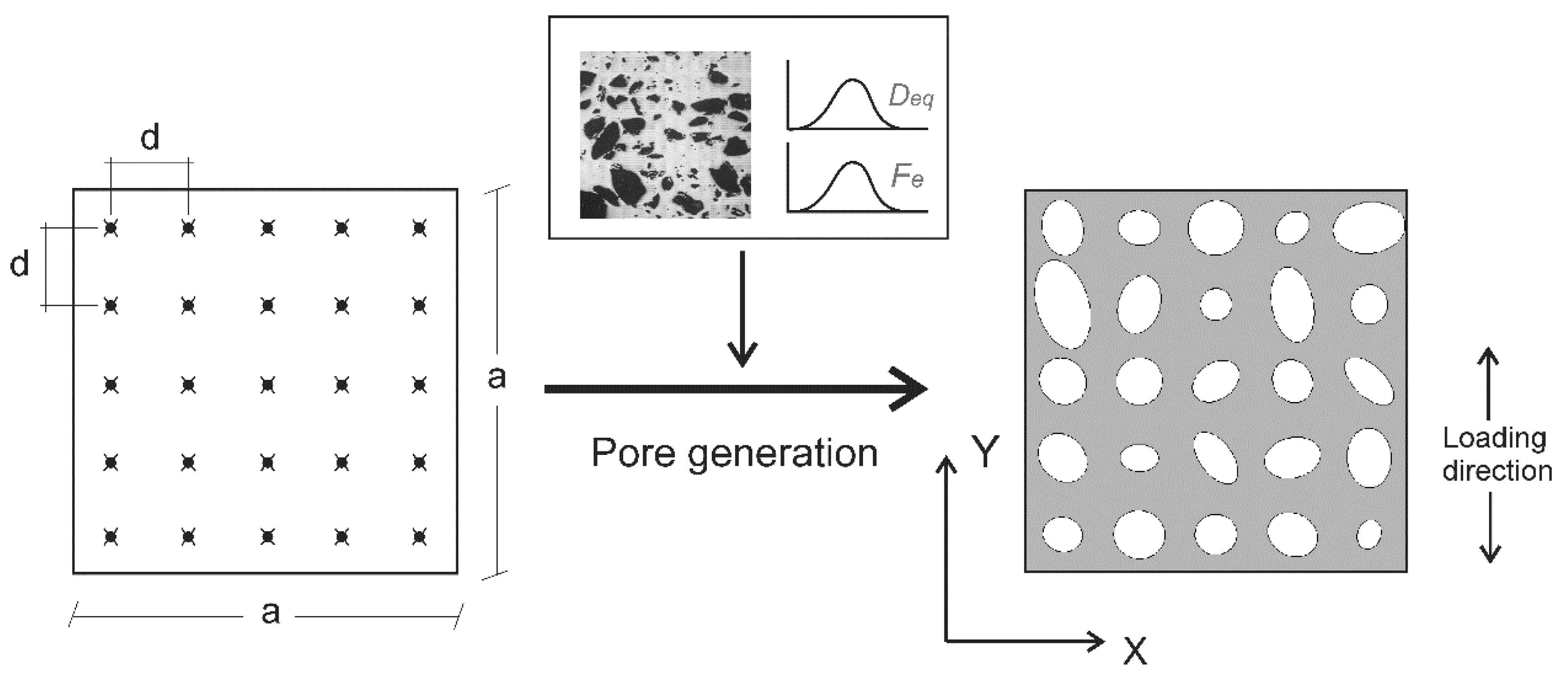
2.4. Finite Element Modelling
3. Results and Discussion
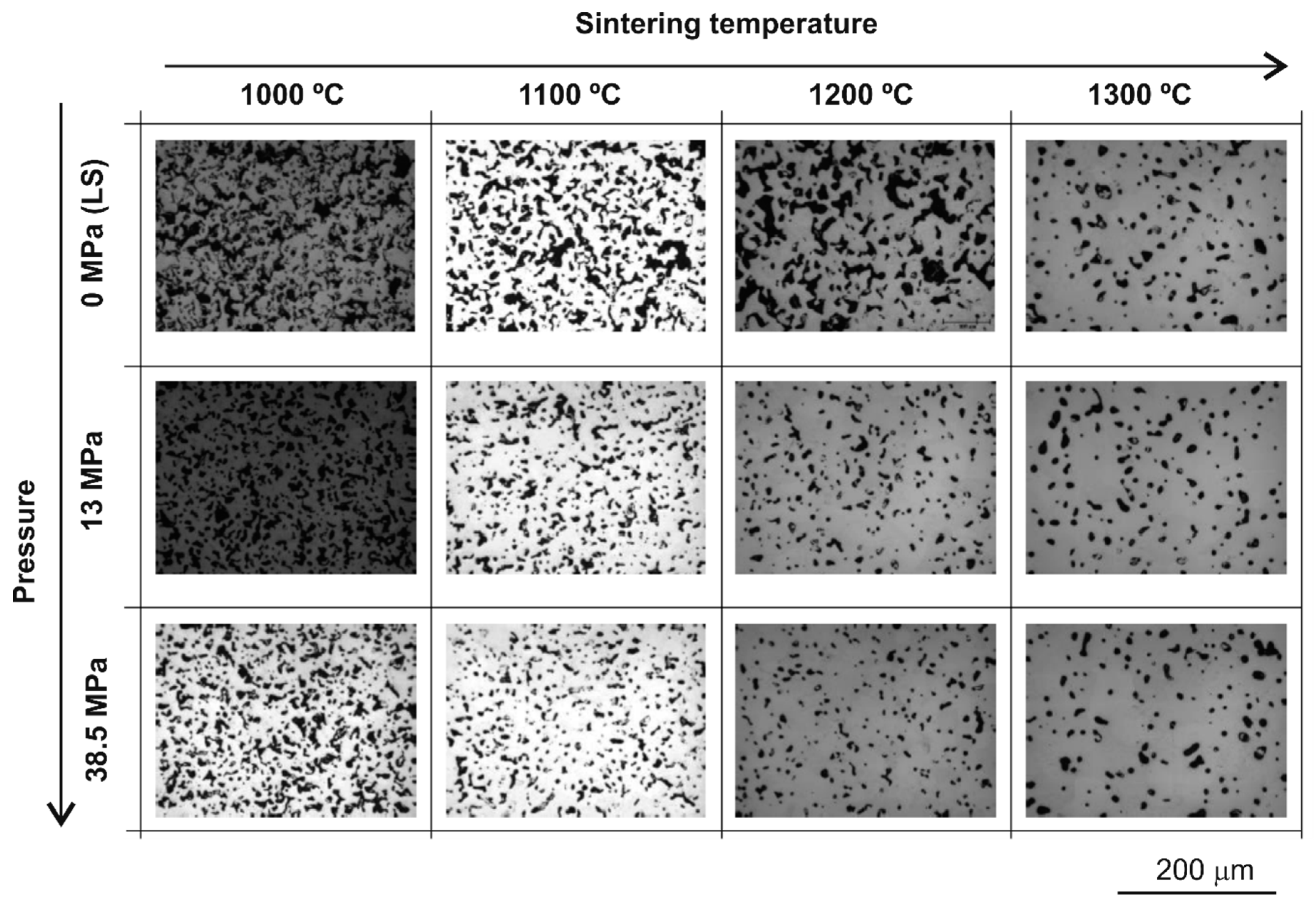
3.1. Porosity Characterisation
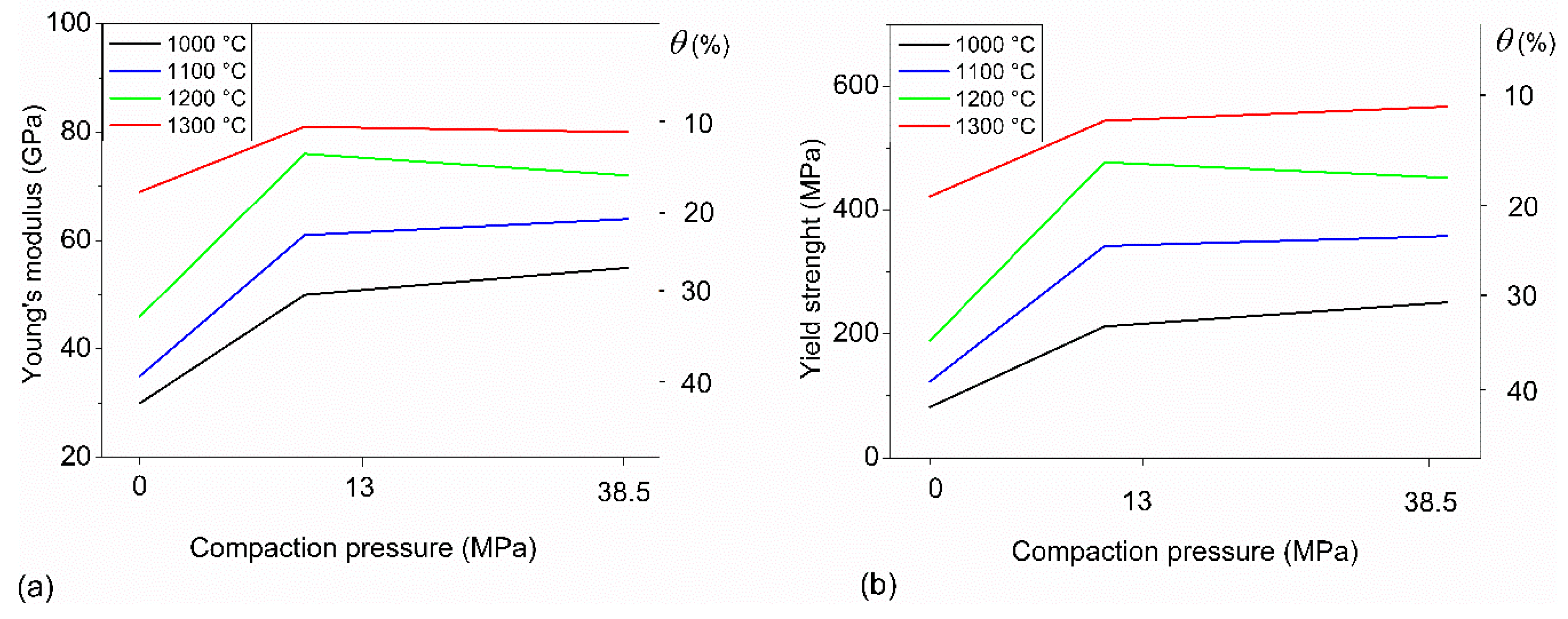
3.2. Mechanical Behavior
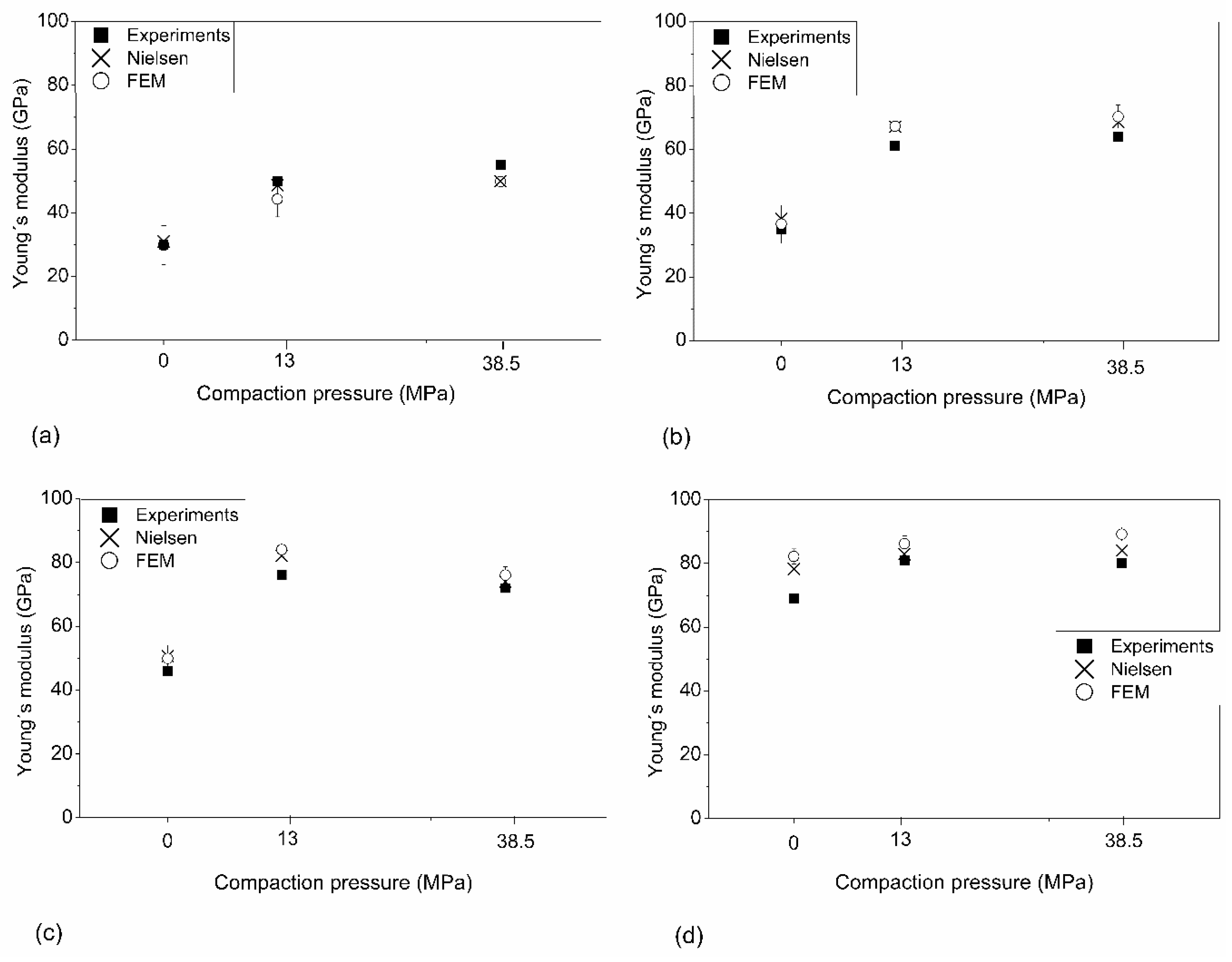
3.3. Finite Element Method Results
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Long, M.; Rack, H.J. Titanium alloys in total joint replacement—A materials science perspective. Biomaterials 1998, 19, 1621–1639. [Google Scholar] [CrossRef]
- Hayashi, Y.S.K.; Uenoyama, K.; Matsuguchi, N. Quantitative-analysis of invivo tissue responses to titanium-oxidecoated and hydroxyapatite-coated titanium-alloy. J. Biomed. Mater. Res. 1991, 25, 515–523. [Google Scholar] [CrossRef] [PubMed]
- Ning, C.; Zhou, L.; Tan, G. Fourth-generation biomedical materials. Mater. Today 2016, 19, 2–3. [Google Scholar] [CrossRef]
- Navarro, M.; Michiardi, A.; Castano, O.; Planell, J.A. Biomaterials in orthopaedics. J. R. Soc. Interface 2008, 5, 1137–1158. [Google Scholar] [CrossRef]
- Goriainov, V.; Cook, R.; Latham, J.M.; Dunlop, D.G.; Oreffo, R.O. Bone and metal: An orthopaedic perspective on osseointegration of metals. Acta Biomater. 2014, 10, 4043–4057. [Google Scholar] [CrossRef]
- Zhang, L.C.; Chen, L.Y. A Review on Biomedical Titanium Alloys: Recent Progress and Prospect. Adv. Eng. Mater. 2019, 21, 1801215. [Google Scholar] [CrossRef]
- Kaur, M.; Singh, K. Review on titanium and titanium based alloys as biomaterials for orthopaedic applications. Mat. Sci. Eng. C Mater. 2019, 102, 844–862. [Google Scholar] [CrossRef]
- Sola, A.; Belluci, D.; Cannillo, V. Functionally graded materials for orthopedic applications—An update on design and manufacturing. Biotechnol. Adv. 2016, 34, 504–531. [Google Scholar] [CrossRef]
- Norwsky, P.A.; Bumgardner, J.D. Biomaterial and antibiotic strategies for peri-implantitis: A review. J. Biomed. Mat. Res. 2009, 88, 530–543. [Google Scholar] [CrossRef]
- Naebe, M.; Shirvanimoghaddam, K. Functionally graded materials: A review of fabrication and properties. Appl. Mater. Today 2016, 5, 223–245. [Google Scholar] [CrossRef]
- Li, F.; Li, J.; Huang, T.; Kou, H.; Zhou, L. Compression fatigue behavior and failure mechanism of porous titanium for biomedical applications. J. Mech. Behav. Biomed. 2017, 65, 814–823. [Google Scholar] [CrossRef] [PubMed]
- Babaee, S.; Jahromi, B.H.; Ajdari, A.; Nayeb-Hashemi, H.; Vaziri, A. Mechanical properties of open-cell rhombic dodecahedron cellular structures. Acta Mater. 2012, 60, 2873–2885. [Google Scholar] [CrossRef]
- Özbilen, S.; Liebert, D.; Beck, T.; Bram, M. Fatigue behavior of highly porous titanium produced by powder metallurgy with temporary space holders. Mat. Sci. Eng. C Mater. 2016, 60, 446–457. [Google Scholar] [CrossRef] [PubMed]
- Yuhua, L.; Yang, C.; Zhao, H.; Qu, S.; Li, X.; Li, Y. New Developments of Ti-Based Alloys for Biomedical Applications. Materials 2014, 7, 1709–1800. [Google Scholar]
- O’Brien, B. Niobium Biomaterials. Charper in Advances in Metallic Biomaterials: Tissues, Materials and Biological Reactions; Niinomi, M., Narushima, T., Nakai, M., Eds.; Springer: Berlin/Heidelberg, Germany, 2015. [Google Scholar]
- Matsuno, H.; Yokoyama, A.; Watari, F.; Uo, M.; Kawasaki, T. Biocompatibility and osteogenesis of refractory metal implants, titanium, hafnium, niobium, tantalum and rhenium. Biomaterials 2001, 22, 1253–1262. [Google Scholar] [CrossRef]
- Niinomi, M.; Liu, Y.; Nakai, M.; Liu, H.; Li, H. Biomedical titanium alloys with Young’s moduli close to that of cortical bone. Regen. Biomater. 2016, 3, 173–185. [Google Scholar] [CrossRef]
- Liu, Y.J.; Li, S.J.; Zhang, L.C.; Hao, Y.L.; Sercombe, T.B. Early plastic deformation behaviour and energy absorption in porous β-type biomedical titanium produced by selective laser melting. Scr. Mater. 2018, 153, 99–103. [Google Scholar] [CrossRef]
- Pałka, K.; Pokrowiecki, R. Porous Titanium implants: A review. Adv. Eng. Mater. 2018, 20, 1700648. [Google Scholar] [CrossRef]
- Jin, Z.M.; Zheng, J.; Li, W.; Zhou, Z.R. Tribology of medical devices. Biosurface Biotribology 2016, 2, 173–192. [Google Scholar] [CrossRef]
- Reis, L.M.; Rodarte, Y.; Falchete, R.; Oliveira, L.G.; Alencastro, M.L.; Alves, C.A. Porous Titanium by Powder Metallurgy for Biomedical Application: Characterization, Cell Citotoxity and in vivo Tests of Osseointegration. In Charper 2 of Biomedical Engineering: Technical Applications in Medicine; Hudak, R., Ed.; InTech: Rijeka, Croatia, 2012. [Google Scholar]
- Khorasani, A.M.; Goldberg, M.; Doeven, E.H.; Littlefair, G. Titanium in biomedical applications—Properties and fabrication: A review. J. Biomater. Tiss. Eng. 2015, 5, 593–619. [Google Scholar] [CrossRef]
- Betts, C. Benefits of metal foams and developments in modelling techniques to assess their materials behaviour: A review. Mater. Sci. Technol. 2012, 28, 129–143. [Google Scholar] [CrossRef]
- Lee, W.H.; Hyun, C.Y. Fabrication of fully porous and porous-surfaced Ti-6Al-4V implants by electro-discharge-sintering of spherical Ti-6Al-4V powders in an one-step process. J. Mater. Process. Tech. 2007, 189, 219–223. [Google Scholar] [CrossRef]
- Su, Y.; Luo, C.; Zhang, Z.; Hermawan, H.; Zhu, D.; Huang, J.; Liang, Y.; Li, G.; Ren, L. Bioinspired surface functionalization of metallic biomaterials. J. Mech. Behav. Biomed. Mater. 2018, 77, 90–105. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Liu, X.; Yeung, K.W.; Liu, C.; Yang, X. Biomimetic porous scaffolds for bone tissue engineering. Mat. Sci. Eng. 2014, 80, 1–36. [Google Scholar] [CrossRef]
- Singh, R.; Lee, P.D.; Dashwood, R.J.; Lindley, T.C. Titanium foams for biomedical applications: A review. Mater. Technol. 2010, 25, 127–136. [Google Scholar] [CrossRef]
- Trueba, P. Desarrollos de Titanio con Porosidad Gradiente Radial y Longitudinal Para Aplicaciones Biomédicas. Ph.D.; Thesis, University of Seville, Seville, Spain, 2017.
- Zhang, L.C.; Liu, Y.; Li, S.; Hao, Y. Additive Manufacturing of Titanium Alloys by Electron Beam Melting: A Review. Adv. Eng. Mater. 2018, 20, 1700842. [Google Scholar] [CrossRef]
- Singh, S.; Ramakrishna, S.; Singh, R. Material issues in additive manufacturing: A review. J. Manuf. Process. 2017, 25, 185–200. [Google Scholar] [CrossRef]
- Orrù, R.; Licheri, R.; Locci, A.M.; Cincotti, A.; Cao, G. Consolidation/synthesis of materials by electric current activated/assisted sintering. Mat. Sci. Eng. 2009, 63, 127–287. [Google Scholar] [CrossRef]
- Asaoka, K.; Kuwayama, N.; Okuno, O.; Miura, I. Mechanical properties and biomechanical compatibility of porous titanium for dental implants. J. Biomed. Mater. Res. 1985, 19, 699–713. [Google Scholar] [CrossRef]
- Wang, J.F.; Liu, X.Y.; Luan, B. Fabrication of Ti/polymer biocomposites for load-bearing implant applications. J. Mater. Process. Tech. 2008, 197, 428–433. [Google Scholar] [CrossRef]
- Oh, I.H.; Nomura, N.; Masahashi, N.; Hanada, S. Mechanical properties of porous titanium compacts prepared by powder sintering. Scr. Mater. 2003, 49, 1197–1202. [Google Scholar] [CrossRef]
- Jamshidinia, M.; Wangb, L.; Tong, W.; Ajlouni, R.; Kovacevic, R. Fatigue properties of a dental implant produced by electron beammelting (EBM). J. Mater. Process. Tech. 2015, 226, 255–263. [Google Scholar] [CrossRef]
- Ren, D.; Li, S.; Wang, H.; Hou, W.; Hao, Y.; Jin, W.; Yang, R.; Misra, R.D.K.; Murr, R.E. Fatigue behavior of Ti-6Al-4V cellular structures fabricated by additive manufacturing technique. J. Mater. Sci. Technol. 2019, 35, 285–294. [Google Scholar] [CrossRef]
- Zhao, D.; Huang, Y.; Ao, Y.; Han, C.; Wang, Q.; Li, Y.; Liu, J.; Wei, Q.; Zhang, Z. Effect of pore geometry on the fatigue properties and cell affinity of porous titanium scaffolds fabricated by selective laser melting. J. Mech. Behav. Biomed. 2018, 88, 478–487. [Google Scholar] [CrossRef]
- Zhao, S.; Li, S.J.; Wang, S.G.; Hou, W.T.; Li, Y.; Zhang, L.C. Compressive and fatigue behaviour of functionally graded Ti-6Al-4V meshes fabricated by electron beam melting. Acta Mater. 2018, 150, 1–15. [Google Scholar] [CrossRef]
- Yavari, S.A.; Ahmadi, S.M.; Wauthle, R.; Pouran, B.; Schrootene, J.; Weinans, H.; Zadpoor, A.A. Relationship between unit cell type and porosity and the fatigue behavior of selective laser melted meta-biomaterials. J. Mech. Behav. Biomed. 2015, 43, 91–100. [Google Scholar] [CrossRef]
- Li, H.; Oppenheimer, S.M.; Stupp, S.I.; Dunand, D.C.; Brinson, L.C. Effects of Pore Morphology and Bone Ingrowth on Mechanical Properties of Microporous Titanium as an Orthopaedic Implant Material. Mater. Trans. 2004, 45, 1124–1131. [Google Scholar] [CrossRef] [Green Version]
- Shen, H.; Oppenheimer, S.M.; Dunand, D.C.; Brinson, L.C. Numerical modeling of pore size and distribution in foamed titanium. Mech. Mater. 2006, 38, 933–944. [Google Scholar] [CrossRef] [Green Version]
- Shen, H.; Brinson, L.C. Finite element modeling of porous titanium. Int. J. Solids Struct. 2007, 44, 320–335. [Google Scholar] [CrossRef] [Green Version]
- Muñoz, S.; Pavón, J.J.; Rodríguez-Ortiz, J.A.; Civantos, A.; Allain, J.P.; Torres, Y. On the influence of space holder in the development of porous titanium implants: Mechanical, computational and biological evaluation. Mater. Charact. 2015, 108, 68–78. [Google Scholar] [CrossRef]
- Muñoz, S.; Castillo, S.M.; Torres, Y. Different models for simulation of mechanical behaviour of porous materials. J. Mech. Behav. Biomed. Mater. 2018, 80, 88–96. [Google Scholar] [CrossRef] [PubMed]
- Torres, Y.; Pavón, J.J.; Nieto, I.; Rodríguez, J.A. Conventional powder metallurgy process and characterization of porous titanium for biomedical applications. Met. Mater. Trans. B 2011, 42, 891–900. [Google Scholar] [CrossRef]
- Torres, Y.; Pavón, J.J.; Rodríguez, J.A. Processing and characterization of porous titanium for implants by using NaCl as space holder. J. Mater. Process. Technol. 2012, 212, 1061–1069. [Google Scholar] [CrossRef]
- Lascano, S.; Arévalo, C.; Montealegre-Melendez, I.; Muñoz, S.; Rodriguez-Ortiz, J.A.; Trueba, P.; Torres, Y. Porous titanium for biomedical applications: Evaluation of the conventional powder metallurgy frontier and space-holder technique. Appl. Sci. 2019, 9, 982. [Google Scholar] [CrossRef] [Green Version]
- Torres, Y.; Lascano, S.; Bris, J.; Pavón, J.J.; Rodriguez-Ortiz, J.A. Development of porous titanium for biomedical applications: A comparison between loose sintering and space-holder techniques. Mater. Sci. Eng. C 2014, 37, 148–155. [Google Scholar] [CrossRef] [PubMed]
- ASTM, C373-88. Standard Test Method for Water Absorption, Bulk Density, Apparent Porosity, and Apparent Specific Gravity of Fired Whiteware Products; AST International: West Conshohocken, PA, USA, 2006. [Google Scholar]
- Kikuchi, M.; Takahashi, M.; Okuno, O. Elastic moduli of cast Ti-Au, Ti-Ag, and Ti- Cu alloys. Dent. Mater. 2006, 22, 641–646. [Google Scholar] [CrossRef]
- Wen, C.E.; Yamada, Y.; Shimojima, K.; Chino, Y.; Asahina, T.; Mabuchi, M. Processing and mechanical properties of autogenous titanium implant materials. Mater. Sci. Mater. Med. 2002, 13, 397–401. [Google Scholar] [CrossRef]
- Dominguez, C.; Beltrán, A.M.; Garvi, M.D.; Salazar-Moya, A.; Lebrato, J.; Hickey, D.J.; Rodriguez-Ortiz, J.A.; Kamm, P.H.; Lebrato, C.; García-Moreno, F. Bacterial behavior on coated porous titanium substrate for biomedical applications. Surf. Coat. Technol. 2019, 357, 896–902. [Google Scholar] [CrossRef]
- Torres, Y.; Rodríguez-Ortiz, J.A.; Arias, S.; Echeverry, M.; Robledo, S.; Amigo, V.; Pavón, J.J. Processing, characterization and biological testing of porous titanium obtained by space-holder technique. J. Mater. Sci. 2012, 47, 6565–6576. [Google Scholar] [CrossRef]
- Civantos, A.; Domínguez, C.; Pino, R.J.; Setti, G.; Pavon, J.J.; Martínez-Campos, E.; Garcia-Garcia, F.J.; Rodriguez-Ortiz, J.A.; Allain, J.P.; Torres, Y. Designing bioactive porous titanium interfaces to balance mechanical properties and in vitro cells behavior towards increased osseointegration. Surf. Coat. Technol. 2019, 368, 162–174. [Google Scholar] [CrossRef]
- Greiner, C.; Oppenheimer, S.M.; Dunand, D.C. High strength, low stiffness, porous NiTi with superelastic properties. Acta Biomater. 2005, 1, 705–716. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, L.F. Elasticity and damping of porous materials and impregnated. J. Am. Ceram. Soc. 1983, 67, 93–98. [Google Scholar] [CrossRef]
- Eudier, M. The mechanical properties of sintered low-alloy steels. Powder Met. 1962, 5, 278–290. [Google Scholar] [CrossRef]
- Hyun, S.; Murakami, K.; Nakajima, H. Anisotropic mechanical properties of porous copper fabricated by unidirectional solidification. Mater. Sci. Eng. A 2001, 299, 241–248. [Google Scholar] [CrossRef]
- Fleck, N.; Smith, R. Effect of Density on Tensile Strength, Fracture Toughness, and Fatigue Crack Propagation Behaviour of Sintered Steel. Powder Met. 1981, 3, 121–125. [Google Scholar] [CrossRef]
- Griffiths, T.J.; Davies, R.; Bassett, M.B. Analytical Study of Effects of Pore Geometry on Tensile Strength of Porous Materials. Powder Met. 1979, 22, 119–123. [Google Scholar] [CrossRef]




| Ts (°C) | Pc (MPa) | Density (g/cm3) | Archimedes’ Method | Images Analysis | |||
|---|---|---|---|---|---|---|---|
| Total Porosity (%) | Interconnected Porosity, θi (%) | θ (%) | Deq (µm) | Fe | |||
| 1000 | 0 (LS) | 2.47 ± 0.23 | 41.4 ± 1.4 | 39.1 ± 1.7 | 43.0 ± 1.2 | 16.9 ± 3.1 | 0.59 ± 0.17 |
| 13 | 3.17 ± 0.02 | 32.2 ± 0.7 | 28.27 ± 0.4 | 30.8 ± 1.6 | 13.8 ± 2.9 | 0.60 ± 0.17 | |
| 38.5 | 3.31 ± 0.03 | 29.4 ± 0.8 | 25.08 ± 0.9 | 29.5 ± 3.3 | 14.9 ± 2.1 | 0.57 ± 0.17 | |
| 1100 | 0 (LS) | 2.79 ± 0.22 | 37.4 ± 1.5 | 36.2 ± 1.5 | 36.9 ± 4.0 | 18.5 ± 3.9 | 0.57 ± 0.17 |
| 13 | 3.37 ± 0.39 | 21.8 ± 0.66 | 22.2 ± 1.9 | 20.7 ± 2.1 | 11.8 ± 2.6 | 0.64 ± 0.18 | |
| 38.5 | 3.65 ± 0.05 | 20.3 ± 0.4 | 14.9 ± 1.1 | 19.9 ± 2.5 | 11.0 ± 2.1 | 0.64 ± 0.18 | |
| 1200 | 0 (LS) | 3.12 ± 0.14 | 30.0 ± 0.6 | 27.9 ± 1.2 | 30.0 ± 6.9 | 15.2 ± 2.4 | 0.62 ± 0.18 |
| 13 | 3.88 ± 0.02 | 13.6 ± 0.4 | 6.2 ± 0.7 | 13.2 ± 1.6 | 10.4 ± 1.5 | 0.70 ± 0.17 | |
| 38.5 | 3.81 ± 0.04 | 17.5 ± 0.6 | 10.1 ± 1.0 | 17.3 ± 2.1 | 12.8 ± 2.9 | 0.70 ± 0.17 | |
| 1300 | 0 (LS) | 3.72 ± 0.07 | 15.1 ± 0.2 | 13.8 ± 1.5 | 14.9 ± 1.8 | 13.7 ± 2.4 | 0.68 ± 0.18 |
| 13 | 3.96 ± 0.08 | 13.4 ± 0.3 | 5.6 ± 0.9 | 12.8 ± 0.6 | 13.4 ± 2.8 | 0.75 ± 0.17 | |
| 38.5 | 3.99 ± 0.04 | 11.8 ± 0.3 | 5.4 ± 0.5 | 12.2 ± 1.3 | 13.0 ± 2.6 | 0.75 ± 0.17 | |
| Ts (°C) | Pc (MPa) | Ultrasound Test | Compression Test | |
|---|---|---|---|---|
| Ed (GPa) | Ec (GPa) | σy (MPa) | ||
| 1000 | 0 (LS) | 29.3 ± 2.2 | 13.4 ± 4.1 | 123 ± 10 |
| 13 | 49.5 ± 0.8 | 40.8 ± 3.6 | 342 ± 8 | |
| 38.5 | 54.6 ± 0.3 | 53.6 ± 5.2 | 358 ± 7 | |
| 1100 | 0 (LS) | 34.9 ± 0.9 | 27.9 ± 3.2 | 189 ± 11 |
| 13 | 61.2 ± 7.0 | 42.3 ± 4.4 | 477 ± 12 | |
| 38.5 | 63.8 ± 3.8 | 34.5 ± 2.8 | 452 ± 9 | |
| 1200 | 0 (LS) | 46.4 ± 5.8 | 40.2 ± 3.7 | 422 ± 8 |
| 13 | 76.3 ± 2.7 | 46.4 ± 3.2 | 544 ± 8 | |
| 38.5 | 72.1 ± 0.3 | 55.2 ± 4.2 | 567 ± 10 | |
| 1300 | 0 (LS) | 69.9 ± 2.2 | 33.2 ± 3.2 | 467 ± 8 |
| 13 | 81.3 ± 6.2 | 56.2 ± 5.2 | 544 ± 7 | |
| 38.5 | 80.7 ± 0.7 | 68.3 ± 5.4 | 567 ± 8 | |
| Ts (°C) | Pc (MPa) | E (GPa) | σy (MPa) |
|---|---|---|---|
| 1000 | 0 (LS) | 29.9 ± 6.1 | 124.4 ± 31.5 |
| 13 | 44.3 ± 5.5 | 240.2 ± 56.0 | |
| 38.5 | 49.8 ± 4.3 | 275.1 ± 42.2 | |
| 1100 | 0 (LS) | 36.6 ± 6.0 | 201.3 ± 28.4 |
| 13 | 67.3 ± 3.7 | 434.4 ± 39.5 | |
| 38.5 | 70.3 ± 3.6 | 481.7 ± 49.1 | |
| 1200 | 0 (LS) | 49.9 ± 4.1 | 271.1 ± 38.9 |
| 13 | 84.9 ± 2.2 | 609.2 ± 21.4 | |
| 38.5 | 76.9 ± 2.6 | 526.5 ± 26.0 | |
| 1300 | 0 (LS) | 82.2 ± 1.4 | 575.8 ± 14.5 |
| 13 | 86.2 ± 1.6 | 615.1 ± 16.5 | |
| 38.5 | 89.2 ± 1.4 | 636.3 ± 14.0 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castillo, S.M.; Muñoz, S.; Trueba, P.; Díaz, E.; Torres, Y. Influence of the Compaction Pressure and Sintering Temperature on the Mechanical Properties of Porous Titanium for Biomedical Applications. Metals 2019, 9, 1249. https://doi.org/10.3390/met9121249
Castillo SM, Muñoz S, Trueba P, Díaz E, Torres Y. Influence of the Compaction Pressure and Sintering Temperature on the Mechanical Properties of Porous Titanium for Biomedical Applications. Metals. 2019; 9(12):1249. https://doi.org/10.3390/met9121249
Chicago/Turabian StyleCastillo, Sandra M., Sergio Muñoz, Paloma Trueba, Eduardo Díaz, and Yadir Torres. 2019. "Influence of the Compaction Pressure and Sintering Temperature on the Mechanical Properties of Porous Titanium for Biomedical Applications" Metals 9, no. 12: 1249. https://doi.org/10.3390/met9121249





