Corrosion Behavior of Fe-Ni-Al Alloy Inert Anode in Cryolite Melts
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Anodes
2.2. Galvanostatic Electrolysis
2.3. Electrochemistry Tests
3. Results and Discussion
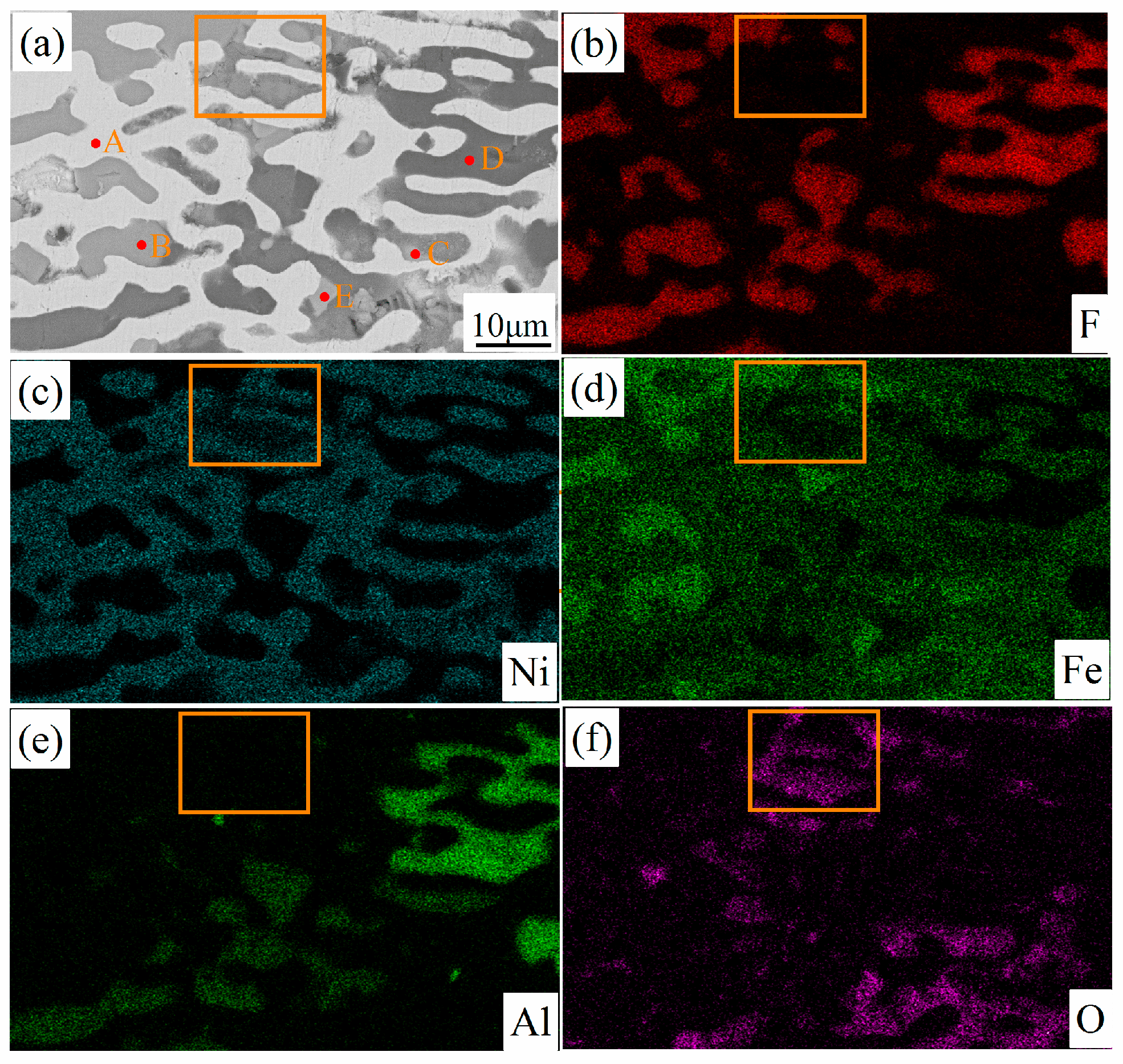
3.1. Material Characterization of Fe-Ni-Al Inert Anodes
3.2. Corrosion Behavior of Fe-Ni-Al Inert Anodes in Al Electrolysis Process
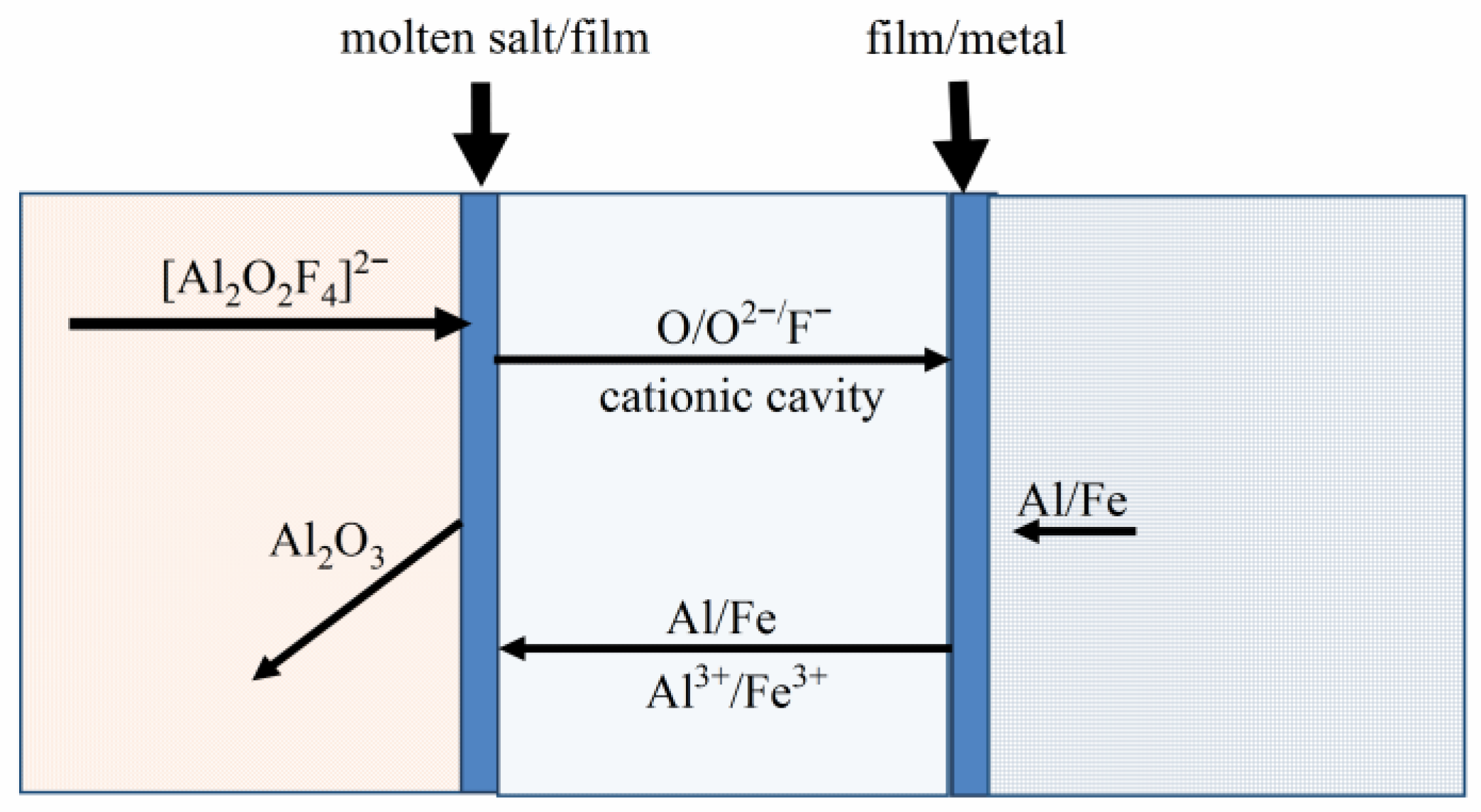
3.3. Corrosion Mechanism of the Fe-Ni-Al Inert Anode
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Marks, J. Methods for calculating PFC emissions from primary aluminum production. Light Met. 2006, 2006, 185–188. [Google Scholar]
- Welch, B.J.; Hyland, M.M.; James, B.J. Future materials requirements for the high-energy-intensity production of aluminum. JOM 2001, 53, 13–18. [Google Scholar] [CrossRef]
- Padamata, S.K.; Yasinskiy, A.S.; Polyakov, P.V. Progress of inert anodes in aluminum industry: Review. J. Siberian Fed. Univ. Chem. 2018, 1, 18–30. [Google Scholar]
- Shi, Z.; Junli, J.; Gao, B.; Hu, X.; Wang, Z. Aluminum electrolysis with Fe-Ni-Al2O3 inert anodes at 850 °C. High Temp. Mater. Proc. 2011, 30, 247–251. [Google Scholar] [CrossRef]
- Sadoway, D.R. Inert anodes for the Hall-Heroult cell: The ultimate materials challenge. JOM 2011, 53, 34–35. [Google Scholar] [CrossRef]
- Kovrov, V.; Khramov, A.; Redkin, A.A.; Zaikov, Y. Oxygen evolving anodes for aluminum electrolysis. ECS Trans. 2009, 16, 7–17. [Google Scholar]
- Chapman, V.; Welch, B.J.; Skyllas-Kazacos, M. Anodic behavior of oxidised Ni-Fe alloys in cryolite-alumina melts. Electrochim. Acta 2011, 56, 1227–1238. [Google Scholar] [CrossRef]
- Chapman, V.; Welch, B.J.; Skyllas-Kazacos, M. High temperature oxidation behavior of Ni-Fe-Co anodes for aluminum electrolysis. Corros. Sci. 2011, 53, 2815–2825. [Google Scholar] [CrossRef]
- Gallino, I.; Kassner, M.E.; Busch, R. Oxidation and corrosion of highly alloyed Cu-Fe-Ni as inert anode material for aluminum electrowinning in as-cast and homogenized conditions. Corros. Sci. 2012, 63, 293–303. [Google Scholar] [CrossRef]
- Sekhar, J.A.; Deng, H.; Liu, J.; Sum, E.; Duruz, J.J.; De, N.V. Micropyretically synthesized porous non-consumable anodes in the Ni-Al-Cu-Fe-X system. Light Met. 1997, 347–354. [Google Scholar]
- Sekhar, J.A.; Liu, J.; Deng, H.; Duruz, J.J.; De, N.V. Graded non-consumable anode materials. Light Met. 1998, 597–604. [Google Scholar]
- Kargin, Y.F.; Samoilov, E.N.; Makarenkov, V.I.; Lysenkov, A.S. Metal-ceramic composites based on iron oxide for low-consumption anode during electrolytic extraction of aluminum. Inorg. Mater. Appl. Res. 2018, 9, 52–56. [Google Scholar] [CrossRef]
- Meyer, P.; Gibilaro, M.; Massot, L.; Pasquet, I.; Tailhades, P.; Bouvet, S.; Chamelot, P. Comparative study on the chemical stability of Fe3O4 and NiFe2O4 in molten salts. Mater. Sci. Eng. B 2018, 228, 117–122. [Google Scholar] [CrossRef]
- Shi, Z.; Xu, J.; Qiu, Z.; Wang, Z.; Gao, B. Copper-nickel superalloys as inert alloy anodes for aluminum electrolysis. JOM 2003, 55, 63–65. [Google Scholar] [CrossRef]
- Cassayre, L.; Chamelot, P.; Aruraul, L.; Massot, L.; Palau, P.; Taxil, P. Electrochemical oxidation of binary copper-nickel alloys in cryolite melts. Corros. Sci. 2007, 49, 3610–3625. [Google Scholar] [CrossRef]
- Sadoway, D.R. A materials systems approach to selection and testing of nonconsumable anodes for the Hall cell. Light Met. 1990, 403–407. [Google Scholar]
- Goupil, G.; Helle, S.; Davis, B.; Guay, D.; Roué, L. Anodic behavior of mechanically alloyed Cu-Ni-Fe and Cu-Ni-Fe-O electrodes for aluminum electrolysis in low-temperature KF-AlF3 electrolyte. Electrochim. Acta 2013, 112, 176–182. [Google Scholar] [CrossRef]
- Helle, S.; Pedron, M.; Assouli, B.; Davis, B.; Guay, D.; Roué, L. Structure and high-temperature oxidation behavior of Cu-Ni-Fe alloys prepared by high-energy ball milling for application as inert anodes in aluminum electrolysis. Corros. Sci. 2010, 52, 3348–3355. [Google Scholar] [CrossRef]
- Helle, S.; Tresse, M.; Davis, B.; Guay, D.; Roué, L. Mechanically alloyed Cu-Ni-Fe-O based materials as oxygen-evolving anodes for aluminum electrolysis. J. Electrochem. Soc. 2012, 159, E62–E68. [Google Scholar] [CrossRef]
- Helle, S.; Davis, B.; Guay, D.; Roué, L. Ball-Milled Cu-Ni-Fe-O materials as inert anodes for aluminum electrolysis in low-temperature KF-AlF3 electrolyte. Light Met. 2012, 1377–1380. [Google Scholar]
- Muñoz-Morris, M.; Morris, D. Microstructure and mechanical behavior of a Fe-Ni-Al alloy. Mater. Sci. Eng. A 2007, 444, 236–241. [Google Scholar] [CrossRef]
- Shi, Z.; Shi, D.; Gao, B.; Xu, J.; Hu, X.; Wang, Z. Oxidation of Fe-Ni alloys in air at 700 °C, 800 °C and 950 °C. High Temp. Mater. Process. 2012, 31, 89–96. [Google Scholar] [CrossRef]
- Alzamani, M.; Jafarzadeh, K. The effect of pre-oxidation treatment on corrosion behavior of Ni-Cu-Fe-Al anode in molten CaCl2 salt. Oxid. Met. 2018, 89, 623–640. [Google Scholar] [CrossRef]
- Cheng, X.; Yin, H.; Wang, D. Rearrangement of oxide scale on Ni-11Fe-10Cu alloy under anodic polarization in molten Na2CO3-K2CO3. Corros. Sci. 2018, 141, 168–174. [Google Scholar] [CrossRef]
- Swartzendruber, L.J.; Itkin, V.P.; Alcock, C.B. Binary Alloy Phase Diagrams; ASM International: Metals Park, OH, USA, 1991. [Google Scholar]
- Dewing, E.; Thonstad, J. Activities in the system cryolite-alumina. Metall. Mater. Trans. B 1997, 28, 1089–1093. [Google Scholar] [CrossRef]







| Anode Samples | Elemental Mass Fraction/wt.% | ||
|---|---|---|---|
| Al | Fe | Ni | |
| 58.2Fe-40.4Ni-1.4Al | 99.68 | 0.28 | 0.04 |
| 57.9Fe-38.2Ni-3.9Al | 98.87 | 1.06 | 0.07 |
| 56.1Fe-39.5Ni-4.4Al | 99.62 | 0.36 | 0.02 |
| 56.8Fe-37.9Ni-5.3Al | 99.61 | 0.34 | 0.05 |
| 52.9Fe-39.2Ni-7.9Al | 98.14 | 1.53 | 0.33 |
| 54.1Fe-37.3Ni-8.6Al | 99.60 | 0.38 | 0.02 |
| Spectral Position | Element Atomic Percentage/Mass Percentage | ||||
|---|---|---|---|---|---|
| Fe | Ni | Al | O | F | |
| A | 21.5/21.3 | 74.0/77.2 | 0.3/0.1 | 1.8/0.5 | 2.2/0.7 |
| B | 36.9/62.2 | 1.3/2.3 | 0.4/0.3 | - | 61.3/35.1 |
| C | 40.6/66.9 | 2.3/4.0 | 6.5/5.1 | 50.5/23.8 | - |
| D | 0.3/0.9 | 0.5/1.5 | 27.6/34.6 | 0.4/0.3 | 71.0/62.6 |
| E | 40.5/67.0 | 1.7/2.9 | 7.5/6.0 | 49.0/23.2 | 1.1/0.6 |
| No. | Alloy Anodes | jp (A/cm2) | Ep (V) | Eb (V) |
|---|---|---|---|---|
| (a) | 58.2Fe-40.4Ni-1.4Al | 0.29 | 1.90 | 2.52 |
| (b) | 57.9Fe-38.2Ni-3.9Al | 0.29 | 1.83 | 2.42 |
| (c) | 56.1Fe-39.5Ni-4.4Al | 0.73 | 2.50 | 2.85 |
| (d) | 56.8Fe-37.9Ni-5.3Al | 0.36 | 2.17 | 2.57 |
| (e) | 52.9Fe-39.2Ni-7.9Al | 0.35 | 2.12 | 2.54 |
| (f) | 54.1Fe-37.3Ni-8.6Al | 0.59 | 2.76 | 3.13 |
| No. | Alloy Anodes | Ecorr (V) | Icorr (A/cm2) × 10−3 |
|---|---|---|---|
| (a) | 58.2Fe-40.4Ni-1.4Al | 1.18 | 3.58 |
| (b) | 57.9Fe-38.2Ni-3.9Al | 1.20 | 5.18 |
| (c) | 56.1Fe-39.5Ni-4.4Al | 1.03 | 2.65 |
| (d) | 56.8Fe-37.9Ni-5.3Al | 1.18 | 1.73 |
| (e) | 52.9Fe-39.2Ni-7.9Al | 1.00 | 1.82 |
| (f) | 54.1Fe-37.3Ni-8.6Al | 0.94 | 2.00 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guan, P.; Liu, A.; Shi, Z.; Hu, X.; Wang, Z. Corrosion Behavior of Fe-Ni-Al Alloy Inert Anode in Cryolite Melts. Metals 2019, 9, 399. https://doi.org/10.3390/met9040399
Guan P, Liu A, Shi Z, Hu X, Wang Z. Corrosion Behavior of Fe-Ni-Al Alloy Inert Anode in Cryolite Melts. Metals. 2019; 9(4):399. https://doi.org/10.3390/met9040399
Chicago/Turabian StyleGuan, Pingping, Aimin Liu, Zhongning Shi, Xianwei Hu, and Zhaowen Wang. 2019. "Corrosion Behavior of Fe-Ni-Al Alloy Inert Anode in Cryolite Melts" Metals 9, no. 4: 399. https://doi.org/10.3390/met9040399
APA StyleGuan, P., Liu, A., Shi, Z., Hu, X., & Wang, Z. (2019). Corrosion Behavior of Fe-Ni-Al Alloy Inert Anode in Cryolite Melts. Metals, 9(4), 399. https://doi.org/10.3390/met9040399





