Study of the Deposition Formation Mechanism in the Heat Exchanger System of RHF
Abstract
:1. Introduction
2. Experimental Procedure
3. Results and Discussion
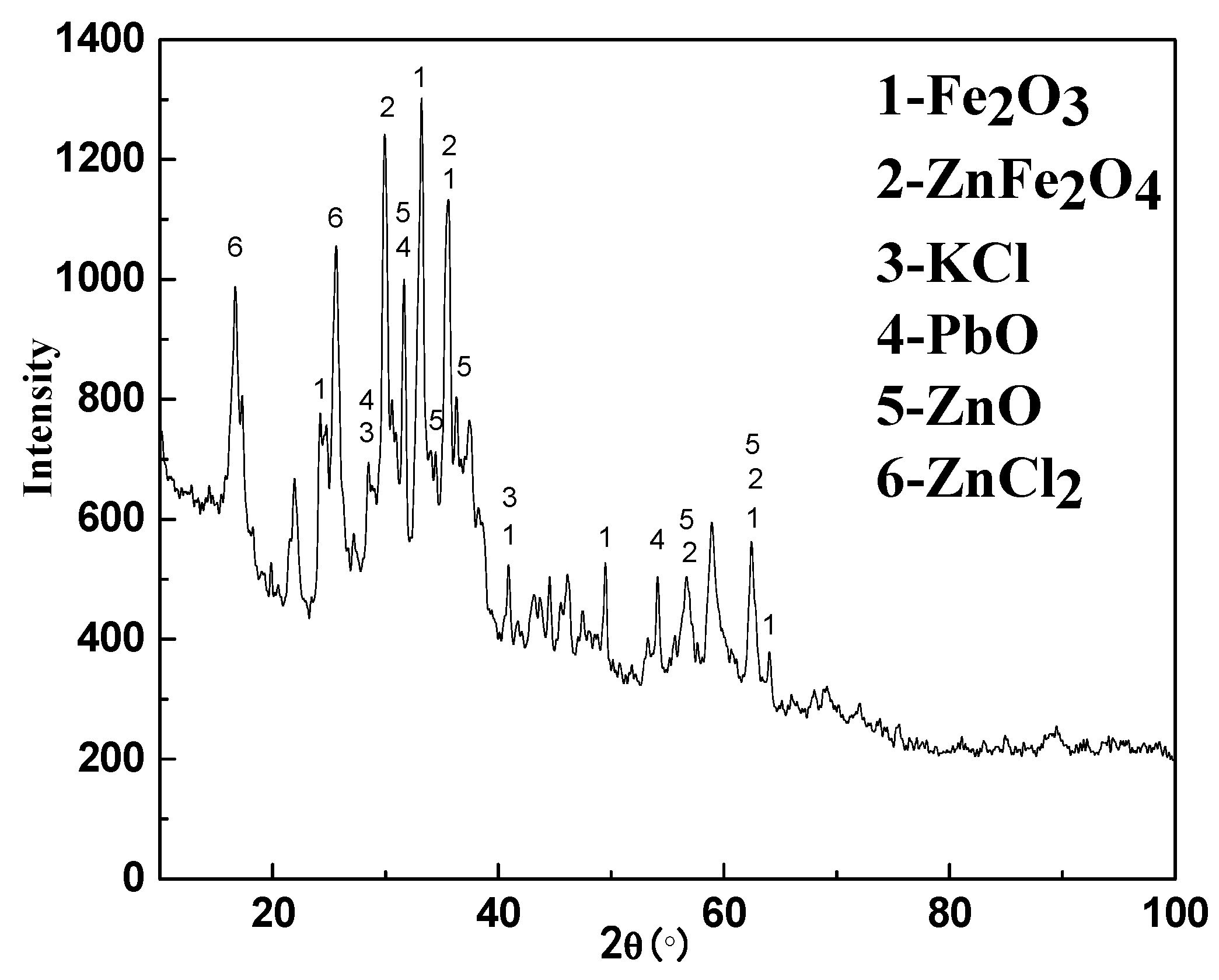
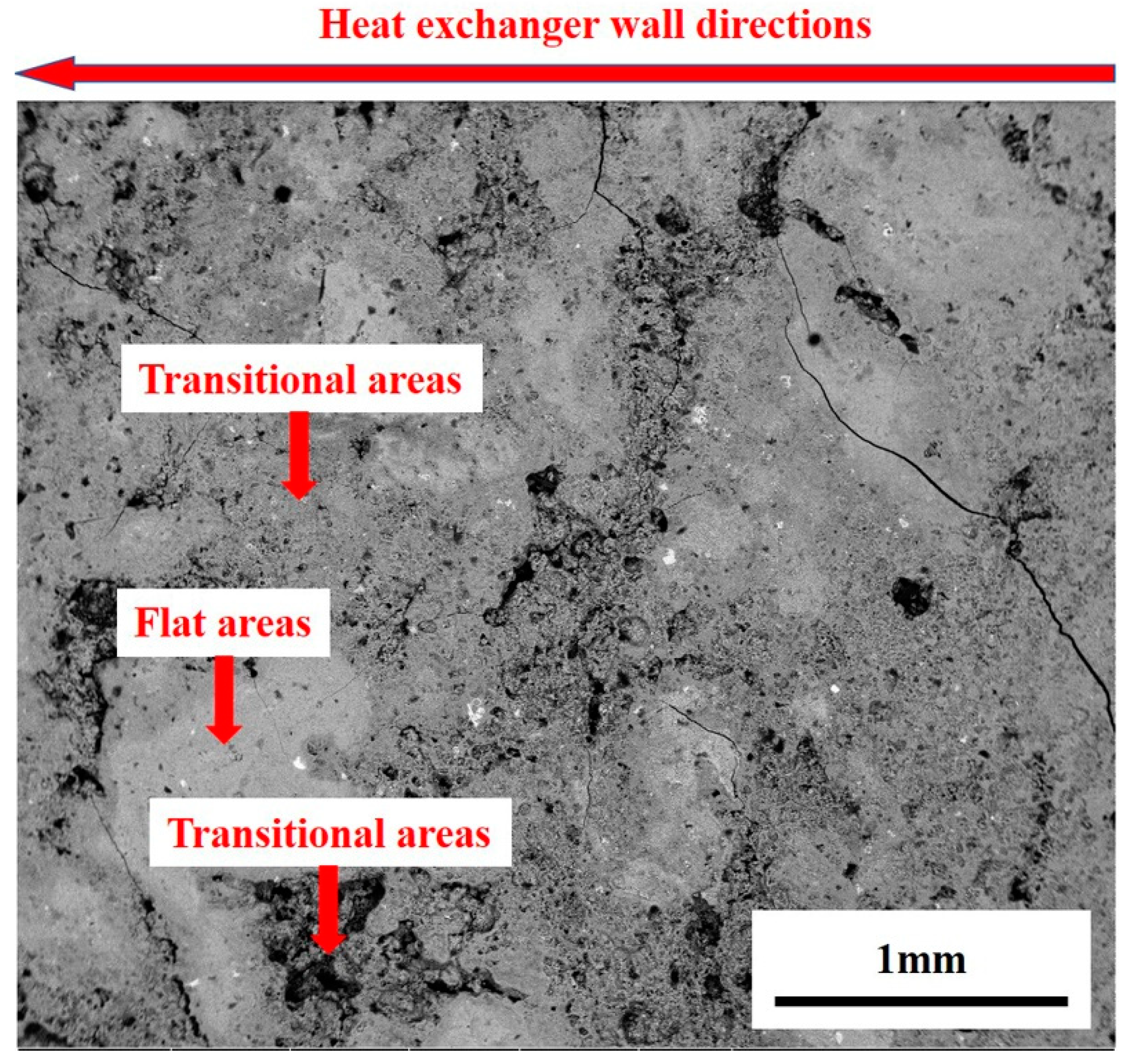
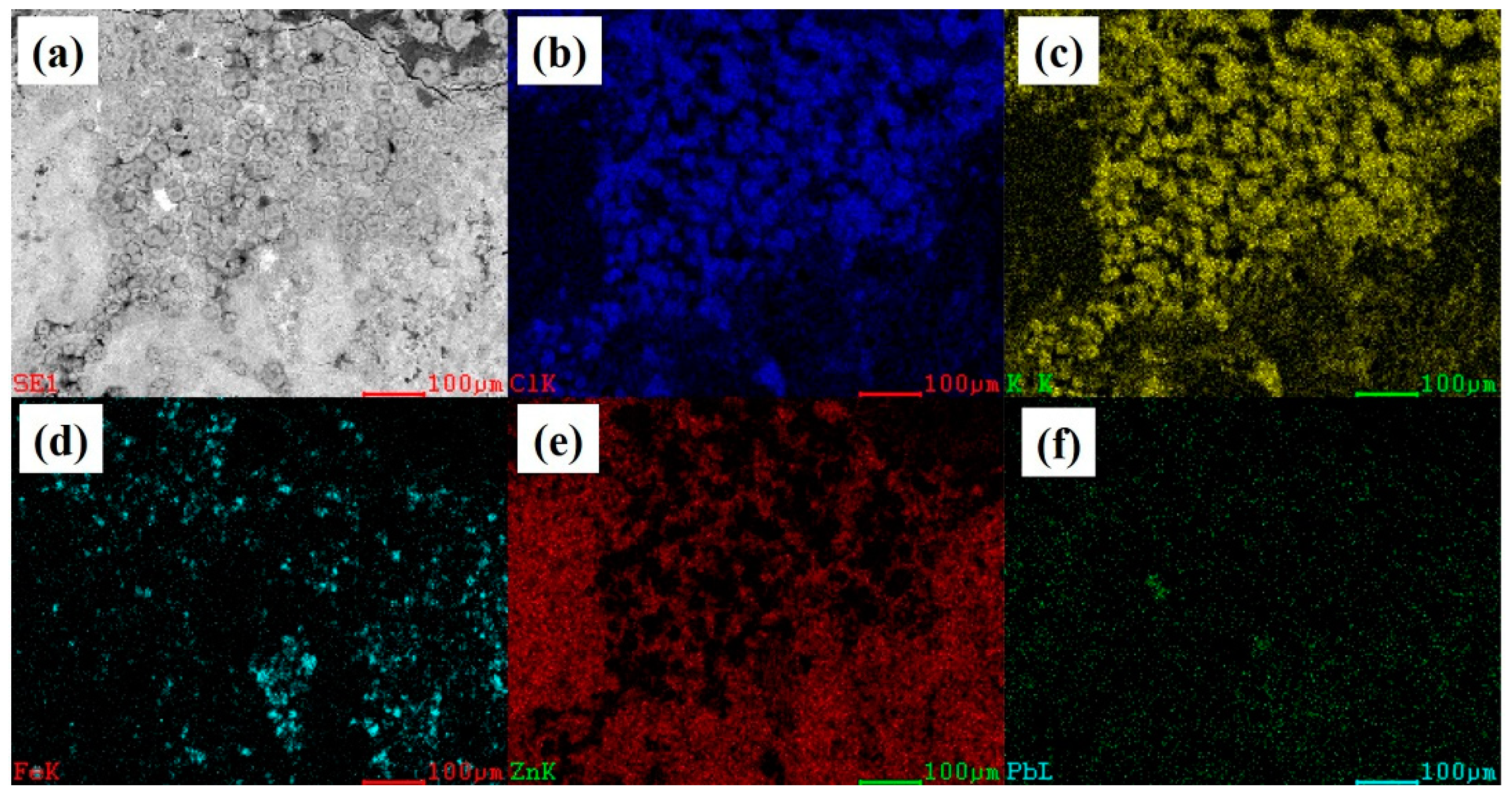
3.1. Analysis Results of Deposition
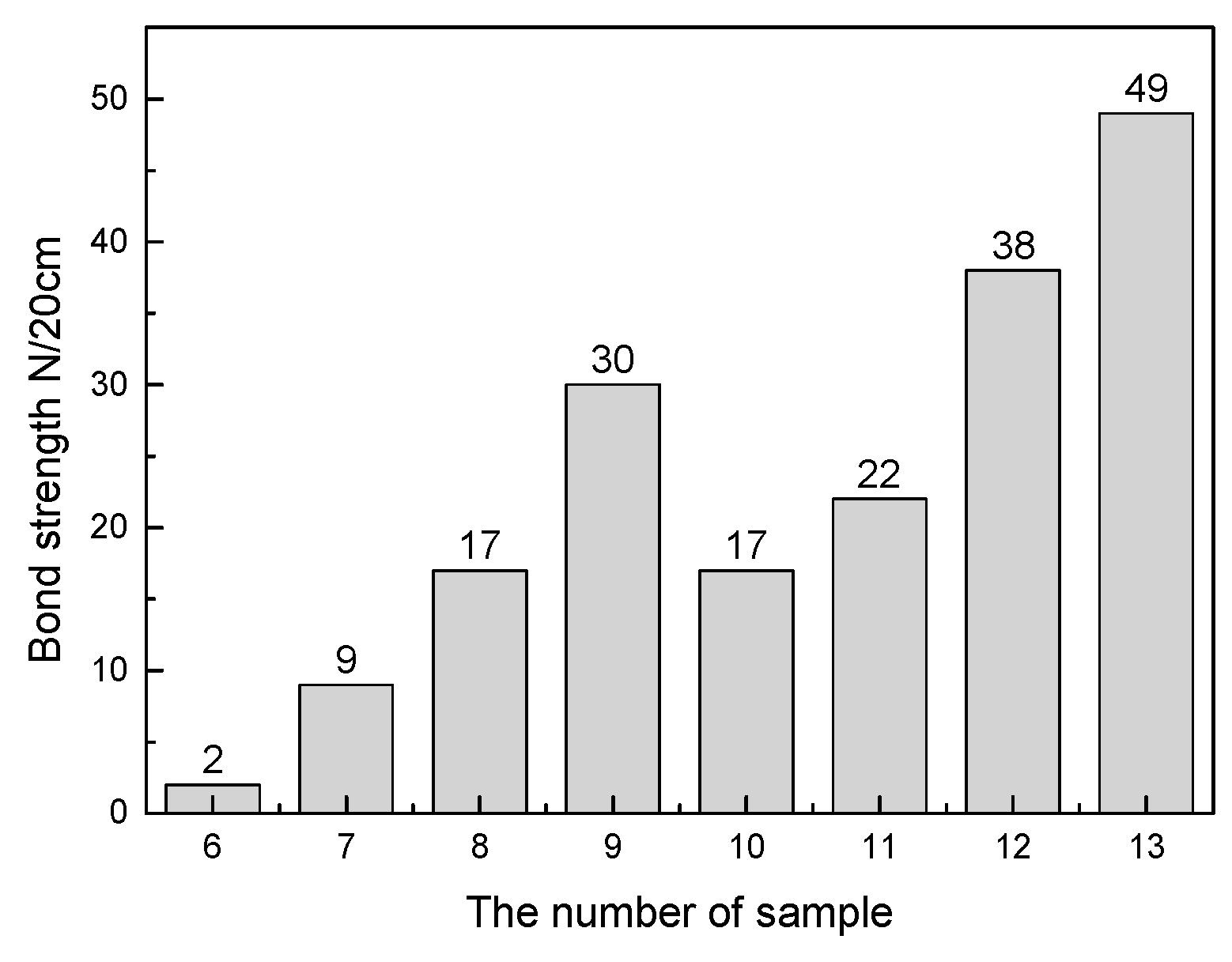
3.2. Experimental Results and Discussion
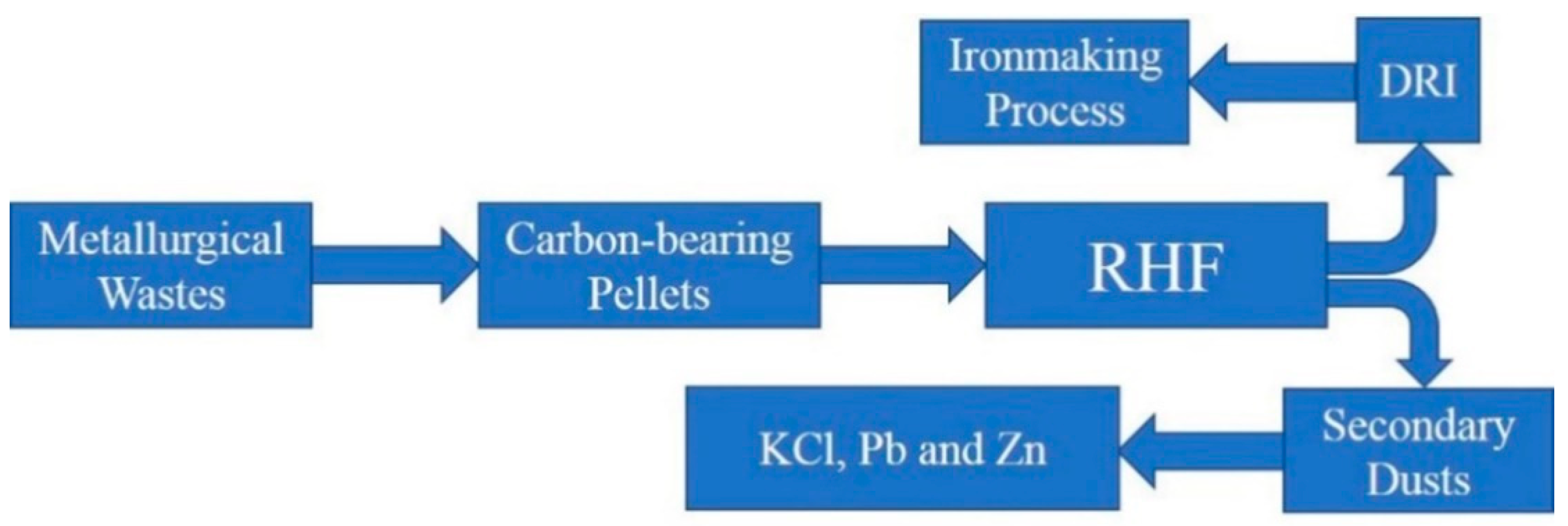
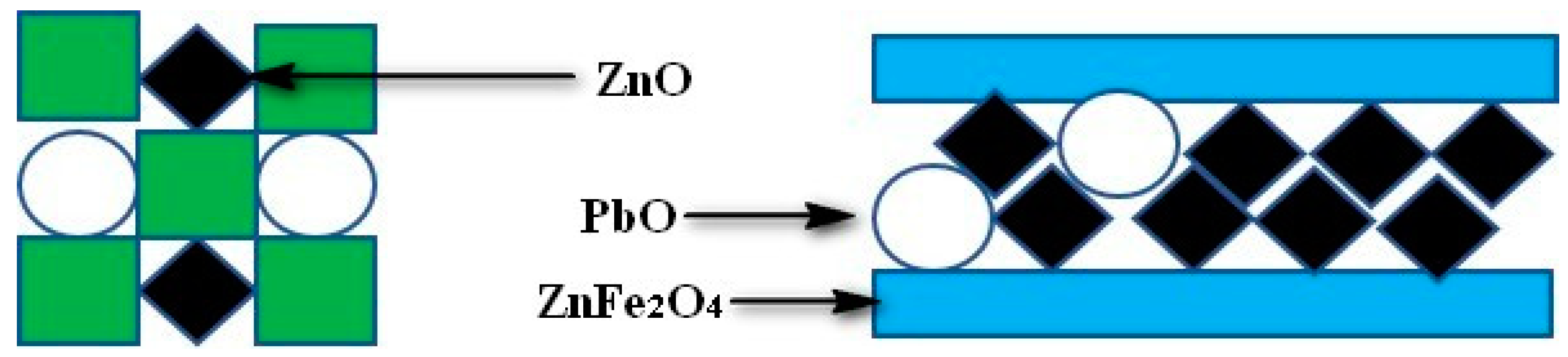
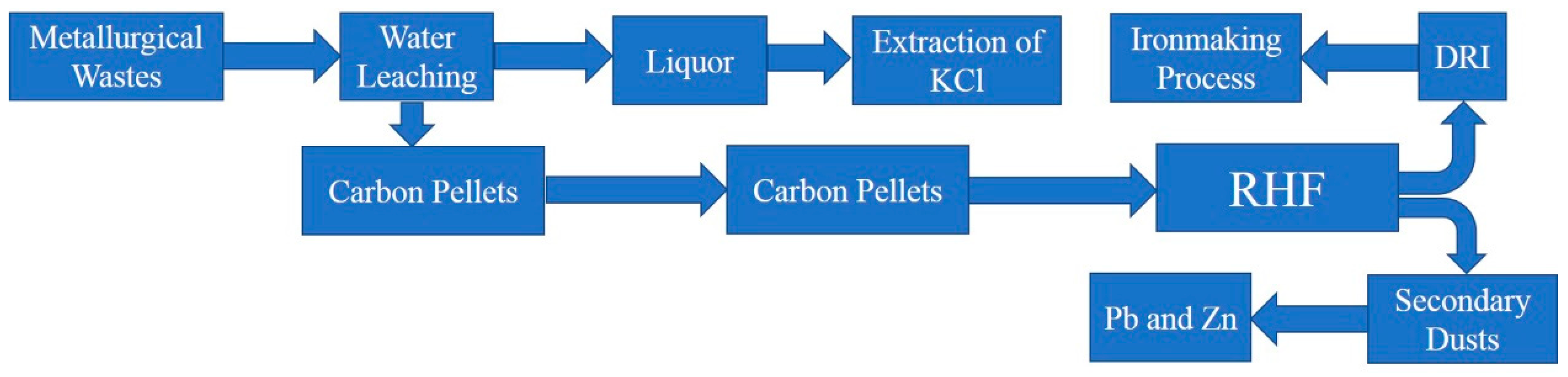
3.3. Discussion on Formation Mechanism and Prevention of Deposition
4. Conclusions
- (1)
- The main cohered phase of the deposition was KCl. The other solid particles were fixed by KCl through the solid-liquid transition of KCl.
- (2)
- The secondary cohered phase of the deposition was ZnFe2O4, forming a porous structure, while the other solid particles were fixed in the porous structure.
- (3)
- The bond strength of ZnFe2O4 was better compared to KCl. Under the combined action of KCl and ZnFe2O4, the deposition could stably occur on the heat exchanger wall.
- (4)
- A new RHF process was proposed to avoid the generation of sediments and to maximize the use of waste from the metallurgical process.
Author Contributions
Funding
Conflicts of Interest
References
- She, X.F.; Wang, J.S.; Han, Y.H.; Zhang, X.X.; Xue, Q.G. Comprehensive mathematical model of direct reduction for rotary hearth furnaces. J. Univ. Sci. Tech. Beijing 2013, 35, 1580–1587. [Google Scholar]
- Peng, C.; Zhang, F.L.; Li, H.F.; Guo, Z.H. Removal behavior of Zn, Pb, K and Na from cold bonded briquettes of metallurgical dust in simulated RHF. ISIJ Int. 2009, 49, 1874–1881. [Google Scholar] [CrossRef]
- Lee, Y.S.; Ri, D.W.; Yi, S.H.; Sohn, I. Relationship between the reduction degree and strength of DRI pellets produced from iron and carbon bearing wastes using an RHF simulator. ISIJ Int. 2012, 52, 1454–1462. [Google Scholar] [CrossRef]
- Han, H.L.; Duan, D.P.; Yuan, P. Binders and bonding mechanism for RHF briquette made from blast furnace dust. ISIJ Int. 2014, 54, 1781–1789. [Google Scholar] [CrossRef]
- Kawaguchi, T.; Kashiwaya, Y. Preface to the special issue on “recycling of wastes and environmental problems”. ISIJ Int. 2000, 40, 211. [Google Scholar] [CrossRef]
- Rutherford, S.D.; Kopfle, J.T. Mesabi Nugget: The world’s first commercial ITmk3® plant. Iron Steel Tech. 2010, 7, 38–43. [Google Scholar]
- She, X.F.; Wang, J.S.; Liu, J.Z.; Zhang, X.X.; Xue, Q.G. Increasing the mixing rate of metalized pellets in blast furnace based on the high-temperature interactivity of iron bearing materials. ISIJ Int. 2014, 54, 2728–2736. [Google Scholar] [CrossRef]
- Fu, J.X.; Zhang, C.; Hwang, W.S.; Liau, Y.T.; Lin, Y.T. Exploration of biomass char for CO2 reduction in RHF process for steel production. Int. J. Greenhouse Gas Control 2012, 8, 143–149. [Google Scholar] [CrossRef]
- Wang, G.; Wang, J.S.; Ding, Y.G.; Ma, S.; Xue, Q.G. New separation method of boron and iron from ludwigite based on carbon bearing pellet reduction and melting technology. ISIJ Int. 2012, 52, 45–51. [Google Scholar] [CrossRef]
- Wang, C.L.; Li, K.Q.; Yang, H.F.; Li, C.H. Probing study on separating Pb, Zn, and Fe from lead slag by coal-based direct reduction. ISIJ Int. 2017, 57, 996–1003. [Google Scholar] [CrossRef]
- Fan, D.C.; Ni, W.; Yan, A.Y.; Wang, J.Y.; Cui, W.H. Orthogonal experiments on direct reduction of carbon-bearing pellets of bayer red mud. J. Iron Steel Res. Int. 2015, 22, 686–693. [Google Scholar] [CrossRef]
- Chung, S.H.; Kim, K.H.; Sohn, I. DRI from recycled iron bearing wastes for lower carbon in the blast furnace. ISIJ Int. 2015, 55, 1157–1164. [Google Scholar] [CrossRef]
- He, Y.W.; Li, Y.C.; Zhang, H.L.; Li, M.; Xu, Z.J.; Zhong, F.; Yang, F. High temperature corrosion behavior of T91 in AlKAli metal chloride medium. Corros. Prot. 2015, 36, 1021–1026. (In Chinese) [Google Scholar]
- Ma, H.T.; Guo, G.F.; Zhao, J.; Wang, L. High temperature corrosion behavior of pure iron in atmosphere O2 containing small amount of KCl vapor. Corros. Sci. Prot. Tech. 2005, 17, 20–23. [Google Scholar]
- Li, Y.S.; Niu, Y.; Liu, G.; Wu, W.T. Corrosion of pure Fe and 310SS beneath ZnCl2-KCl salt film at 450 °C. Acta Metall. Sin. 2002, 36, 1183–1186. [Google Scholar]
- Zhang, M.; Fu, Z.G.; Wu, B.; Lv, N.; Zeng, L.L.; Yang, Y.Q. Recovery of potassium chloride from sintering EAF dust. Chin. J. Process Eng. 2014, 14, 979–983. [Google Scholar]
- Zhan, G.; Guo, Z.C. Water leaching kinetics and recovery of potassium salt from sintering dust. Trans. Nonferr. Met. Soc. China 2013, 23, 3770–3779. [Google Scholar] [CrossRef]
- Tang, H.H.; Sun, W.; Han, H.S. A novel method for comprehensive utilization of sintering dust. Trans. Nonferr. Met. Soc. China 2015, 25, 4192–4200. [Google Scholar] [CrossRef]
- Pei, B.; Zhan, G.; Chen, P.Z.; Guo, Z.C.; Gao, J.T. Preparation of potassium chloride and spherical calcium carbonate particles from leaching solution of electrostatic precipitator dust of iron ore sintering. Chin. J. Process Eng. 2015, 15, 137–146. [Google Scholar]

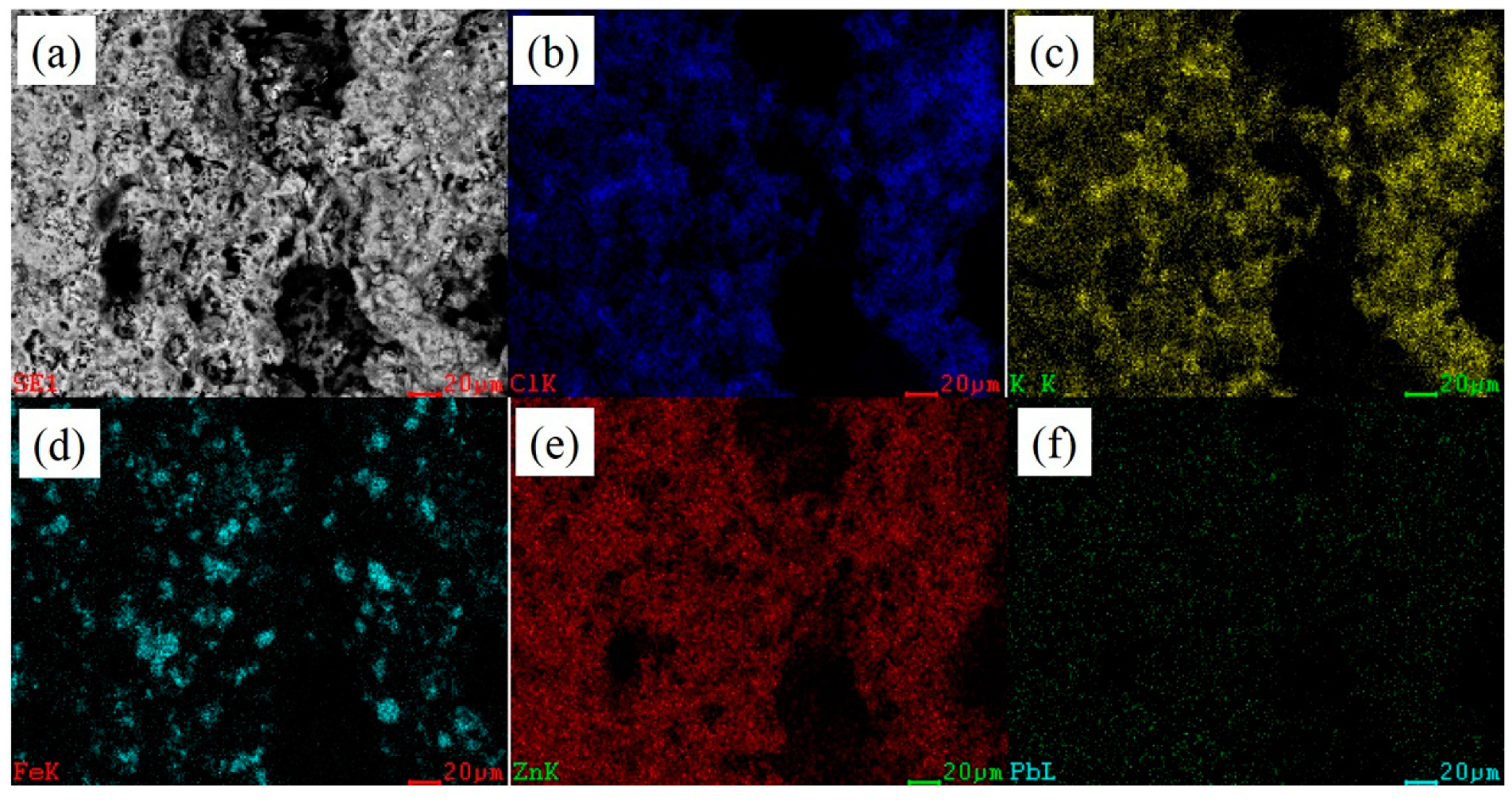
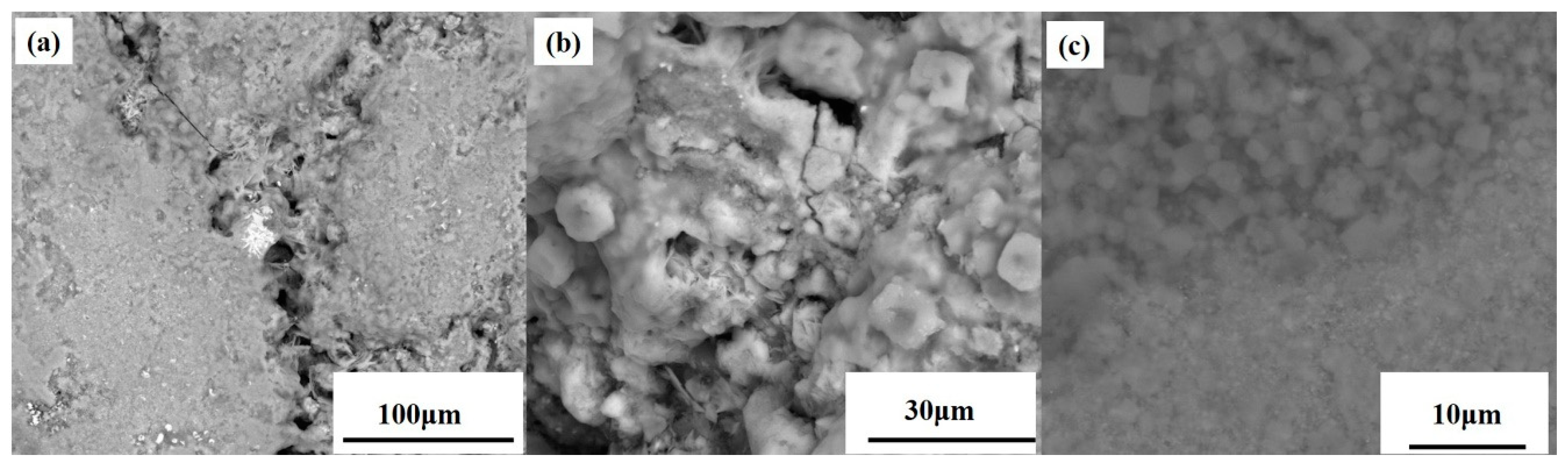
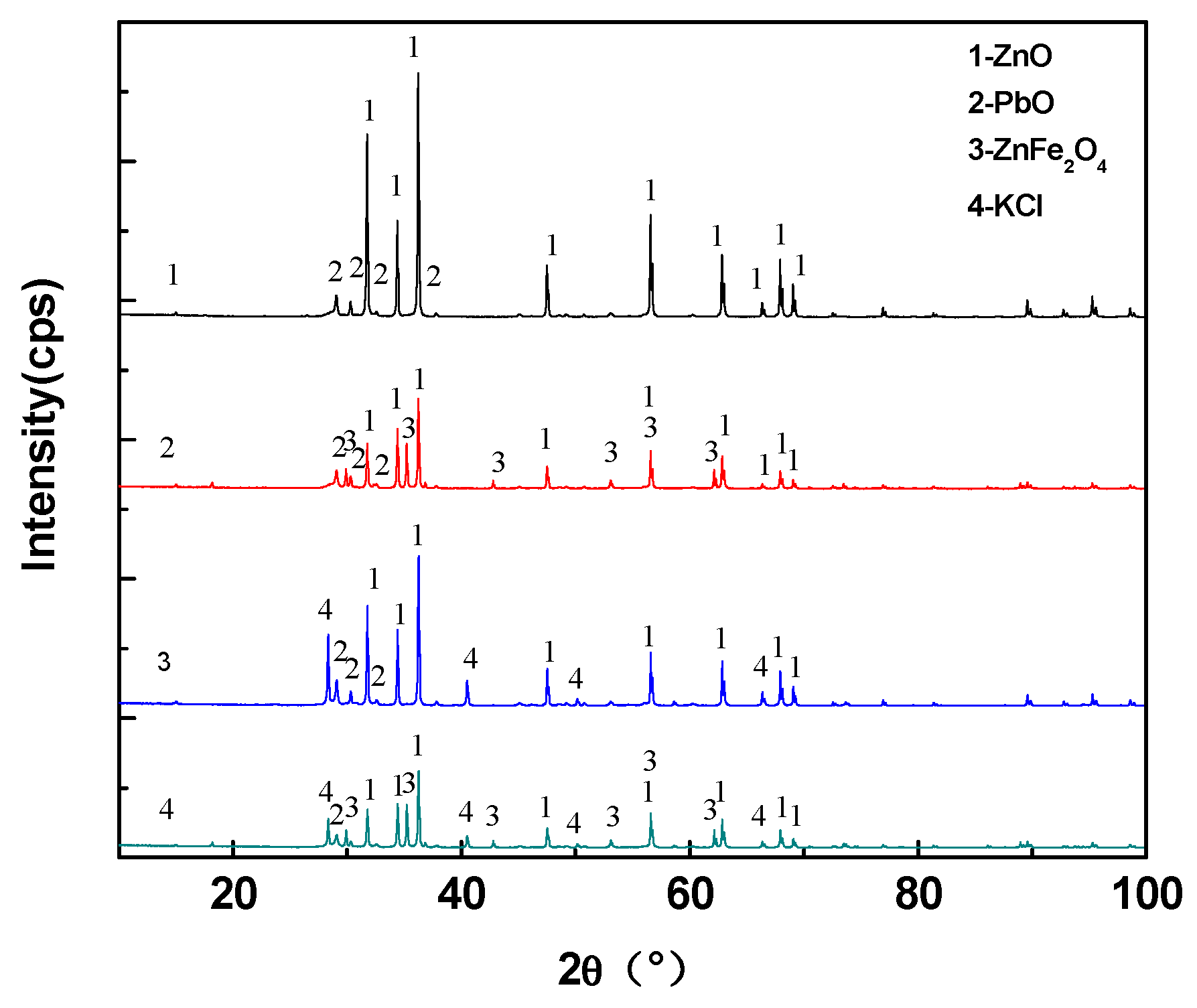
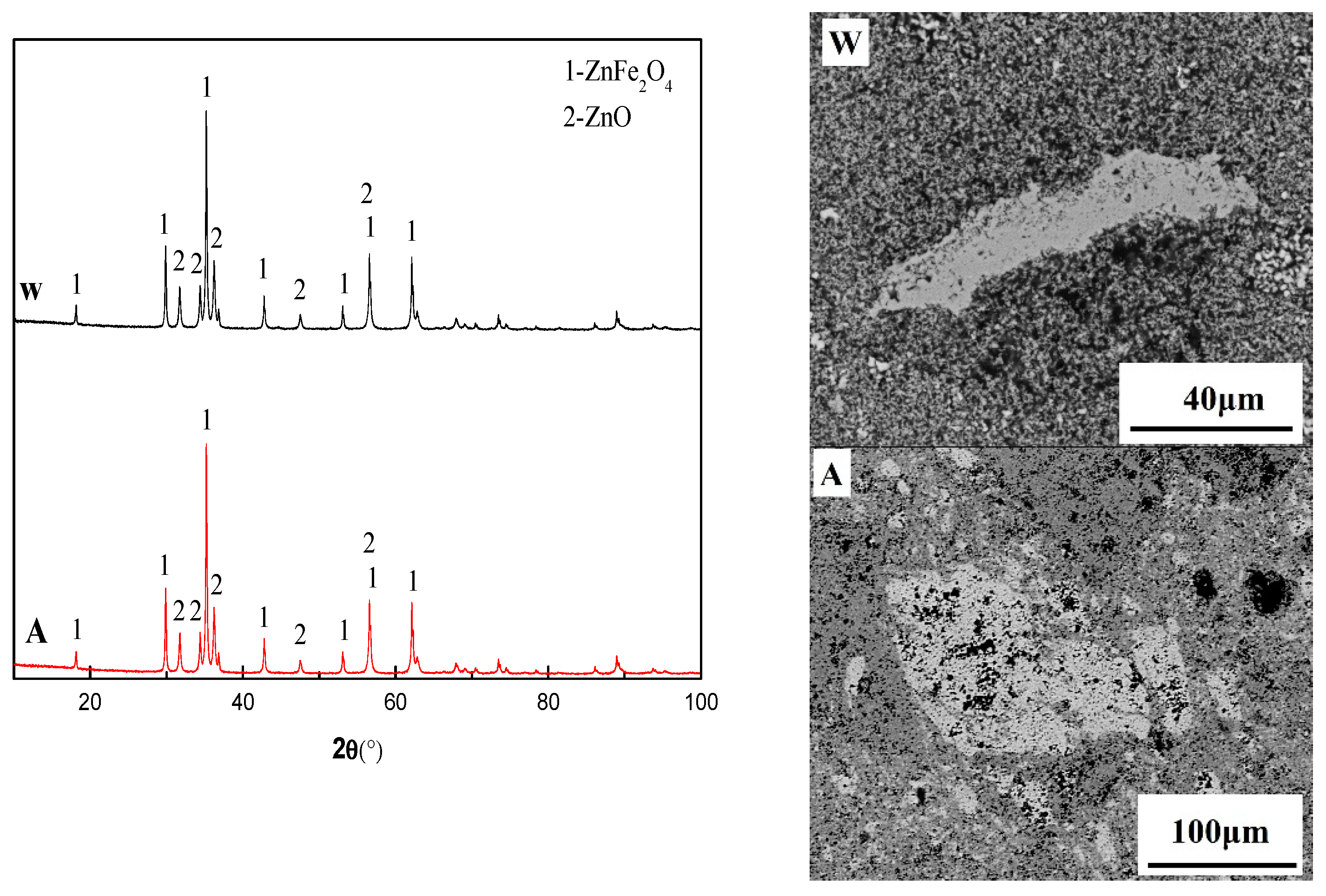

| Chemical Compositions % | CaO | SiO2 | Zn | Pb | K | C | TFe |
|---|---|---|---|---|---|---|---|
| Deposition | 1.62 | 1.08 | 36.5 | 8.4 | 11.57 | 0.66 | 12.84 |
| Experiments | ZnO | PbO | Fe2O3 | KCl |
|---|---|---|---|---|
| 1 | 84.38 | 15.62 | - | - |
| 2 | 62.89 | 11.63 | 25.48 | - |
| 3 | 63.94 | 11.83 | - | 24.22 |
| 4 | 50.81 | 9.4 | 20.53 | 19.26 |
| 5 | 50.31 | - | 49.46 | - |
| 6 | 80.78 | 14.22 | - | 5 |
| 7 | 76.43 | 13.57 | - | 10 |
| 8 | 72.29 | 12.71 | - | 15 |
| 9 | 67.86 | 12.14 | - | 20 |
| 10 | 80.78 | 14.22 | 5 | - |
| 11 | 76.43 | 13.57 | 10 | - |
| 12 | 72.29 | 12.71 | 15 | - |
| 13 | 67.86 | 12.14 | 20 | - |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pan, Y.; She, X.; Wang, J.; Liu, Y. Study of the Deposition Formation Mechanism in the Heat Exchanger System of RHF. Metals 2019, 9, 443. https://doi.org/10.3390/met9040443
Pan Y, She X, Wang J, Liu Y. Study of the Deposition Formation Mechanism in the Heat Exchanger System of RHF. Metals. 2019; 9(4):443. https://doi.org/10.3390/met9040443
Chicago/Turabian StylePan, Yuzhu, Xuefeng She, Jingsong Wang, and Yingli Liu. 2019. "Study of the Deposition Formation Mechanism in the Heat Exchanger System of RHF" Metals 9, no. 4: 443. https://doi.org/10.3390/met9040443





