Metal Oxide Nanoparticle-Based Coating as a Catalyzer for A-TIG Welding: Critical Raw Material Perspective
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. Particle Size Fractions
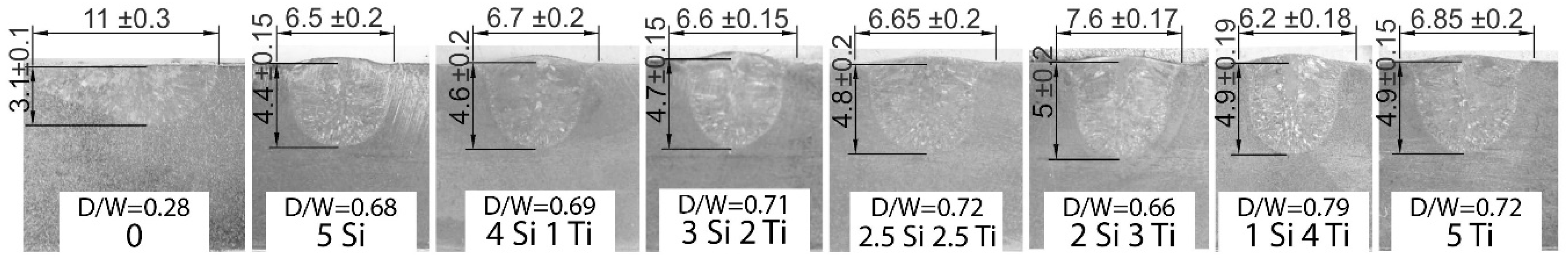
3.2. Macro and Weld Bead Dimensions
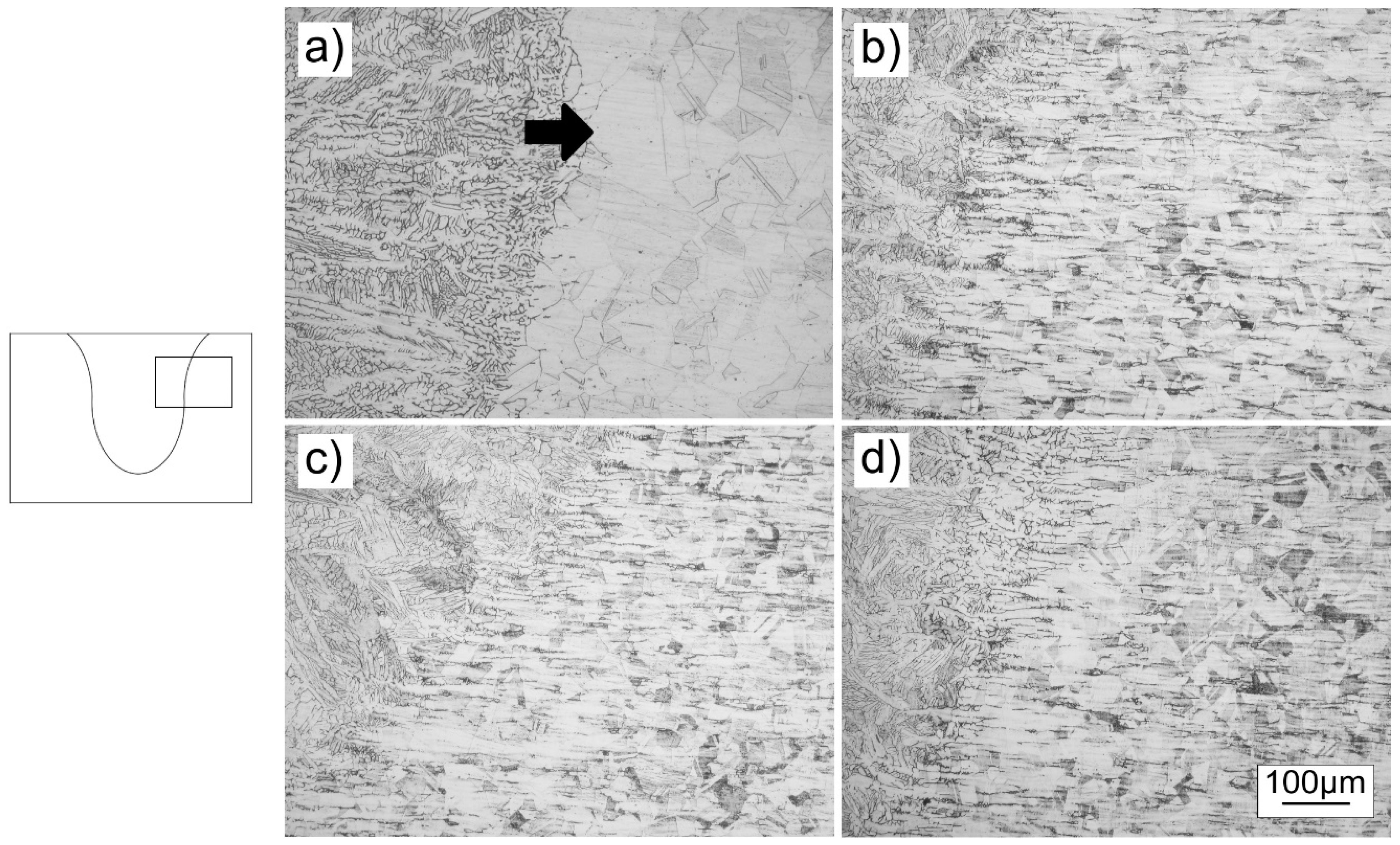
3.3. Microstructures
3.4. Vickers Microhardness Results
4. Discussion
- Improved penetration, along with all accompanying benefits of a lower shielding gas consumption, an increased productivity, a lower environmental impact, and lower costs;
- A lower negative influence on the material’s microstructure and subsequently higher tensile strength, which is proportional to the hardness of the material;
- A completely eliminated consumable material (i.e., welding wire) containing CRMs such as silicon and a near-CRM such as chromium.
5. Conclusions
- Coatings containing metallic oxide nanoparticles offer a higher A-TIG welding penetration compared to the specimen without the coating;
- The added consumable material in TIG results in a higher hardness of the weld metal compared to the specimens that were re-melted—that is, welded without the consumable material (A-TIG);
- The penetration of A-TIG specimens depends primarily on the particle size in the coating solution, not the primary particle size, due to the agglomeration of nanoparticles;
- smaller size of agglomerates in coatings with an increased TiO2 content caused a higher coating effectiveness, leading to an increased penetration that reached a value of 61% over that of the TIG weld without the coating;
- The presence of the reversed Marangoni convection was proved by the recrystallization of certain areas of the base metal near the melt line: near the surface in TIG specimen and under the weld in A-TIG specimens. These results were proved by microhardness measurements, with marked drops in microhardness values in recrystallized areas.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gulicovski, J.J.; Bajat, J.; Miskovic-Stankovic, V.; Jokic, B.; Panic, V.; Milonjic, S. Cerium oxide as conversion coating for the corrosion protection of aluminum. J. Electrochem. Sci. Eng. 2017, 3, 151–156. [Google Scholar] [CrossRef]
- Bieber, J. Trivalent chrome conversion coating for zinc and zinc alloys. Mater. Lett. 2007, 105, 425–435. [Google Scholar]
- Ana-Maria, L.; Wolfgang, P.Y.; Sabrina, M.; Nadine, P.; Diane, S.; Tendero, C.; Vahlas, C. Corrosion protection of 304L stainless steel by chemical vapour deposited alumina coatings. Corros. Sci. 2014, 81, 125–131. [Google Scholar]
- Cellarda, A.; Garniera, V.; Fantozzia, G.; Baretb, G.; Fort, P. Wear resistance of chromium oxide nanostructured coatings. Ceram. Int. 2009, 35, 913–916. [Google Scholar] [CrossRef]
- Hwanga, B.; Leea, S.; Ahn, J. Effect of oxides on wear resistance and surface roughness of ferrous coated layers fabricated by atmospheric plasma spraying. Mat. Sci. Eng. A 2002, 335, 268–280. [Google Scholar] [CrossRef]
- Fomin, A.A.; Rodionov, I.V. Handbook of Nanoceramic and Nanocomposite Coatings and Materials, 1st ed.; Butterworth-Heinemann: Oxford, UK, 2015; pp. 403–424. [Google Scholar]
- Curran, J.A.; Clyne, T.W. The thermal conductivity of plasma electrolytic oxide coatings on aluminium and magnesium. Surf. Coat. Technol. 2005, 199, 177–183. [Google Scholar] [CrossRef]
- Narottam, P.B.; Dongming, Z. Thermal Properties of Oxides with Magnetoplumbite Structure for Advanced Thermal Barrier Coatings. Surf. Coat. Technol. 2008, 202, 2698–2703. [Google Scholar]
- Calnan, S. Applications of Oxide Coatings in Photovoltaic Devices. Coatings 2014, 4, 162–202. [Google Scholar] [CrossRef]
- Sang, Y.J.; Jaesun, S.; Sanghan, L. Photoelectrochemical Device Designs toward Practical Solar Water Splitting: A Review on the Recent Progress of BiVO4 and BiFeO3 Photoanodes. Appl. Sci. 2018, 8, 1388–1404. [Google Scholar]
- Thakur, P.P.; Chapgaon, A.N. A Review on Effects of GTAW Process Parameters on weld. IJRASET 2016, 4, 136–140. [Google Scholar]
- German Development Service-GIZ, Gas Tungsten Arc Welding-GTAW. 2000. Available online: https://www.giz.de/expertise/downloads/Fachexpertise/en-metalwork-gas-tungsten-arc-welding.pdf (accessed on 15 March 2019).
- Cibi, A.J.; Thilagham, K.T. High Frequency Gas Tungsten Arc Welding Process for Dressing of Weldment. IJAERS 2017, 4, 229–235. [Google Scholar]
- Robert, W.; Messler, J. Principles of Welding: Processes, Physics, Chemistry, and Metallurgy; Wiley-VCH: Weinheim, Germany, 2004; p. 55. [Google Scholar]
- Ramkumar, K.D.; Goutham, P.S.; Radhakrishna, V.S.; Tiwari, A.; Anirudh, S. Studies on the structure–property relationships and corrosion behaviour of the activated flux TIG welding of UNS S32750J. Manuf. Process. 2016, 23, 231–241. [Google Scholar] [CrossRef]
- Yangchuan, C.; Zhen, L.; Zunyue, H.; Yida, Z. Effect of cerium oxide flux on active flux TIG welding of 800 MPa super steel. J. Mater. Process. Technol. 2016, 230, 80–87. [Google Scholar]
- Vora, J.J.; Badheka, J.B. Experimental investigation on mechanism and weld morphology ofactivated TIG welded bead-on-plate weldments of reduced activationferritic/martensitic steel using oxide fluxes. J. Manuf. Process. 2015, 20, 224–233. [Google Scholar] [CrossRef]
- Jurica, M.; Kozuh, Z.; Garasic, I.; Busic, M. Optimization of the A-TIG welding for stainless steels. IOP Conf. Ser. Mater. Sci. Eng. 2018, 329, 1–9. [Google Scholar] [CrossRef]
- Klobcar, D.; Tusek, J.; Bizjak, M.; Simoncic, S.; Lešer, V. Active flux tungsten inert gas welding of austenitic stainless steel AISI 304. METABK 2016, 55, 617–620. [Google Scholar]
- Vidyarthy, R.S.; Dwivedi, D.K. Activating flux tungsten inert gas welding for enhanced weld penetration. J. Manuf. Process. 2016, 22, 211–228. [Google Scholar] [CrossRef]
- Vora, J.J.; Badheka, J.B. Improved Penetration with the Use of Oxide Fluxes in Activated TIG Welding of Low Activation Ferritic/Martensitic Steel. Trans. Indian Inst. Met. 2016, 69, 1755–1764. [Google Scholar] [CrossRef]
- Venkatesan, G.; George, J.; Sowmysari, M.; Muthupandi, V. Effect of ternary fluxes on depth of penetration in A-TIG welding AISI 409 ferritic stainless steel. Procedia Mater. Sci. 2014, 5, 2402–2410. [Google Scholar] [CrossRef]
- Takeuchi, Y.; Takagi, R.; Shinoda, T. Effect of bismuth on weld joint pen-etration in austenitic stainless steel. Weld. Res. Suppl. 1992, 71, 283–290. [Google Scholar]
- Mills, K.C.; Keene, B.J.; Brooks, R.F.; Shirali, A. Marangoni effects in welding. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 1998, 356, 911–925. [Google Scholar] [CrossRef]
- Skvortsov, E.A. Role of electronegative elements in contraction of thearc discharge. Weld Int. 1998, 12, 471–475. [Google Scholar] [CrossRef]
- Tanaka, M.; Shimizu, T.; Terasaki, T.; Ushio, M.; Koshiishi, F.; Terasaki, H. Effects of activating flux on arc phenomena in gas tungstenarc welding. Sci. Technol. Weld Join. 2000, 5, 397–402. [Google Scholar] [CrossRef]
- Kuang-Hung, T.; Po-Yu, L. UNS S31603 Stainless Steel Tungsten Inert Gas Welds Made with Microparticle and Nanoparticle Oxides. Materials. 2014, 7, 4755–4772. [Google Scholar]
- International Standard ISO 9692-1:2013. Welding and Allied Processes—Types of Joint Preparation—Part 1: Manual Metal-arc Welding, Gas-Shielded Metal-arc Welding, Gas Welding, TIG Welding and Beam Welding of Steels; ISO: Geneva, Switzerland, 2013. [Google Scholar]
- EUROPEAN TYRE & RUBBER MANUFACTURERS’ ASSOCIATION. Available online: http://www.etrma.org/uploads/Modules/Documentsmanager/20170913---2017-list-of-critical-raw-materials-for-the-eu.pdf (accessed on 24 November 2018).
- Report on Critical Raw Materials for EU, Report of the Ad-Hoc Working Group on Defining Critical Raw Materials for EU. May 2014. Available online: http://mima.geus.dk/report-on-critical-raw-materials_en.pdf (accessed on 24 November 2018).
- Venkatesan, G.; Muthupandi, V.; Justine, J. Activated TIG welding of AISI 304L using mono- and tri-component fluxes. Int. J. Adv. Manuf. Technol. 2017, 93, 329–336. [Google Scholar] [CrossRef]
- Housecroft, C.E.; Sharpe, A.G. Inorganic Chemistry, 2nd ed.; Pearson, Prentice Hall, Pearson Education Limited: Edinburgh, UK, 2005; p. 38. [Google Scholar]
- Tseng, K.H.; Chen, K.L. Comparisons between TiO2- and SiO2-flux assisted TIG welding processes. J. Nanosci. Nanotechnol. 2012, 12, 6359–6367. [Google Scholar] [CrossRef] [PubMed]
- Trunec, M. Dispersion of nanoparticles in solvents, polymer solutions and melts, principles and possibilities. In Proceedings of the COST Action MP0701 Workshop, Novi Sad, Serbia, 23–24 September 2010. [Google Scholar]
- Efimov, A.; Lizunova, A.; Sukharev, V.; Ivanov, V. Synthesis and Characterization of TiO2, Cu2O and Al2O3 Aerosol Nanoparticles Produced by the Multi-Spark Discharge Generator. Korean J. Mater. Res. 2016, 26, 123–129. [Google Scholar] [CrossRef]




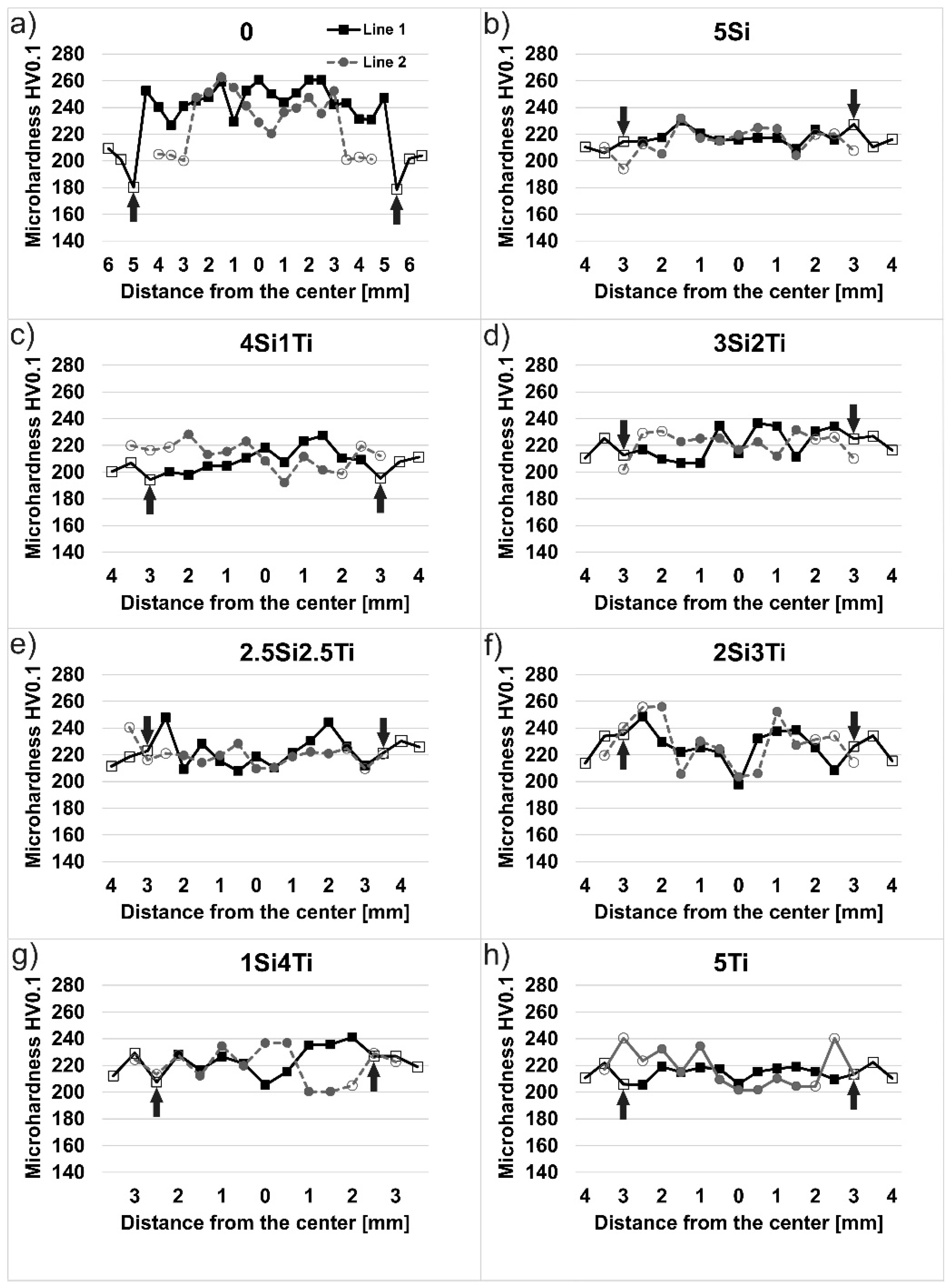


| Measurement Area | 0 | 5Si | 4Si1Ti | 3Si2Ti | 2.5Si2.5Ti | 2Si3Ti | 1Si4Ti | 5Ti |
|---|---|---|---|---|---|---|---|---|
| Line 1 | 246 | 218 | 210 | 221 | 223 | 226 | 225 | 214 |
| Line 2 | 243 | 218 | 212 | 222 | 218 | 225 | 220 | 214 |
| Line 3 | 245 | 215 | 214 | 220 | 221 | 227 | 223 | 212 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balos, S.; Dramicanin, M.; Janjatovic, P.; Zabunov, I.; Klobcar, D.; Busic, M.; Grilli, M.L. Metal Oxide Nanoparticle-Based Coating as a Catalyzer for A-TIG Welding: Critical Raw Material Perspective. Metals 2019, 9, 567. https://doi.org/10.3390/met9050567
Balos S, Dramicanin M, Janjatovic P, Zabunov I, Klobcar D, Busic M, Grilli ML. Metal Oxide Nanoparticle-Based Coating as a Catalyzer for A-TIG Welding: Critical Raw Material Perspective. Metals. 2019; 9(5):567. https://doi.org/10.3390/met9050567
Chicago/Turabian StyleBalos, Sebastian, Miroslav Dramicanin, Petar Janjatovic, Ivan Zabunov, Damjan Klobcar, Matija Busic, and Maria Luisa Grilli. 2019. "Metal Oxide Nanoparticle-Based Coating as a Catalyzer for A-TIG Welding: Critical Raw Material Perspective" Metals 9, no. 5: 567. https://doi.org/10.3390/met9050567
APA StyleBalos, S., Dramicanin, M., Janjatovic, P., Zabunov, I., Klobcar, D., Busic, M., & Grilli, M. L. (2019). Metal Oxide Nanoparticle-Based Coating as a Catalyzer for A-TIG Welding: Critical Raw Material Perspective. Metals, 9(5), 567. https://doi.org/10.3390/met9050567







