Detection and Estimation of Retained Austenite in a High Strength Si-Bearing Bainite-Martensite-Retained Austenite Micro-Composite Steel after Quenching and Bainitic Holding (Q&B)
Abstract
:1. Introduction
2. Materials and Experimental Procedures
2.1. Primary Specimen Preparation
2.2. Microstructural Characterization and Analyzing
3. Results and Discussion
3.1. Light and Electron Microscopic Observations
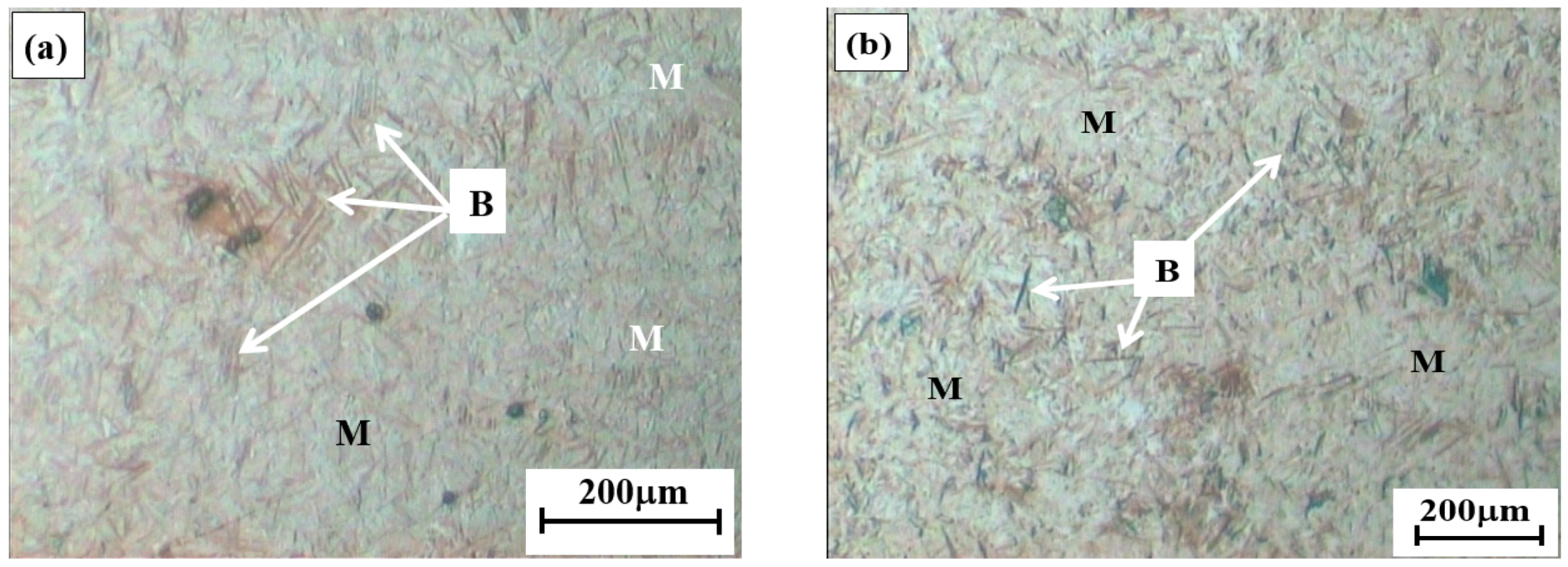
3.1.1. Optical Micrographs
3.1.2. FE-SEM Micrographs
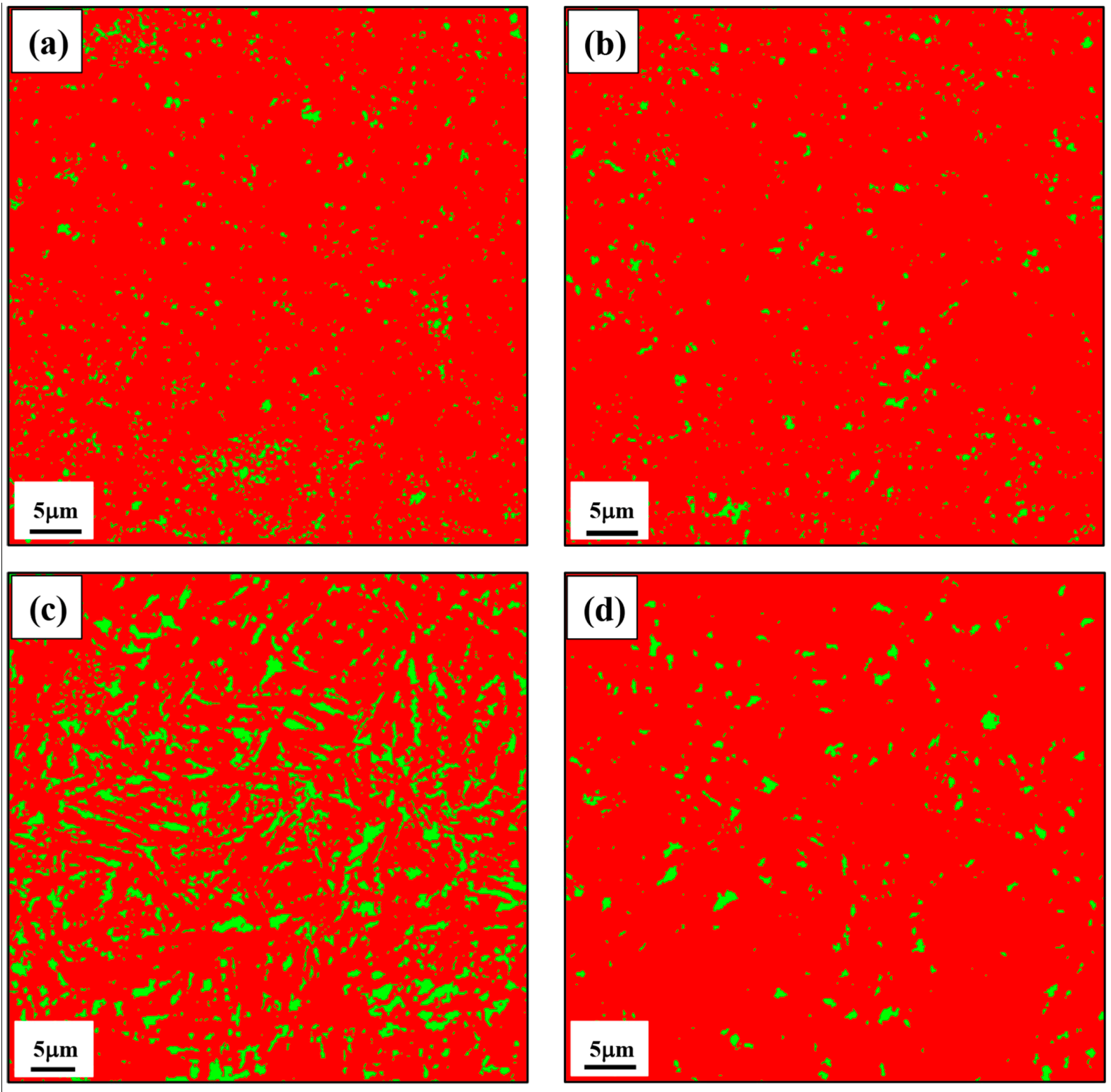
3.1.3. EBSD Micrographs
3.2. Thermal Stability of Retained Austenite
3.3. Magnetic Analysis
3.4. XRD Confirmations
4. Conclusions
- The LePera’s etching reagent was used to reveal bainite, martensite and retained austenite by colorizing these microphases in the microstructures of Q&B samples isothermally heat-treated at 350 °C for shorter than 30 s. In holding times longer than 30 s, bainite was still distinguished from other microphases, but it was not possible to make a good contrasting resolution between martensite and retained austenite formed in the M/RA as they appeared as white blocky discrete islands or thin films.
- In comparison to the possibility of identifying different phases by color light metallography, the FE-SEM microscopy was unable to clearly detect different microphases from each other. However, it could reveal the bainite morphologies including granular and/or degenerate upper bainite in the microstructures.
- The EBSD analysis was able to reveal the FCC retained austenite from rest of the BCC bainite/martensite microconstituents. In addition, the volume fraction, morphology and distribution of retained austenite were clearly determined by this method. The volume fraction of retained austenite increased from 3.1 to 15.3 vol.% with increase in isothermal holding time from 5 to 200 s. However, it decreased to 3.1 vol.% following isothermal holding for 1h due to extensive austenite decomposition and carbon redistribution from bainite into the adjacent prior austenite locations. The retained austenite appeared as blocky grains and thin film morphologies in the micro-composite microstructures.
- The volume fractions of retained austenite measured by EBSD technique were largely in accordance with those obtained by color light metallography and XRD. The quantitative measurements of retained austenite performed by Rietveld process using Maud software were slightly higher than the values measured by both EBSD and color light metallography methods apparently due to the ability of the technique for getting diffraction even from finely divided retained austenite.
- The DTA technique was used to determine the thermal stability of retained austenite in the micro-composite samples. The exothermic peaks on the DTA curves occurred in the temperature range of 139 to 285 °C and this range of decomposition temperature was attributed to the retained austenite decomposition and carbide formation.
- The carbon concentration of retained austenite was continuously increased from 0.57 to 1.34 wt% with increasing isothermal bainitic holding time from 1s to 1h, respectively. The higher carbon concentration of retained austenite with longer isothermal bainitic holding time was related to more redistribution of carbon from bainite to the adjacent prior austenite areas developed with the progress of bainite formation on longer isothermal holding.
- The thermal stability of retained austenite was justified with an abnormal trending by increasing isothermal bainitic holding times from 10s to 1h. The decomposition temperature of austenite was continuously increased from 151 to 285 °C and then surprisingly decreased to 139 °C with increasing isothermal bainitic holding times from 10 to 200 s and then to 1h, respectively. This abnormal trending of thermal stability for retained austenite was related to its level of solute carbon concentration.
- The magnetic measurement was successfully carried out to qualitatively detect retained austenite in the Q&B heat-treated micro-composite samples by measuring the saturation magnetization and coercivity in comparison to the associated values taken from full annealed samples.
Author Contributions
Funding
Conflicts of Interest
References
- Nakagawa, A.H.; Thomas, G. Microstructure-mechanical property relationships of dual-phase steel wire. Metall. Trans. A 1985, 16, 831–840. [Google Scholar] [CrossRef] [Green Version]
- Guo, H.; Gao, G.; Gui, X.; Misra, R.D.K.; Bai, B. Structure-property relation in a quenched-partitioned low alloy steel involving bainite transformation. Mater. Sci. Eng. A 2016, 667, 224–231. [Google Scholar] [CrossRef]
- Huyghe, P.; Malet, L.; Caruso, M.; Georges, C.; Godet, S. On the relationship between the multiphase microstructure and the mechanical properties of a 0.2C quenched and partitioned steel. Mater. Sci. Eng. A 2017, 701, 254–263. [Google Scholar] [CrossRef]
- Yan, S.; Liu, X.; Liu, W.J.; Liang, T.; Zhang, B.; Liu, L.; Zhao, Y. Comparative study on microstructure and mechanical properties of a C-Mn-Si steel treated by quenching and partitioning (Q&P) processes after a full and intercritical austenitization. Mater. Sci. Eng. A 2017, 684, 261–269. [Google Scholar] [CrossRef]
- Emadoddin, E.; Akbarzadeh, A.; Daneshi, G. Effect of intercritical annealing on retained austenite characterization in textured TRIP-assisted steel sheet. Mater. Charact. 2006, 57, 408–413. [Google Scholar] [CrossRef]
- Santofimia, M.J.; Zhao, L.; Petrov, R.; Sietsma, J. Characterization of the microstructure obtained by the quenching and partitioning process in a low-carbon steel. Mater. Charact. 2008, 59, 1758–1764. [Google Scholar] [CrossRef]
- Xie, Z.J.; Han, G.; Zhou, W.H.; Zeng, C.Y.; Shang, C.J. Study of retained austenite and nano-scale precipitation and their effects on properties of a low alloyed multi-phase steel by the two-step intercritical treatment. Mater. Charact. 2016, 113, 60–66. [Google Scholar] [CrossRef]
- Rao, B.V.N.; Rashid, M.S. Direct Observations of Deformation-Induced Retained Austenite Transformation in a Vanadium-Containing Dual-Phase Steel. Mater. Charact. 1997, 39, 435–453. [Google Scholar] [CrossRef]
- Navarro-López, A.; Hidalgo, J.; Sietsma, J.; Santofimia, M.J. Characterization of bainitic/martensitic structures formed in isothermal treatments below the Ms temperature. Mater. Charact. 2017, 128, 248–256. [Google Scholar] [CrossRef]
- Arbor, A. Correlation of measurements of retained austenite in carburized steels by X-ray diffraction and quantitative metallography. J. Heat Treat. 1980, 1, 24–30. [Google Scholar]
- Gotoh, Y.; Koga, K.; Sasaguri, N.; Takahashi, N. Magnetic nondestructive inspection of retained austenite in cast iron. IEEE Trans. Magn. 2006, 42, 3180–3182. [Google Scholar] [CrossRef]
- Zhao, L.; van Dijk, N.H.; Brück, E.; Sietsma, J.; van der Zwaag, S. Magnetic and X-ray diffraction measurements for the determination of retained austenite in TRIP steels. Mater. Sci. Eng. A 2001, 313, 145–152. [Google Scholar] [CrossRef]
- Ladriere, J.H.; He, X.J. Mosbauer study on retained austenite in an Fe Mn C dual-phase steel. Mater. Sci. Eng. 1986, 77, 133–138. [Google Scholar] [CrossRef]
- Mostafapour, A.; Ebrahimpour, A.; Saied, T. Identification of retained austenite, ferrite, bainite and martensite in the microstructure of TRIP steel. Int. J. Iron Steel Soc. Iran. 2016, 13, 1–6. [Google Scholar]
- Marder, A.R.; Benscoter, A.O. Quantitative microanalysis of dual-phase steels. Metallography 1982, 15, 73–85. [Google Scholar] [CrossRef]
- Voort, G.F.V.; Manilova, E.P. Hint for imaging phases in steels. Adv. Mater. Process. 2005, 163, 32–37. [Google Scholar]
- Stormvinter, A. Low Temperature Austenite Decomposition in Carbon Steels. Doctoral Dissertation, KTH Royal Institute of Technology, Stockholm, Sweden, September 2012. [Google Scholar]
- Kučerová, L.; Opatová, K.; Jandová, A. Metallography of AHSS steels with retained austenite. In Microscopy and Imaging Science: Practical Approaches to Applied Research and Education; Formatex Research Center: Badajoz, Spain, 2016; pp. 455–463. [Google Scholar]
- Zhao, H.S.; Zhu, X.; Li, W.; Jin, X.J.; Wang, L.; Jiao, H.; Jiang, D.M. Austenite stability for quenching and partitioning treated steel revealed by colour tint-etching method. Mater. Sci. Technol. 2014, 30, 1008–1013. [Google Scholar] [CrossRef]
- Primig, S.; Leitner, H. Separation of overlapping retained austenite decomposition and cementite precipitation reactions during tempering of martensitic steel by means of thermal analysis. Thermochim. Acta 2011, 526, 111–117. [Google Scholar] [CrossRef]
- Jacques, P.; Delannay, F.; Cornet, X.; Harlet, P.; Ladriere, J. Enhancement of the mechanical properties of a low-carbon, low-silicon steel by formation of a multiphased microstructure containing retained austenite. Metall. Mater. Trans. A 1998, 29, 2383–2393. [Google Scholar] [CrossRef]
- Mijovilovich, A.G.; Vieira, R.; Paniago, H.D.; Pfannes, B.M. Gonzalez, Mössbauer study of the retained austenitic phase in multiphase steels. Mater. Sci. Eng. A. 2000, 283, 65–69. [Google Scholar] [CrossRef]
- Avishan, B.; Garcia-Mateo, C.; Yazdani, S.; Caballero, F.G. Retained austenite thermal stability in a nanostructured bainitic steel. Mater. Charact. 2013, 81, 105–110. [Google Scholar] [CrossRef] [Green Version]
- Hossain, R.; Pahlevani, F.; Sahajwalla, V. Effect of small addition of Cr on stability of retained austenite in high carbon steel. Mater. Charact. 2017, 125, 114–122. [Google Scholar] [CrossRef]
- Chen, J.; Lv, M.; Tang, S.; Liu, Z.; Wang, G. Correlation between mechanical properties and retained austenite characteristics in a low-carbon medium manganese alloyed steel plate. Mater. Charact. 2015, 106, 108–111. [Google Scholar] [CrossRef]
- Witte, M.; Lesch, C. On the improvement of measurement accuracy of retained austenite in steel with X-ray diffraction. Mater. Charact. 2018, 139, 111–115. [Google Scholar] [CrossRef]
- Sicupira, F.L.; Sandim, M.J.R.; Sandim, H.R.Z.; Santos, D.B.; Renzetti, R.A. Quantification of retained austenite by X-ray diffraction and saturation magnetization in a supermartensitic stainless steel. Mater. Charact. 2016, 115, 90–96. [Google Scholar] [CrossRef]
- Hidalgo, J.; Findley, K.O.; Santofimia, M.J. Thermal and mechanical stability of retained austenite surrounded by martensite with different degrees of tempering. Mater. Sci. Eng. A 2017, 690, 337–347. [Google Scholar] [CrossRef]
- De Moor, E.; Lacroix, S.; Samek, L.; Penning, J.; Speer, J.G. Dilatometric study of the quench and partitioning process. In Proceedings of the 3rd International Conference on Advanced Structural Steels, Gyeongju, Korea, 22–24 August 2006; pp. 873–878. [Google Scholar]
- Caballero, F.G.; García-Mateo, C.; de Andrés, C.G. Dilatometric study of reaustenitisation of high silicon bainitic steels: Decomposition of retained austenite. Mater. Trans. 2005, 46, 581–586. [Google Scholar] [CrossRef]
- Lerchbacher, C.; Zinner, S.; Leitner, H. Retained austenite decomposition and carbide formation during tempering a hot-work tool steel X38CrMoV5-1 studied by dilatometry and atom probe tomography. Metall. Mater. Trans. A Phys. Metall. Mater. Sci. 2012, 43, 4989–4998. [Google Scholar] [CrossRef]
- Ouda, K.; Danninger, H. Christian Gierl-Mayer, Magnetic measurement of retained austenite in sintered steels—Benefits and limitations. Powder Metall. 2018, 6, 358–368. [Google Scholar] [CrossRef]
- Ajus, C.; Tavares, S.S.M.; Silva, M.R.; Corte, R.R.A. Magnetic properties and retained austenite quantification in SAE 4340 steel. Matéria 2009, 14, 993–999. [Google Scholar] [CrossRef] [Green Version]
- Mumtaz, K.; Takahashi, S.; Echigoya, J.; Kamada, Y.; Zhang, L.F.; Kikuchi, H.; Ara, K.; Sato, M. Magnetic measurements of the reverse martensite to austenite transformation in a rolled austenitic stainless steel. J. Mater. Sci. 2004, 39, 1997–2010. [Google Scholar] [CrossRef]
- Shi, W.; Li, L.; de Cooman, B.C.; Wollants, P.; Yang, C.X. Thermal stability of retained austenite in TRIP steel after different treatments. J. Iron Steel Res. Int. 2008, 15, 61–64. [Google Scholar] [CrossRef]
- Wierszyllowski, I.; Rys, J. Application of the DTA to the analysis of the residual austenite transformation during tempering. In Nondestructive Characterization of Materials II; Bussière, J.F., Monchalin, J.-P., Ruud, C.O., Green, R.E., Eds.; Springer: Boston, MA, USA, 1987; pp. 309–315. [Google Scholar] [CrossRef]
- Lin, L.; de Cooman, B.C.; Wollants, P.; Yang, C. Thermal Stability of Retained Austenite in Quenching & Partitioning Steels. Master’s Thesis, Mechanical Engineering, Delft University of Technology, Delft, The Netherlands, July 2015. [Google Scholar]
- Wu, R.M.; Wang, L.; Jin, X.J. Thermal Stability of Austenite and Properties of Quenching & Partitioning (Q&P) Treated AHSS. Phys. Procedia 2013, 50, 8–12. [Google Scholar] [CrossRef] [Green Version]
- Gao, G.; Zhang, H.; Gui, X.; Luo, P.; Tan, Z.; Bai, B. Enhanced ductility and toughness in an ultrahigh-strength Mn–Si–Cr–C steel: The great potential of ultrafine filmy retained austenite. Acta Mater. 2014, 76, 425–433. [Google Scholar] [CrossRef]
- Petrov, R.; Kestens, L.; Wasilkowska, A.; Houbaert, Y. Microstructure and texture of a lightly deformed TRIP-assisted steel characterized by means of the EBSD technique. Mater. Sci. Eng. A 2007, 447, 285–297. [Google Scholar] [CrossRef]
- Peng, F.; Xu, Y.; Gu, X.; Wang, Y.; Liu, X.; Li, J. The relationships of microstructure-mechanical properties in quenching and partitioning (Q&P) steel accompanied with microalloyed carbide precipitation. Mater. Sci. Eng. A 2018, 723, 247–258. [Google Scholar] [CrossRef]
- Kwon, E.P.; Fujieda, S.; Shinoda, K.; Suzuki, S. Characterization of transformed and deformed microstructures in transformation induced plasticity steels using electron backscattering diffraction. Mater. Sci. Eng. A 2011, 528, 5007–5017. [Google Scholar] [CrossRef]
- Tan, X.; Xu, Y.; Yang, X.; Liu, Z.; Wu, D. Effect of partitioning procedure on microstructure and mechanical properties of a hot-rolled directly quenched and partitioned steel. Mater. Sci. Eng. A 2014, 594, 149–160. [Google Scholar] [CrossRef]
- Abbasi, M.; Kim, D.-I.; Nelson, T.W.; Abbasi, M. EBSD and reconstruction of pre-transformation microstructures, examples and complexities in steels. Mater. Charact. 2014, 95, 219–231. [Google Scholar] [CrossRef]
- Williamson, D.L.; Schupmann, R.G.; Materkowski, J.P.; Krauss, G. Determination of small amounts of austenite and carbide in hardened medium carbon steels by Mössbauer spectroscopy. Metall. Trans. A 1979, 10, 379–382. [Google Scholar] [CrossRef]
- Giordano, L.; Matteazzi, P.; Tiziani, A.; Zambon, A. Retained austenite variation in dual-phase steel after mechanical stressing and heat treatment. Mater. Sci. Eng. A 1991, 131, 215–219. [Google Scholar] [CrossRef]
- Da Silva, A.K.; Inden, G.; Kumar, A.; Ponge, D.; Gault, B.; Raabe, D. Competition between formation of carbides and reversed austenite during tempering of a medium-manganese steel studied by thermodynamic-kinetic simulations and atom probe tomography. Acta Mater. 2018, 147, 165–175. [Google Scholar] [CrossRef]
- Magner, S.H.; de Angleis, R.J.; Weins, W.N.; Makinson, J.D. A historical review of retained austenite and its measurement by X-Ray diffraction. Adv. X-Ray Anal. 2002, 45, 92–97. [Google Scholar]
- Rodrigues, T.A.; Duarte, V.; Avila, J.A.; Santos, T.G.; Miranda, R.M.; Oliveira, J.P. Wire and arc additive manufacturing of HSLA steel: Effect of thermal cycles on microstructure and mechanical properties. Addit. Manuf. 2019, 27, 440–450. [Google Scholar] [CrossRef]
- Tavares, S.S.M.; Mello, S.R.; Gomes, A.M.; Neto, J.M.; da Silva, M.R.; Pardal, J.M. X-ray diffraction and magnetic characterization of the retained austenite in a chromium alloyed high carbon steel. J. Mater. Sci. 2006, 41, 4732–4736. [Google Scholar] [CrossRef]
- Hufenbach, J.; Kunze, K.; Giebeler, L.; Gemming, T.; Wendrock, H.; Baldauf, C.; Kühn, U.; Hufenbach, W.; Eckert, J. The effect of boron on microstructure and mechanical properties of high-strength cast FeCrVC. Mater. Sci. Eng. A 2013, 586, 267–275. [Google Scholar] [CrossRef]
- Torboli, A. Retained Austenite: Non-Destructive Analysis by XRD and ASTM E975-03; Anallytical Instruments Group: Agrate Conturbia, Novara, Italy, 2014; pp. 1–27. [Google Scholar]
- ASTM E975-13. Standard Practice for X-ray Determination of Retained Austenite in Steel with near Random Crystallographic Orientation; ASTM: West Conshohocken, PA, USA, 2009; pp. 1–7. [Google Scholar] [CrossRef]
- Jacques, P.J.; Allain, S.; Bouaziz, O.; De, A.; Gourgues, A.-F.; Hance, B.M.; Houbaert, Y.; Huang, J.; Iza-Mendia, A.; Kruger, S.E.; et al. On measurement of retained austenite in multiphase TRIP steels—Results of blind round robin test involving six different techniques. Mater. Sci. Technol. 2009, 25, 567–574. [Google Scholar] [CrossRef]
- Escobar, J.D.; Faria, G.A.; Wu, L.; Oliveira, J.P.; Mei, P.R.; Ramirez, A.J. Austenite reversion kinetics and stability during tempering of a Ti-stabilized supermartensitic stainless steel: Correlative in situ synchrotron x-ray diffraction and dilatometry. Acta Mater. 2017, 138, 92–99. [Google Scholar] [CrossRef]
- Garcia-Mateo, C.; Caballero, F.G.; Bhadeshia, H.K.D.H. Development of Hard Bainite. ISIJ Int. 2003, 43, 1238–1243. [Google Scholar] [CrossRef] [Green Version]
- Li, H. Microstructure and mechanical properties of 50SiMnNiNb steel by a novel quenching-partitioning-austempering heat treatment. Chin. J. Mech. Eng. 2009, 22, 645. [Google Scholar] [CrossRef]
- Bagliani, E.P.; Santofimia, M.J.; Zhao, L.; Sietsma, J.; Anelli, E. Microstructure, tensile and toughness properties after quenching and partitioning treatments of a medium-carbon steel. Mater. Sci. Eng. A 2013, 559, 486–495. [Google Scholar] [CrossRef]
- Bhadeshia, H.K.D.H. Bainite in Steels: Transformations, Microstructure and Properties, 2nd ed.; IOM Communications Ltd.: London, UK, 2001. [Google Scholar]
- Clayton, P.; Sawley, K.J.; Bolton, P.J.; Pell, G.M. Wear behavior of bainitic steels. Wear 1987, 120, 199–220. [Google Scholar] [CrossRef]
- Huda, N.; Lazor, R.; Gerlich, A.P. Study of MA Effect on Yield Strength and Ductility of X80 Linepipe Steels Weld. Metall. Mater. Trans. A 2017, 48, 4166–4179. [Google Scholar] [CrossRef]
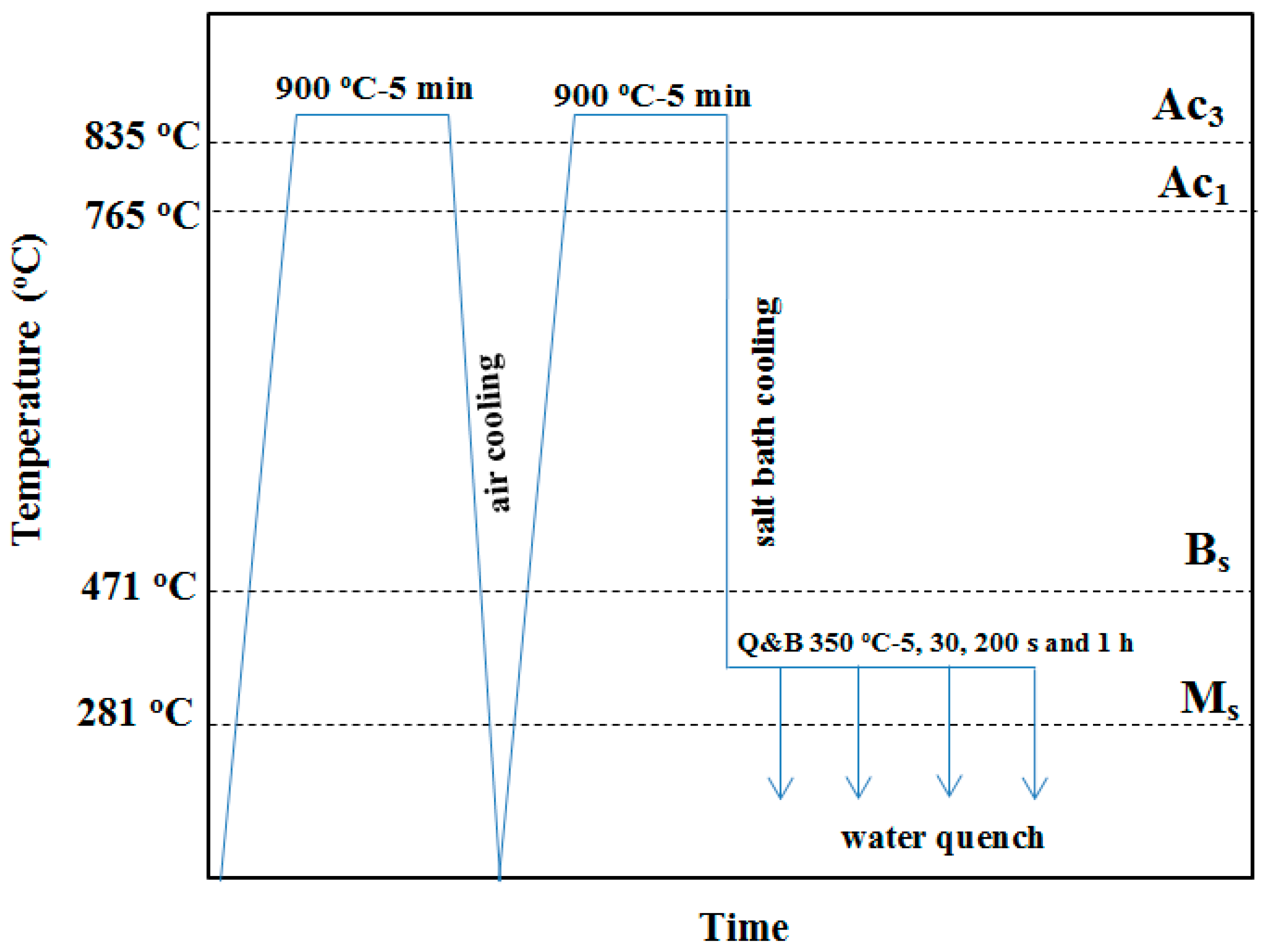







| Item | Empirical Equation | Calculated Values (°C) | Ref. |
|---|---|---|---|
| Martensite start temperature, Ms | Ms (°C) = 539 − 423C − 30.4Mn − 17.7Ni − 12.1Cr − 7.5Mo | 281 | [56] |
| Bainite start temperature, Bs | Bs (°C) = 656 − 58C − 35Mn − 75Si − 15Ni − 34Cr − 41Mo | 471 | [57] |
| Etching Reagent | Chemical Composition |
|---|---|
| 2% nital | 2 mL nitric acid, 98 mL ethanol |
| 5% picral | 5 g picric acid, 100 mL ethanol |
| Sodium metabisulfite | 7 g Na2S2O5, 100 mL distilled water |
| Glyceregia | 9 mL glyceregia, 6 mL HCl, 3 mL nitric acid |
| LePera | 50 mL Na2S2O5 1% in aqueous solution, 50 mL picric acid 4% in ethanol |
| Q&B Samples | Volume Fractions of Retained Austenite (vol.%) | ||
|---|---|---|---|
| Optical Microscopy (±4) | XRD (±1) | BSD (±2) | |
| 350 °C-5 s | 3.6 | 3.1 | 3.1 |
| 350 °C-30 s | 3.4 | 4.5 | 4.4 |
| 350 °C-200 s | - | 18.0 | 15.3 |
| 350 °C-1 h | - | 6.9 | 3.1 |
| Q&B Samples | DTA Data | XRD Measurements | ||
|---|---|---|---|---|
| RA Decomposition Temperature (°C) | RA Peak Intensity (Micro-volts) | RA Volume Fraction (vol.%) | RA Carbon Content (wt%) | |
| Q&B 350 °C-10 s | 151 | 3.2 | 4.8 | 0.57 |
| Q&B 350 °C-200 s | 285 | 11.2 | 18 | 1.12 |
| Q&B 350 °C-500 s | 222 | 7.4 | 13.4 | 1.29 |
| Q&B 350 °C-1 h | 139 | 2.5 | 6.9 | 1.34 |
| Heat-Treated Sample | Saturation Magnetization (Ms) (emu/g) | Coercivity (Hc) |
|---|---|---|
| Full annealed | 210.18 | 9.27 |
| Q&B at 350 °C-200 s | 156.59 | 35.68 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pashangeh, S.; Karimi Zarchi, H.R.; Ghasemi Banadkouki, S.S.; Somani, M.C. Detection and Estimation of Retained Austenite in a High Strength Si-Bearing Bainite-Martensite-Retained Austenite Micro-Composite Steel after Quenching and Bainitic Holding (Q&B). Metals 2019, 9, 492. https://doi.org/10.3390/met9050492
Pashangeh S, Karimi Zarchi HR, Ghasemi Banadkouki SS, Somani MC. Detection and Estimation of Retained Austenite in a High Strength Si-Bearing Bainite-Martensite-Retained Austenite Micro-Composite Steel after Quenching and Bainitic Holding (Q&B). Metals. 2019; 9(5):492. https://doi.org/10.3390/met9050492
Chicago/Turabian StylePashangeh, Shima, Hamid Reza Karimi Zarchi, Seyyed Sadegh Ghasemi Banadkouki, and Mahesh C. Somani. 2019. "Detection and Estimation of Retained Austenite in a High Strength Si-Bearing Bainite-Martensite-Retained Austenite Micro-Composite Steel after Quenching and Bainitic Holding (Q&B)" Metals 9, no. 5: 492. https://doi.org/10.3390/met9050492
APA StylePashangeh, S., Karimi Zarchi, H. R., Ghasemi Banadkouki, S. S., & Somani, M. C. (2019). Detection and Estimation of Retained Austenite in a High Strength Si-Bearing Bainite-Martensite-Retained Austenite Micro-Composite Steel after Quenching and Bainitic Holding (Q&B). Metals, 9(5), 492. https://doi.org/10.3390/met9050492






