CuAAC-Based Assembly and Characterization of a New Molecular Dyad for Single Material Organic Solar Cell
Abstract
:1. Introduction
2. Materials and Methods
2.1. Device Fabrication and Testing
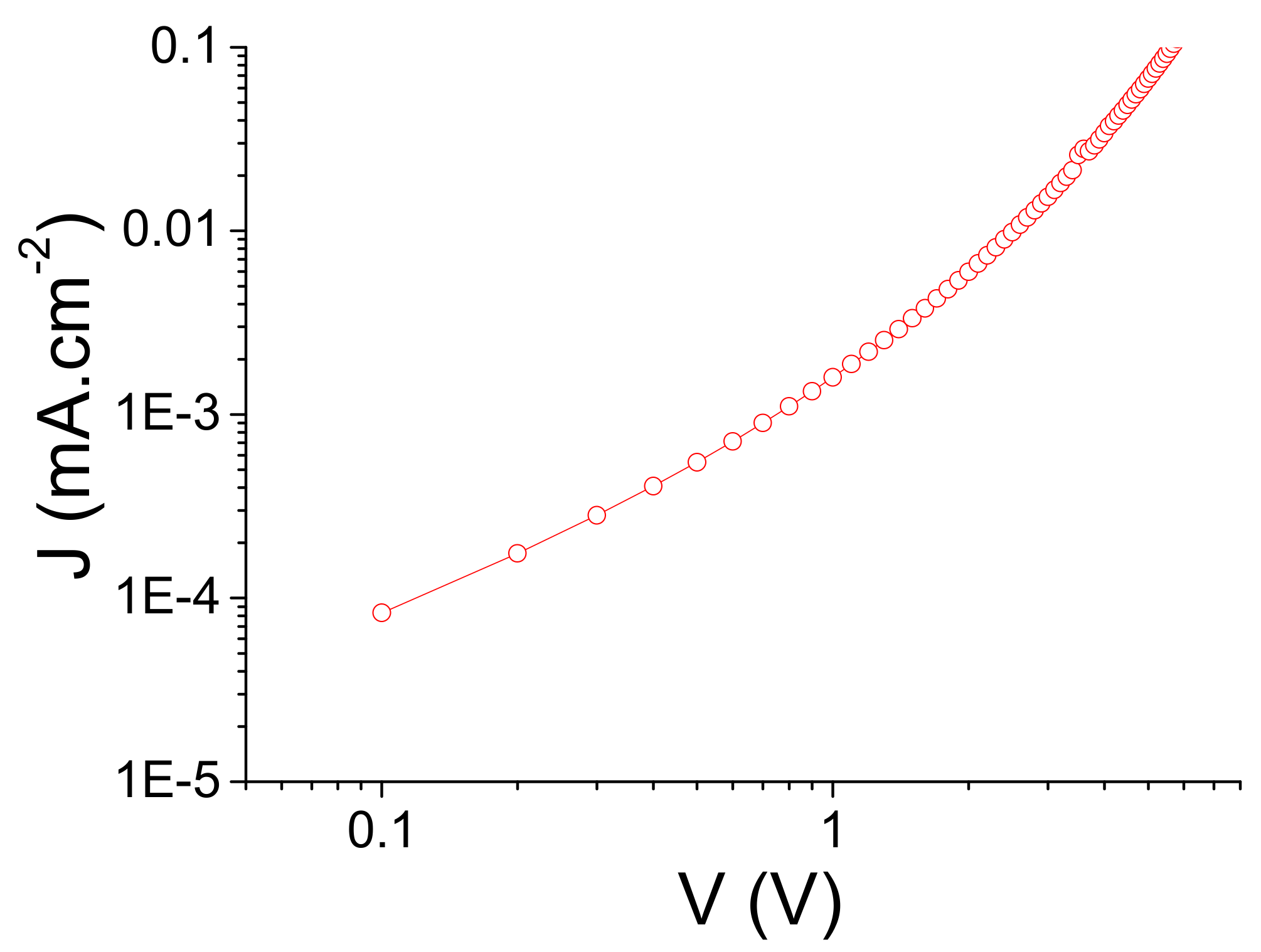
2.2. Space Charge Limited Current (SCLC) Measurements
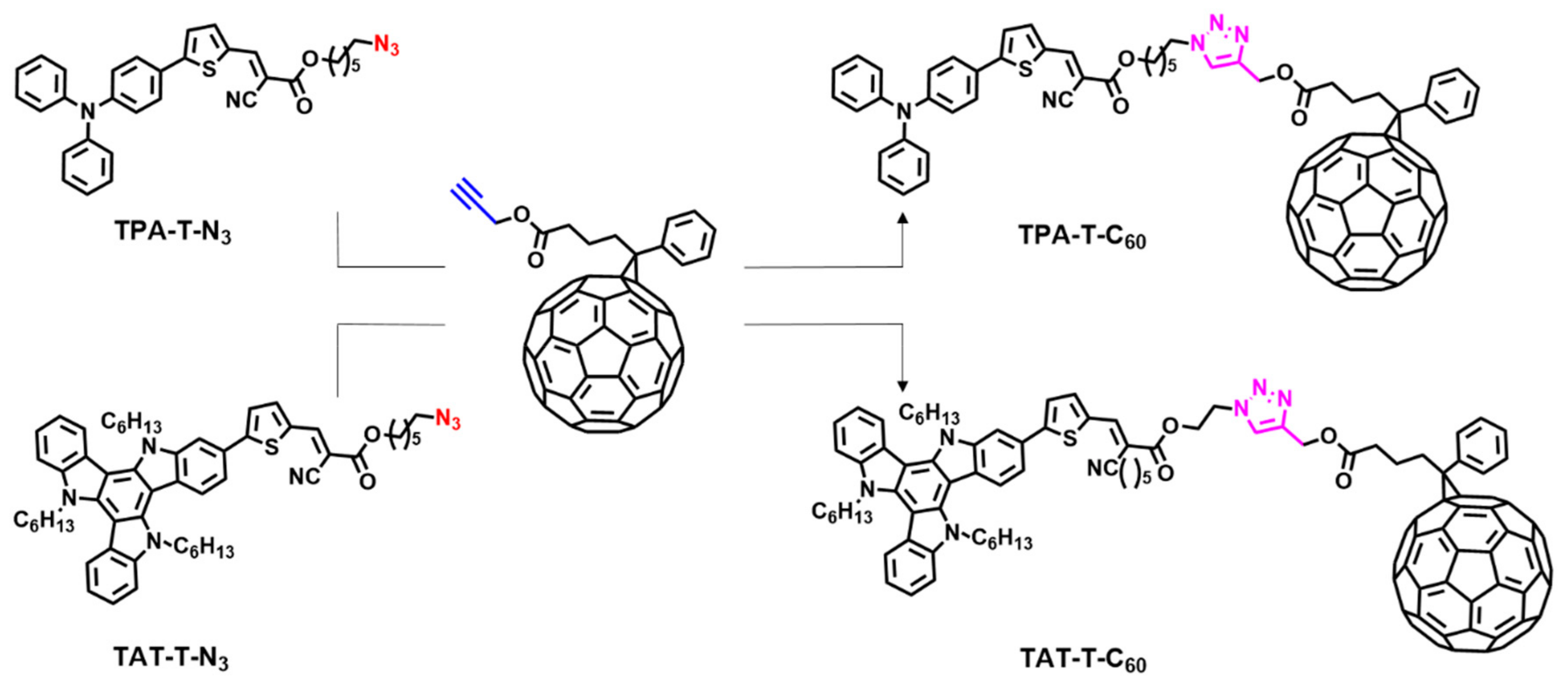
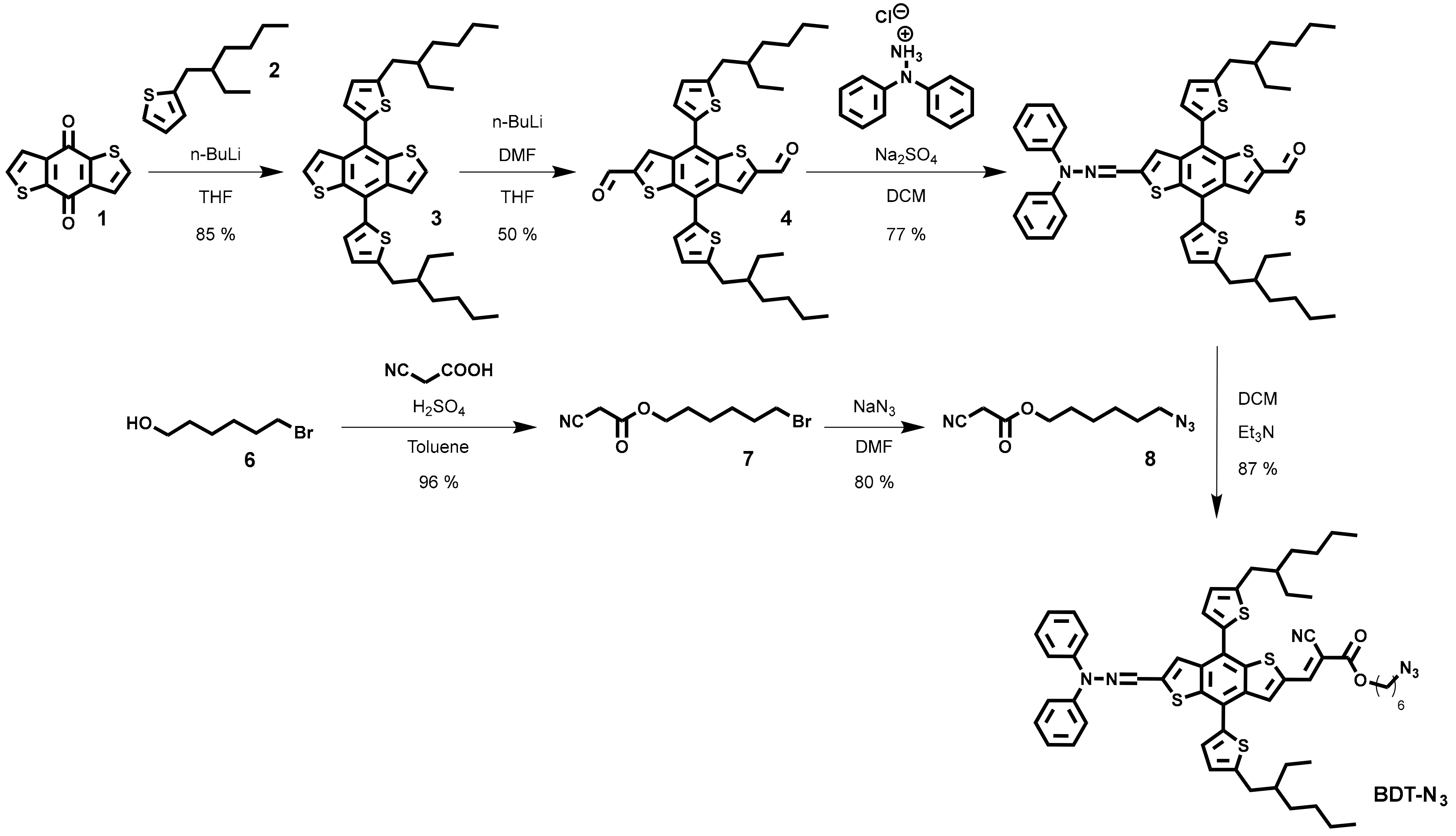
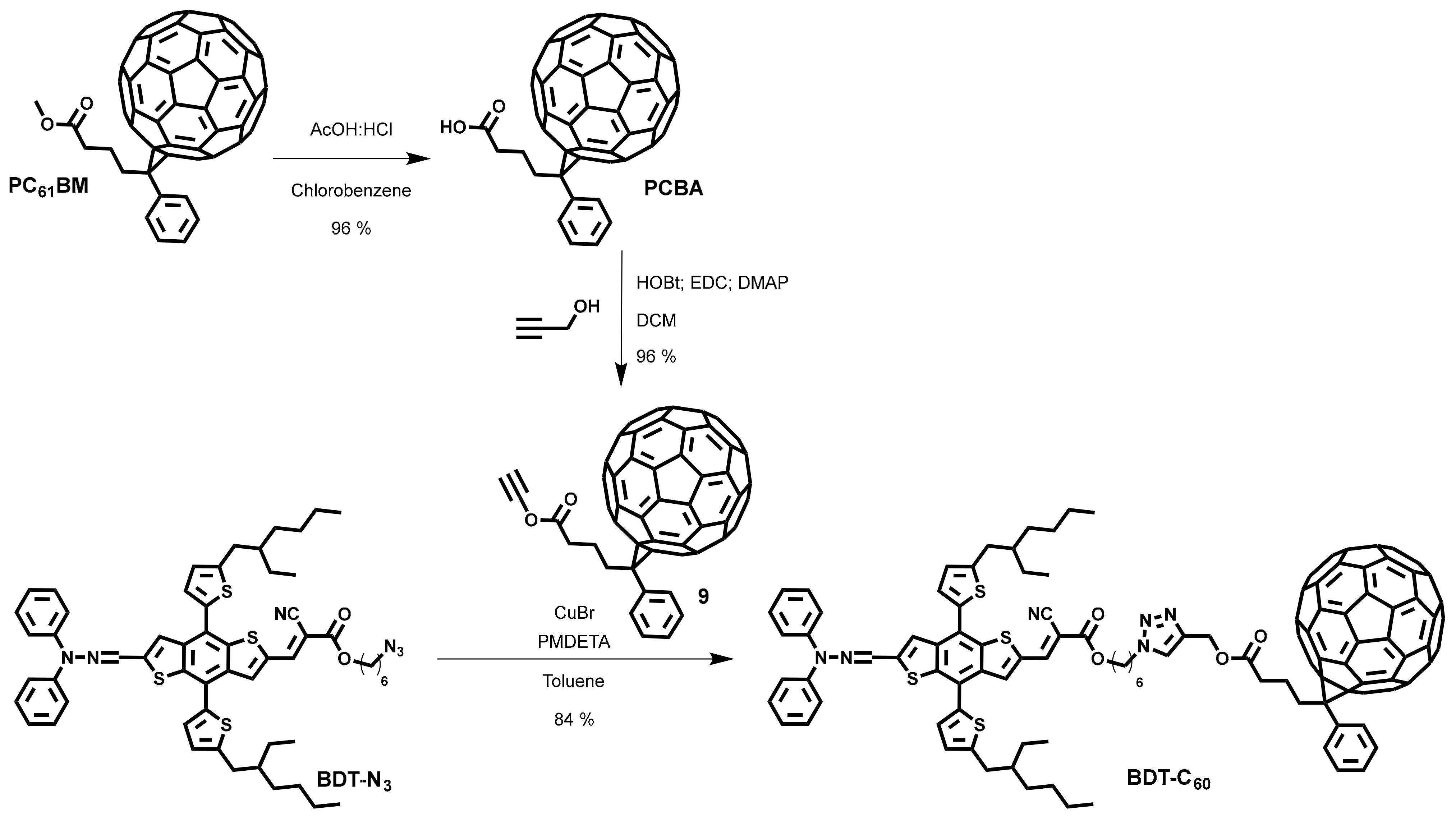
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yuan, J.; Zhang, Y.; Zhou, L.; Zhang, G.; Yip, H.-L.; Lau, T.-K.; Lu, X.; Zhu, C.; Peng, H.; Johnson, P.A.; et al. Single-Junction Organic Solar Cell with over 15% Efficiency Using Fused-Ring Acceptor with Electron-Deficient Core. Joule 2019, 3, 1140–1151. [Google Scholar] [CrossRef]
- Marszalek, T.; Li, M.; Pisula, W. Design directed self-assembly of donor–acceptor polymers. Chem. Commun. 2016, 52, 10938–10947. [Google Scholar] [CrossRef] [PubMed]
- Duan, C.; Willems, R.E.M.; van Franeker, J.J.; Bruijnaers, B.J.; Wienk, M.M.; Janssen, R.A.J. Effect of side chain length on the charge transport, morphology, and photovoltaic performance of conjugated polymers in bulk heterojunction solar cells. J. Mater. Chem. A 2016, 4, 1855–1866. [Google Scholar] [CrossRef] [Green Version]
- Liang, Y.; Feng, D.; Wu, Y.; Tsai, S.-T.; Li, G.; Ray, C.; Yu, L. Highly Efficient Solar Cell Polymers Developed via Fine-Tuning of Structural and Electronic Properties. J. Am. Chem. Soc. 2009, 131, 7792–7799. [Google Scholar] [CrossRef] [PubMed]
- Beaujuge, P.M.; Fréchet, J.M.J. Molecular Design and Ordering Effects in π-Functional Materials for Transistor and Solar Cell Applications. J. Am. Chem. Soc. 2011, 133, 20009–20029. [Google Scholar] [CrossRef]
- Labrunie, A.; Josse, P.; Dabos-Seignon, S.; Blanchard, P.; Cabanetos, C. Pentaerythritol based push–pull tetramers for organic photovoltaics. Sustain. Energy Fuels 2017, 1, 1921–1927. [Google Scholar]
- Josse, P.; Dayneko, S.; Zhang, Y.; Dabos-Seignon, S.; Zhang, S.; Blanchard, P.; Welch, G.C.; Cabanetos, C. Direct (Hetero)Arylation Polymerization of a Spirobifluorene and a Dithienyl-Diketopyrrolopyrrole Derivative: New Donor Polymers for Organic Solar Cells. Molecules 2018, 23, 962. [Google Scholar] [CrossRef] [PubMed]
- Josse, P.; Favereau, L.; Shen, C.; Dabos-Seignon, S.; Blanchard, P.; Cabanetos, C.; Crassous, J. Enantiopure versus Racemic Naphthalimide End-Capped Helicenic Non-fullerene Electron Acceptors: Impact on Organic Photovoltaics Performance. Chem. Eur. J. 2017, 23, 6277–6281. [Google Scholar] [CrossRef] [Green Version]
- Swinnen, A.; Haeldermans, I.; vande Ven, M.; D’Haen, J.; Vanhoyland, G.; Aresu, S.; D’Olieslaeger, M.; Manca, J. Tuning the Dimensions of C60-Based Needlelike Crystals in Blended Thin Films. Adv. Funct. Mater. 2006, 16, 760–765. [Google Scholar] [CrossRef]
- Dang, M.T.; Hirsch, L.; Wantz, G. P3HT: PCBM, Best Seller in Polymer Photovoltaic Research. Adv. Mater. 2011, 23, 3597–3602. [Google Scholar] [CrossRef]
- Liao, H.-C.; Ho, C.-C.; Chang, C.-Y.; Jao, M.-H.; Darling, S.B.; Su, W.-F. Additives for morphology control in high-efficiency organic solar cells. Mater. Today 2013, 16, 326–336. [Google Scholar] [CrossRef]
- Roncali, J.; Grosu, I. The Dawn of Single Material Organic Solar Cells. Adv. Sci. 2019, 6, 1801026. [Google Scholar] [CrossRef]
- Roncali, J. Single Material Solar Cells: The Next Frontier for Organic Photovoltaics? Adv. Energy Mater. 2011, 1, 147–160. [Google Scholar] [CrossRef]
- Segura, J.L.; Martín, N.; Guldi, D.M. Materials for organic solar cells: The C60/π-conjugated oligomer approach. Chem. Soc. Rev. 2005, 34, 31–47. [Google Scholar] [CrossRef]
- Chen, T.L.; Zhang, Y.; Smith, P.; Tamayo, A.; Liu, Y.; Ma, B. Diketopyrrolopyrrole-Containing Oligothiophene-Fullerene Triads and Their Use in Organic Solar Cells. ACS Appl. Mater. Interfaces 2011, 3, 2275–2280. [Google Scholar] [CrossRef] [PubMed]
- Izawa, S.; Hashimoto, K.; Tajima, K. Efficient charge generation and collection in organic solar cells based on low band gap dyad molecules. Chem. Commun. 2011, 47, 6365–6367. [Google Scholar] [CrossRef] [PubMed]
- Bu, L.; Guo, X.; Yu, B.; Qu, Y.; Xie, Z.; Yan, D.; Geng, Y.; Wang, F. Monodisperse Co-oligomer Approach toward Nanostructured Films with Alternating Donor−Acceptor Lamellae. J. Am. Chem. Soc. 2009, 131, 13242–13243. [Google Scholar] [CrossRef]
- Bu, L.; Guo, X.; Yu, B.; Fu, Y.; Qu, Y.; Xie, Z.; Yan, D.; Geng, Y.; Wang, F. Donor–acceptor liquid crystalline conjugated cooligomers for the preparation of films with the ideal morphology for bulk heterojunction solar cells. Polymer 2011, 52, 4253–4260. [Google Scholar] [CrossRef]
- Qu, J.; Gao, B.; Tian, H.; Zhang, X.; Wang, Y.; Xie, Z.; Wang, H.; Geng, Y.; Wang, F. Donor–spacer–acceptor monodisperse conjugated co-oligomers for efficient single-molecule photovoltaic cells based on non-fullerene acceptors. J. Mater. Chem. A 2014, 2, 3632–3640. [Google Scholar] [CrossRef]
- Labrunie, A.; Londi, G.; Dayneko, S.V.; Blais, M.; Dabos-Seignon, S.; Welch, G.C.; Beljonne, D.; Blanchard, P.; Cabanetos, C. A triazatruxene-based molecular dyad for single-component organic solar cells. Chem. Squared 2018, 2, 3. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, T.L.; Lee, T.H.; Gautam, B.; Park, S.Y.; Gundogdu, K.; Kim, J.Y.; Woo, H.Y. Single Component Organic Solar Cells Based on Oligothiophene-Fullerene Conjugate. Adv. Funct. Mater. 2017, 27, 1702474. [Google Scholar] [CrossRef]
- Narayanaswamy, K.; Venkateswararao, A.; Nagarjuna, P.; Bishnoi, S.; Gupta, V.; Chand, S.; Singh, S.P. An Organic Dyad Composed of Diathiafulvalene-Functionalized Diketopyrrolopyrrole–Fullerene for Single-Component High-Efficiency Organic Solar Cells. Angew. Chem. Int. Ed. 2016, 55, 12334–12337. [Google Scholar] [CrossRef] [PubMed]
- Labrunie, A.; Gorenflot, J.; Babics, M.; Alévêque, O.; Dabos-Seignon, S.; Balawi, A.H.; Kan, Z.; Wohlfahrt, M.; Levillain, E.; Hudhomme, P.; et al. Triphenylamine-Based Push–Pull σ–C60 Dyad As Photoactive Molecular Material for Single-Component Organic Solar Cells: Synthesis, Characterizations, and Photophysical Properties. Chem. Mater. 2018, 30, 3474–3485. [Google Scholar] [CrossRef]
- Warnan, J.; Cabanetos, C.; Bude, R.; El Labban, A.; Li, L.; Beaujuge, P.M. Electron-Deficient N-Alkyloyl Derivatives of Thieno[3,4-c]pyrrole-4,6-dione Yield Efficient Polymer Solar Cells with Open-Circuit Voltages > 1 V. Chem. Mater. 2014, 26, 2829–2835. [Google Scholar] [CrossRef]
- Warnan, J.; El Labban, A.; Cabanetos, C.; Hoke, E.T.; Shukla, P.K.; Risko, C.; Brédas, J.-L.; McGehee, M.D.; Beaujuge, P.M. Ring Substituents Mediate the Morphology of PBDTTPD-PCBM Bulk-Heterojunction Solar Cells. Chem. Mater. 2014, 26, 2299–2306. [Google Scholar] [CrossRef]
- Cabanetos, C.; El Labban, A.; Bartelt, J.A.; Douglas, J.D.; Mateker, W.R.; Fréchet, J.M.J.; McGehee, M.D.; Beaujuge, P.M. Linear Side Chains in Benzo[1,2-b:4,5-b′]dithiophene–Thieno[3,4-c]pyrrole-4,6-dione Polymers Direct Self-Assembly and Solar Cell Performance. J. Am. Chem. Soc. 2013, 135, 4656–4659. [Google Scholar] [CrossRef] [PubMed]
- Graham, K.R.; Cabanetos, C.; Jahnke, J.P.; Idso, M.N.; El Labban, A.; Ngongang Ndjawa, G.O.; Heumueller, T.; Vandewal, K.; Salleo, A.; Chmelka, B.F.; et al. Importance of the Donor:Fullerene Intermolecular Arrangement for High-Efficiency Organic Photovoltaics. J. Am. Chem. Soc. 2014, 136, 9608–9618. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Cabanetos, C.; Allain, M.; Liu, P.; Roncali, J. Molecular electron-acceptors based on benzodithiophene for organic photovoltaics. Tetrahedron Lett. 2015, 56, 2324–2328. [Google Scholar] [CrossRef]
- Wang, Z.B.; Helander, M.G.; Greiner, M.T.; Qiu, J.; Lu, Z.H. Carrier mobility of organic semiconductors based on current-voltage characteristics. J. Appl. Phys. 2010, 107, 034506. [Google Scholar] [CrossRef]
- Diac, A.; Demeter, D.; Allain, M.; Grosu, I.; Roncali, J. Simple and Versatile Molecular Donors for Organic Photovoltaics Prepared by Metal-Free Synthesis. Chem. Eur. J. 2015, 21, 1598–1608. [Google Scholar] [CrossRef]
- Huisgen, R.; Blaschke, H. 1.3-Dipolare Cycloadditionen, XX: 2-Äthoxy-oxazole aus Äthoxycarbonyl-azen und Alkinen. Chem. Ber. 1965, 98, 2985–2997. [Google Scholar] [CrossRef]
- Huisgen, R. 1,3-Dipolar Cycloadditions. Past and Future. Angew. Chem. Int. Ed. 1963, 2, 565–598. [Google Scholar] [CrossRef]
- Kahle, F.-J.; Saller, C.; Köhler, A.; Strohriegl, P. Crosslinked Semiconductor Polymers for Photovoltaic Applications. Adv. Energy Mater. 2017, 7, 1700306. [Google Scholar] [CrossRef]
- Labrunie, A.; Jiang, Y.; Baert, F.; Leliège, A.; Roncali, J.; Cabanetos, C.; Blanchard, P. Small molecular push–pull donors for organic photovoltaics: Effect of the heterocyclic π-spacer. RSC Adv. 2015, 5, 102550–102554. [Google Scholar] [CrossRef]
- Jiang, Y.; Allain, M.; Gindre, D.; Dabos-Seignon, S.; Blanchard, P.; Cabanetos, C.; Roncali, J. Thermally induced crystallization, hole-transport, NLO and photovoltaic activity of a bis-diarylamine-based push-pull molecule. Sci. Rep. 2017, 7, 8317. [Google Scholar] [CrossRef] [PubMed]

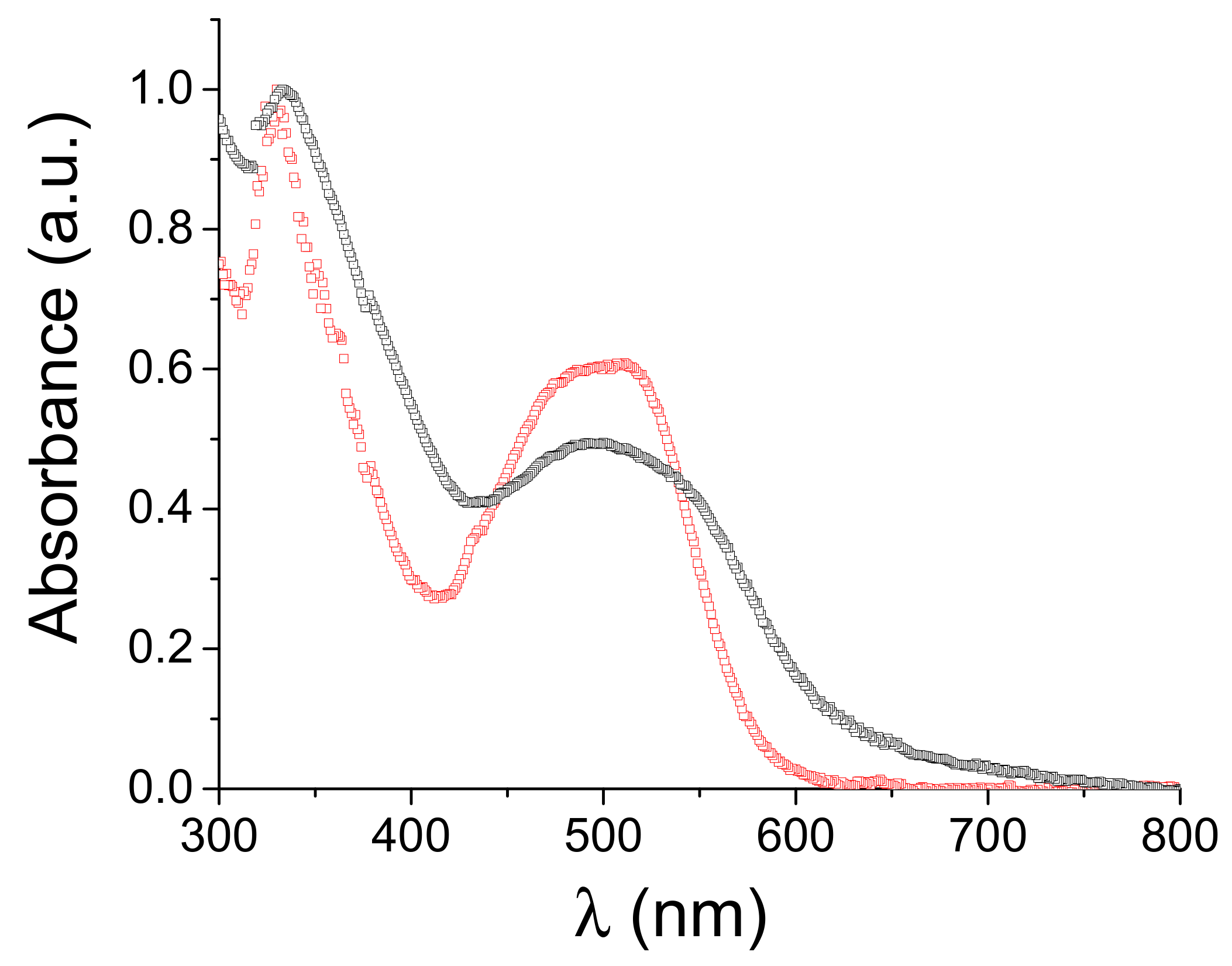
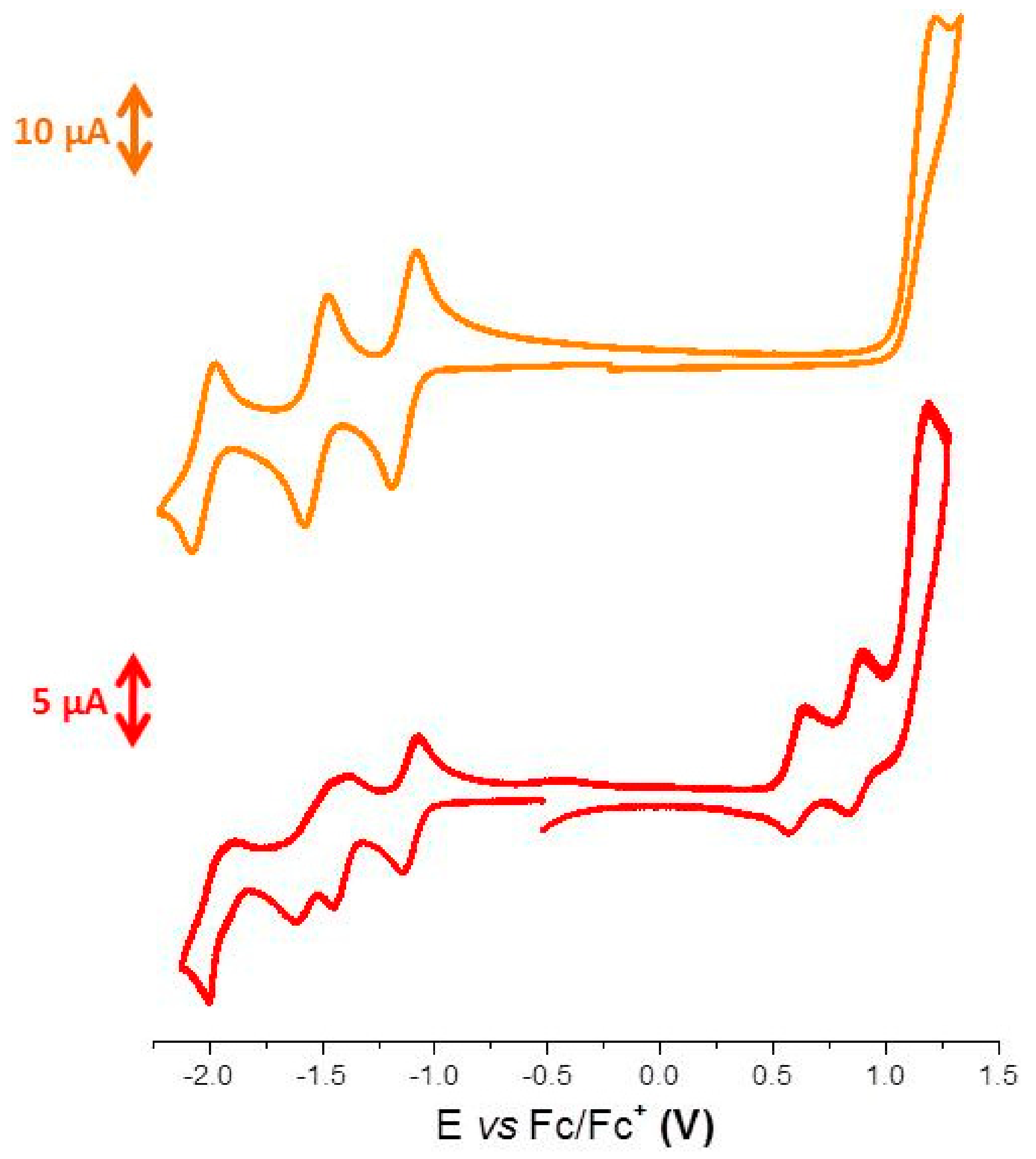
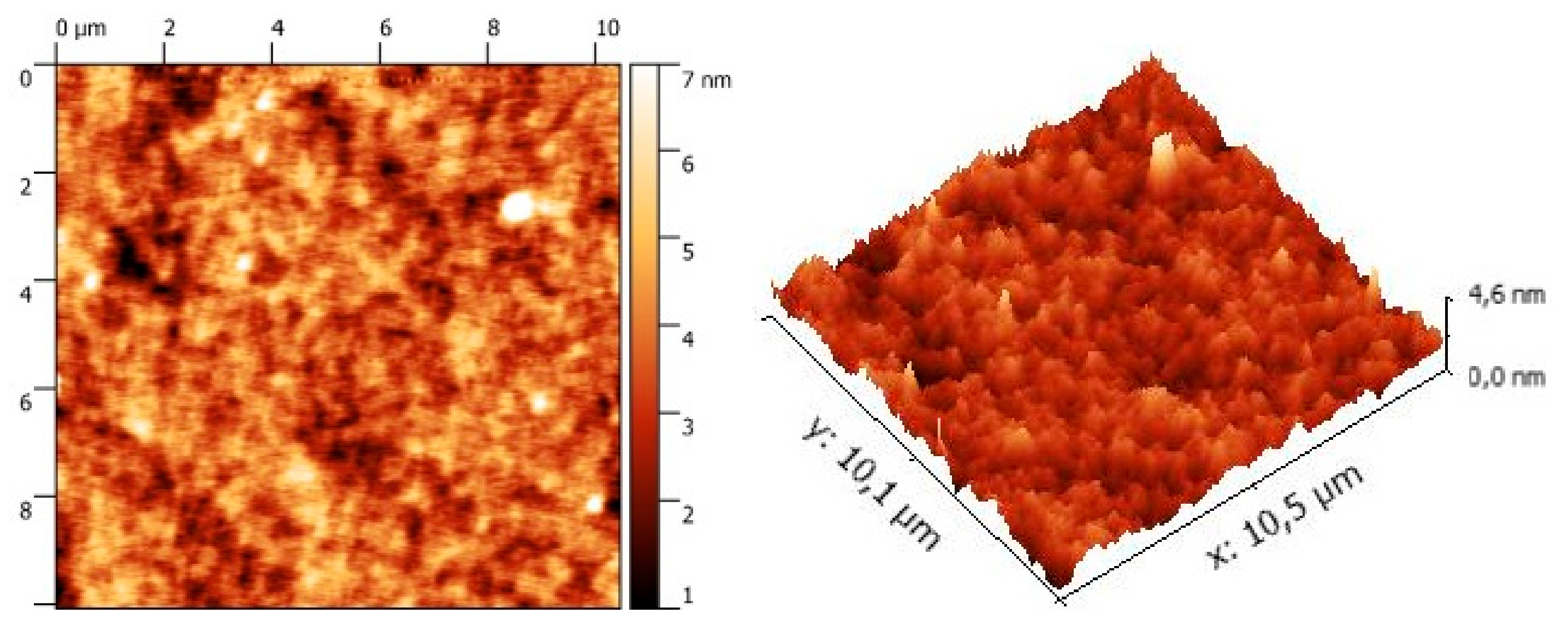

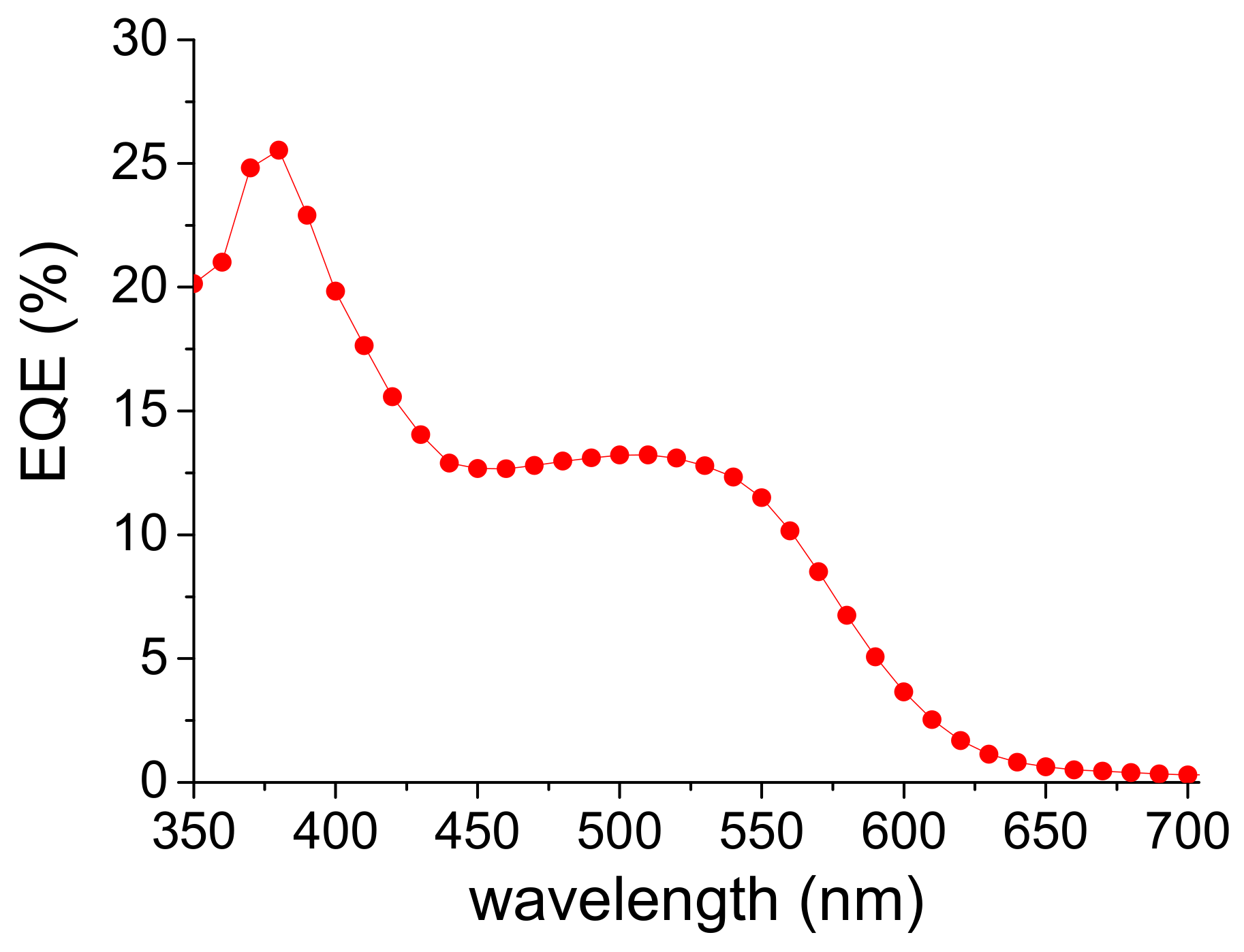
| λmax Solution (nm) a | ε (L·mol−1·cm−1) a | λmax Thin Films (nm) b | λonset Thin Films (nm) b | ΔEopt (eV) c | Epa1 (V) c | Epa2 (V) c | Epc1 (V) c | Epc2 (V) c | Epc3 (V) c | Epc4 (V) c |
|---|---|---|---|---|---|---|---|---|---|---|
| 511 330 | 43800 71950 | 500 335 | 635 | 1.95 | 0.64 | 0.90 | −1.13 | −1.46 | −1.62 | −2.01 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Labrunie, A.; Lebailly, T.; Habibi, A.H.; Dalinot, C.; Jiang, Y.; Dabos-Seignon, S.; Roncali, J.; Blanchard, P.; Cabanetos, C. CuAAC-Based Assembly and Characterization of a New Molecular Dyad for Single Material Organic Solar Cell. Metals 2019, 9, 618. https://doi.org/10.3390/met9060618
Labrunie A, Lebailly T, Habibi AH, Dalinot C, Jiang Y, Dabos-Seignon S, Roncali J, Blanchard P, Cabanetos C. CuAAC-Based Assembly and Characterization of a New Molecular Dyad for Single Material Organic Solar Cell. Metals. 2019; 9(6):618. https://doi.org/10.3390/met9060618
Chicago/Turabian StyleLabrunie, Antoine, Teddy Lebailly, Amir Hossein Habibi, Clément Dalinot, Yue Jiang, Sylvie Dabos-Seignon, Jean Roncali, Philippe Blanchard, and Clément Cabanetos. 2019. "CuAAC-Based Assembly and Characterization of a New Molecular Dyad for Single Material Organic Solar Cell" Metals 9, no. 6: 618. https://doi.org/10.3390/met9060618