Exposure Risk to Medical Staff in a Nasopharyngeal Swab Sampling Cabin under Four Different Ventilation Strategies
Abstract
:1. Introduction
2. Materials and Methods
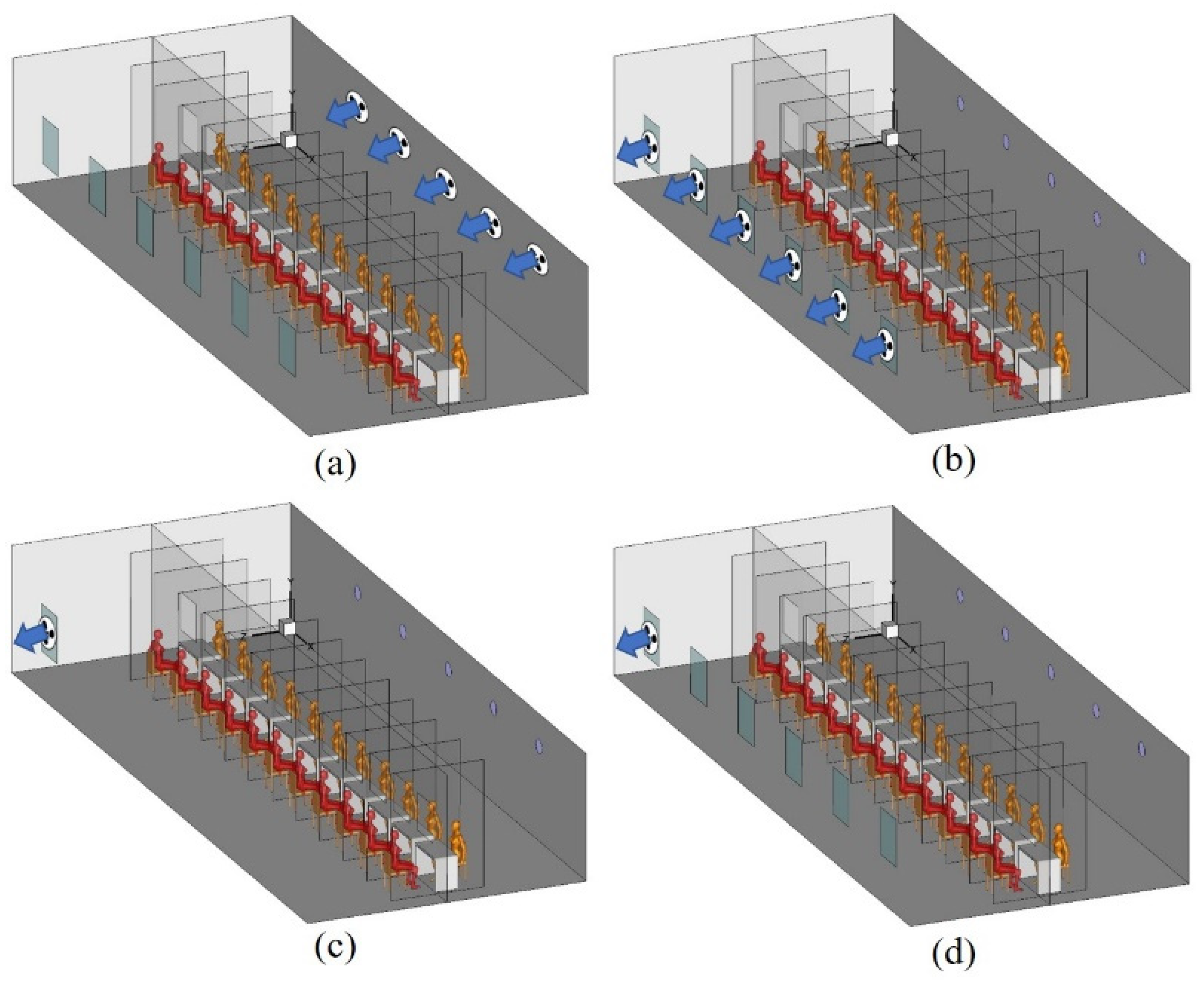
2.1. Physical Model
2.2. Boundary Conditions
2.3. Numerical Methods and Grid Independency
2.4. Evaluation Indexes
3. Model Validation
4. Results
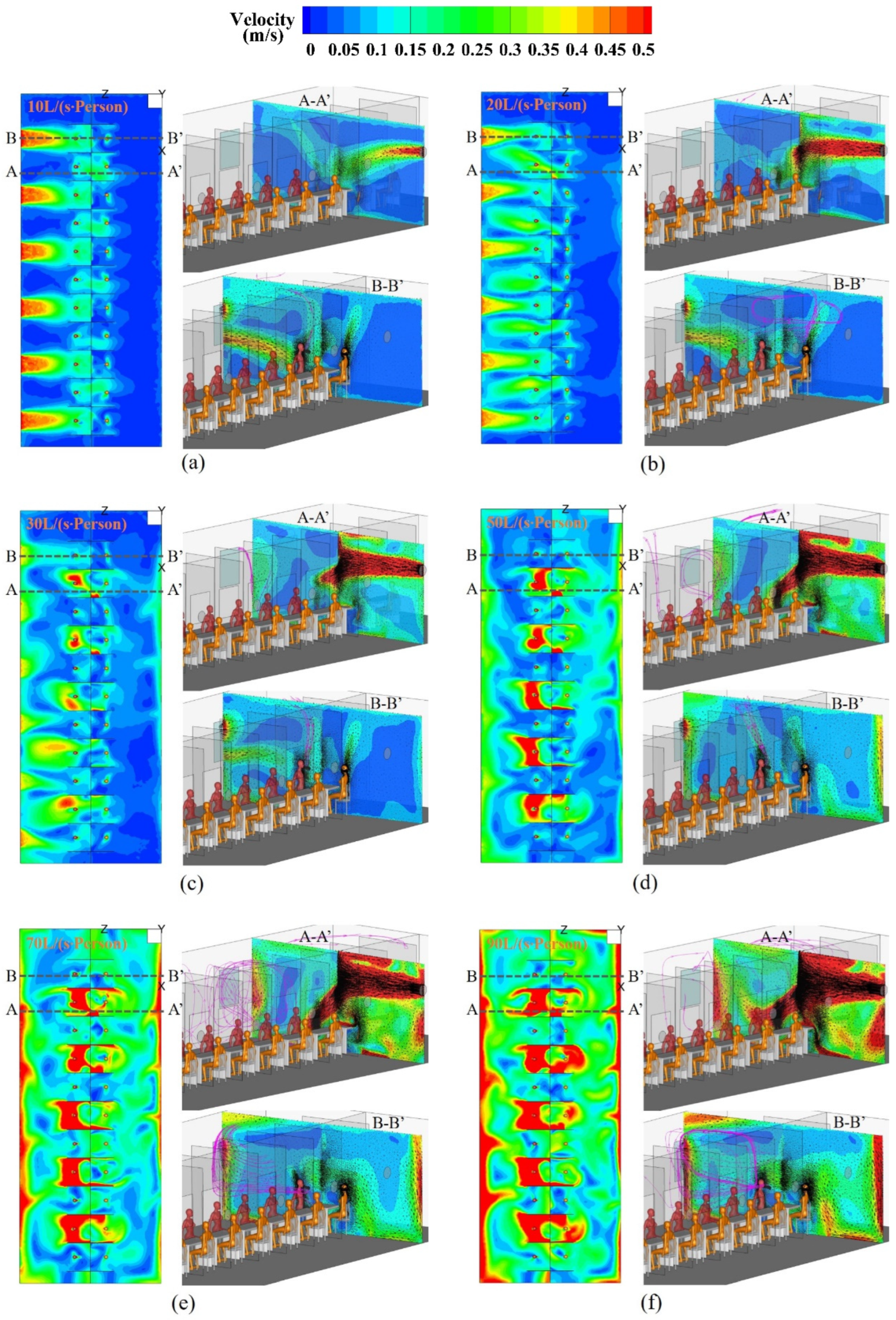
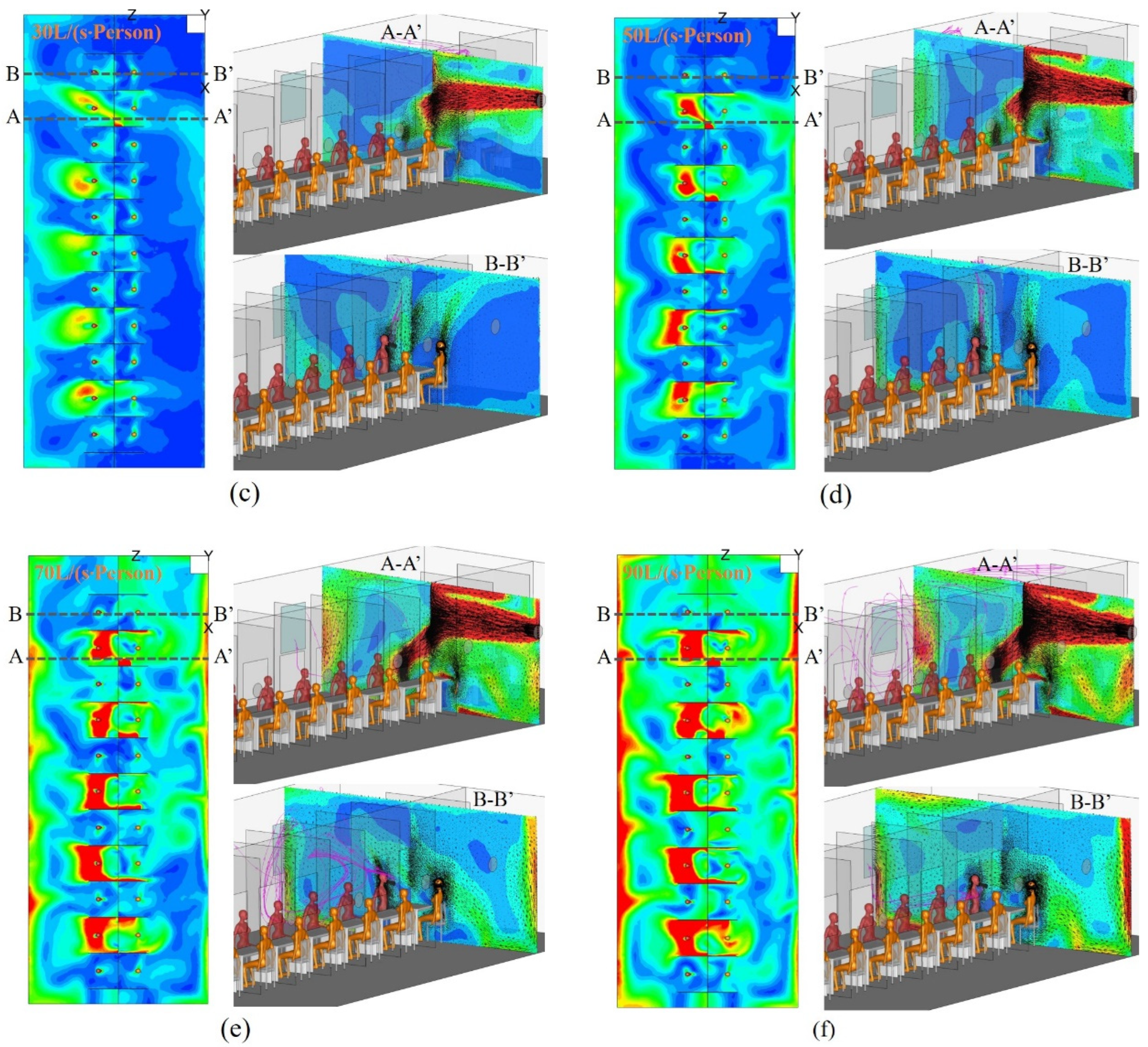
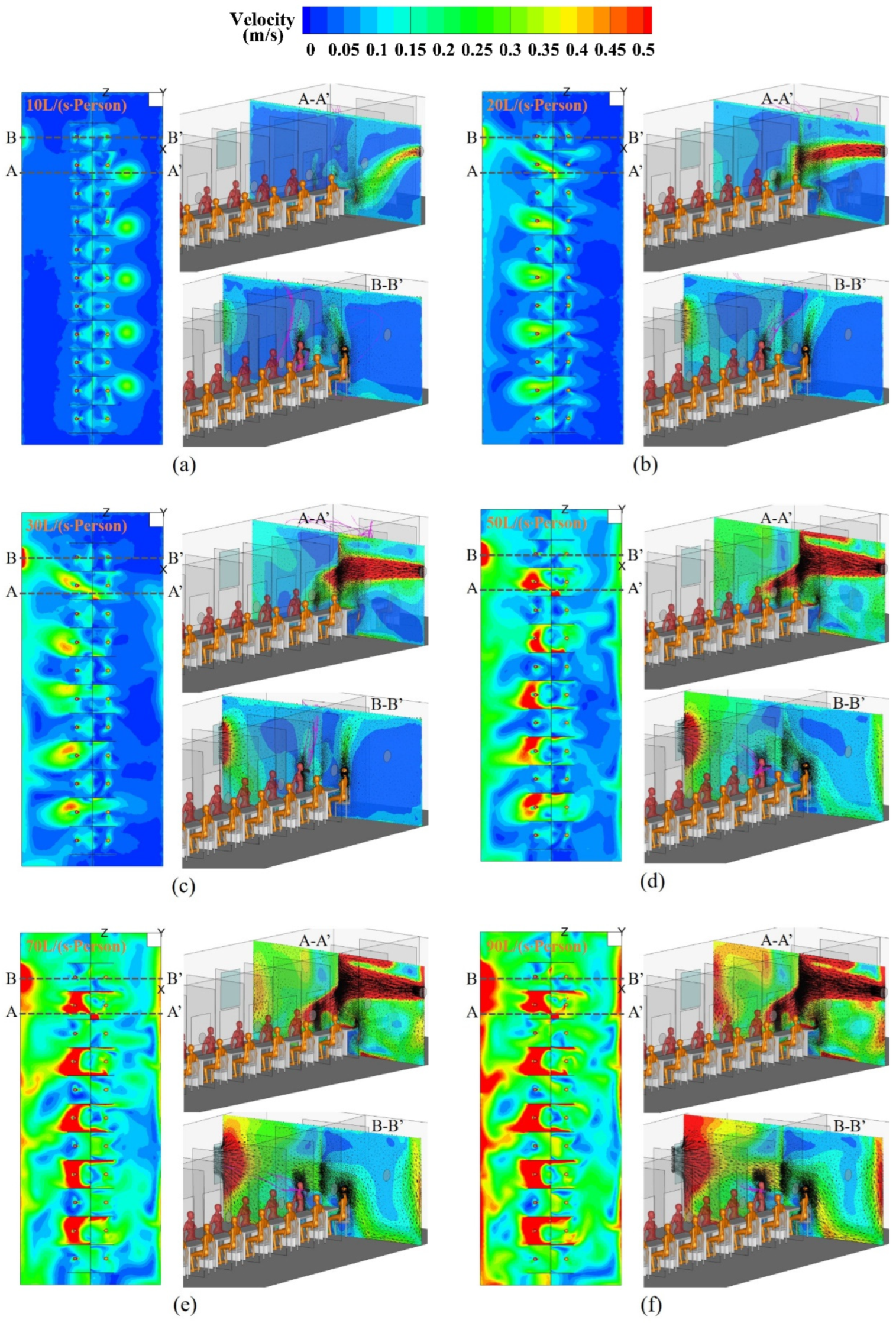
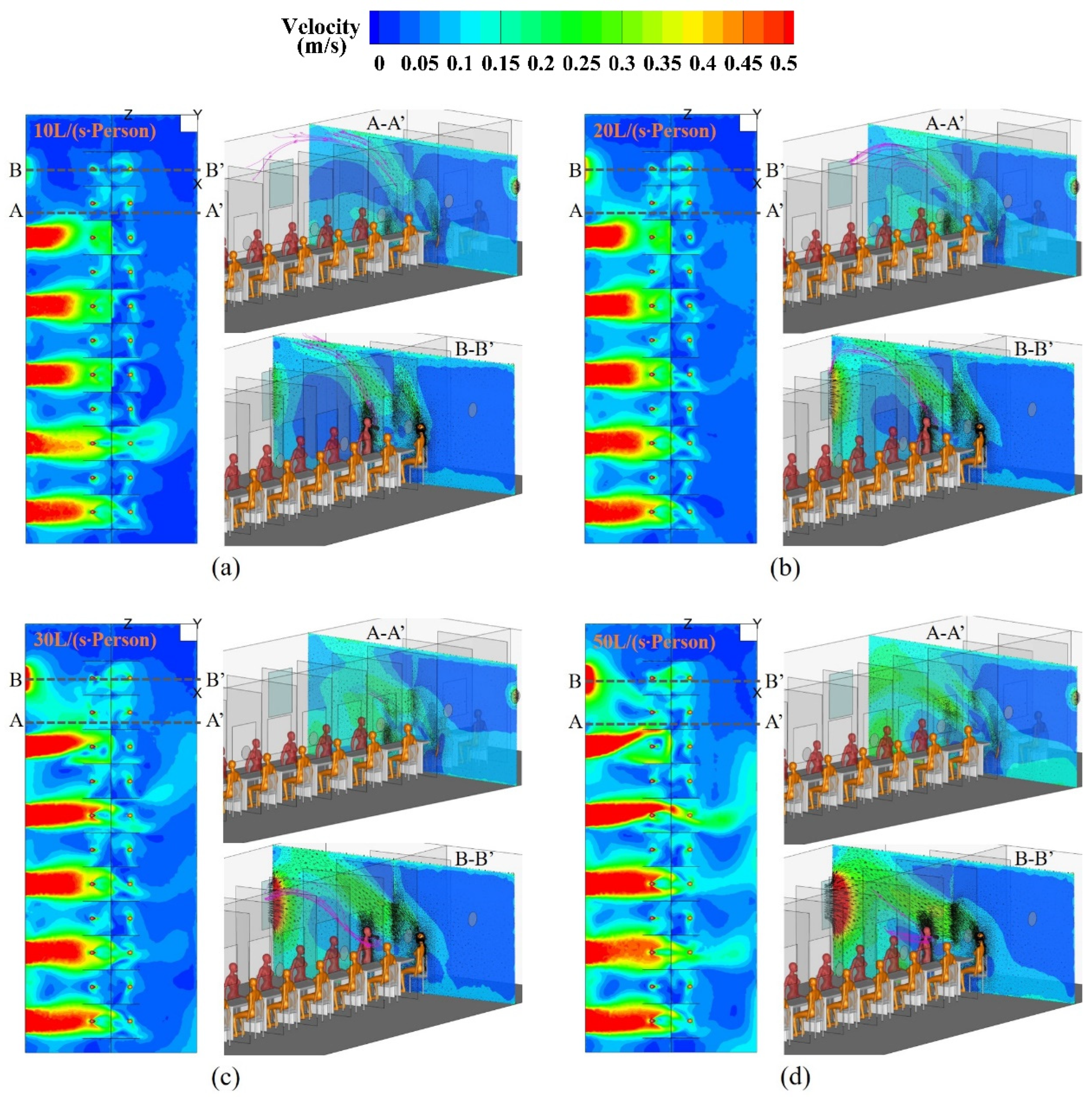
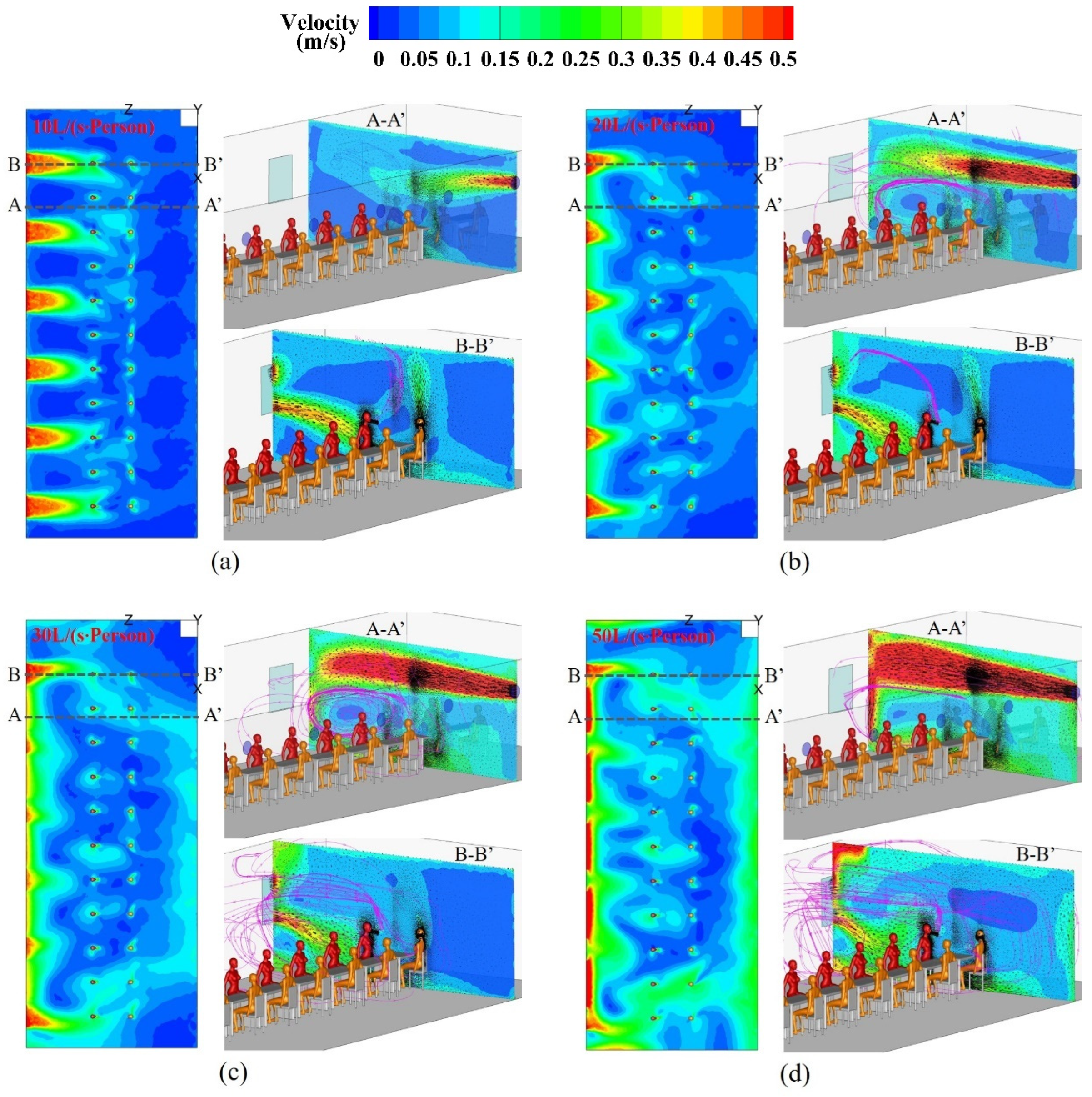
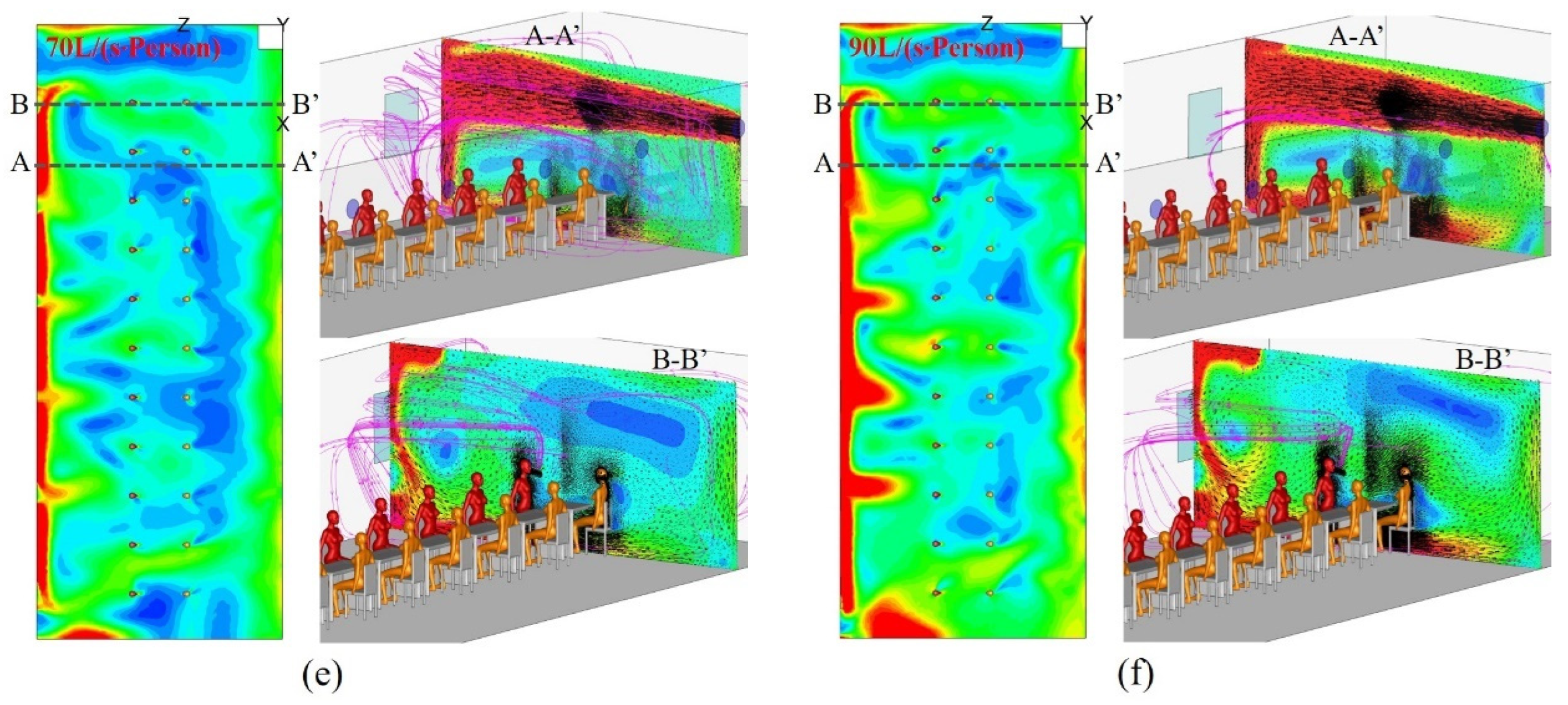
4.1. Airflow Patterns in the Sampling Cabin
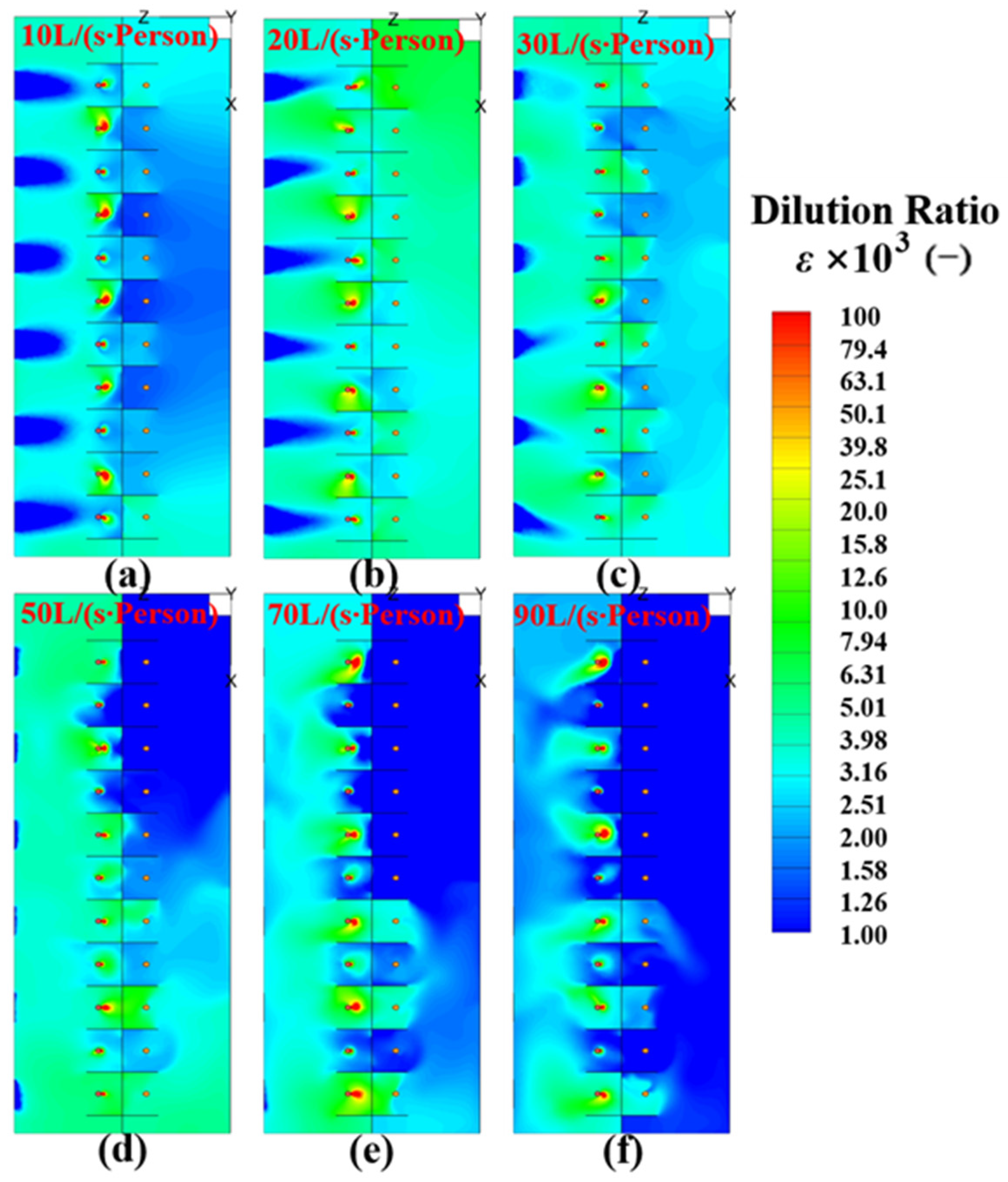
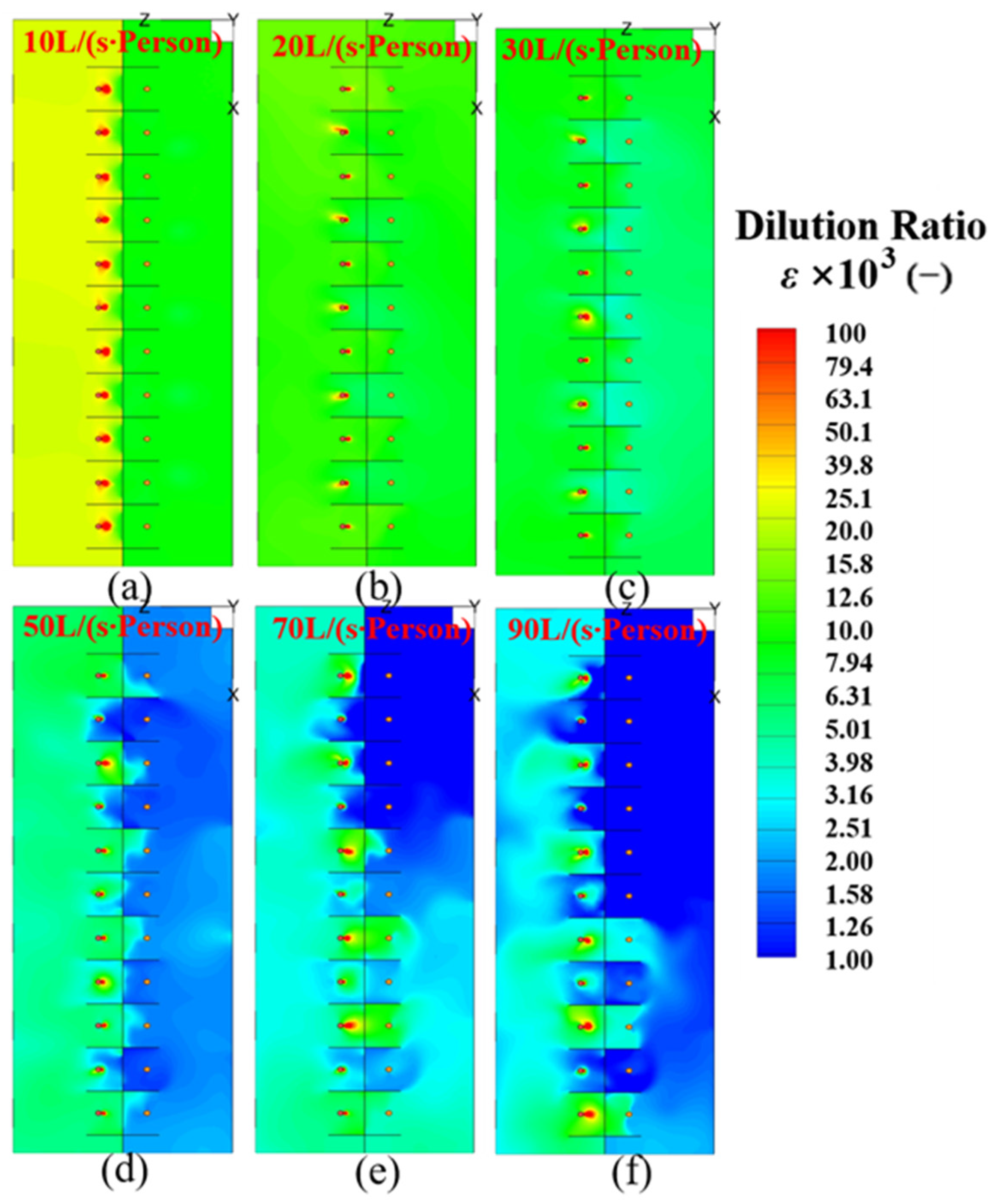
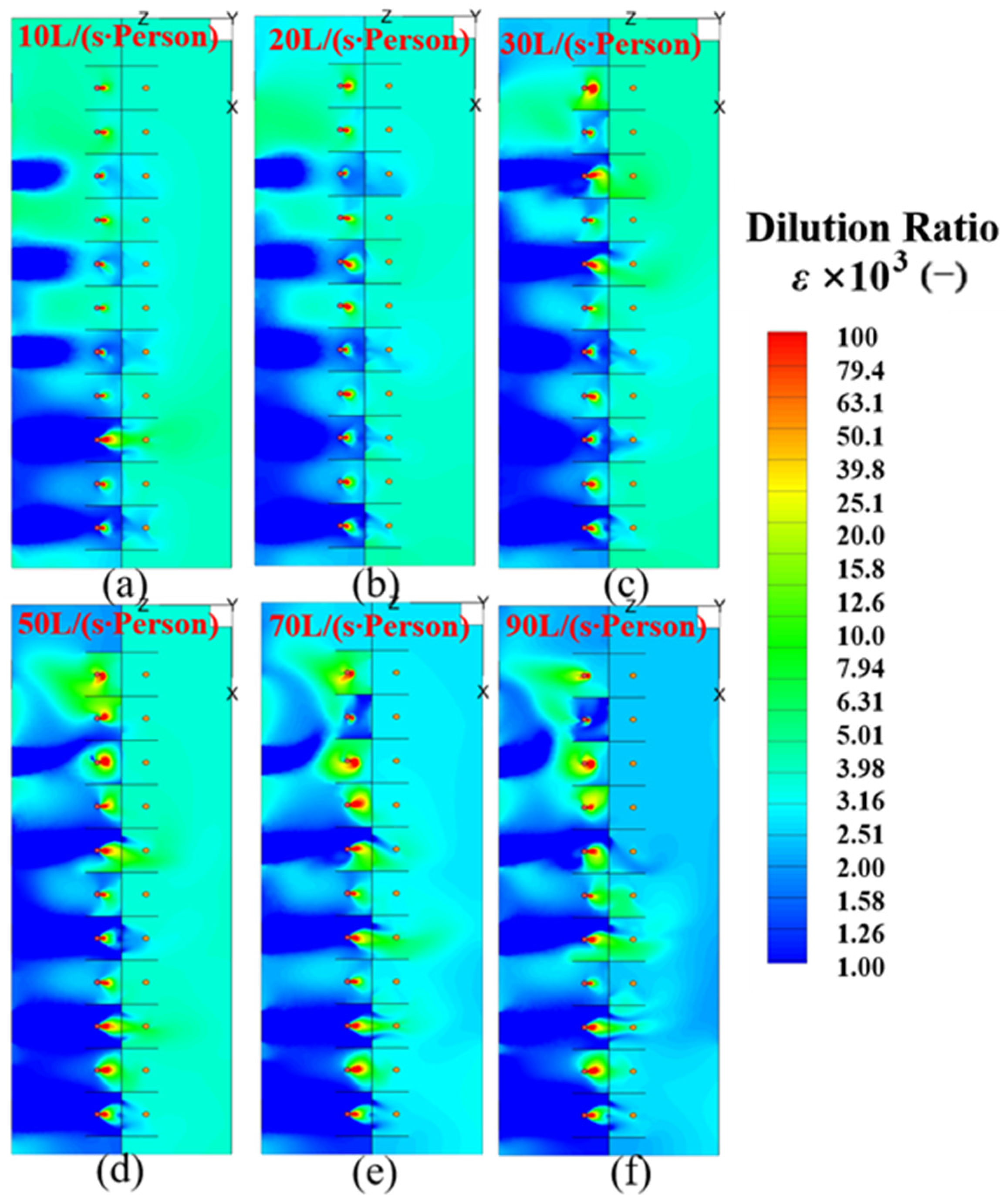
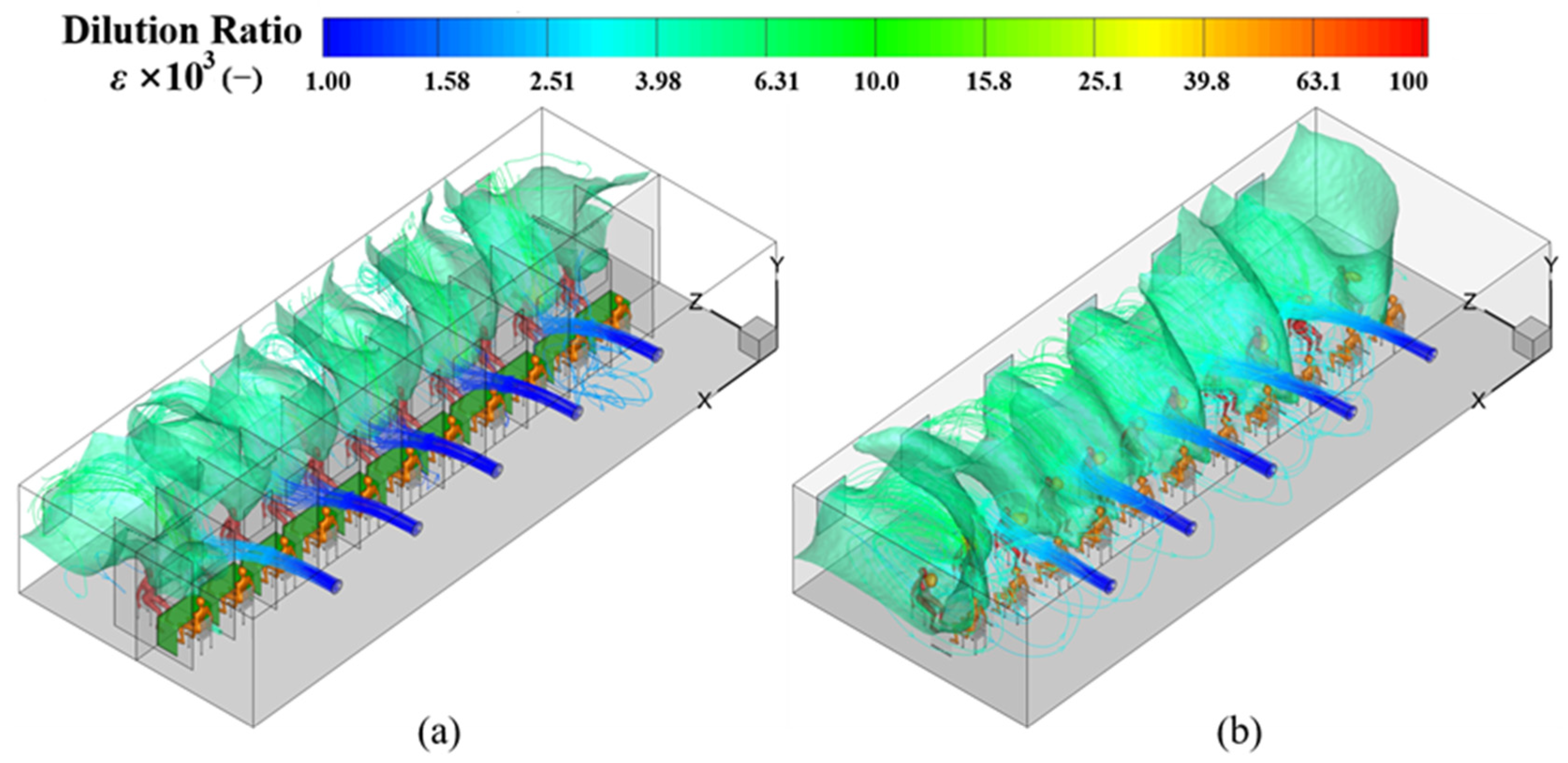
4.2. Dilution Ratio (ε) at the Breathing Height in the MSZ
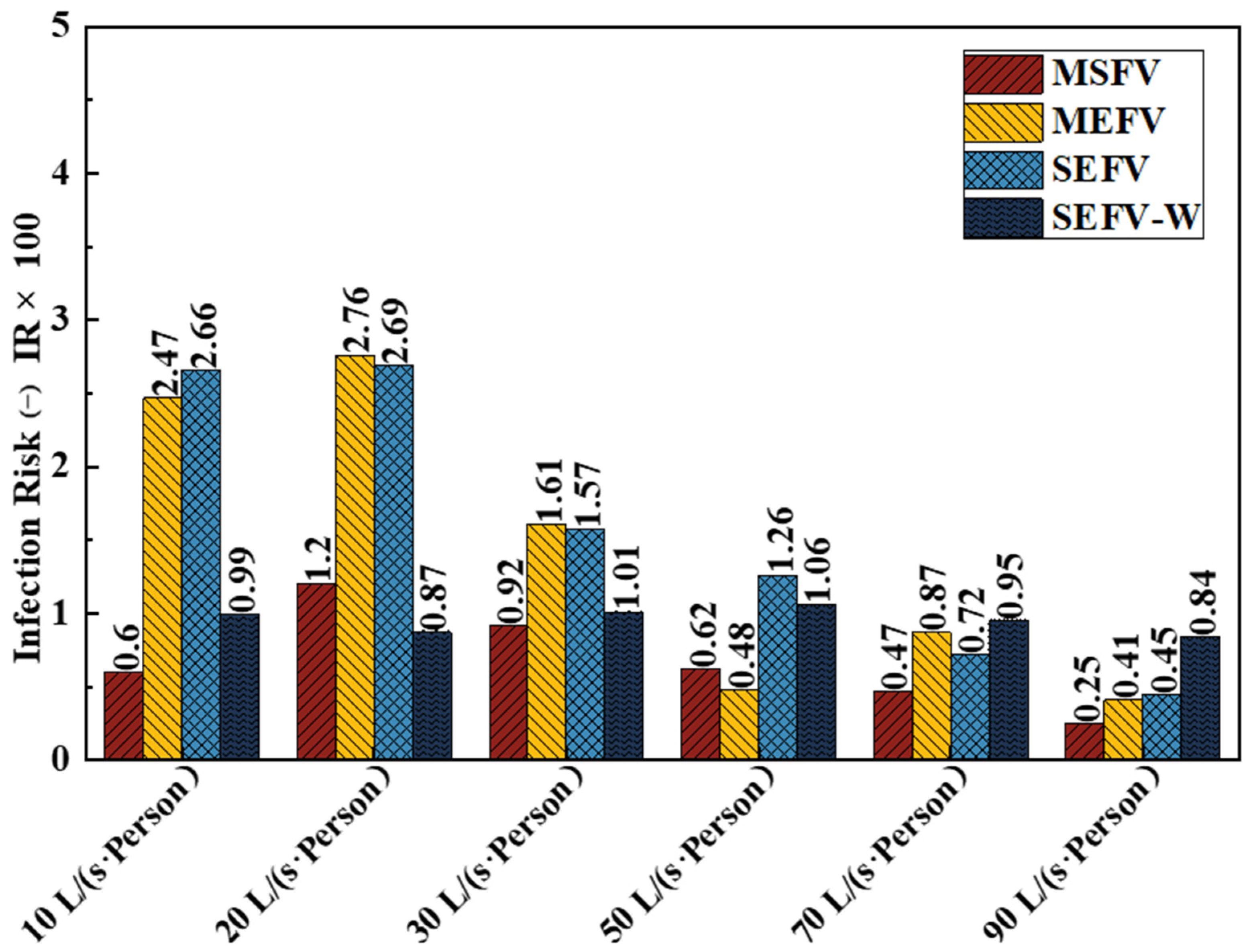
4.3. Inhaled Fraction (IF) and Infection Risk (IR) to the Medical Staff
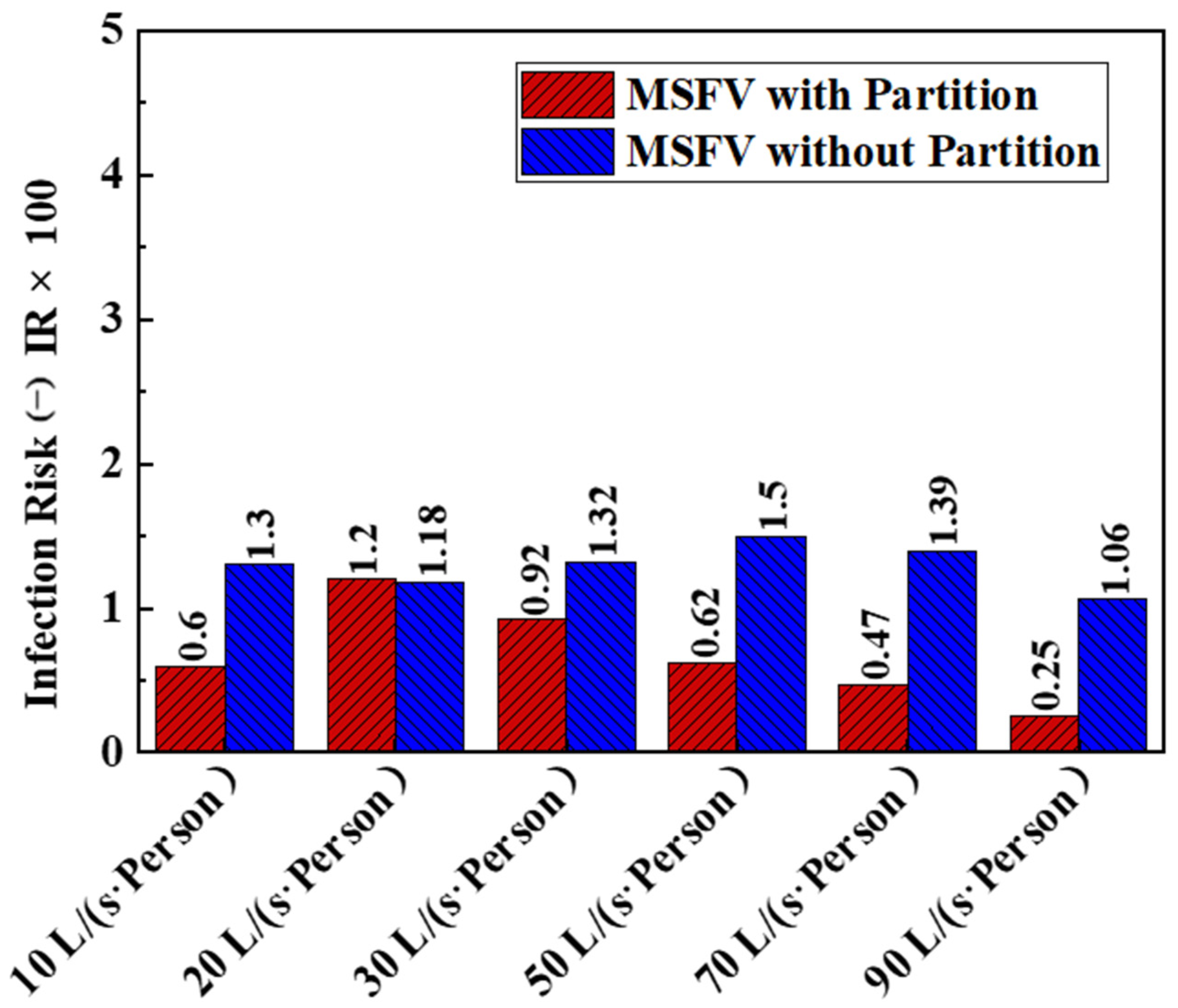
4.4. Influence of the Partitions on the Exposure Risk to Medical Staff
5. Discussion and Conclusions
- Among the four ventilation strategies, multiple supply fans ventilation (MSFV) performed best in reducing the exposure risk to medical staff in the sampling cabin in most cases. It is therefore recommended for ventilation of sampling cabins.
- The exposure risk to medical staff did not decrease linearly with the increase in the ventilation rate. Considering both the exposure risk to medical staff and the energy consumption of the ventilation, a ventilation rate of 10 L/(s∙Person) is recommended for the MSFV.
- For the MSFV, installing partitions was beneficial to reduce the risk of exposure in most cases. Under the recommended ventilation flow rate of 10 L/(s∙Person), the medical staff’s inhaled fraction and exposure risk were reduced by more than 50%.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Acter, T.; Uddin, N.; Das, J.; Akhter, A.; Choudhury, T.R.; Kim, S. Evolution of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) as coronavirus disease 2019 (COVID-19) pandemic: A global health emergency. Sci. Total Environ. 2020, 730, 138996. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, L.H.; Drew, D.A.; Graham, M.S.; Joshi, A.D.; Guo, C.G.; Ma, W.J.; Mehta, R.S.; Warner, E.T.; Sikavi, D.R.; Lo, C.H.; et al. Risk of COVID-19 among front-line health-care workers and the general community: A prospective cohort study. Lancet Public Health 2020, 5, E475–E483. [Google Scholar] [CrossRef]
- Bandyopadhyay, S.; Baticulon, R.E.; Kadhum, M.; Alser, M.; Ojuka, D.K.; Badereddin, Y.; Kamath, A.; Parepalli, S.A.; Brown, G.; Iharchane, S.; et al. Infection and mortality of healthcare workers worldwide from COVID-19: A systematic review. BMJ Global Health 2020, 5, e003097. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention (CDC). How 2019-nCoV Spreads. 2019 Novel Coronavirus; U.S. Department of Health & Human Services: Washington DC, USA, 2020.
- World Health Organization. Coronavirus Disease (COVID-19): How Is It Transmitted? WHO: Geneva, Switzerland, 2020; Available online: https://www.who.int/news-room/q-a-detail/coronavirus-disease-covid-19-how-is-it-transmitted (accessed on 23 December 2021).
- Cravero, C.; Marsano, D. Simulation of COVID-19 Indoor Emissions from Coughing and Breathing with Air Conditioning and Mask Protection Effects. Indoor Built. Environ. 2021. Available online: https://journals.sagepub.com/doi/abs/10.1177/1420326X211039546 (accessed on 23 December 2021). [CrossRef]
- Lidia, M.; Donald, K.M. It Is Time to Address Airborne Transmission of Coronavirus Disease 2019 (COVID-19). Clin. Infect. Dis. 2020, 71, 2311–2313. [Google Scholar]
- Vuorinen, V.; Aarnio, M.; Alava, M.; Alopaeus, V.; Atanasova, N.; Auvinen, M.; Balasubramanian, N.; Bordbar, H.; Erästö, P.; Grande, R.; et al. Modelling aerosol transport and virus exposure with numerical simulations in relation to SARS-CoV-2 transmission by inhalation indoors. Saf. Sci. 2020, 130, 104866. [Google Scholar] [CrossRef]
- Li, H.; Leong, F.Y.; Xu, G.; Kang, C.W.; Lim, K.H.; Tan, B.H.; Loo, C.M. Airborne dispersion of droplets during coughing: A physical model of viral transmission. Sci. Rep. 2021, 11, 4617. [Google Scholar] [CrossRef]
- Leung, N.H.L. Transmissibility and transmission of respiratory viruses. Nat. Rev. Microbiol. 2021, 19, 528–545. [Google Scholar] [CrossRef] [PubMed]
- Qian, H.; Li, Y.; Nielsen, P.V.; Hyldgaard, C.E.; Wong, T.W.; Chwang, A. Dispersion of exhaled droplet nuclei in a two-bed hospital ward with three different ventilation systems. Indoor Air 2006, 16, 111–128. [Google Scholar] [CrossRef] [PubMed]
- Qian, H.; Li, Y.; Nielsen, P.V.; Hyldgaard, C.E. Dispersion of exhalation pollutants in a two-bed hospital ward with a downward ventilation system. Build. Environ. 2008, 43, 344–354. [Google Scholar] [CrossRef]
- Yang, X.; Ou, C.Y.; Yang, H.Y.; Liu, L.; Song, T.; Kang, M.; Lin, H.; Hang, J. Transmission of pathogen-laden expiratory droplets in a coach bus. J. Hazard. Mater. 2020, 397, 122609. [Google Scholar] [CrossRef] [PubMed]
- Cho, J.; Woo, K.; Kim, B.S. Removal of airborne contamination in airborne infectious isolation rooms. Ashrae J. 2019, 61, 8–21. [Google Scholar]
- Somsen, G.A.; van Rijn, C.; Kooij, S.; Bem, R.A.; Bonn, D. Small droplet aerosols in poorly ventilated spaces and SARS-CoV-2 transmission. Lancet Resp. Med. 2020, 8, 658–659. [Google Scholar] [CrossRef]
- Li, Y.; Qian, H.; Hang, J.; Chen, X.; Cheng, P.; Ling, H.; Wang, S.; Liang, P.; Li, J.; Xiao, S.; et al. Probable airborne transmission of SARS-CoV-2 in a poorly ventilated restaurant. Build. Environ. 2021, 196, 107788. [Google Scholar] [CrossRef]
- Ou, C.; Hu, S.; Luo, K.; Yang, H.; Hang, J.; Cheng, P.; Hai, Z.; Xiao, S.; Qian, H.; Xiao, S.; et al. Insufficient ventilation led to a probable long-range airborne transmission of SARS-CoV-2 on two buses. Build. Environ. 2022, 207, 108414. [Google Scholar] [CrossRef]
- Qian, H.; Miao, T.; Liu, L.; Zheng, X.H.; Luo, D.T.; Li, Y.G. Indoor transmission of SARS-CoV-2. Indoor Air 2021, 31, 639–645. [Google Scholar] [CrossRef]
- Satheesan, M.K.; Mui, K.W.; Wong, L.T. A numerical study of ventilation strategies for infection risk mitigation in general inpatient wards. Build. Simul. 2020, 13, 887–896. [Google Scholar] [CrossRef] [Green Version]
- Ye, J.; Qian, H.; Ma, J.; Zhou, R.; Zheng, X. Using air curtains to reduce short-range infection risk in consulting ward: A numerical investigation. Build. Simul. 2021, 14, 325–335. [Google Scholar] [CrossRef]
- Wang, F.; Chaerasari, C.; Rakshit, D.; Permana, I. Performance Improvement of a Negative-Pressurized Isolation Room for Infection Control. Healthcare 2021, 9, 1081. [Google Scholar] [CrossRef] [PubMed]
- Ichai, P.; Saliba, F.; Baune, P.; Daoud, A.; Coilly, A.; Samuel, D. Impact of negative air pressure in ICU rooms on the risk of pulmonary aspergillosis in COVID-19 patients. Crit. Care 2020, 24, 538. [Google Scholar] [CrossRef]
- Crawford, C.; Vanoli, E.; Decorde, B.; Lancelot, M.; Duprat, C.; Josserand, C.; Jilesen, J.; Bouadma, L.; Timsit, J.-F. Modeling of aerosol transmission of airborne pathogens in ICU rooms of COVID-19 patients with acute respiratory failure. Sci. Rep. 2021, 11, 11778. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Liu, H.; Yin, H.; Rong, R.; Cao, G.; Deng, Q. Prevention of surgical site infection under different ventilation systems in operating room environment. Front. Environ. Sci. Eng. 2020, 15, 36. [Google Scholar] [CrossRef] [PubMed]
- Agirman, A.; Cetin, Y.E.; Avci, M.; Aydin, O. Effect of air exhaust location on surgical site particle distribution in an operating room. Build. Simul. 2020, 13, 979–988. [Google Scholar] [CrossRef]
- Cheng, K.K.; Lam, T.H.; Leung, C.C. Wearing Face Masks in the Community during the COVID-19 Pandemic: Altruism and Solidarity. Lancet 2020. Available online: https://www.thelancet.com/journals/lancet/article/PIIS0140-6736(20)30918-1/fulltext (accessed on 16 April 2020). [CrossRef]
- Liu, F.; Luo, Z.; Li, Y.; Zheng, X.; Zhang, C.; Qian, H. Revisiting physical distancing threshold in indoor environment using infection-risk-based modeling. Environ. Int. 2021, 153, 106542. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Infection Prevention and Control during Health Care when Coronavirus Disease (COVID-19) Is Suspected or Confirmed: Interim Guidance; WHO: Geneva, Switzerland, 2021. [Google Scholar]
- Centers for Disease Control and Prevention (CDC). What Airport Retail or Food Service Workers Need to Know about COVID-19; U.S. Department of Health & Human Services: Washington, DC, USA, 2020.
- Nielsen, P.V.; Li, Y.; Buus, M.; Winther, F.V. Risk of cross-infection in a hospital ward with downward ventilation. Build. Environ. 2010, 45, 2008–2014. [Google Scholar] [CrossRef]
- Liu, Z.; Li, R.; Wu, Y.; Ju, R.; Gao, N. Numerical study on the effect of diner divider on the airborne transmission of diseases in canteens. Energy Build. 2021, 248, 111171. [Google Scholar] [CrossRef]
- Ren, C.; Xi, C.; Wang, J.; Feng, Z.; Nasiri, F.; Cao, S.-J.; Haghighat, F. Mitigating COVID-19 infection disease transmission in indoor environment using physical barriers. Sustain. Cities Soc. 2021, 74, 103175. [Google Scholar] [CrossRef] [PubMed]
- Fennelly, K.P. Particle sizes of infectious aerosols: Implications for infection control. Lancet Respir. Med. 2020, 8, 914–924. [Google Scholar] [CrossRef]
- Tang, J.; Noakes, C.; Nielsen, P.; Eames, I.; Nicolle, A.; Li, Y.; Settles, G. Observing and quantifying airflows in the infection control of aerosol-and airborne-transmitted diseases: An overview of approaches. J. Hosp. Infect. 2011, 77, 213–222. [Google Scholar] [CrossRef]
- Ai, Z.; Mak, C.M.; Gao, N.; Niu, J. Tracer gas is a suitable surrogate of exhaled droplet nuclei for studying airborne transmission in the built environment. Build. Simul. 2020, 13, 489–496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, C.; Nielsen, P.V.; Gong, G.; Liu, L.; Jensen, R.L. Measuring the exhaled breath of a manikin and human subjects. Indoor Air 2015, 25, 188–197. [Google Scholar] [CrossRef] [PubMed]
- GB 50189-2015; Design Standard for Energy Efficiency of Public Buildings: GB50189-2015. China Academy of Building Research: Beijing, China, 2015.
- ASHRAE Standarad 62.1—2019; Ventilation for Acceptable Indoor Air Quality. American Society of Heating, Refrigerating and Air-Conditioning Engineers: Atlanta, GA, USA, 2019.
- GB 50736-2012; Design Code for Heating Ventilation and Air Conditioning of Civil Buildings: 2012. China Architecture and Building Press: Beijing, China, 2012.
- Launder, B.E.; Spalding, D.B. Computer Methods in Applied Mechanics and Engineering; Elsevier: Amsterdam, The Netherlands, 1974; pp. 269–289. [Google Scholar]
- Wang, H.; Qian, H.; Zhou, R.; Zheng, X. A novel circulated air curtain system to confine the transmission of exhaled contaminants: A numerical and experimental investigation. Build. Simul. 2020, 13, 1425–1437. [Google Scholar] [CrossRef] [PubMed]
- ANSYS. ANSYS Fluent User’s Guide, Release 17.0.; ANSYS, Inc.: Canonsburg, PA, USA, 2016. [Google Scholar]
- Patankar, S. Numerical Heat Transfer and Fluid Flow; Taylor & Francis: Abingdon, VA, USA, 1980. [Google Scholar]
- Ma, J.; Qian, H.; Nielsen, P.V.; Liu, L.; Li, Y.; Zheng, X. What dominates personal exposure? Ambient airflow pattern or local human thermal plume. Build. Environ. 2021, 196, 107790. [Google Scholar] [CrossRef]
- GAMBIT. GAMBIT 6.2 User’s Guide; Fluent, Inc.: New York, NY, USA, 2005. [Google Scholar]
- Riley, E.; Murphy, G.; Riley, R. Airborne spread of measles in a suburban elementary school. Am. J. Epidemiol 1978, 107, 421–432. [Google Scholar] [CrossRef] [PubMed]
- Shao, X.; Li, X. COVID-19 transmission in the first presidential debate in 2020. Phys. Fluids 2020, 32, 115125. [Google Scholar] [CrossRef] [PubMed]
- Gupta, J.K.; Lin, C.H.; Chen, Q. Risk assessment of airborne infectious diseases in aircraft cabins. Indoor Air 2012, 22, 388–395. [Google Scholar] [CrossRef]
- Dai, H.; Zhao, B. Association of the infection probability of COVID-19 with ventilation rates in confined spaces. Build. Simul. 2020, 13, 1321–1327. [Google Scholar] [CrossRef]
- Qian, Y.; Zeng, T.; Wang, H.; Xu, M.; Chen, J.; Hu, N.; Chen, D.; Liu, Y. Safety management of nasopharyngeal specimen collection from suspected cases of coronavirus disease 2019. Int. J. Nurs. Sci. 2020, 7, 153–156. [Google Scholar] [CrossRef]
- Posner, J.; Buchanan, C.; Dunn-Rankin, D. Measurement and prediction of indoor air flow in a model room. Energy Build. 2003, 35, 515–526. [Google Scholar] [CrossRef]
- Yuan, X.; Chen, Q.; Glicksman, L.R.; Hu, Y.; Yang, X. Measurements and computations of room airflow with displacement ventilation. Ashrae Trans. 1999, 105, 340. [Google Scholar]
- Hui, D.S.; Chow, B.; Chu, L.; Ng, S.; Gin, T.; Chan, M. Exhaled air dispersion during coughing with and without wearing a surgical or N95 mask. PLoS ONE 2012, 7, e50845. [Google Scholar] [CrossRef] [PubMed]
- Ai, Z.; Huang, T.; Melikov, A.K. Airborne transmission of exhaled droplet nuclei between occupants in a room with horizontal air distribution. Build. Environ. 2019, 163, 106328. [Google Scholar] [CrossRef]
- Licina, D.; Melikov, A.; Sekhar, C.; Tham, K.W. Human convective boundary layer and its interaction with room ventilation flow. Indoor Air 2015, 25, 21–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grosskopf, K. Balancing ventilation and energy use in hospitals: A case study of bioaerosol transport in healthcare environments. Antimicrob. Resist. Infect. Control 2013, 2, P396. [Google Scholar] [CrossRef] [Green Version]
- Cheng, P.; Luo, K.; Xiao, S.; Yang, H.; Hang, J.; Ou, C.; Cowling, B.J.; Yen, H.L.; Hui, D.S.; Hu, S.; et al. Predominant airborne transmission and insignificant fomite transmission of SARS-CoV-2 in a two-bus COVID-19 outbreak originating from the same pre-symptomatic index case. J. Hazard. Mater. 2022, 425, 128051. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Li, Y.; Zhou, H.; Lin, J.; Zheng, Z.; Xu, H.; Lin, B.; Lin, M.; Liu, L. Affordable measures to monitor and alarm nosocomial SARS-CoV-2 infection due to poor ventilation. Indoor Air 2021, 31, 1833–1842. [Google Scholar] [CrossRef] [PubMed]
- Epple, P.; Steppert, M.; Florschütz, M.; Dahlem, P. Partition Walls as Effective Protection from Bio-Aerosols in Classrooms—An Experimental Investigation. Gms. Hyg. Infect. Control 2021, 16. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7982998/ (accessed on 26 February 2021).
- Nishimura, H.; Sakata, S. Development of positive/negative pressure booth generating airflow for protection of medical staff from contagious respiratory pathogens. J. Thorac. Dis. 2020, 12, 4633. [Google Scholar] [CrossRef] [PubMed]
- Fanger, P.O. Thermal Comfort Analysis and Applications in Environment Engineering; Danish Technical Press: Copenhagen, Denmark, 1972; 244p. [Google Scholar]







| Ventilation Strategy | Supply Openings | Outer Windows |
|---|---|---|
| MSFV | Velocity Inlet (10, 20, 30, 50, 70, 90 L/(s∙Person)) | W1–W6: Pressure Outlet |
| MEFV | Pressure Outlet | W1–W6: Velocity Inlet (−10, −20, −30, −50, −70, −90 L/(s∙Person)) |
| SEFV | Pressure Outlet | W1: Velocity Inlet (−10, −20, −30, −50, −70, −90 L/(s∙Person)); W2–W6: Wall |
| SEFV-W | Pressure Outlet | W1: Velocity Inlet (−10, −20, −30, −50, −70, −90 L/(s∙Person)); W2–W6: Pressure Outlet |
| Flow Rate | MSFV | MEFV | SEFV | SEFV-W | ||||
|---|---|---|---|---|---|---|---|---|
| L/(s·Person) | Without Mask | With Mask | Without Mask | With Mask | Without Mask | With Mask | Without Mask | With Mask |
| 10 | 0.23% | 0.02% | 0.94% | 0.09% | 1.01% | 0.10% | 0.38% | 0.04% |
| 20 | 0.46% | 0.05% | 1.05% | 0.10% | 1.02% | 0.10% | 0.33% | 0.03% |
| 30 | 0.35% | 0.03% | 0.61% | 0.06% | 0.59% | 0.06% | 0.38% | 0.04% |
| 50 | 0.23% | 0.02% | 0.18% | 0.02% | 0.48% | 0.05% | 0.40% | 0.04% |
| 70 | 0.18% | 0.02% | 0.33% | 0.03% | 0.27% | 0.03% | 0.36% | 0.04% |
| 90 | 0.10% | 0.01% | 0.16% | 0.02% | 0.17% | 0.02% | 0.32% | 0.03% |
| Flow Rate | MSFV with Partition | MSFV without Partition | ||
|---|---|---|---|---|
| L/(s·Person) | Without Mask | With Mask | Without Mask | With Mask |
| 10 | 0.23% | 0.02% | 0.49% | 0.05% |
| 20 | 0.46% | 0.05% | 0.45% | 0.04% |
| 30 | 0.35% | 0.03% | 0.50% | 0.05% |
| 50 | 0.23% | 0.02% | 0.57% | 0.06% |
| 70 | 0.18% | 0.02% | 0.40% | 0.04% |
| 90 | 0.10% | 0.01% | 0.40% | 0.04% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, J.; Qian, H.; Liu, F.; Sui, G.; Zheng, X. Exposure Risk to Medical Staff in a Nasopharyngeal Swab Sampling Cabin under Four Different Ventilation Strategies. Buildings 2022, 12, 353. https://doi.org/10.3390/buildings12030353
Ma J, Qian H, Liu F, Sui G, Zheng X. Exposure Risk to Medical Staff in a Nasopharyngeal Swab Sampling Cabin under Four Different Ventilation Strategies. Buildings. 2022; 12(3):353. https://doi.org/10.3390/buildings12030353
Chicago/Turabian StyleMa, Jianchao, Hua Qian, Fan Liu, Guodong Sui, and Xiaohong Zheng. 2022. "Exposure Risk to Medical Staff in a Nasopharyngeal Swab Sampling Cabin under Four Different Ventilation Strategies" Buildings 12, no. 3: 353. https://doi.org/10.3390/buildings12030353






