Application of the Hazardous Waste Vitreous Enamel Generated in the Production Process of Heating Devices as a Partial Replacement for Cement
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sampling
2.2. Physical-Chemical Analyses
2.3. Testing of the Pozzolanic Activity of Waste Vitreous Enamel
2.4. Mortar and Concrete Preparation
2.5. Physical-Mechanical Characterization of Mortar and Concrete Samples
3. Results and Discussion
3.1. Physical-Chemical Characterization of the Waste Vitreous Enamel Samples
3.1.1. Determination of Chemical Composition and Heavy Metal Content
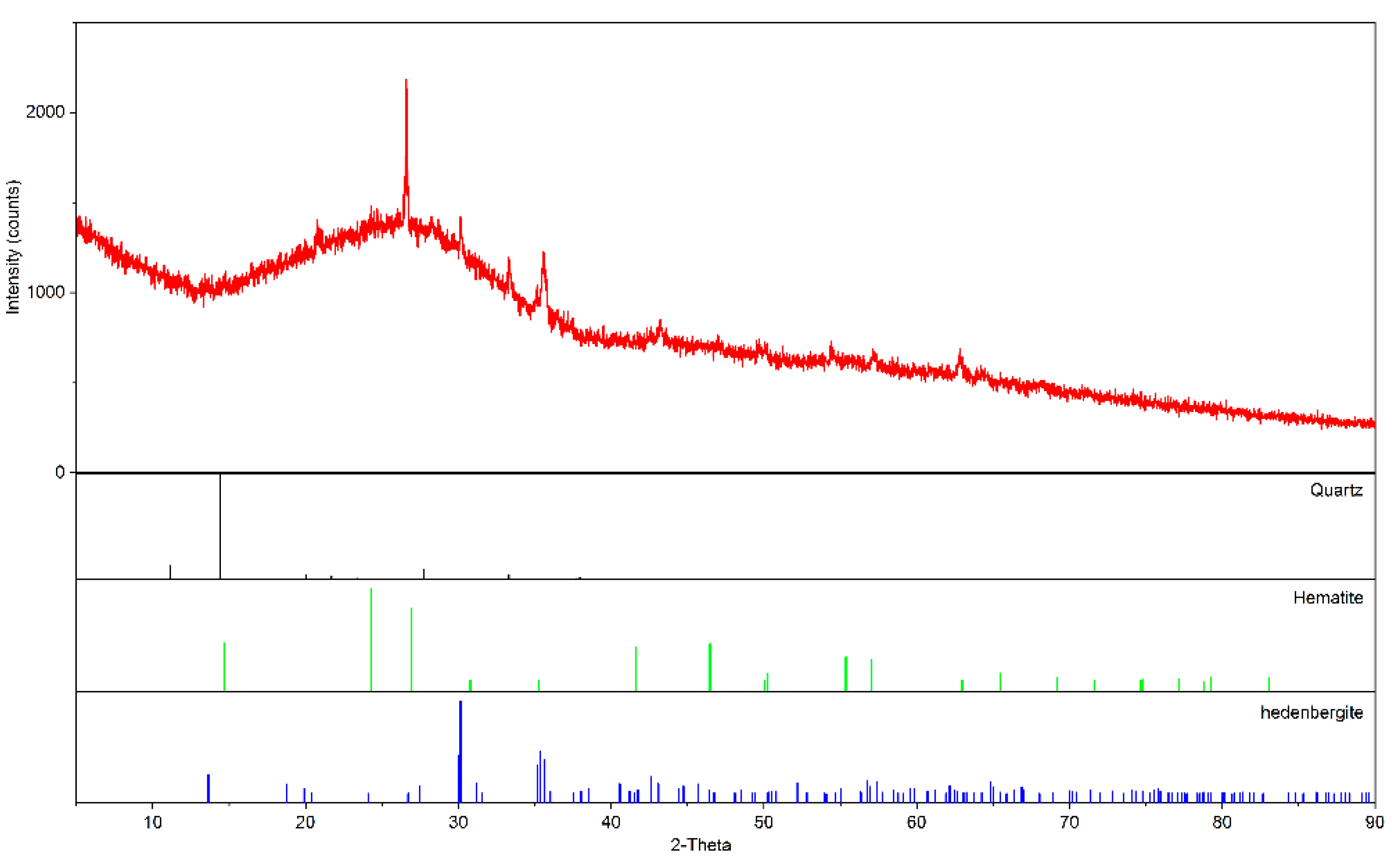
3.1.2. X-ray Powder Diffraction Analysis (XRD)
3.1.3. Textural Properties
3.2. Testing of the Pozzolanic Activity of Waste Vitreous Enamel
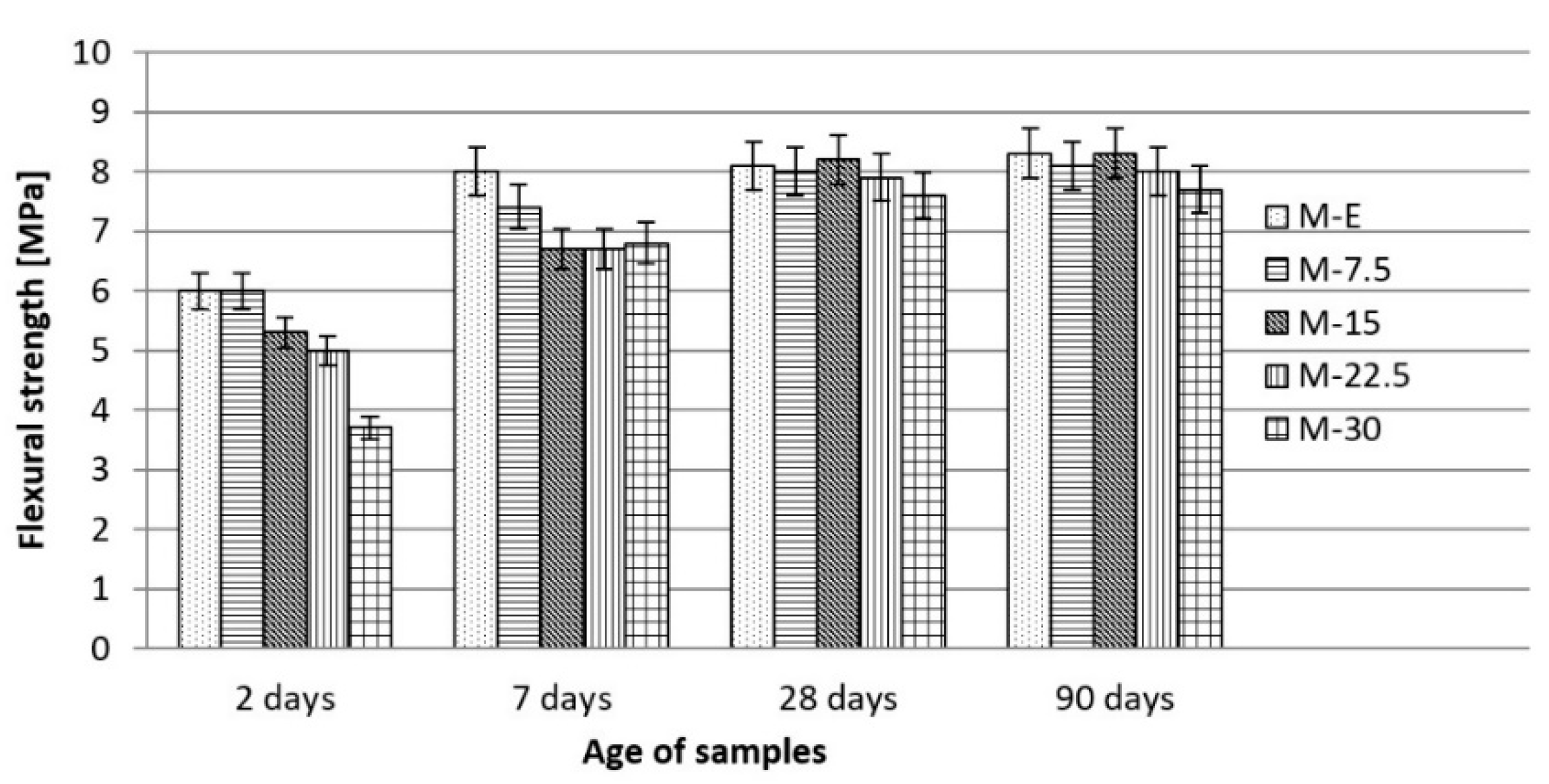
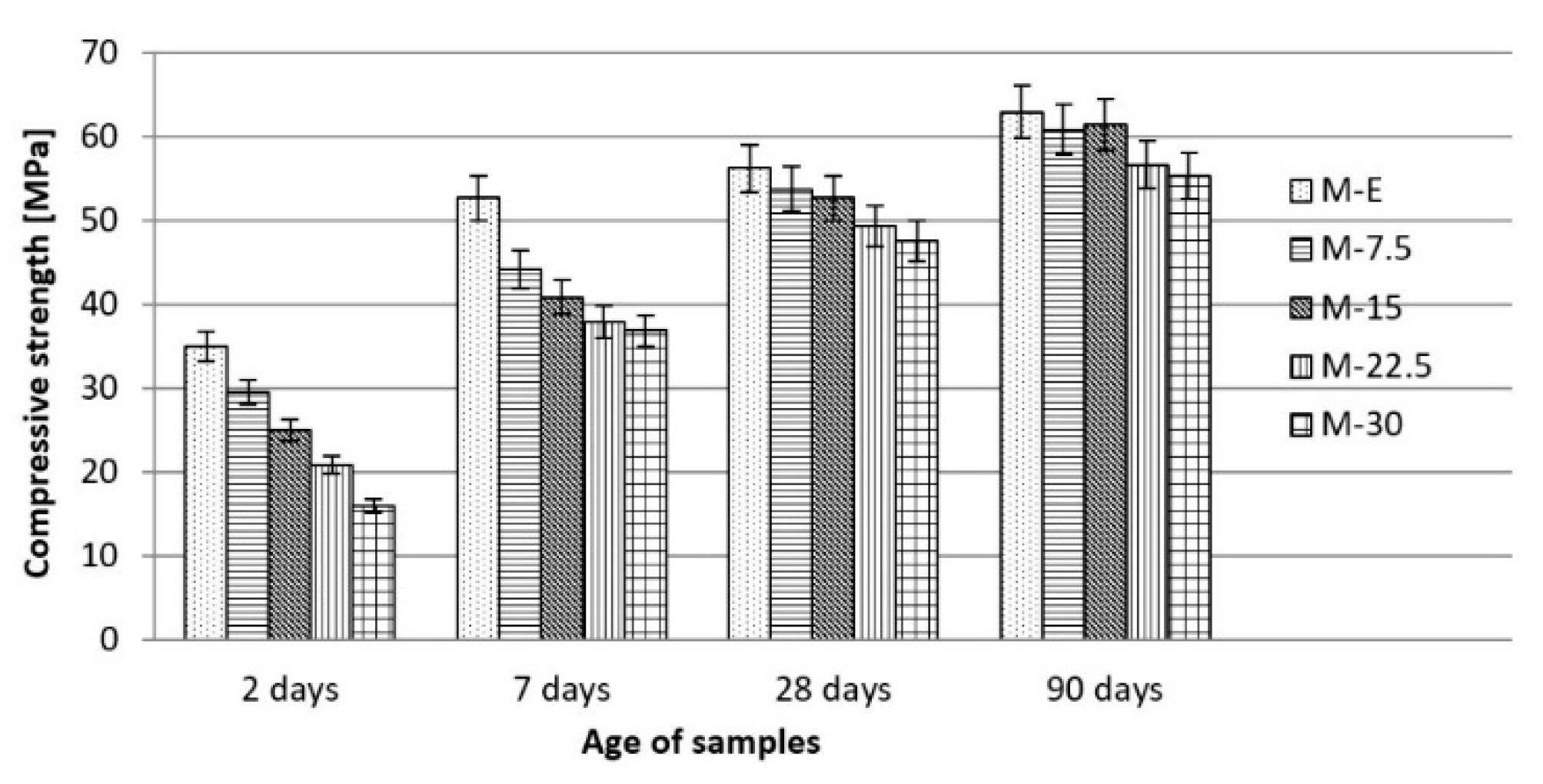
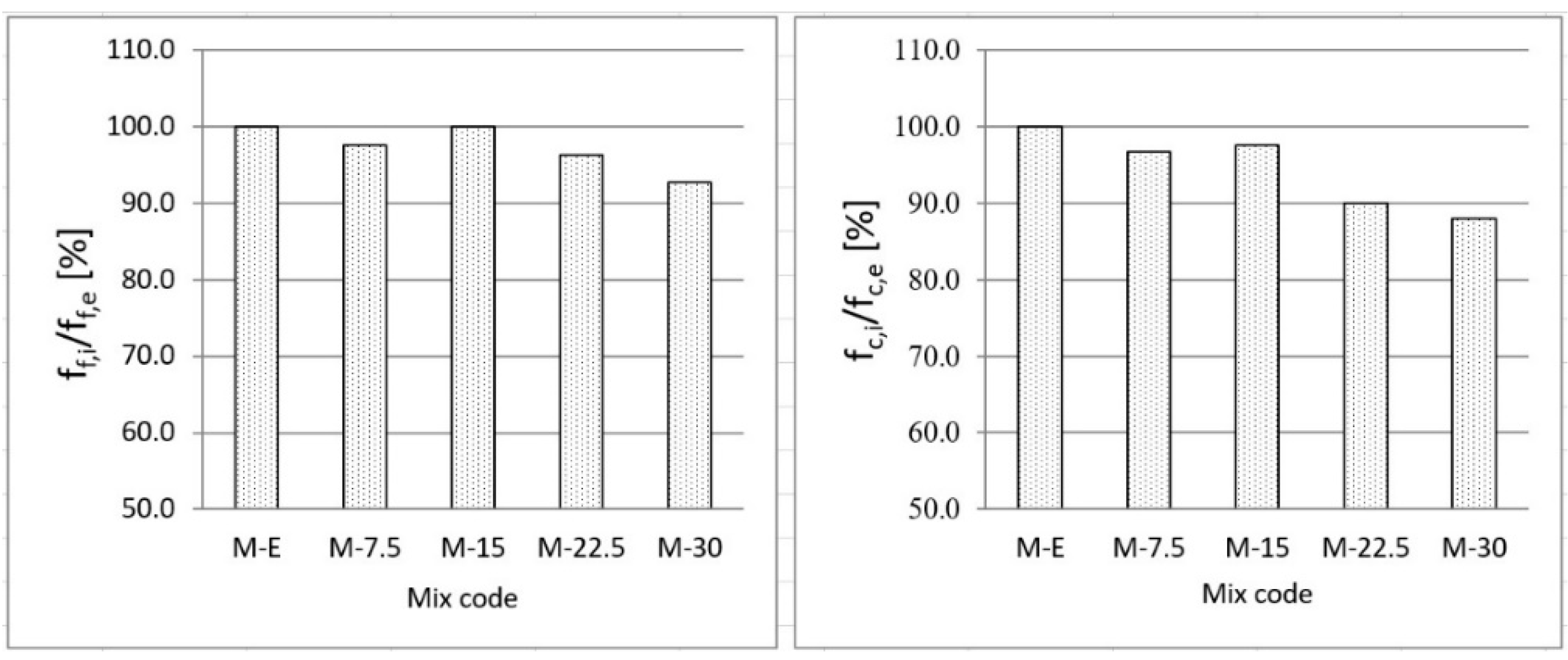
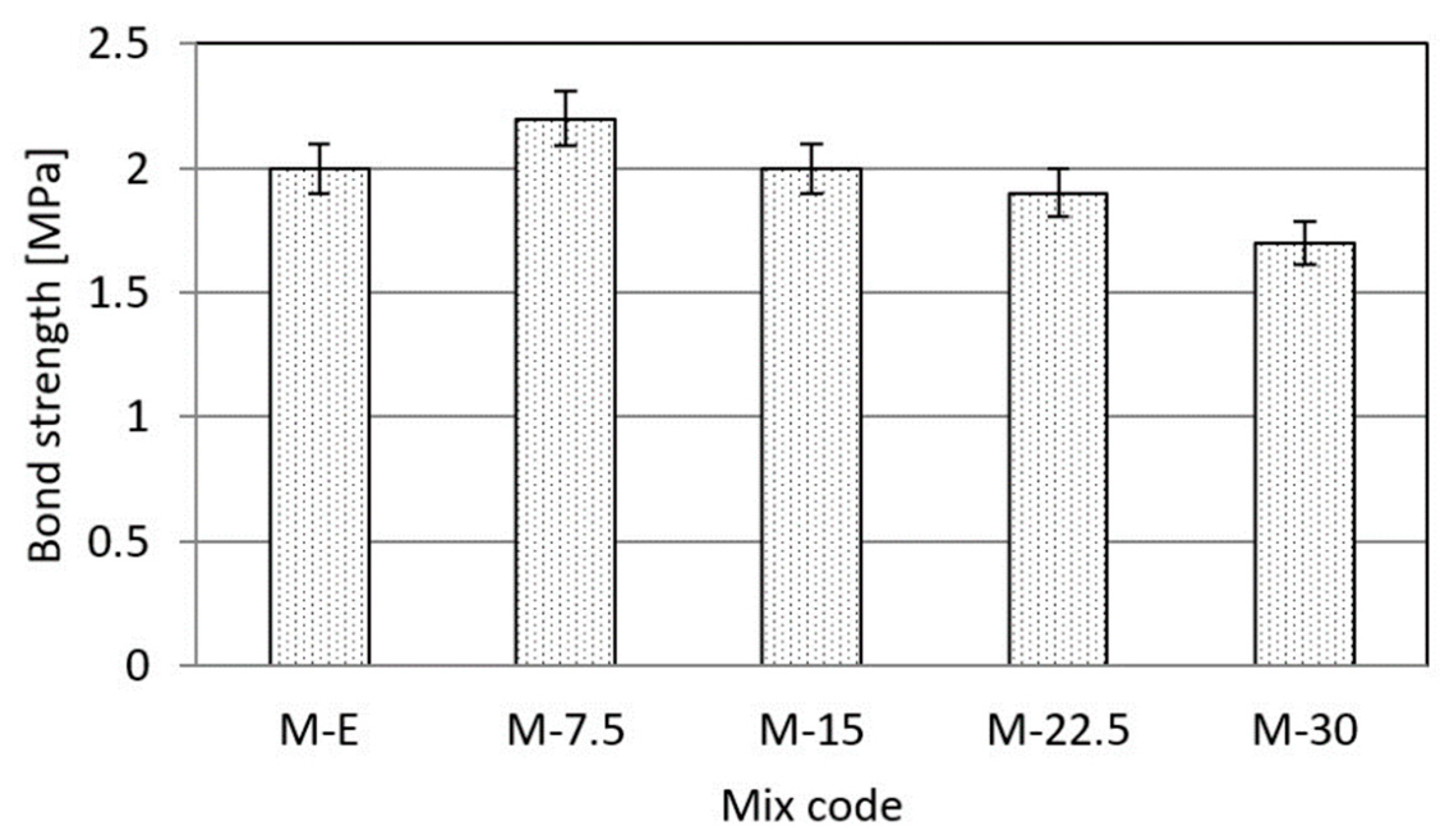
3.3. Effects of Waste Vitreous Enamel on Mortar Properties
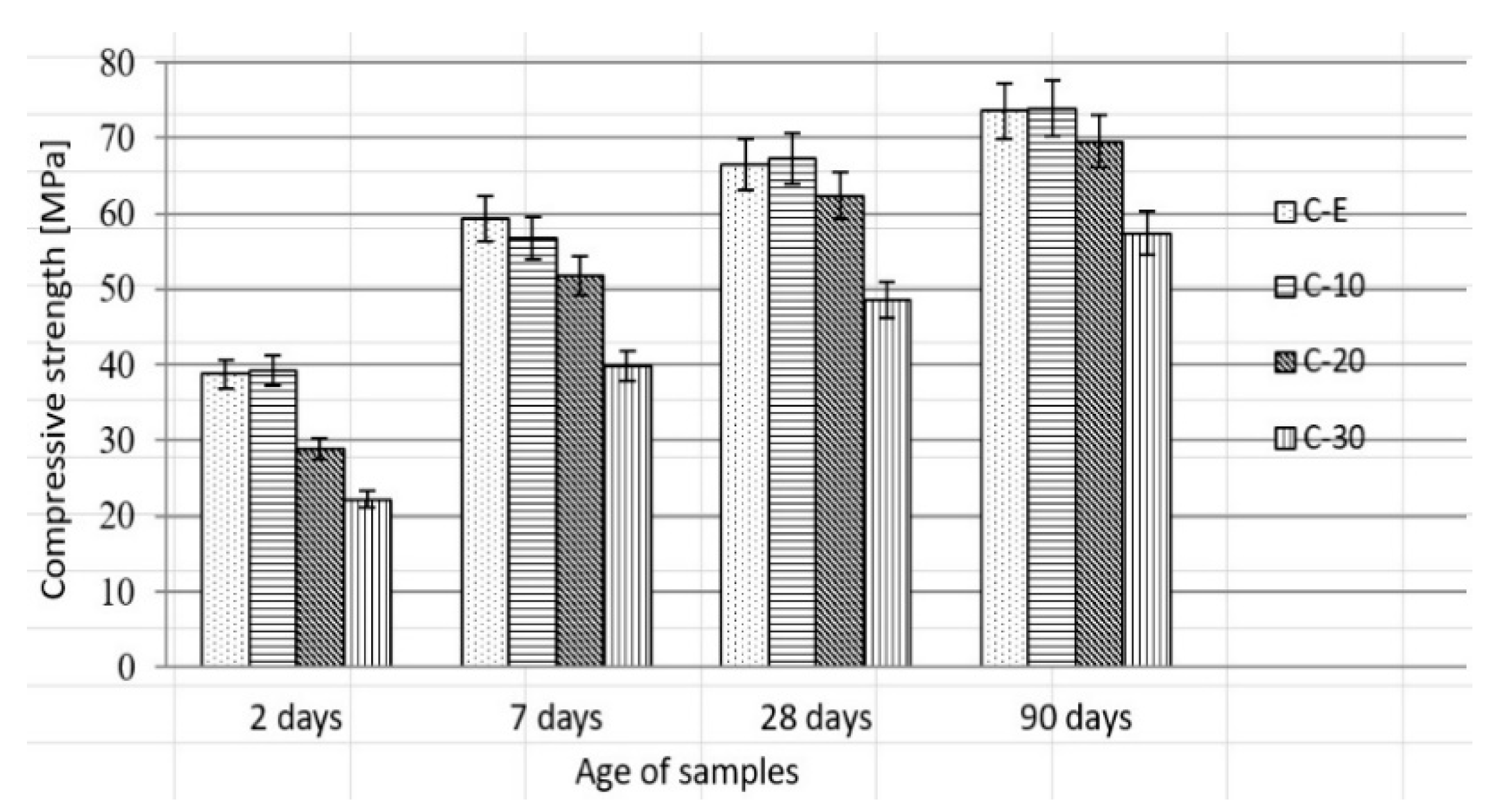
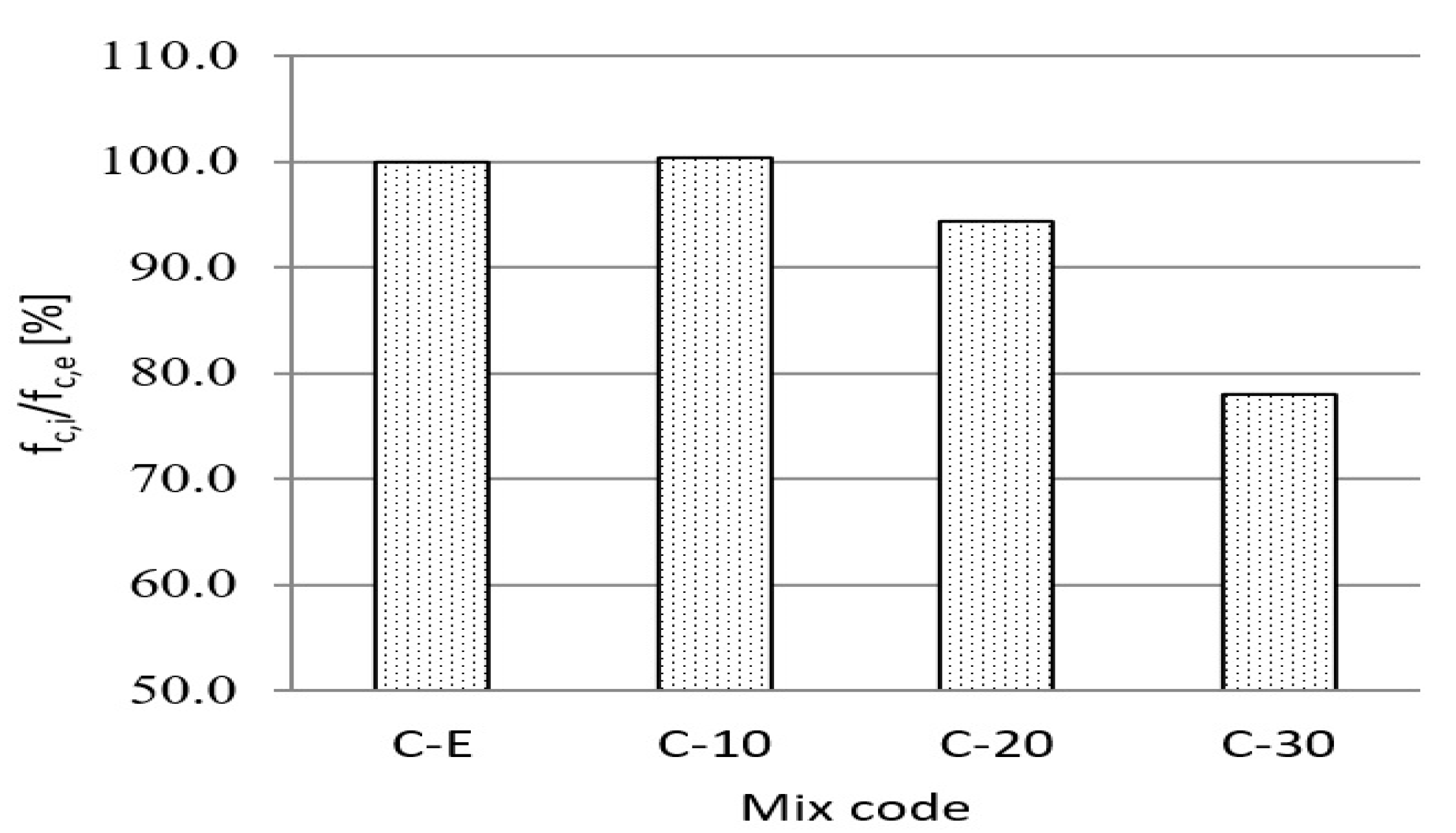
3.4. Effect of Waste Vitreous Enamel on Concrete Properties
3.5. Leaching Test
4. Conclusions
- The waste vitreous enamel possesses pozzolanic activity and belongs to class 5 of pozzolanic materials. Additionally, the activity index, water requirement, setting time, and soundness possess values that completely satisfied standard requirements, which means it is possible to use a type II admixture for the production of concrete in accordance with EN 206;
- Replacing cement with waste material contributed to a reduction in compressive strength of up to 12% and flexural strength of up to 7%, and also contributed to a reduction in shrinkage due to drying and water absorption. Due to its glassy structure, waste enamel has a positive effect on the consistency of the mortar by increasing its workability by approximately 20%. Generally, replacement of cement with waste vitreous enamel in the amount of up to 20% in mortar does not greatly reduce its physical and mechanical characteristics compared to the characteristics of the reference mortar made with 100% cement;
- The use of waste vitreous enamel in concrete, as a partial replacement of cement, contributes to a slight decrease in mechanical properties, while on the other hand it does not compromise the durability of the concrete. Replacement of cement with waste vitreous enamel contributes to the improvement of concrete consistency (increases settlement by 10–30 mm) and reduction of entrained air content by 20–50% compared to reference concrete. Compressive strength decreases by 6% at 20% replacement of cement, i.e., 22% at 30% replacement, while flexural strength decreases by a maximum of 13%. The depth of penetration of water under pressure in hardened concrete and freeze/thaw resistance with de-icing salt of concrete with waste material are in range of reference concrete for replacement of cement to 20%. Generally, the physical and mechanical properties of concrete mixed with up to 20% of cement replacement with waste vitreous enamel do not significantly differ from the reference concrete;
- Bearing in mind that the use of waste vitreous enamel solves the problem of its disposal, and it can be used as a replacement for cement, it can be used in the production of mortar and concrete whose quality is slightly lower than cement composites made only with cement as a binder;
- The application of the hazardous waste vitreous enamel as a replacement for cement in mortar and concrete productions is completely acceptable from ecological as well as human health safety aspects;
- Further research should be focused on the study of the effect of pozzolanic reaction of waste vitreous enamel on the characteristics of the interfacial transition zone and the porous system of concrete composites in general.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Blackman, W.C., Jr. Basic Hazardous Waste Management; CRC Press Lewis Publishers: Boca Raton, FL, USA, 1996. [Google Scholar]
- Sharma, H.D.; Lewis, S.P. Waste Containment Systems, Waste Stabilization, and Landfills: Design and Evaluation; John Wiley and Sons: New York, NY, USA, 1994. [Google Scholar]
- Millano, E.F. Hazardous waste: Storage, disposal, remediation, and closure. Water Environ. Res. 1996, 68, 586–607. [Google Scholar] [CrossRef]
- Misra, V.; Pandey, S.D. Hazardous waste, impact on health and environment for development of better waste management strategies in future in India. Environ. Int. 2005, 31, 417–431. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Tong, K.; Xu, F.; Xue, M.; Chen, H.; Chen, Q.; Wang, D.; Xu, Y. Development of supercritical water oxidation technology for application to hazardous waste treatment: An extreme case study. J. Environ. Chem. Eng. 2021, 9, 105296. [Google Scholar] [CrossRef]
- Ali, B.H.; Baghdadi, M.; Torabian, A. Application of nickel foam as an effective electrode for the electrochemical treatment of liquid hazardous wastes of COD analysis containing mercury, silver, and chromium (VI). Environ. Technol. Innov. 2021, 23, 101617. [Google Scholar] [CrossRef]
- Gupt, C.B.; Bordoloi, S.; Sekharan, S.; Sarmah, A.K. Adsorption characteristics of Barmer bentonite for hazardous waste containment application. J. Hazard. Mater. 2020, 396, 122594. [Google Scholar] [CrossRef]
- Je, C.H.; Stone, R.; Oberg, S.G. Development and application of a multi-channel monitoring system for near real-time VOC measurement in a hazardous waste management facility. Sci. Total Environ. 2007, 382, 364–374. [Google Scholar] [CrossRef]
- Duncan, C.M.; Mainhagu, J.; Virgone, K.; Ramírez, D.M.; Brusseau, M.L. Application of phytoscreening to three hazardous waste sites in Arizona. Sci. Total Environ. 2017, 609, 951–955. [Google Scholar] [CrossRef]
- Andrade, L.C.; Míguez, C.G.; Gómez, M.T.; Bugallo, P.B. Management strategy for hazardous waste from atomised SME: Application to the printing industry. J. Clean. Prod. 2012, 35, 214–229. [Google Scholar] [CrossRef]
- Otwong, A.; Jongmeewasin, S.; Phenrat, T. Legal obstacles for the circular economy in Thailand: Illegal dumping of recyclable hazardous industrial waste. J. Clean. Prod. 2021, 302, 126969. [Google Scholar] [CrossRef]
- Liu, J.; Fan, X.; Li, Z.; Zhang, W.; Jin, H.; Xing, F.; Tang, L. Novel recycling application of high volume municipal solid waste incineration bottom ash (MSWIBA) into sustainable concrete. Sci. Total Environ. 2022, 838, 156124. [Google Scholar] [CrossRef]
- Liu, J.; Hu, L.; Tang, L.; Ren, J. Utilisation of municipal solid waste incinerator (MSWI) fly ash with metakaolin for preparation of alkali-activated cementitious material. J. Hazard. Mater. 2021, 402, 123451. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Liang, Z.; Jin, H.; Kastiukas, G.; Tang, L.; Xing, F.; Ren, J. Alkali-activated binders based on incinerator bottom ash combined with limestone-calcined clay or fly ash. Constr. Build. Mater. 2022, 320, 126306. [Google Scholar] [CrossRef]
- Ren, J.; Hu, L.; Dong, Z.; Tang, L.; Xing, F.; Liu, J. Effect of silica fume on the mechanical property and hydration characteristic of alkali-activated municipal solid waste incinerator (MSWI) fly ash. J. Clean. Prod. 2021, 295, 126317. [Google Scholar] [CrossRef]
- Kragović, M.; Ristić, N.; Gulicovski, J.; Nedeljković, A.; Pašalić, S.; Ristović, I.; Stojmenović, M. Application of Lignite Combustion Waste Slag Generated in Heating Plants as a Partial Replacement for Cement. Part II: Physical–Mechanical and Physical–Chemical Characterization of Mortar and Concrete. Minerals 2021, 11, 925. [Google Scholar] [CrossRef]
- Nedeljković, A.; Stojmenović, M.; Gulicovski, J.; Ristić, N.; Milićević, S.; Krstić, J.; Kragović, M. Waste Slag from Heating Plants as a Partial Replacement for Cement in Mortar and Concrete Production. Part I—Physical–Chemical and Physical–Mechanical Characterization of Slag. Minerals 2020, 10, 992. [Google Scholar] [CrossRef]
- Mymrin, V.; Presotto, P.; Alekseev, K.; Avanci, M.A.; Rolim, P.H.; Petukhov, V.; Taskin, A.; Gidarakos, E.; Valouma, A.; Yu, G. Application of hazardous serpentine rocks’ extraction wastes in composites with glass waste and clay-sand mix to produce environmentally clean construction materials. Constr. Build. Mater. 2020, 234, 117319. [Google Scholar] [CrossRef]
- Pitarch, A.; Reig, L.; Gallardo, A.; Soriano, L.; Borrachero, M.; Rochina, S. Reutilisation of hazardous spent fluorescent lamps glass waste as supplementary cementitious material. Constr. Build. Mater. 2021, 292, 123424. [Google Scholar] [CrossRef]
- Najm, O.; El-Hassan, H.; El-Dieb, A. Ladle slag characteristics and use in mortar and concrete: A comprehensive review. J. Clean. Prod. 2020, 288, 125584. [Google Scholar] [CrossRef]
- Lieberman, R.N.; Knop, Y.; Izquierdo, M.; Palmerola, N.M.; de la Rosa, J.; Cohen, H.; Muñoz-Quirós, C.; Cordoba, P.; Querol, X. Potential of hazardous waste encapsulation in concrete with coal fly ash and bivalve shells. J. Clean. Prod. 2018, 185, 870–881. [Google Scholar] [CrossRef]
- Long, W.-J.; Peng, J.-K.; Gu, Y.-C.; Li, J.-L.; Dong, B.; Xing, F.; Fang, Y. Recycled use of municipal solid waste incinerator fly ash and ferronickel slag for eco-friendly mortar through geopolymer technology. J. Clean. Prod. 2021, 307, 127281. [Google Scholar] [CrossRef]
- Kazmina, O.; Borovoy, V.; Semenova, V. White vitreous enamel for ferrous metals with preliminary thermal activation of frit. Ceram. Int. 2021, 47, 28471–28478. [Google Scholar] [CrossRef]
- Rossi, S.; Russo, F.; Gasparre, N.; Fontanari, V. Influence of graphene addition on the mechanical and surface properties of vitreous enamel coatings. Surf. Coat. Technol. 2020, 398, 126071. [Google Scholar] [CrossRef]
- Rossi, S.; Calovi, M.; Velez, D.; Munoz, J. Influence of addition of hard particles on the mechanical and chemical behavior of vitreous enamel. Surf. Coat. Technol. 2019, 357, 69–77. [Google Scholar] [CrossRef]
- Rossi, S.; Bergamo, L.; Fontanari, V. Fire resistance and mechanical properties of enamelled aluminium foam. Mater. Des. 2017, 132, 129–137. [Google Scholar] [CrossRef]
- Rossi, S.; Quaranta, A.; Tavella, L.; Deflorian, F.; Compagnoni, A.M. Innovative Luminescent Vitreous Enameled Coatings; Elsevier Inc.: Amsterdam, The Netherlands, 2015. [Google Scholar]
- Liu, W.; Wang, N.; Han, J.; Xu, J.; Li, Z.; Qin, W.; Liu, W.; Wang, N.; Han, J.; Xu, J.; et al. Thermal degradation behaviors and evolved products analysis of polyester paint and waste enameled wires during pyrolysis. Waste Manag. 2020, 107, 82–90. [Google Scholar] [CrossRef]
- Gregg, S.J.; Sing, K.S.W. Adsorption, Surface Area and Porosity; Academic Press: London, UK, 1982; pp. 35–120. [Google Scholar]
- Dubinin, M.M. Physical adsorption Caden head of gases and vapors in microspores. In Progress in Surface Press, and Membrane Science; Cadenhead, D.A., Danielli, J.F., Rosenberg, M.D., Eds.; Academic Press: New York, NY, USA, 1975; Volume 9, pp. 1–70. [Google Scholar]
- Dollimore, D.; Heal, G.R. An improved distribution method for the calculation of pore size from adsorption data. J. Appl. Chem. 1964, 14, 109–114. [Google Scholar] [CrossRef]
- Grdić, D.; Topličić-Ćurčić, G.; Grdić, Z.; Ristić, N. Durability Properties of Concrete Supplemented with Recycled CRT Glass as Cementitious Material. Materials 2021, 14, 4421. [Google Scholar] [CrossRef]
- Al-Amoudi, O.; Maslehuddin, M.; Shameem, M.; Ibrahim, M. Shrinkage of plain and silica fume cement concrete under hot weather. Cem. Concr. Compos. 2007, 29, 690–699. [Google Scholar] [CrossRef]
- Roz-Ud-Din, N.; Soroushian, P. Strength and durability of recycled aggregate concrete containing milled glass as partial replacement for cement. Constr. Build. Mater. 2012, 29, 368–377. [Google Scholar]
- Islam, G.M.S.; Rahman, M.H.; Kazi, N. Waste glass powder as partial replacement of cement for sustainable concrete practice. Int. J. Sustain. Built Environ. 2017, 6, 37–44. [Google Scholar] [CrossRef] [Green Version]
- Neville, A.M.; Brooks, J.J. Concrete Technology, 2nd ed.; England Prentice Hall: Harlow, UK, 2010. [Google Scholar]
- Jihwan, K.; Jae-Heum, M.; Shim, J.W.; Jongsung, S.; Hyeon-Gi, L.; Goangseup, Z. Durability properties of a concrete with waste glass sludge exposed to freeze-and-thaw condition and de-icing salt. Constr. Build. Mater. 2014, 66, 398–402. [Google Scholar]

| Chemical Composition | ||||||||
|---|---|---|---|---|---|---|---|---|
| SiO2 | Fe2O3 | Al2O3 | CaO | MgO | SO3 | Na2O | K2O | LOI |
| 21.62 | 2.60 | 7.00 | 60.16 | 2.34 | 2.55 | 0.33 | 0.66 | 2.68 |
| Mineral properties | ||||||||
| brownmillerite; calcium-silicate-oxide; calcite; larnite; magnesium-silicate; calcium-hydroxide | ||||||||
| Component | M-E | M-7.5 | M-15 | M-22.5 | M-30 |
|---|---|---|---|---|---|
| Cement [g (cm3)] | 450.00 (142.86) | 416.25 (132.15) | 382.5 (121.43) | 348.75 (110.71) | 331.96 (100.00) |
| Waste enamel [g (cm3)] | - | 33.75 (11.63) | 67.5 (23.28) | 101.25 (34.91) | 135.00 (46.55) |
| River sand 0/2 mm [g (cm3)] | 1350.00 (517.24) | 1350 (517.24) | 1350 (517.24) | 1350 (517.24) | 1350 (517.24) |
| Water [g] | 225.00 (225.00) | 225 (225) | 225 (225) | 225 (225) | 225 (225) |
| Superplasticizer [g (cm3)] | 1.00 (0.92) | 1.00 (0.92) | 1.00 (0.92) | 1.00 (0.92) | 1.00 (0.92) |
| SUM [g (cm3)] | 2026.00 (886.02) | 2026.0 (886.94) | 2026.0 (887.87) | 2026.0 (888.78) | 2026.0 (889.71) |
| Component | C-E | C-10 | C-20 | C-30 |
|---|---|---|---|---|
| Cement [kg (m3)] | 380.00 (0.121) | 342.00 (0.109) | 304.00 (0.097) | 266.00 (0.084) |
| Waste enamel [kg (m3)] | - | 38.00 (0.013) | 76.00 (0.026) | 114.00 (0.039) |
| River sand 0/4 mm [kg (m3)] | 808.00 (0.308) | 808.00 (0.308) | 808.00 (0.308) | 808.00 (0.308) |
| Crushed aggr. (4/8 mm) [kg (m3)] | 376.00 (0.130) | 376.00 (0.130) | 376.00 (0.130) | 376.00 (0.130) |
| Crushed aggr. (8/16 mm) [kg (m3)] | 696.00 (0.240) | 696.00 (0.240) | 696.00 (0.240) | 696.00 (0.240) |
| Water [kg (m3)] | 180.00 (0.180) | 180.00 (0.180) | 180.00 (0.180) | 180.00 (0.180) |
| Superplasticizer [kg (m3)] | 3.04 (0.002) | 3.04 (0.002) | 3.04 (0.002) | 3.04 (0.002) |
| SUM | 2443.04 (0.981) | 2443.04 (0.982) | 2443.04 (0.983) | 2443.04 (0.983) |
| Content, mg/kg | |||
|---|---|---|---|
| Element | Determined Amount | Limits for Disposal of Non-Hazardous Waste | Limits for Disposal of Hazardous Waste |
| Mo | 15.0 | 10 | 30 |
| Hg | <0.15 | 0.2 | 2.0 |
| Sb | 9.5 | 0.7 | 5.0 |
| Se | 4.5 | 0.5 | 7.0 |
| Sr | 222.0 | - | - |
| Ba | 35.5 | 100 | 300 |
| Ca | 6854.0 | - | - |
| Mg | 234.0 | - | - |
| Ti | 620.0 | - | - |
| V | 18.5 | - | - |
| Mn | 7353.0 | - | - |
| Fe | 10,153.0 | - | - |
| Co | 2872.0 | - | - |
| Cu | 2463.0 | 50 | 100 |
| Zn | 312.0 | 50 | 200 |
| Ni | 68.5 | 10 | 40 |
| Cd | 4.0 | 1 | 5.0 |
| Al | 622.0 | - | - |
| Si | 53,380.0 | - | - |
| Pb | 14.0 | 10 | 50 |
| As | <0.5 | 2 | 25 |
| Be | <0.05 | - | - |
| Cr | 59.0 | 10 | 70 |
| Tl | 48.0 | - | - |
| Sn | <1.0 | - | - |
| Sample | SBET, m2/g | Vtotal, cm3/g | Vmeso, cm3/g | Vmicro, cm3/g |
|---|---|---|---|---|
| Vitreous enamel | 15.3 | 0.0794 | 0.0654 | 0.0140 |
| Property | Standard | Parameters/Results | Requirement | Conclusion |
|---|---|---|---|---|
| Class of pozzolanic materials | SRPS B.C1.018 | Flexural strength: 2.02 MPa Compressive strength: 5.50 MPa | >2.0 MPa >5.0 MPa | class 5 of pozzolanic materials |
| Activity index | EN 450-1 | After 28 days: 79.42% After 90 days—94.75% | >75 %>85% | satisfies satisfies |
| Water requirement | EN 450-1 Annex B | 94% | <95% | satisfies |
| Initial setting time Final setting time | EN 196-3 | 135 min 165 min | <230 min not prescribed | satisfies - |
| Soundness | EN 196-3 | 1.0 mm | <10 mm | satisfies |
| Property | Unit | M-E | M-7.5 | M-15 | M-22.5 | M-30 |
|---|---|---|---|---|---|---|
| Consistency—by flow table | mm | 135 ± 2.0 | 144 ± 2.5 | 147 ± 3.0 | 153 ± 2.5 | 164 ± 3.0 |
| Bulk density of fresh mortar | kg/m3 | 2299 ± 8 | 2294 ± 6 | 2289 ± 7 | 2281 ± 9 | 2275 ± 8 |
| Bulk density of hardened mortar | kg/m3 | 2294 ± 7 | 2290 ± 8 | 2285 ± 6 | 2278 ± 8 | 2270 ± 9 |
| Water abs. at atm. pressure | % | 7.54 ± 0.12 | 7.45 ± 0.10 | 7.36 ± 0.09 | 7.25 ± 0.11 | 7.16 ± 0.08 |
| Water abs. due to capillary action of hardened mortar: for mortars other than renovation mortars | m2 × min−0.5 | 0.24 ± 0.02 | 0.23 ± 0.01 | 0.23 ± 0.02 | 0.22 ± 0.01 | 0.21 ± 02 |
| Water absorption due to capillary action of hardened mortar: for renovation mortars | kg/m2 | 5.50 ± 0.03 | 5.53 ± 0.04 | 5.57 ± 0.05 | 5.62 ± 0.04 | 5.65 ± 0.03 |
| Designation of Mortar | M-E | M-7.5 | M-15 | M-22.5 | M-30 |
|---|---|---|---|---|---|
| Age [days] | |||||
| 3 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| 4 | 0.25 ± 0.02 | 0.23 ± 0.03 | 0.20 ± 0.03 | 0.18 ± 0.02 | 0.16 ± 0.03 |
| 7 | 0.38 ± 0.03 | 0.36 ± 0.02 | 0.33 ± 0.01 | 0.30 ± 0.03 | 0.28 ± 0.02 |
| 14 | 0.59 ± 0.01 | 0.59 ± 0.02 | 0.58 ± 0.02 | 0.57 ± 0.02 | 0.56 ± 0.02 |
| 21 | 0.69 ± 0.03 | 0.70 ± 0.03 | 0.70 ± 0.03 | 0.71 ± 0.03 | 0.72 ± 0.03 |
| 28 | 0.91 ± 0.02 | 0.90 ± 0.03 | 0.88 ± 0.02 | 0.85 ± 0.02 | 0.84 ± 0.02 |
| 56 | 0.93 ± 0.02 | 0.92 ± 0.02 | 0.90 ± 0.02 | 0.87 ± 0.01 | 0.86 ± 0.01 |
| 90 | 0.94 ± 0.03 | 0.93 ± 0.01 | 0.92 ± 0.01 | 0.89 ± 0.02 | 0.88 ± 0.02 |
| Property | Unit | C-E | C-10 | C-20 | C-30 |
|---|---|---|---|---|---|
| Consistency—slump test | mm | 200 ± 10 | 210 ± 12 | 220 ± 9 | 240 ± 11 |
| Density of fresh concrete | kg/m3 | 2466 ± 12 | 2495 ± 9 | 2488 ± 10 | 2481 ± 12 |
| Air content in fresh concrete | % | 2.6 ± 0.19 | 1.9 ± 0.16 | 1.5 ± 0.18 | 1.3 ± 0.15 |
| Density of hardened concrete (water saturated) | kg/m3 | 2455 ± 10 | 2490 ± 12 | 2485 ± 8 | 2477 ± 11 |
| Determination of ultrasonic pulse velocity | km/s | 5.21 ± 0.022 | 5.23 ± 0.020 | 5.20 ± 0.018 | 5.18 ± 0.015 |
| Property | Unit | C-E | C-10 | C-20 | C-30 |
|---|---|---|---|---|---|
| Flexural strength | MPa | 28 days: 7.0 ± 0.2 | 28 days: 6.4 ± 0.3 | 28 days: 5.9 ± 0.1 | 28 days: 5.6 ± 0.3 |
| Compressive strength | MPa | 90 days: 7.4 ± 0.3 | 90 days: 6.8 ± 0.2 | 90 days: 6.4 ± 0.2 | 90 days: 6.1 ± 0.2 |
| See Figure 6 | |||||
| Tensile splitting strength | MPa | 28 days: 3.9 ± 0.2 | 28 days: 3.5 ± 0.2 | 28 days: 3.0 ± 0.3 | 28 days: 2.8 ± 0.2 |
| Secant modulus of elasticity | GPa | 28 days: 33.0 ± 0.3 | 28 days: 33.5 ± 0.2 | 28 days: 33.2 ± 0.2 | 28 days: 33.0 ± 0.3 |
| Property | Unit | C-E | C-10 | C-20 | C-30 |
|---|---|---|---|---|---|
| Depth of penetration of water under pressure | mm | 12 | 10 | 14 | 16 |
| Freeze-thaw resistance with de-icing salts—Scaling | kg/m2 | 0.14 | 0.11 | 0.15 | 0.19 |
| Released Element | Concentration, µg/dm3 | * Allowed Values, µg/dm3 |
|---|---|---|
| Cu | 4 | <100 |
| Zn | 17 | <1000 |
| Ni | 20 | <100 |
| Cd | 2 | <10 |
| Pb | 30 | <100 |
| Cr | 3 | <500 |
| Hg | <0.1 | <1 |
| As | <5 | <50 |
| Mg | 5 | - |
| Fe | 27 | - |
| Co | 8 | - |
| Al | 47,500 | - |
| Sn | <10 | - |
| Si | 665 | - |
| Mo | <1 | - |
| Sr | <1 | - |
| Ca | 140 | - |
| Mn | <1 | - |
| V | <10 | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kragović, M.; Stojmenović, M.; Ristić, N.; Milićević, S.; Živković, S.; Liu, S.; Gulicovski, J. Application of the Hazardous Waste Vitreous Enamel Generated in the Production Process of Heating Devices as a Partial Replacement for Cement. Buildings 2022, 12, 1287. https://doi.org/10.3390/buildings12081287
Kragović M, Stojmenović M, Ristić N, Milićević S, Živković S, Liu S, Gulicovski J. Application of the Hazardous Waste Vitreous Enamel Generated in the Production Process of Heating Devices as a Partial Replacement for Cement. Buildings. 2022; 12(8):1287. https://doi.org/10.3390/buildings12081287
Chicago/Turabian StyleKragović, Milan, Marija Stojmenović, Nenad Ristić, Sonja Milićević, Sanja Živković, Shanke Liu, and Jelena Gulicovski. 2022. "Application of the Hazardous Waste Vitreous Enamel Generated in the Production Process of Heating Devices as a Partial Replacement for Cement" Buildings 12, no. 8: 1287. https://doi.org/10.3390/buildings12081287
APA StyleKragović, M., Stojmenović, M., Ristić, N., Milićević, S., Živković, S., Liu, S., & Gulicovski, J. (2022). Application of the Hazardous Waste Vitreous Enamel Generated in the Production Process of Heating Devices as a Partial Replacement for Cement. Buildings, 12(8), 1287. https://doi.org/10.3390/buildings12081287








