Evaluation of Mineral Carbonation of Asbestos-Tex and Analysis of Airborne Asbestos Concentrations
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Mineral Carbonation
2.2.1. Asbestos-Tex Slurry
2.2.2. Carbonation Experiments
2.2.3. Characterization
2.3. Airborne Asbestos Concentration
3. Results
3.1. Mineral Carbonation
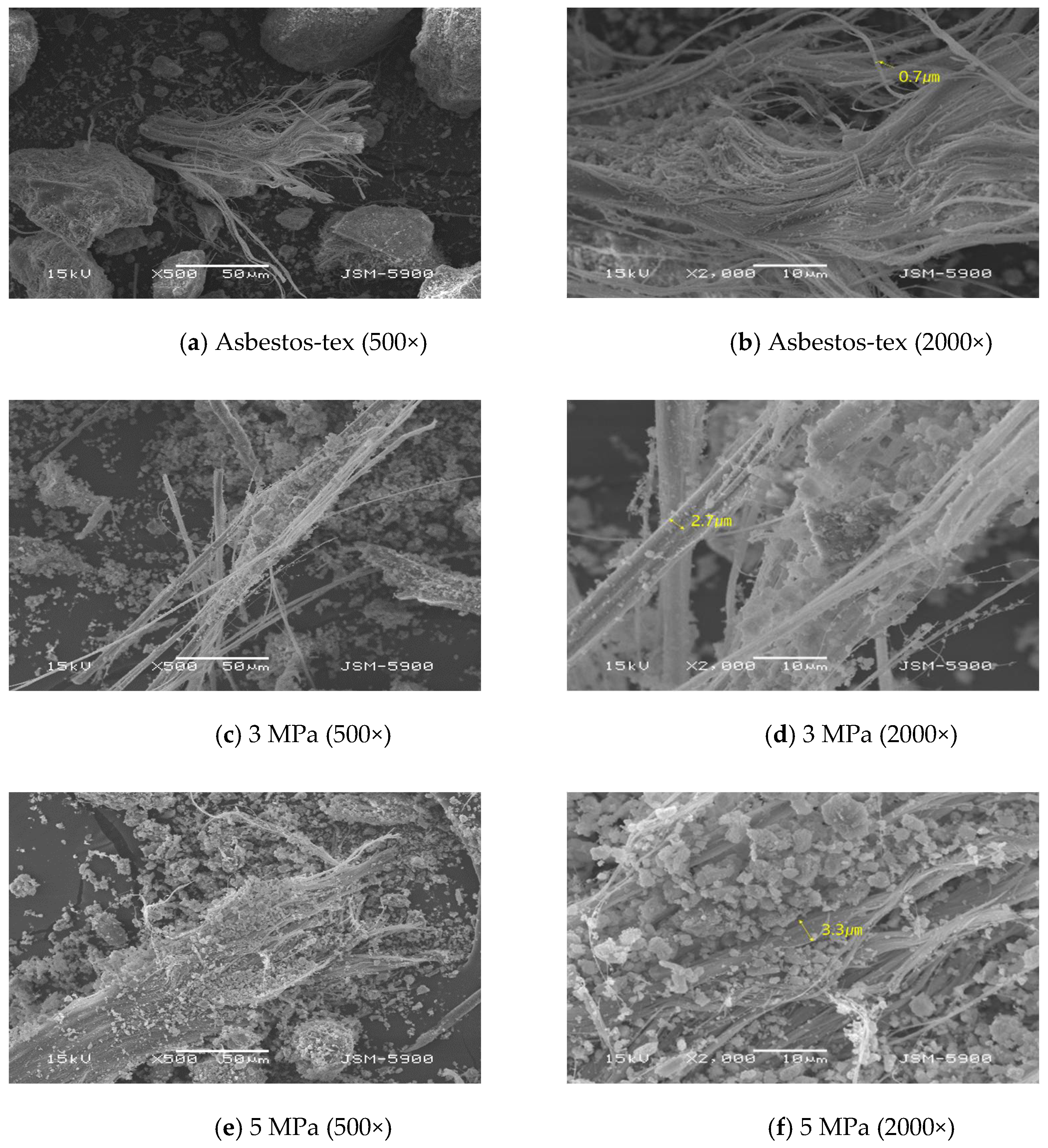
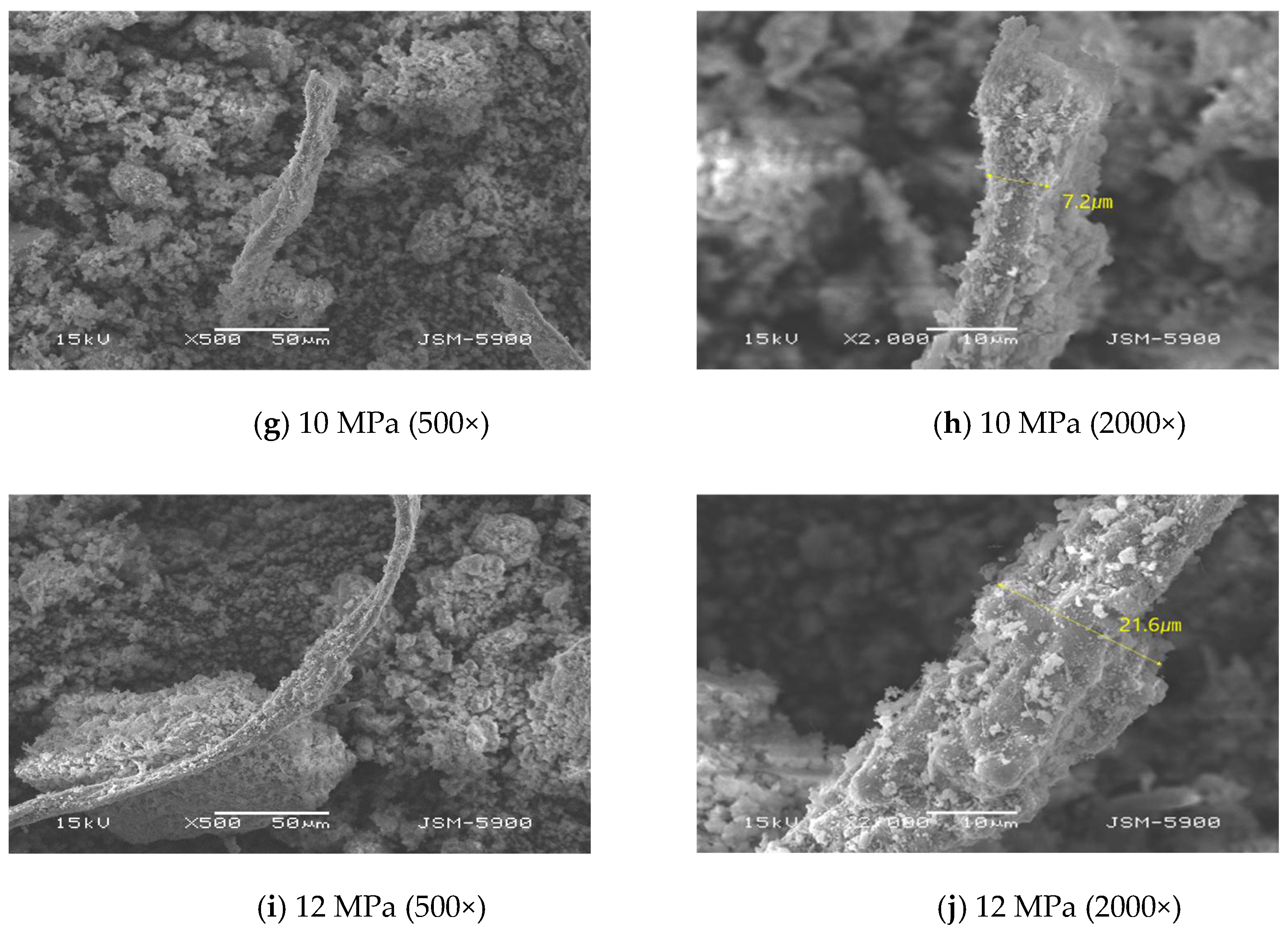
3.1.1. Microstructure
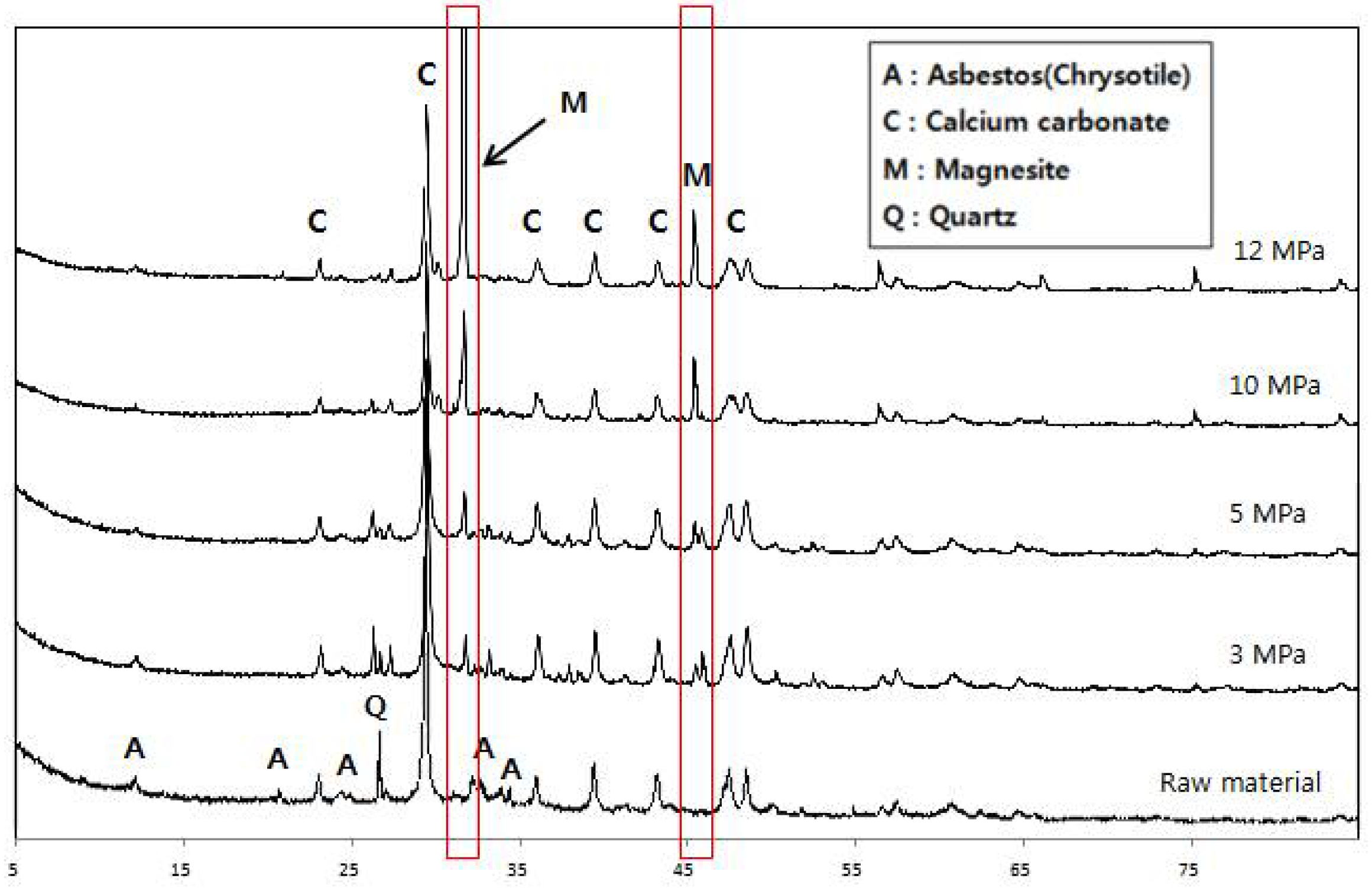
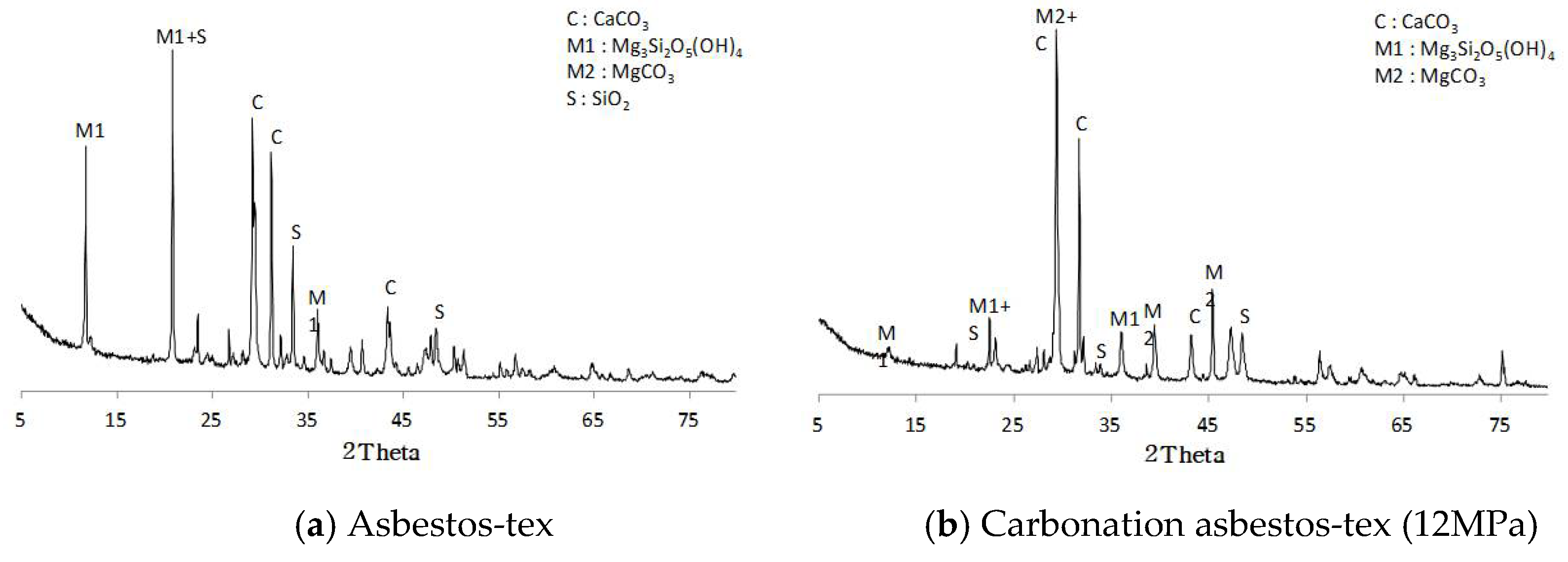
3.1.2. X-ray Diffraction Analysis
3.2. Airborne Asbestos Concentration Analysis
4. Conclusions
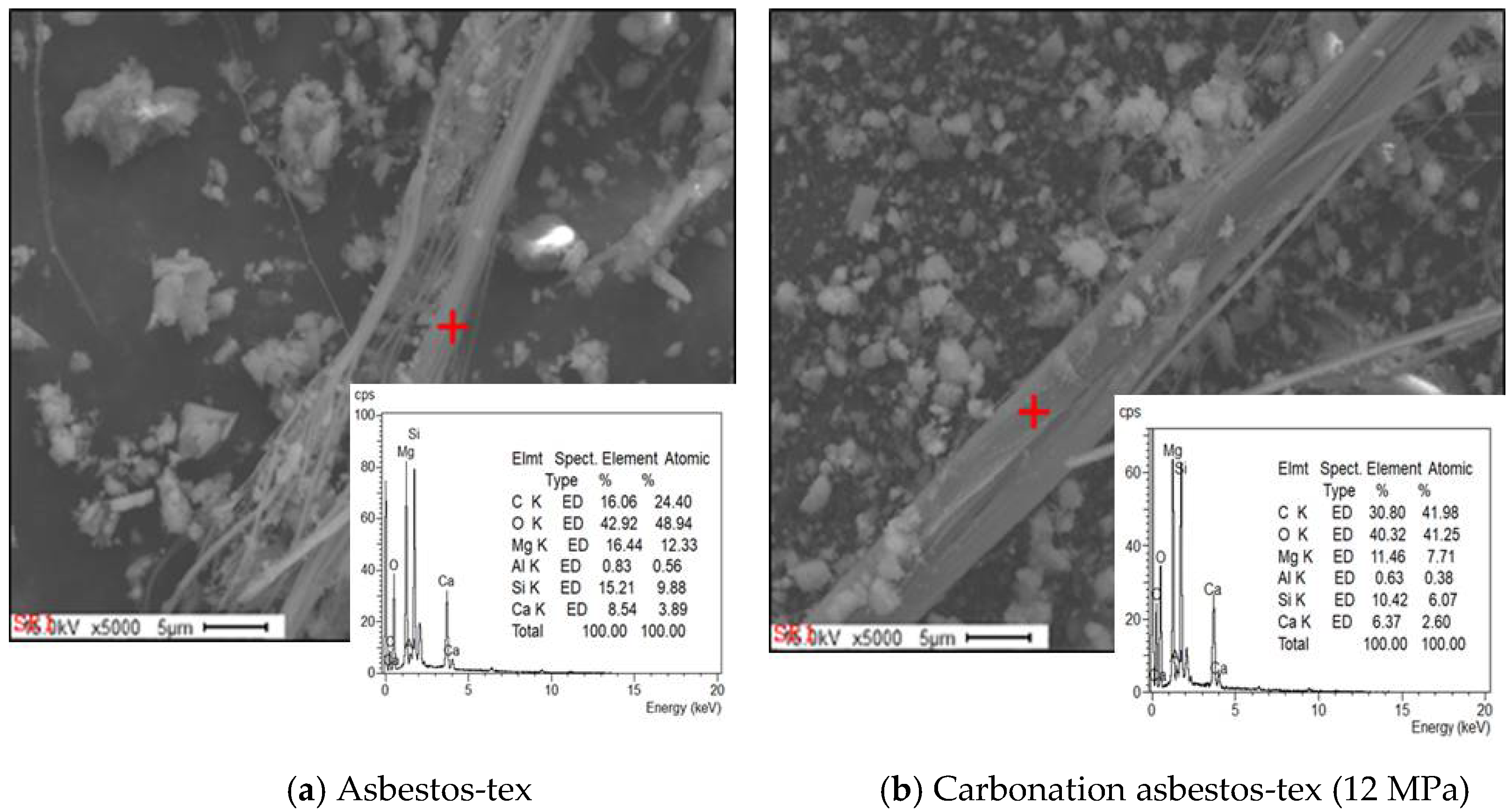
- SEM analysis revealed that the asbestos-tex raw material was in the form of needle-like fibrous minerals. At 3 MPa, the mineral form was not significantly different from the raw material. However, at 5 MPa, carbonate particles were generated. At 10 MPa or above, it was transformed into thick rod-like minerals following mineral carbonation.
- Asbestos-tex is a mixture of calcium carbonate, quartz, and chrysotile. At the CO2 partial pressures of 3 and 5 MPa, only some parts of the chrysotile were transformed into magnesite. At CO2 partial pressures of 10 MPa or above, most of the chrysotile was transformed into magnesite; at 12 MPa, a large growth of magnesite crystals was observed.
- The analysis of airborne asbestos concentration did not show any significant difference between the carbonated samples (at 3 or 5 MPa) and the raw material. However, at 10 MPa or above, it was significantly reduced to one-seventh of that of the raw material. This confirmed that the asbestos-type fibrous material was transformed into a mineral through the carbonation process.
- Mineral carbonation of asbestos-tex was conducted at a constant temperature of 100 °C and a range of CO2 partial pressures. Only a fraction of the chrysotile of asbestos-tex was transformed into magnesite at low-partial pressures. However, at CO2 partial pressures in excess of 10 MPa, the detoxification of asbestos-tex was achieved up to a level that made it more significant than 3 µm.
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- WHO/EURO Technical Committee for Monitoring and Evaluating MMMF. The WHO/EURO man-made mineral fiber reference scheme. Scand. J. Work Environ. Health 1985, 11, 123–129. [Google Scholar] [CrossRef]
- Becklake, M.R.; Gibbs, G.W.; Arhirii, M.; Hurwitz, S. Exposure to asbestos and respiratory abnormality: The influence of fibre type and nature of exposure. In Biological Effects of Mineral Fibers; Wagner, J.C., Ed.; International Agency for Resarch on Cancer Scientific Publication: Genova, Switzerland, 1980; Volume 30, pp. 763–768. [Google Scholar]
- Stayner, L.; Welch, L.S.; Lemen, R. The worldwide pandemic of asbestos related diseases. Annu. Rev. Public Health 2013, 34, 205–216. [Google Scholar] [CrossRef] [PubMed]
- Tossavainen, A. Global use of asbestos and the incidence of mesothelioma. Int. J. Occup. Environ. Health 2004, 10, 22–25. [Google Scholar] [CrossRef]
- David, J.B.; Celeste, M.B.; David, P.C.; Cardozo-Pelaez, F.; Pfau, J.C. Internalization of Libby Amphibole Asbestos and Induction of Oxidative Stress in Murine Macrophages. Toxicol. Sci. 2007, 19, 277–288. [Google Scholar]
- Vijayalakshmi, P.; Sigmund, A.W.; Navdeep, S.C.; David, W.K. Mitochondrial-derived free radicals mediate asbestos-induced alveolar epithelial cell apoptosis. Am. J. Physiol. Lung Cell Mol. Physiol. 2004, l286, 1220–1227. [Google Scholar]
- Ministry of Environment. Asbestos Management Overview. Korea Ministry of Environment, 2009; p. 456. Available online: http://me.go.kr/home/web/main.do (accessed on 5 January 2021).
- Kim, T.H.; Song, T.H.; Shin, H.G.; Jang, K.P. A Study on the Recycling of Detoxified Waste Asbestos. J. Rec. Const. Resour. 2020, 8, 161–166. [Google Scholar] [CrossRef]
- Li, J.; Dong, Q.; Yu, K.; Liu, L. Asbestos and asbestos waste management in the Asian-Pacific region: Trends, challenges and solutions. J. Clean Prod. 2014, 81, 218–226. [Google Scholar] [CrossRef]
- Yoo, K.K.; Kim, B.S.; Kim, M.S.; Lee, J.C.; Jeong, J. Dissolution of magnesium from serpentine mineral in sulfuric acid solution. Mater. Trans. 2009, 50, 1225–1230. [Google Scholar] [CrossRef]
- Fedoročková, A.; Hreus, M.; Paschman, P.; Sučik, G. Dissolution of magnesium from calcined serpentinite in hydrochloric acid. Miner. Eng. 2012, 32, 1–4. [Google Scholar] [CrossRef]
- Rozalen, M.; Huertas, F.J. Comparative effect of chrysotile leaching in nitric, sulfuric and oxalic acids at room temperature. Chem. Geol. 2013, 352, 134–142. [Google Scholar] [CrossRef]
- Chung, Y.H.; Han, J.H.; Sung, J.H.; Song, K.S.; Lim, K.T.; Yu, I.J. Investigation of Asbestos and MMMF Containing Construction Material in Korean Buildings. J. Odor. Indoor Environ. 2005, 1, 156–165. [Google Scholar]
- Shafer, R.; Throwe, S.; Salgado, O.; Garlow, C.; Hoerath, E.; Asbestos/NESHAP Adequately Wet Guidance’. North Carolina: Alliance Technologies Corporation. 1990. Available online: https://s23893.pcdn.co/wp-content/uploads/2013/09/Pamphlet-–-AsbestosNESHAP-Adequately-Wet-Guidance-December-1990.pdf (accessed on 13 September 2021).
- Gualtieri, A.F.; Tartaglia, A. Thermal decomposition of asbestos and recycling in traditional ceramics. J. Eur. Ceram. Soc. 1999, 20, 1409–1418. [Google Scholar] [CrossRef]
- Zaremba, T.; Peszko, M. Investigation of the thermal modification of asbestos wastes for potential use in ceramic formulation. J. Therm. Anal. Calorim. 2008, 92, 873–877. [Google Scholar] [CrossRef]
- O’Connor, W.K.; Dahlin, D.C.; Turner, P.C.; Walters, R.P. Carbon Dioxide Sequestration by Ex-Situ Mineral Carbonation; Albany Research Center: Albany, Oregon, 1999. Available online: https://www.osti.gov/servlets/purl/r875354 (accessed on 5 October 2015).
- Schulze, R.K.; Hill, M.A.; Field, R.D.; Papin, P.A.; Hanrahan, R.J.; Byler, D.D. Characterization of carbonated serpentine using XPS and TEM. Energy Conv. Manag. 2004, 45, 3169–3179. [Google Scholar] [CrossRef]
- Fouda, M.F.R.; Amin, R.E.; Abd-Elzaher, M.K. Extraction of magnesia from Egyptian serpentine ore via reaction with different acids. I. Reaction with sulfuric acid, Bull. Chem. Soc. Jpn. 1996, 69, 1907–1912. [Google Scholar] [CrossRef]
- Teir, S.; Revitzer, H.; Eloneva, S.; Fogelholm, C.J.; Zevenhoven, R. Dissolution of natural serpentinite in mineral and organic acids. Int. J. Miner. Process. 2007, 83, 36–46. [Google Scholar] [CrossRef]
- Azdarpour, A.; Asadullah, M.; Junin, R.; Mohammadian, E.; Hamidi, H.; Daud, A.R.M.; Manan, M. Extraction of calcium from red gypsum for calcium carbonate production. Fuel Process. Technol. 2015, 130, 12–19. [Google Scholar] [CrossRef]
- Rahmani, O. CO2 sequestration by indirect mineral carbonation of industrial waste red gypsum. J. CO2 Util. 2018, 27, 374–380. [Google Scholar] [CrossRef]
- Gao, J.; Li, C.; Liu, W.; Hu, J.; Wang, L.; Liu, Q.; Liang, B.; Yue, H.; Zhang, G.; Luo, D.; et al. Process simulation and energy integration in the mineral carbonation of blast furnace slag. Chin. J. Chem. Eng. 2019, 27, 157–167. [Google Scholar] [CrossRef]
- Gerdermann, S.J.; O’Connor, W.K.; Dahlin, D.C.; Penner, L.R.; Rush, H. Ex situ aqueous mineral carbonation. Environ. Sci. Technol. 2007, 41, 2587–2593. [Google Scholar] [CrossRef]
- Wang, F.; Dreisinger, D.; Jarvis, M.; Hitchins, T. Kinetics and mechanism of mineral carbonation of olivine for CO2 sequestration. Miner. Eng. 2019, 131, 185–197. [Google Scholar] [CrossRef]
- Huijgen, W.J.J.; Witkamp, G.; Comans, R.N.J. Mechanisms of aqueous wollastonite carbonation as a possible CO2 sequestration process. Chem. Eng. Sci. 2006, 61, 4242–4251. [Google Scholar] [CrossRef]
- National Institute of Occupational Safety and Health (NIOSH). Asbestos and Other Fibers by PCM 7400′. In NIOSH Manual of Analytical Methods, 4th ed.; DHHS (NIOSH) Publication: Cincinnati, OH, USA, 1994; pp. 133–147. [Google Scholar]
- Commins, B.T. The Significance of Asbestos and Other Mineral Fibres in Environmental Ambient Air; Commins Associates: Berkshire, UK, 1985. [Google Scholar]
- Rom, W.N.; Markowitz, S.B. Environmental and Occupational Medicine, 4th ed.; Lippincott-Raven Publishers: Philadelphia, PA, USA, 2007. [Google Scholar]
- National Research Council. Committee on Nonoccupational Health Risks of Asbestiform Fibers. In Asbestiform Fibers: Nonoccupational Health Risks; National Academy Press: Washington, DC, USA, 1984. [Google Scholar] [CrossRef]
- Jo, H.J.; Jang, Y.N.; Jo, J.H. A low temperature detoxification method for treatment of Chrysotile-containing waste roofing slate. Minerals 2017, 7, 144. [Google Scholar] [CrossRef] [Green Version]


| Type | Oxide Composition (%) | |||||||
|---|---|---|---|---|---|---|---|---|
| CaO | SiO2 | MgO | Fe2O3 | Al2O3 | SO3 | K2O | Others | |
| Asbestos-Tex | 59.4 | 20.6 | 6.86 | 4.79 | 3.88 | 3.21 | 0.65 | 0.61 |
| Exposure Pressures | Observed Field Count | Number of Fibers (1 f) | Number of Fibers (1/2 f) | Number of Fibers | Number of Fibers per Unit Area (f/mm2) | Asbestos Concentration (f/cc) |
|---|---|---|---|---|---|---|
| Atmospheric pressure | 100 | 3 | 1 | 3.5 | 4.459 | 0.00046 |
| 3 MPa | 3 | 1 | 3.5 | 4.459 | 0.00046 | |
| 5 MPa | 2 | 2 | 3 | 3.821 | 0.00039 | |
| 10 MPa | 0 | 1 | 0.5 | 0.637 | 0.000065 | |
| 12 MPa | 0 | 1 | 0.5 | 0.637 | 0.000065 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lim, Y.; Jang, H.; So, S. Evaluation of Mineral Carbonation of Asbestos-Tex and Analysis of Airborne Asbestos Concentrations. Buildings 2022, 12, 1372. https://doi.org/10.3390/buildings12091372
Lim Y, Jang H, So S. Evaluation of Mineral Carbonation of Asbestos-Tex and Analysis of Airborne Asbestos Concentrations. Buildings. 2022; 12(9):1372. https://doi.org/10.3390/buildings12091372
Chicago/Turabian StyleLim, Yongtaek, Hongseok Jang, and Seungyoung So. 2022. "Evaluation of Mineral Carbonation of Asbestos-Tex and Analysis of Airborne Asbestos Concentrations" Buildings 12, no. 9: 1372. https://doi.org/10.3390/buildings12091372
APA StyleLim, Y., Jang, H., & So, S. (2022). Evaluation of Mineral Carbonation of Asbestos-Tex and Analysis of Airborne Asbestos Concentrations. Buildings, 12(9), 1372. https://doi.org/10.3390/buildings12091372






