Degradation Mechanisms of Early Strength for High-Fluidization Cement Mortar under Magnesium Sulfate Corrosion
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Cement and Admixture
2.1.2. Chemical Reagent
2.2. Methods
2.2.1. Preparation of the HECM
- Calculate and weigh the quality of water, cement, accelerating agent, expansion agent, and water-reducing agent according to the base mix ratio.
- Mix the accelerating agent, expansion agent, and water-reducing agent evenly, and then mix evenly with cement. Pour in the water while stirring. Before pouring the mold, stir the cement mortar for two minutes.
- Pour the cement mortar into a mold that has been brushed with kerosene. Vibrate the mold to discharge bubbles and scrape the surface of the mold with a scraper.
- Put the test specimens into the curing box for one day.
2.2.2. Preparation and Corrosion Methods of MgSO4 Solution
- Calculate and weigh the MgSO4 powder mass and water volume that correspond to different concentrations.
- Pour the MgSO4 powder into the water and stir until the powder is dissolved. MgSO4 solution is prepared.
- Pour the prepared solution into the plastic bottle which corroded the microscopic specimens and the bucket which corroded the macroscopic specimens.
- Put the test specimens into the MgSO4 solution and record the corrosion time.
2.2.3. Preparation of the Microscopic Test Specimens
- Prepare the standard cuboid specimens (4 cm × 4 cm × 16 cm) according to the Chinese test standard “Test Method of Cement and Concrete for Highway Engineering” (JTG E30-2005) [25].
- Cut the cuboid specimens into some cubic specimens (1 cm × 1 cm × 1 cm) and put the cubic specimens into the corrosion solution until they reach the target corrosion age.
- Put the cubic specimens after corrosion into anhydrous ethanol for seven days to stop the hydration. The anhydrous ethanol must be replaced at least three times in seven days.
- Break the cubic specimens into pieces. The outer and inner specimens of SEM tests can be prepared via selection from these pieces. The outer and inner specimens of XRD and DSC tests can be prepared by pulverizing the selected pieces.
- The prepared SEM, XRD, and DSC specimens should be placed in the oven at 60 °C for at least 48 h.
2.2.4. Strength Test
2.2.5. SEM Experiment
2.2.6. XRD Experiment
2.2.7. DSC Experiment
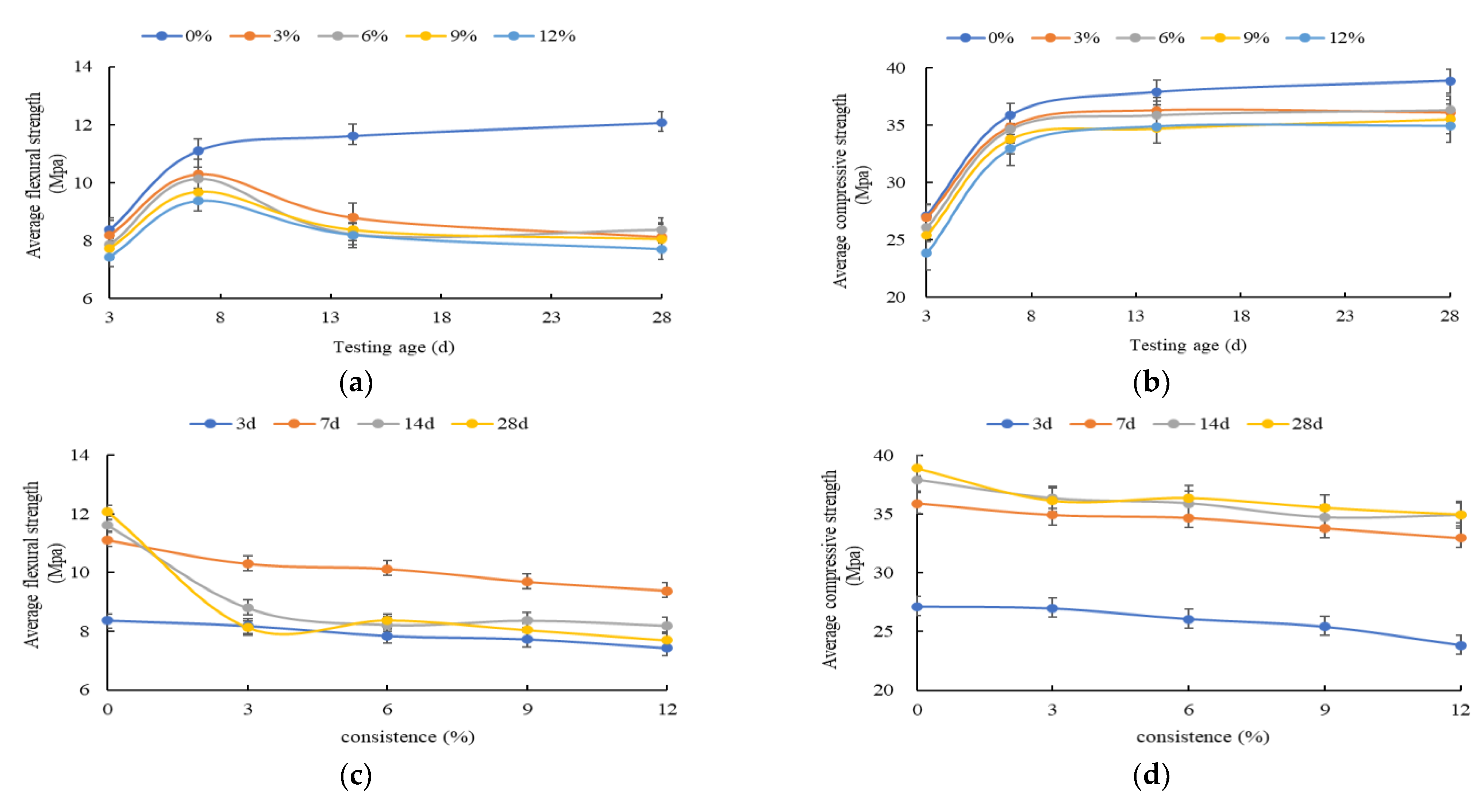
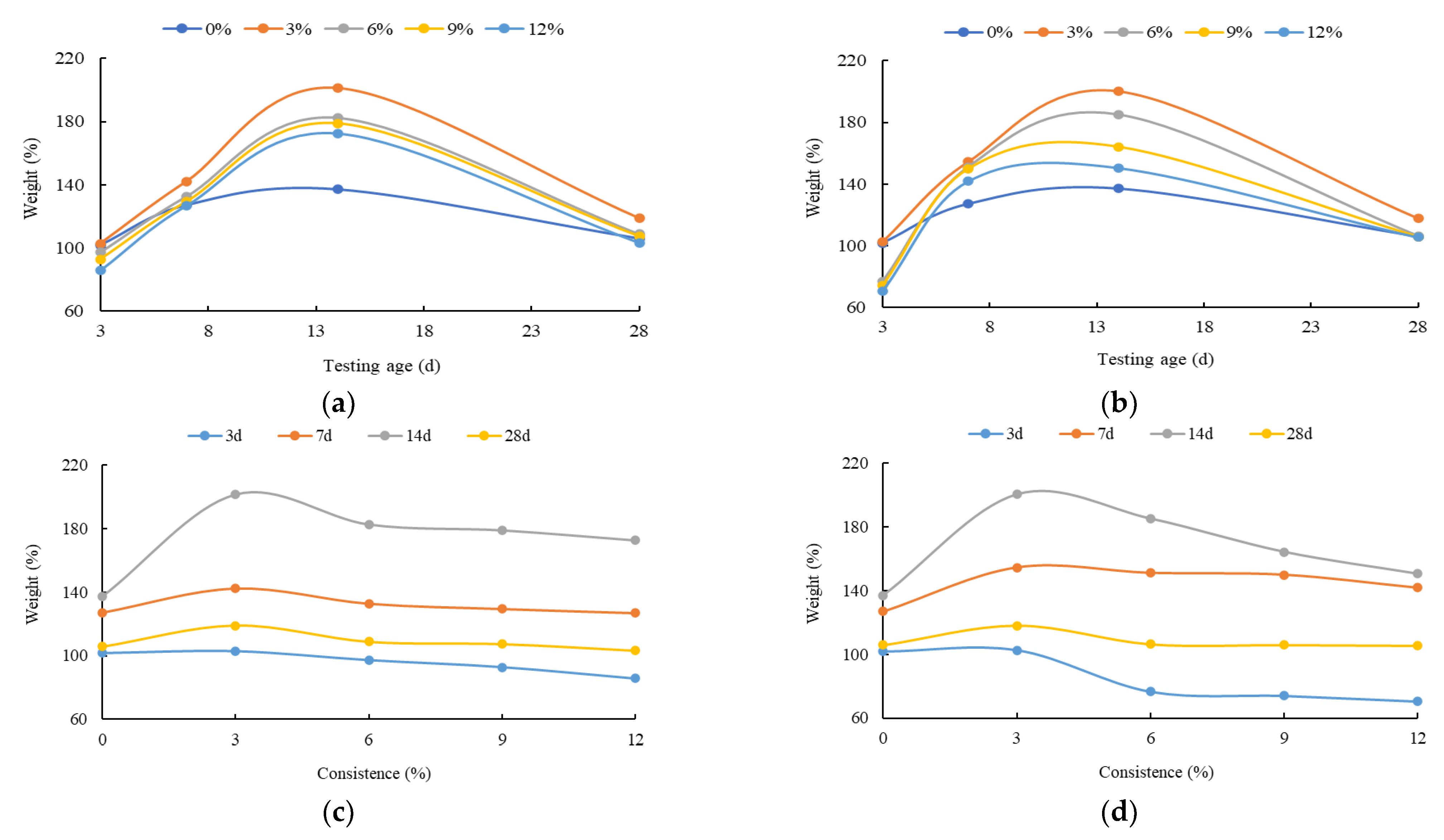
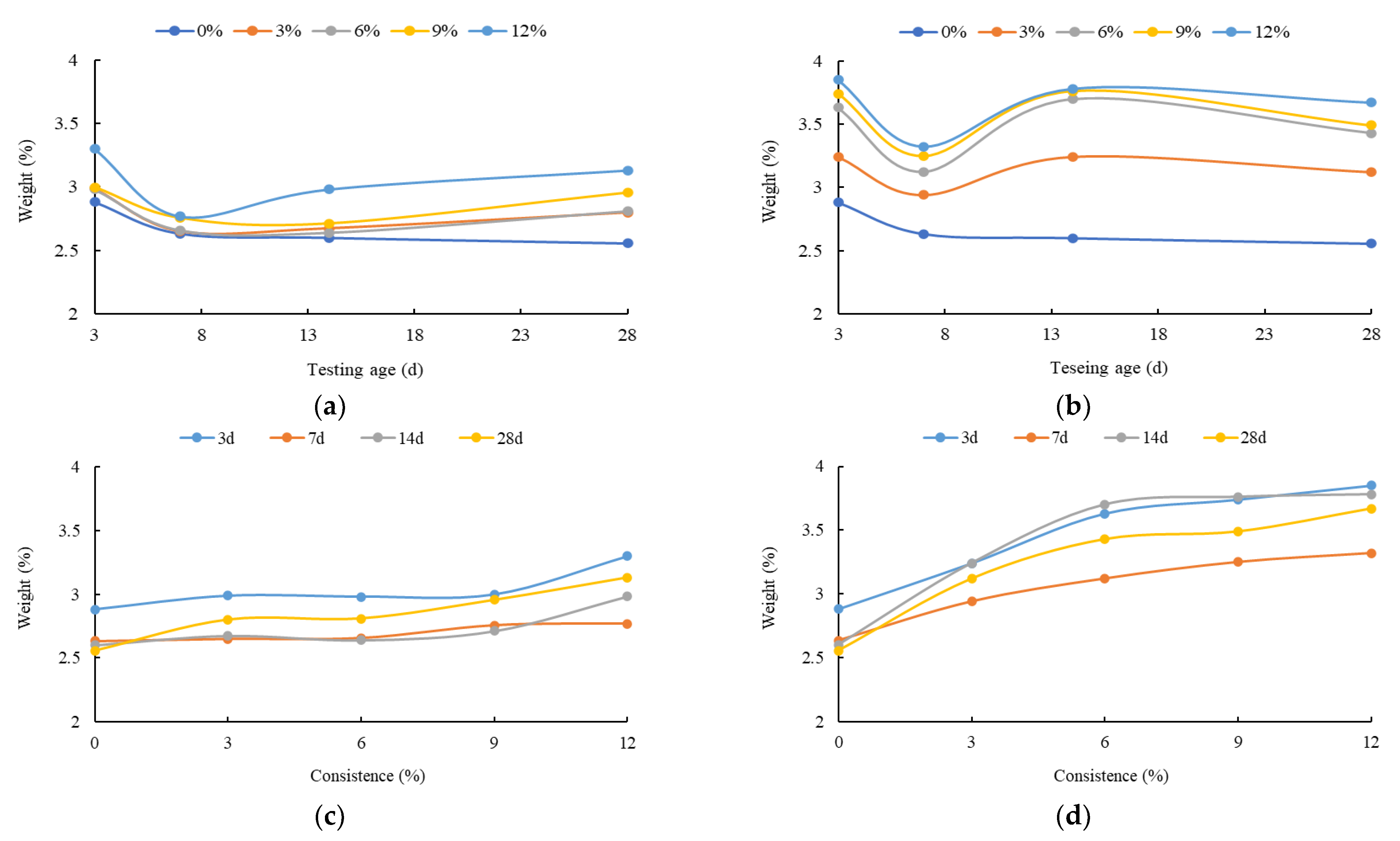
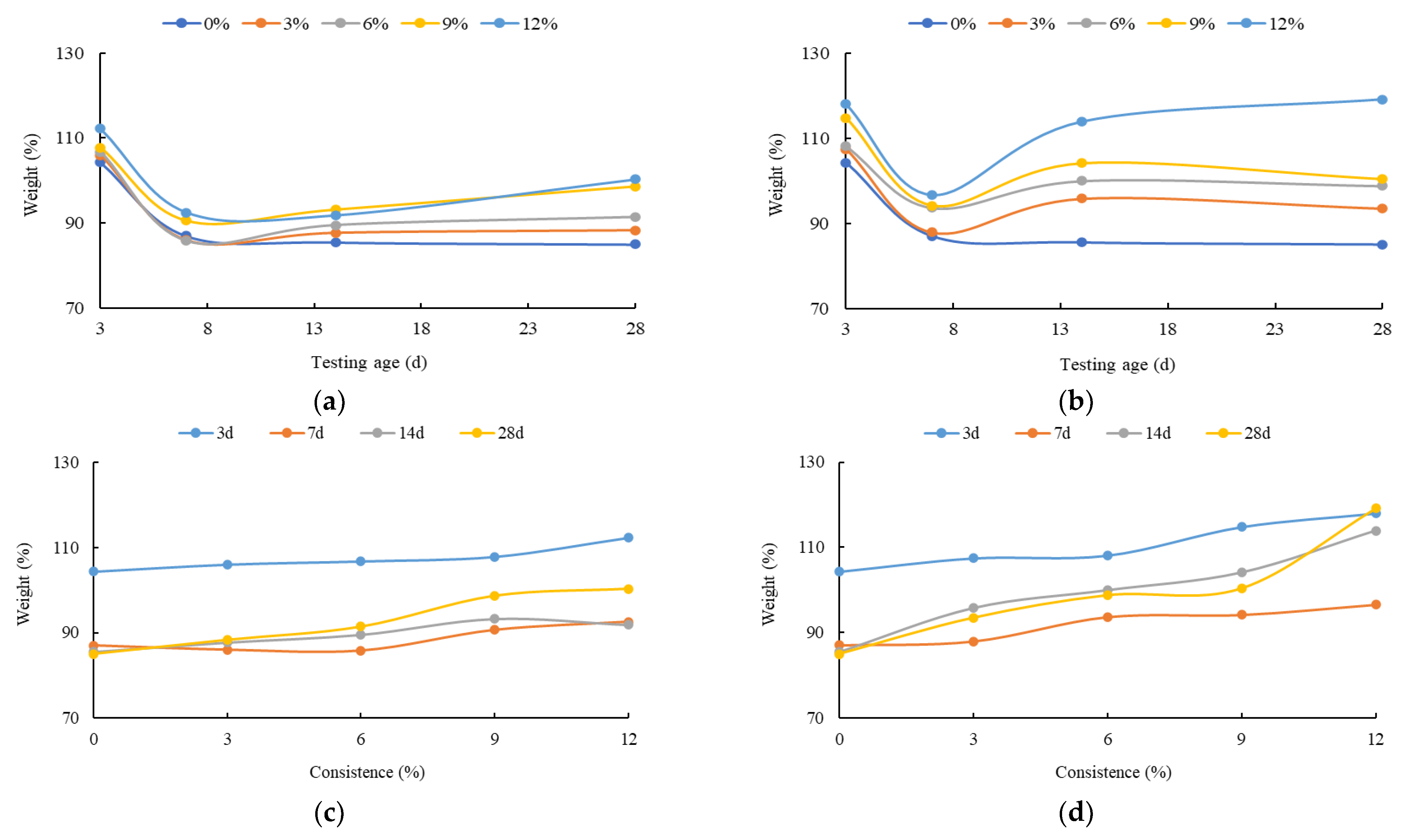
3. The Mechanical Strength Variation Law of the HECM
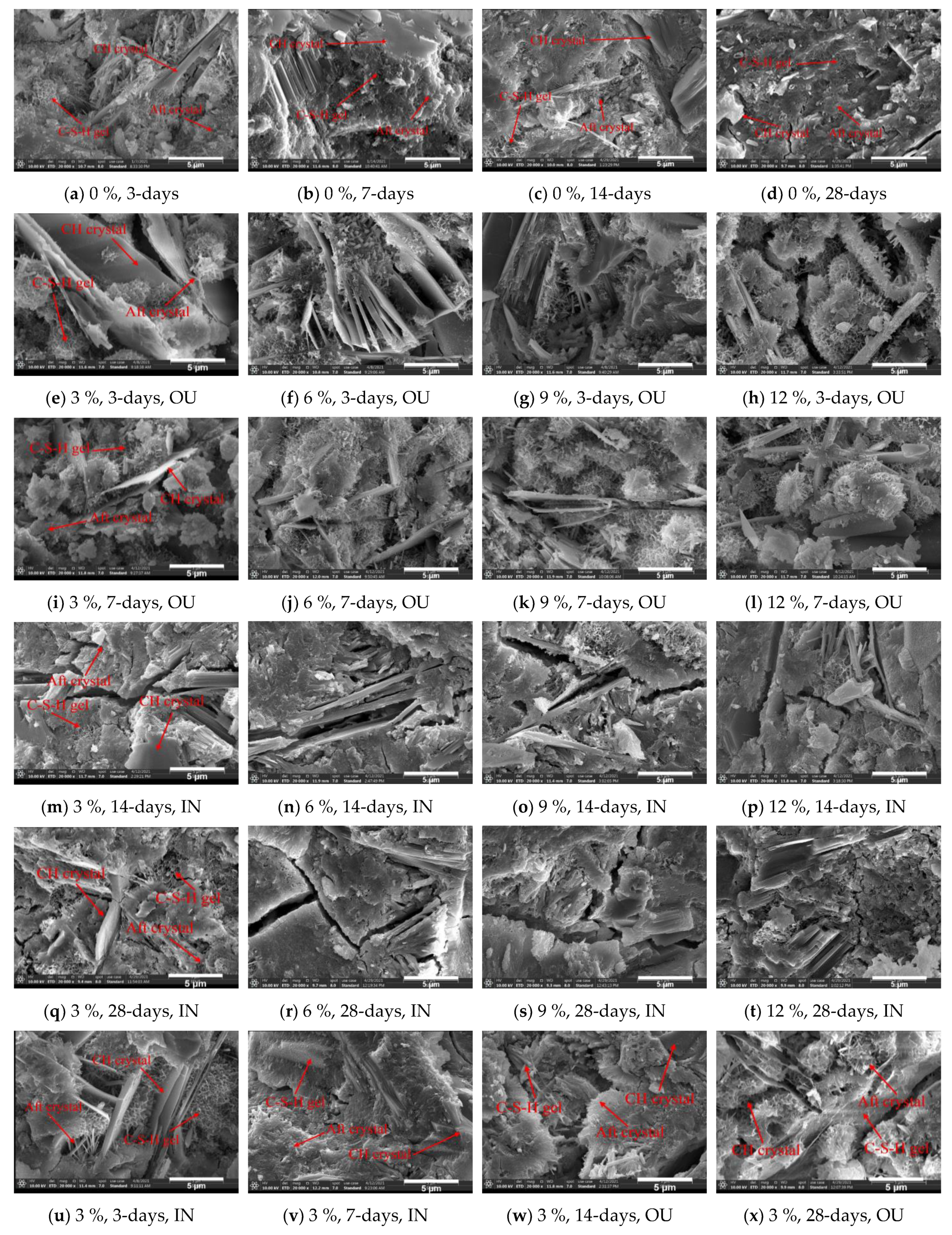
4. The Microstructure Changes in the HECM
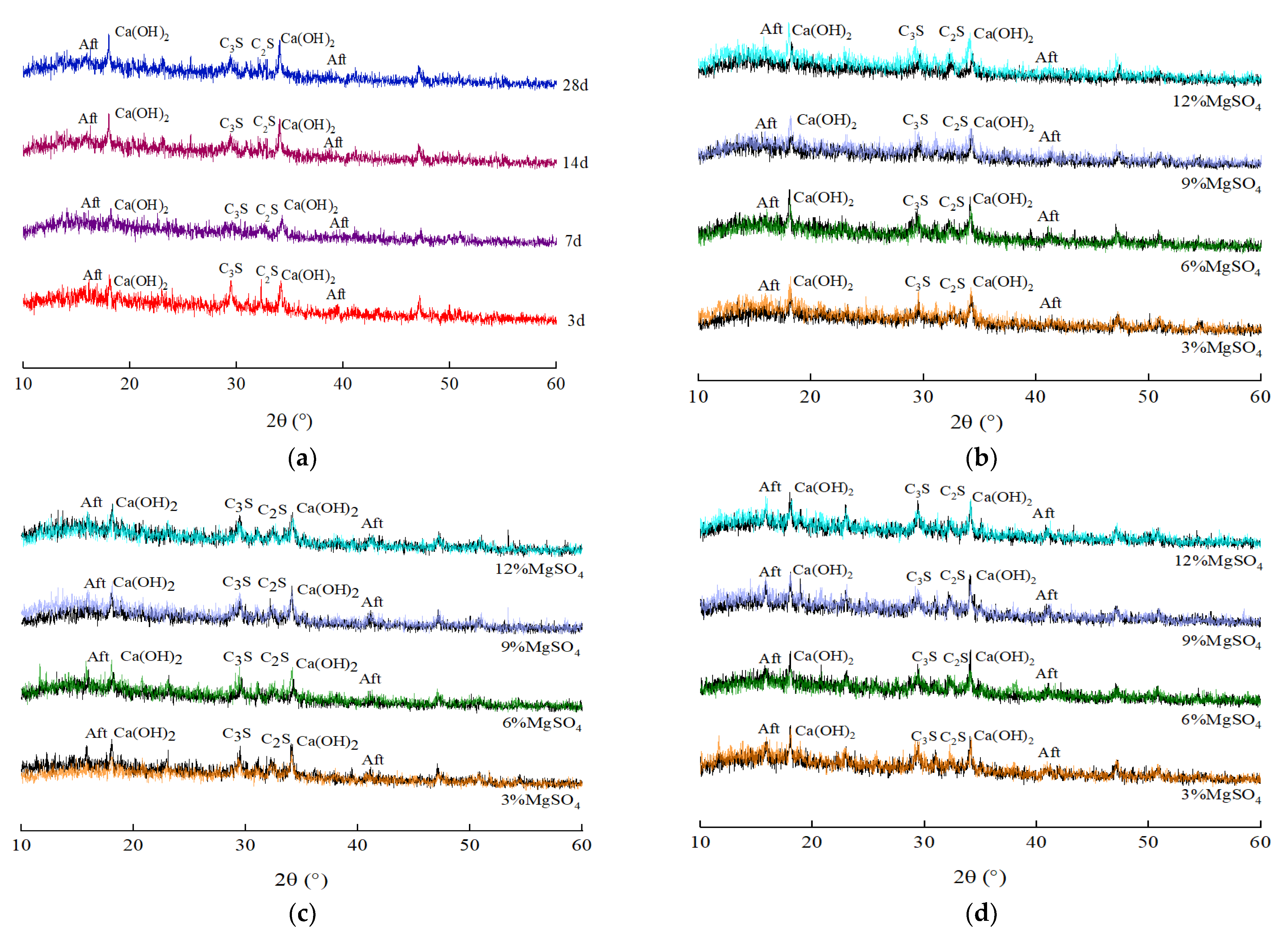
5. The Changes in Hydration Products in the HECM
5.1. XRD
5.2. DSC
6. Conclusions
- The flexural and compressive strength of the HECM steadily decrease as the solution concentration increases. This is due to the CH crystal generating the insoluble magnesium hydroxide with Mg2+. The insoluble magnesium not only consumes the CH crystal to influence the hydration process, but also prompts the C-S-H gel to transform into the M-S-H gel. The contribution to the macro strength of the M-S-H gel is lower than that of the C-S-H gel, because the cementitious of the M-S-H gel is weaker than the C-S-H gel. In addition, the SO42− will also interfere with the generation of the C-S-H gel and destroy the CH crystal. The negative impact of the insoluble magnesium, M-S-H gel, and SO42− on the macro strength of the HECM becomes more significant as the solution concentration increases.
- The flexural and compressive strength of the HECM first increase and then decrease as the corrosion time increases, especially for the flexural strength. The increased strength at the early hydration stage comes from the gypsum generated by CH crystal and SO42− and the insoluble magnesium attached to the outside of the HECM. With the increase in corrosion time, the above composite impact of the insoluble magnesium, M-S-H gel, and SO42− becomes more superior to weaken the strength.
- This study is conducive for pinpointing the evolution process of mechanical strength and hydration products of the HECM under magnesium sulfate corrosion. It is helpful to specifically adjust and control the composition of the HECM during the material design, aiming at some special engineering conditions.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ren, J.; Zhao, H.; Zhang, L.; Zhao, Z.; Xu, Y.; Cheng, Y.; Wang, M.; Chen, J.; Wang, J. Design optimization of cement grouting material based on adaptive boosting algorithm and simplicial homology global optimization. J. Build. Eng. 2022, 49, 104049. [Google Scholar] [CrossRef]
- Stewart, M.G.; Mullard, J.A. Spatial time-dependent reliability analysis of corrosion damage and the timing of first repair for RC structures. Eng. Struct. 2007, 29, 1457–1464. [Google Scholar] [CrossRef]
- Ren, J.; Zhao, Z.; Xu, Y.; Wang, S.; Chen, H.; Huang, J.; Xue, B.; Wang, J.; Chen, J.; Yang, C. High-fluidization, early strength cement grouting material enhanced by Nano-SiO2: Formula and mechanisms. Materials 2021, 14, 6144. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, Y.; She, W.; Liu, N.; Liu, Z. In situ observing the erosion process of cement pastes exposed to different sulfate solutions with X-ray computed tomography. Constr. Build. Mater. 2018, 176, 556–565. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, W.; Li, Z.; Jin, H.; Tang, L. Influence of deicing salt on the surface properties of concrete specimens after 20 years. Constr. Build. Mater. 2021, 295, 123643. [Google Scholar] [CrossRef]
- Ding, Z.; Li, Z.; Xing, F. Chemical durability investigation of magnesium phosphosilicate cement. Environ. Ecol. Technol. Concr. 2006, 302, 275–281. [Google Scholar]
- Li, J.; Ji, Y.; Huang, G.; Zhang, L. Microstructure evolution of a magnesium phosphate protective layer on concrete structures in a sulfate environment. Coatings 2018, 8, 140. [Google Scholar] [CrossRef]
- Li, J.; Ji, Y.; Zhang, L.; Liu, B. Resistance to sulfate attack of magnesium phosphate cement-coated concrete. Constr. Build. Mater. 2019, 195, 156–164. [Google Scholar]
- Cheng, S.; Shui, Z.; Sun, T.; Gao, X.; Guo, C. Effects of sulfate and magnesium ion on the chloride transportation behavior and binding capacity of Portland cement mortar. Constr. Build. Mater. 2019, 204, 265–275. [Google Scholar] [CrossRef]
- Zhao, G.; Guo, M.; Cui, J.; Li, J.; Xu, L. Partially-exposed cast-in-situ concrete degradation induced by internal-external sulfate and magnesium multiple coupled attack. Constr. Build. Mater. 2021, 294, 123560. [Google Scholar] [CrossRef]
- Pinto, S.R.; da Luz, C.A.; Munhoz, G.S.; Medeiros, R.A. Durability of phosphogypsum-based supersulfated cement mortar against external attack by sodium and magnesium sulfate. Constr. Build. Mater. 2020, 136, 106172. [Google Scholar] [CrossRef]
- Cheng, S.; Shui, Z.; Gao, X.; Yu, R.; Sun, T.; Guo, C.; Huang, Y. Degradation mechanisms of portland cement mortar under seawater attack and drying-wetting cycles. Constr. Build. Mater. 2020, 230, 116934. [Google Scholar] [CrossRef]
- Yu, H.; Tan, Y.; Yang, L. Microstructural evolution of concrete under the attack of chemical, salt crystallization, and bending stress. J. Mater. Civ. Eng. 2007, 29, 7. [Google Scholar] [CrossRef]
- Wang, D.; Liu, G.; Wang, Z.; Jia, X.; Luo, S. The role of limestone powder in the corrosion products of cement mortars under magnesium sulfate solution. J. Mater. Civ. Eng. 2022, 34, 04022021. [Google Scholar] [CrossRef]
- Yao, J.; Chen, J. Sensitivity analysis of the deterioration of concrete strength in marine environment to multiple corrosive ions. Front. Struct. Civ. Eng. 2022, 16, 175–190. [Google Scholar] [CrossRef]
- Lee, S.T.; Park, D.W.; Ann, K.Y. Mitigating effect of chloride ions on sulfate attack of cement mortars with or without silica fume. Can. J. Civ. Eng. 2008, 35, 1210–1220. [Google Scholar] [CrossRef]
- Li, X.; Shui, Z.; Yu, R.; Wang, X. Magnesium induced hydration kinetics of ultra-high performance concrete (UHPC) served in marine environment: Experiments and modelling. Constr. Build. Mater. 2019, 224, 1056–1068. [Google Scholar] [CrossRef]
- Xu, J.; Mo, R.; Wang, P.; Zhou, J.; Dong, X.; She, W. Coupled transport of sulfate and chloride ions with adsorption effect: A numerical analysis. Front. Mater. 2020, 7, 536517. [Google Scholar] [CrossRef]
- Li, J.; Xu, Y.; Tian, Z.; Ma, J.; Jing, P.; Song, Z. Study on leaching damage mechanism of calcium ions of reactive powder concrete (RPC) under ion corrosion. Constr. Build. Mater. 2021, 269, 121303. [Google Scholar] [CrossRef]
- Zhang, W.; Gong, S.; Kang, B. Surface Corrosion and microstructure degradation of calcium sulfoaluminate cement subjected to wet-dry cycles in sulfate solution. Adv. Mater. Sci. Eng. 2017, 2017, 1464619. [Google Scholar] [CrossRef]
- Wu, M.; Zhang, Y.; Ji, Y.; She, W.; Yang, L.; Liu, G. A comparable study on the deterioration of limestone powder blended cement under sodium sulfate and magnesium sulfate attack at a low temperature. Constr. Build. Mater. 2020, 243, 118279. [Google Scholar] [CrossRef]
- Sun, H.; Jiang, C.; Cao, K.; Yu, D.; Liu, W.; Zhang, X.; Xing, X.; Zhao, D. Monitoring of steel corrosion and cracking in cement paste exposed to combined sulfate-chloride attack with X-ray microtomography. Constr. Build. Mater. 2021, 302, 124345. [Google Scholar] [CrossRef]
- Zhang, G.; Wu, C.; Hou, D.; Yang, J.; Sun, D.; Zhang, X. Effect of environmental pH values on phase composition and microstructure of portland cement paste under sulfate attack. Compos. Part B-Eng. 2021, 216, 108862. [Google Scholar] [CrossRef]
- T\CECS G:D51-01-2019; Technical Specification for Road Semi-Flexible Pavement. China Association for Engineering Construction Standardization: Beijing, China, 2019.
- JTG E30-2005; Test Method of Cement and Concrete for Highway Engineering. Ministry of Transport of the People’s Public of China: Beijing, China, 2005.
- Kong, D.; Pan, H.; Wang, L.; Corr, D.J.; Yang, Y.; Shah, S.P.; Sheng, J. Effect and mechanism of colloidal silica sol on properties and microstructure of the hardened cement-based materials as compared to nano-silica powder with agglomerates in micron-scale. Cem. Concr. Compos. 2019, 98, 137–149. [Google Scholar] [CrossRef]
- Zhu, N.; Jin, F.; Kong, X.; Xu, Y.; Zhou, J.; Wang, B.; Wu, H. Interface and anti-corrosion properties of sea-sand concrete with fumed silica. Constr. Build. Mater. 2018, 188, 1085–1091. [Google Scholar] [CrossRef]






| Type | Fluidity (s) | Flexural Strength (MPa) | Compressive Strength (MPa) | Shrinkage Rate (%) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 day | 3 days | 7 days | 14 days | 28 days | 1 day | 3 days | 7 days | 14 days | 28 days | 7 days | 28 days | ||
| HECM | 11.3 | 3.89 | 8.37 | 11.10 | 11.61 | 12.06 | 13.97 | 27.11 | 35.89 | 37.90 | 38.89 | 0.017 | 0.088 |
| Standard | 9–13 | -- | -- | ≥2 | -- | -- | -- | -- | ≥30 | -- | -- | -- | <0.5 |
| Molecular Formula | Molecular Weight | Content | Purity Level |
|---|---|---|---|
| MgSO4·7H2O | 246.47 | >99% | Industrial-grade |
| Testing Age (Days) | Concentration (%) | Flexural Strength (MPa) | Compressive Strength (MPa) |
|---|---|---|---|
| 3 | 0 | 8.37 | 27.11 |
| 3 | 8.19 | 26.95 | |
| 6 | 7.85 | 26.04 | |
| 9 | 7.73 | 25.39 | |
| 12 | 7.43 | 23.82 | |
| 7 | 0 | 11.10 | 35.89 |
| 3 | 10.29 | 34.91 | |
| 6 | 10.12 | 34.66 | |
| 9 | 9.68 | 33.79 | |
| 12 | 9.37 | 32.96 | |
| 14 | 0 | 11.61 | 37.90 |
| 3 | 8.79 | 36.33 | |
| 6 | 8.22 | 35.90 | |
| 9 | 8.36 | 34.71 | |
| 12 | 8.19 | 34.91 | |
| 28 | 0 | 12.06 | 38.89 |
| 3 | 8.12 | 36.12 | |
| 6 | 8.37 | 36.36 | |
| 9 | 8.04 | 35.52 | |
| 12 | 7.69 | 34.95 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Wang, S.; Zhao, L.; Tang, G.; Wang, C.; Ren, J. Degradation Mechanisms of Early Strength for High-Fluidization Cement Mortar under Magnesium Sulfate Corrosion. Buildings 2022, 12, 1376. https://doi.org/10.3390/buildings12091376
Zhang X, Wang S, Zhao L, Tang G, Wang C, Ren J. Degradation Mechanisms of Early Strength for High-Fluidization Cement Mortar under Magnesium Sulfate Corrosion. Buildings. 2022; 12(9):1376. https://doi.org/10.3390/buildings12091376
Chicago/Turabian StyleZhang, Xiangquan, Siyuan Wang, Lihong Zhao, Gangting Tang, Chunguang Wang, and Jiaolong Ren. 2022. "Degradation Mechanisms of Early Strength for High-Fluidization Cement Mortar under Magnesium Sulfate Corrosion" Buildings 12, no. 9: 1376. https://doi.org/10.3390/buildings12091376





