Synthesis of Aragonite Whiskers by Co-Carbonation of Waste Magnesia Slag and Magnesium Sulfate: Enhancing Microstructure and Mechanical Properties of Portland Cement Paste
Abstract
:1. Introduction
2. Experimental Design
2.1. Raw Materials
2.2. Preparation of C-MS and Curing of Cement Samples
2.3. Test Methods
2.3.1. pH Changes
2.3.2. X-ray Diffraction Analysis (XRD)
2.3.3. Thermal Analysis (TG-DTG)
2.3.4. Fournier Transform Infrared Analysis (FTIR)
2.3.5. Scanning Electron Microscopy Analysis (SEM)
3. Results and Discussion
3.1. pH Monitoring during MS Carbonation
3.2. Characterization of MS Carbonation Products
3.2.1. Phase Compositions of Carbonated MS
3.2.2. Thermal Analysis
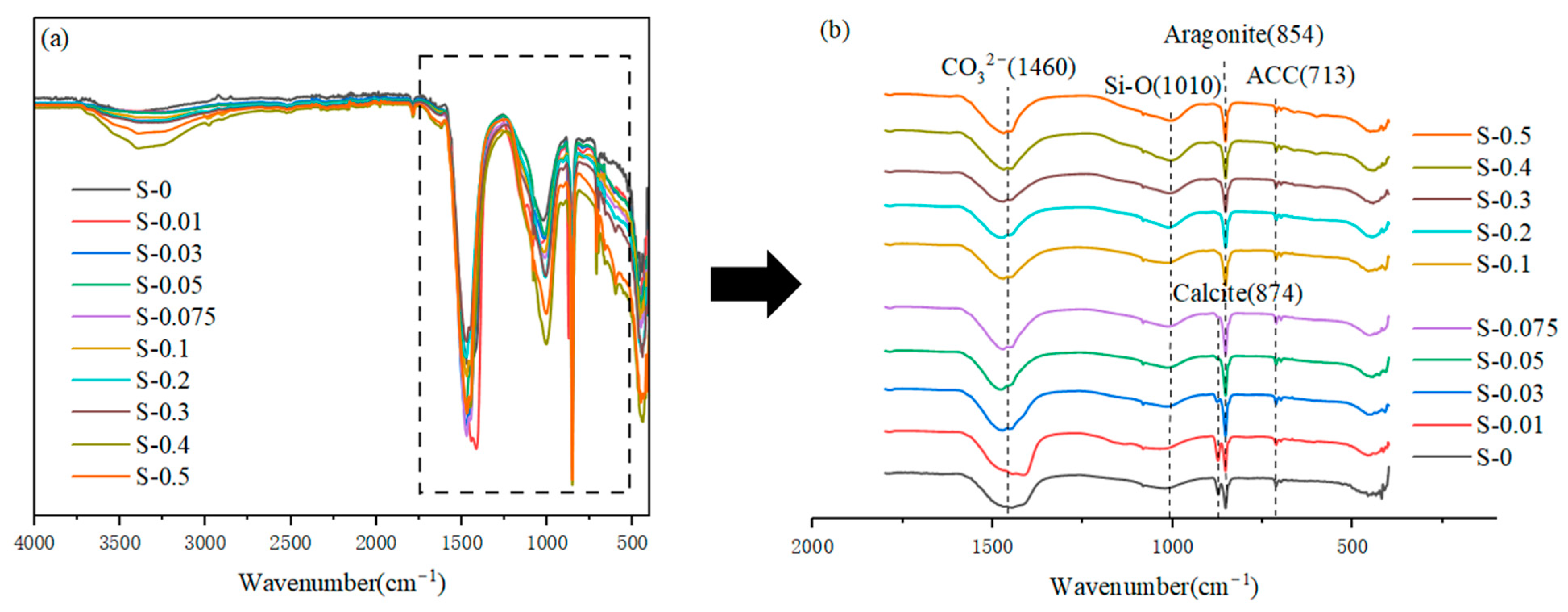
3.2.3. Evolution of the Siliceous Phase
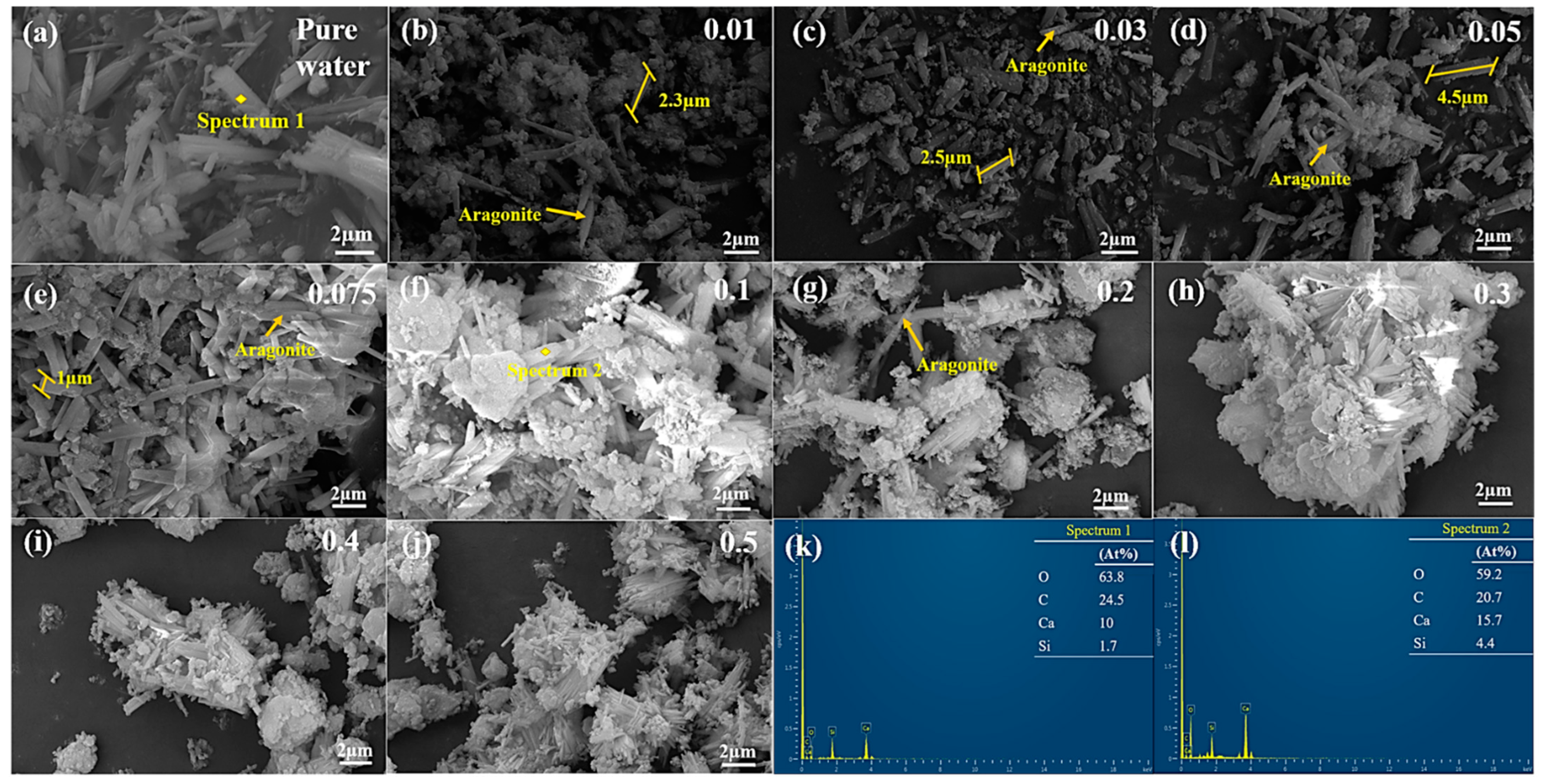
3.2.4. Microstructure
3.3. Effect of Aragonite Whisker on Cement Paste
3.3.1. Compressive Strength
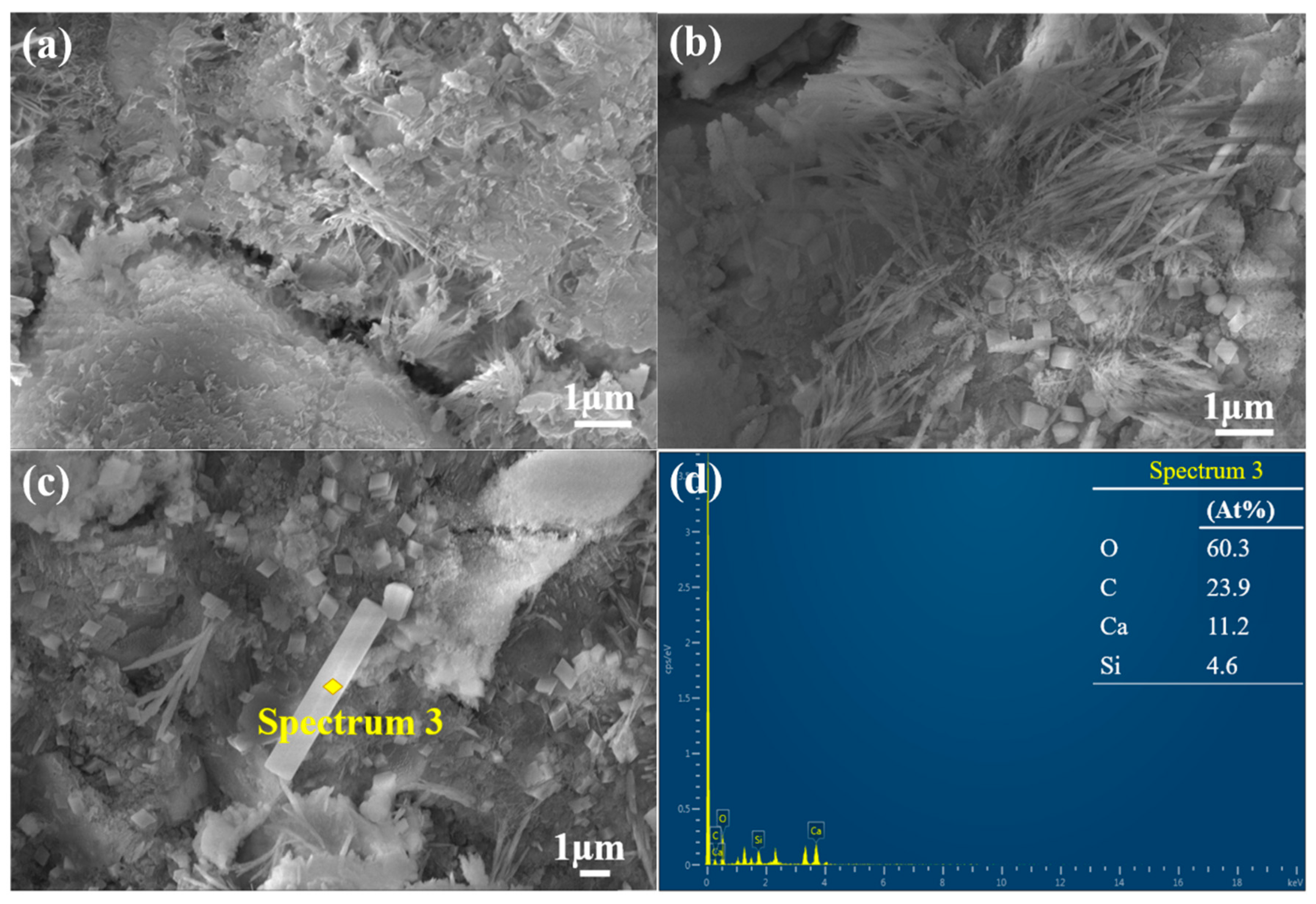
3.3.2. Fracture Surface Micromorphology
3.4. CO2 Sequestration
4. Conclusions
- The presence of Mg2+ and SO42− simultaneously affects the phase composition of carbonation products. When the MgSO4 concentration is less than 0.1 M, the carbonation products mainly consist of aragonite, calcite, gel, and amorphous calcium carbonate. The content of aragonite increases with increasing concentration. When the MgSO4 concentration exceeds 0.1 M, calcite disappears, and a new phase, dihydrate gypsum, appears with its content increasing with higher concentration.
- The presence of Mg2+ and SO42− simultaneously affects the morphology and size of the formed calcite crystals. When the MgSO4 concentration is less than 0.075 M, the formed calcite crystals appear as elongated rods. When the concentration exceeds 0.1 M, the size of calcite crystals increases while the diameter decreases, forming slender strips with aggregation phenomena.
- Incorporating the optimal concentration (0.075 M) of C-MS at a substitution rate of 5% into OPC enhances the compressive strength of the cement paste by 37.5% compared to the control group at an early stage (seven days).
- MS subjected to wet carbonation demonstrates excellent CO2 sequestration capacity. The CO2 sequestration efficiency of the S-0.075 experimental group reaches 19.62 g CO2 per 100 g MS.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Santos, R.L.; Horta, R.B.; Pereira, J.; Nunes, T.G.; Rocha, P.; Canongia Lopes, J.N.; Colaço, R. Microstructural control and hydration of novel micro-dendritic clinkers with CaO/SiO2 = 1.4. Cem. Concr. Res. 2015, 76, 212–221. [Google Scholar] [CrossRef]
- Shia, C.; Qua, B.; Provis, J.L. Recent progress in low-carbon binders. Cem. Concr. Res. 2019, 122, 227–250. [Google Scholar] [CrossRef]
- Liao, Y.; Wang, S.; Wang, K.; Qunaynah, S.; Wan, S.; Yuan, Z.; Xu, P.; Tang, S. A study on the hydration of calcium aluminate cement pastes containing silica fume using non-contact electrical resistivity measurement. J. Mater. Res. Technol. 2023, 24, 8135–8149. [Google Scholar] [CrossRef]
- Huang, J.; Li, W.; Huang, D.; Wang, L.; Chen, E.; Wu, C.; Wang, B.; Deng, H.; Tang, S.; Shi, Y.; et al. Fractal Analysis on Pore Structure and Hydration of Magnesium Oxysulfate Cements by First Principle, Thermodynamic and Microstructure-Based Methods. Fractal Fract. 2021, 5, 164. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, H.; Huang, M.; Yin, H.; Jiang, K.; Xiao, K.; Tang, S. Influence of Different Alkali Sulfates on the Shrinkage, Hydration, Pore Structure, Fractal Dimension and Microstructure of Low-Heat Portland Cement, Medium-Heat Portland Cement and Ordinary Portland Cement. Fractal Fract. 2021, 5, 79. [Google Scholar] [CrossRef]
- Shen, W.; Cao, L.; Li, Q.; Zhang, W.; Wang, G.; Li, C. Quantifying CO2 emissions from China’s cement industry. Renew. Sustain. Energy Rev. 2015, 50, 1004–1012. [Google Scholar] [CrossRef]
- Ashraf, W.; Olek, J. Carbonation activated binders from pure calcium silicates: Reaction kinetics and performance controlling factors. Cem. Concr. Compos. 2018, 93, 85–98. [Google Scholar] [CrossRef]
- Liao, Y.; Yao, J.; Deng, F.; Li, H.; Wang, K.; Tang, S. Hydration behavior and strength development of supersulfated cement prepared by calcined phosphogypsum and slaked lime. J. Build. Eng. 2023, 80, 108075. [Google Scholar] [CrossRef]
- Liao, Y.; Jiang, G.; Wang, K.; Al Qunaynah, S.; Yuan, W. Effect of steel slag on the hydration and strength development of calcium sulfoaluminate cement. Constr. Build. Mater. 2020, 265, 120301. [Google Scholar] [CrossRef]
- Hasanbeigi, A.; Price, L.; Lin, E. Emerging energy-efficiency and CO2 emission-reduction technologies for cement and concrete production: A technical review. Renew. Sustain. Energy Rev. 2012, 16, 6220–6238. [Google Scholar] [CrossRef]
- Liu, S.; Rong, P.; Zhang, C.; Lu, J.; Guan, X.; Shi, C.; Zhu, J. Preparation and carbonation hardening of low calcium CO2 sequestration materials from waste concrete powder and calcium carbide slag. Cem. Concr. Compos. 2023, 141, 105151. [Google Scholar] [CrossRef]
- Panesar, D.K.; Mo, L. Properties of binary and ternary reactive MgO mortar blends subjected to CO2 curing. Cem. Concr. Compos. 2013, 38, 40–49. [Google Scholar] [CrossRef]
- Knight, K.A.; Cunningham, P.R.; Miller, S.A. Optimizing supplementary cementitious material replacement to minimize the environmental impacts of concrete. Cem. Concr. Compos. 2023, 139, 105049. [Google Scholar] [CrossRef]
- Liu, S.; Shen, P.; Xuan, D.; Li, L.; Sojobi, A.; Zhan, B.; Poon, C. A comparison of liquid-solid and gas-solid accelerated carbonation for enhancement of recycled concrete aggregate. Cem. Concr. Compos. 2021, 118, 103988. [Google Scholar] [CrossRef]
- Zhu, C.; Fang, Y.; Wei, H. Carbonation-cementation of recycled hardened cement paste powder. Constr. Build. Mater. 2018, 192, 224–232. [Google Scholar] [CrossRef]
- Zhang, D.; Ghouleh, Z.; Shao, Y. Review on carbonation curing of cement-based materials. J. CO2 Util. 2017, 21, 119–131. [Google Scholar] [CrossRef]
- Jiang, K.; Ashworth, P.; Zhang, S.; Hu, G. Print media representations of carbon capture utilization and storage (CCUS) technology in China. Renew. Sustain. Energy Rev. 2022, 155, 111938. [Google Scholar] [CrossRef]
- Liu, Z.; Van den Heede, P.; Zhang, C.; Shi, X.; Wang, L.; Li, J.; Yao, Y.; Lothenbach, B.; De Belie, N. Carbonation of blast furnace slag concrete at different CO2 concentrations: Carbonation rate, phase assemblage, microstructure and thermodynamic modelling. Cem. Concr. Res. 2023, 169, 107161. [Google Scholar] [CrossRef]
- Chang, J.; Wang, D.; Fang, Y. Effects of mineralogical changes in BOFS during carbonation on pH and Ca and Si leaching. Constr. Build. Mater. 2018, 192, 584–592. [Google Scholar] [CrossRef]
- Humbert, P.S.; Castro-Gomes, J. CO2 activated steel slag-based materials: A review. J. Clean. Prod. 2019, 208, 448–457. [Google Scholar] [CrossRef]
- Shah, V.; Scrivener, K.; Bhattacharjee, B.; Bishnoi, S. Changes in microstructure characteristics of cement paste on carbonation. Cem. Concr. Res. 2018, 109, 184–197. [Google Scholar] [CrossRef]
- Zajac, M.; Skibsted, J.; Skocek, J.; Durdzinski, P.; Bullerjahn, F.; Ben Haha, M. Phase assemblage and microstructure of cement paste subjected to enforced, wet carbonation. Cem. Concr. Res. 2020, 130, 105990. [Google Scholar] [CrossRef]
- Liu, P.; Yu, Z.; Chen, Y. Carbonation depth model and carbonated acceleration rate of concrete under different environment. Cem. Concr. Compos. 2020, 114, 103736. [Google Scholar] [CrossRef]
- Sevelsted, T.F.; Skibsted, J. Carbonation of C–S–H and C–A–S–H samples studied by 13 C, 27 Al and 29 Si MAS NMR spectroscopy. Cem. Concr. Res. 2015, 71, 56–65. [Google Scholar] [CrossRef]
- Wang, D.; Fang, Y.; Zhang, Y.; Chang, J. Changes in mineral composition, growth of calcite crystal, and promotion of physico-chemical properties induced by carbonation of β-C2S. J. CO2 Util. 2019, 34, 149–162. [Google Scholar] [CrossRef]
- Wu, F.; You, X.; Wang, M.; Liu, T.; Lu, B.; Hou, G.; Jiang, R.; Shi, C. Increasing flexural strength of CO2 cured cement paste by CaCO3 polymorph control. Cem. Concr. Compos. 2023, 141, 105128. [Google Scholar] [CrossRef]
- Peng, L.; Shen, P.; Poon, C.; Zhao, Y.; Wang, F. Development of carbon capture coating to improve the durability of concrete structures. Cem. Concr. Res. 2023, 168, 107154. [Google Scholar] [CrossRef]
- Ketrane, R.; Leleyter, L.; Baraud, F.; Jeannin, M.; Gil, O.; Saidani, B. Characterization of natural scale deposits formed in southern Algeria groundwater. Effect of its major ions on calcium carbonate precipitation. Desalination 2010, 262, 21–30. [Google Scholar] [CrossRef]
- Reddy, M.M.; Wang, K.K. Crystallization of calcium carbonate in the presence of metal ions. J. Cryst. Growth 1980, 50, 470–480. [Google Scholar] [CrossRef]
- Ren, D.; Feng, Q.; Bourrat, X. Effects of additives and templates on calcium carbonate mineralization in vitro. Micron 2011, 42, 228–245. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Xuan, D.; Zhan, B.; Li, W.; Poon, C. Characterization and optimization of a two-step carbonation process for valorization of recycled cement paste fine powder. Constr. Build. Mater. 2021, 278, 122343. [Google Scholar] [CrossRef]
- Cizer, Ö.; Van Balen, K.; Elsen, J.; Van Gemert, D. Real-time investigation of reaction rate and mineral phase modifications of lime carbonation. Constr. Build. Mater. 2012, 35, 741–751. [Google Scholar] [CrossRef]
- Xu, X.; Guo, H.; Cheng, X.; Li, M. The promotion of magnesium ions on aragonite precipitation in MICP process. Constr. Build. Mater. 2020, 263, 120057. [Google Scholar] [CrossRef]
- Shen, P.; Jiang, Y.; Zhang, Y.; Liu, S.; Xuan, D.; Lu, J.; Zhang, S.; Poon, C. Production of aragonite whiskers by carbonation of fine recycled concrete wastes: An alternative pathway for efficient CO2 sequestration. Renew. Sustain. Energy Rev. 2023, 173, 113079. [Google Scholar] [CrossRef]
- You, X.; Hu, X.; Xiao, Z.; Saleh Bairq, Z.; Chen, W.; Shi, C. Thermodynamic modelling of CaCO3 polymorphs during CO2 sequestration by cement slurry with the addition of MgCl2. J. Clean. Prod. 2023, 410, 137294. [Google Scholar] [CrossRef]
- Waly, T.; Waly, T.; Kennedy, M.; Witkamp, G.; Amy, G.; Schippers, J. The role of inorganic ions in the calcium carbonate scaling of seawater reverse osmosis systems. Desalination 2012, 284, 279–287. [Google Scholar] [CrossRef]
- Ye, J.; Liu, S.; Zhao, Y.; Li, Y.; Fang, J.; Zhang, H.; Guan, X. Development of Ultrafine Mineral Admixture from Magnesium Slag and Sequestration of CO2. Buildings 2023, 13, 204. [Google Scholar] [CrossRef]
- Xie, G.; Suo, Y.; Liu, L.; Zhu, M.; Xie, L.; Qu, H.; Sun, W. Mechanical grinding activation of modified magnesium slag and its use as backfilling cementitious material. Case Stud. Constr. Mater. 2023, 18, e01778. [Google Scholar] [CrossRef]
- Ruan, S.; Liu, L.; Shao, C.; Xie, L.; Zhu, M.; Wang, R. Study on the source of activity and differences between modified and unmodified magnesium slag as a filling cementitious materials. Constr. Build. Mater. 2023, 392, 132019. [Google Scholar] [CrossRef]
- Ruan, S.; Liu, L.; Zhu, M.; Shao, C.; Xie, L. Development and field application of a modified magnesium slag-based mine filling cementitious material. J. Clean. Prod. 2023, 419, 138269. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, S.; Tang, P.; Guan, X.; Shi, C. Enhancing the hardening properties and microstructure of magnesium slag blocks by carbonation-hydration sequential curing. J. Build. Eng. 2023, 76, 107414. [Google Scholar] [CrossRef]
- Mo, L.; Panesar, D.K. Accelerated carbonation—A potential approach to sequester CO2 in cement paste containing slag and reactive MgO. Cem. Concr. Compos. 2013, 43, 69–77. [Google Scholar] [CrossRef]
- Mo, L.; Hao, Y.; Liu, Y.; Wang, F.; Deng, M. Preparation of calcium carbonate binders via CO2 activation of magnesium slag. Cem. Concr. Res. 2019, 121, 81–90. [Google Scholar] [CrossRef]
- Aryal, M.; Liakopoulou-Kyriakides, M. Equilibrium, kinetics and thermodynamic studies on phosphate biosorption from aqueous solutions by Fe(III)-treated Staphylococus xylosus biomass: Common ion effect. Colloids Surf. A Physicochem. Eng. Asp. 2011, 387, 43–49. [Google Scholar] [CrossRef]
- Hao, J.; Cheng, G.; Hu, T.; Guo, B.; Li, X. Preparation of high-performance building gypsum by calcining FGD gypsum adding CaO as crystal modifier. Constr. Build. Mater. 2021, 306, 124910. [Google Scholar] [CrossRef]
- Wu, C.; He, J.; Wang, K.; Yang, L.; Wang, F. Enhance the mechanical and water resistance performance of flue gas desulfurization gypsum by quaternary phase. Constr. Build. Mater. 2023, 387, 131565. [Google Scholar] [CrossRef]
- Chen, Q.; Ding, W.; Sun, H.; Peng, T. Synthesis of anhydrite from red gypsum and acidic wastewater treatment. J. Clean. Prod. 2021, 278, 124026. [Google Scholar] [CrossRef]
- Shen, P.; Lu, J.; Zhang, Y.; Jiang, Y.; Zhang, S.; Poon, C. Preparation aragonite whisker-rich materials by wet carbonation of cement: Towards yielding micro-fiber reinforced cement and sequestrating CO2. Cem. Concr. Res. 2022, 159, 106891. [Google Scholar] [CrossRef]
- Zhan, B.J.; Xuan, D.; Poon, C.; Scrivener, K. Characterization of interfacial transition zone in concrete prepared with carbonated modeled recycled concrete aggregates. Cem. Concr. Res. 2020, 136, 106175. [Google Scholar] [CrossRef]
- Lu, B.; Drissi, S.; Liu, J.; Hu, X.; Song, B.; Shi, C. Effect of temperature on CO2 curing, compressive strength and microstructure of cement paste. Cem. Concr. Res. 2022, 157, 106827. [Google Scholar] [CrossRef]
- Wang, X.; Ni, W.; Wei, X.; Zhang, S.; Li, J.; Hu, W. Promotion effects of gypsum on carbonation of aluminates in medium Al ladle furnace refining slag. Constr. Build. Mater. 2022, 336, 127567. [Google Scholar] [CrossRef]
- Jian, S.; Yang, X.; Gao, W.; Li, B.; Gao, X.; Huang, W.; Tan, H.; Lei, Y. Study on performance and function mechanisms of whisker modified flue gas desulfurization (FGD) gypsum. Constr. Build. Mater. 2021, 301, 124341. [Google Scholar] [CrossRef]
- Ma, F.; Chen, C.; Wang, Y. Mechanical behavior of calcium sulfate whisker-reinforced paraffin/gypsum composites. Constr. Build. Mater. 2021, 305, 124795. [Google Scholar] [CrossRef]
- Ding, X.; Wei, B.; Deng, M.; Chen, H.; Shan, Z. Effect of protein peptides with different molecular weights on the setting and hydration process of gypsum. Constr. Build. Mater. 2022, 318, 126185. [Google Scholar] [CrossRef]
- Li, L.; Cao, M.; Yin, H. Comparative roles between aragonite and calcite calcium carbonate whiskers in the hydration and strength of cement paste. Cem. Concr. Compos. 2019, 104, 103350. [Google Scholar] [CrossRef]
- Zajac, M.; Skocek, J.; Durdzinski, P.; Bullerjahn, F.; Skibsted, J.; Ben Haha, M. Effect of carbonated cement paste on composite cement hydration and performance. Cem. Concr. Res. 2020, 134, 106090. [Google Scholar] [CrossRef]
- Li, M.; Yang, Y.; Liu, M.; Guo, X.; Zhou, S. Hybrid effect of calcium carbonate whisker and carbon fiber on the mechanical properties and microstructure of oil well cement. Constr. Build. Mater. 2015, 93, 995–1002. [Google Scholar] [CrossRef]
- Lu, B.; Shi, C.; Zhang, J.; Wang, J. Effects of carbonated hardened cement paste powder on hydration and microstructure of Portland cement. Constr. Build. Mater. 2018, 186, 699–708. [Google Scholar] [CrossRef]
- Cao, M.; Zhang, C.; Lv, H.; Xu, L. Characterization of mechanical behavior and mechanism of calcium carbonate whisker-reinforced cement mortar. Constr. Build. Mater. 2014, 66, 89–97. [Google Scholar] [CrossRef]
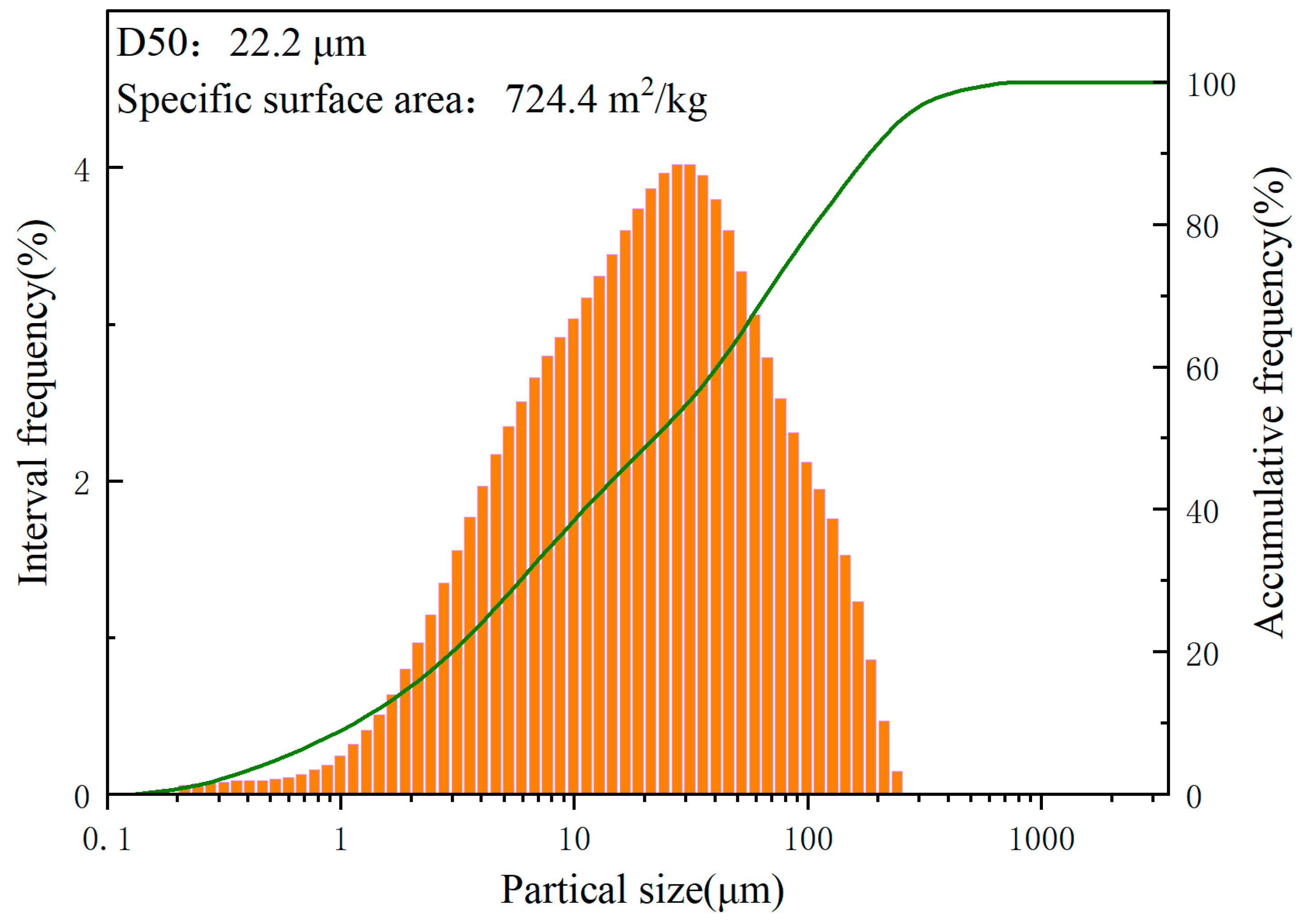

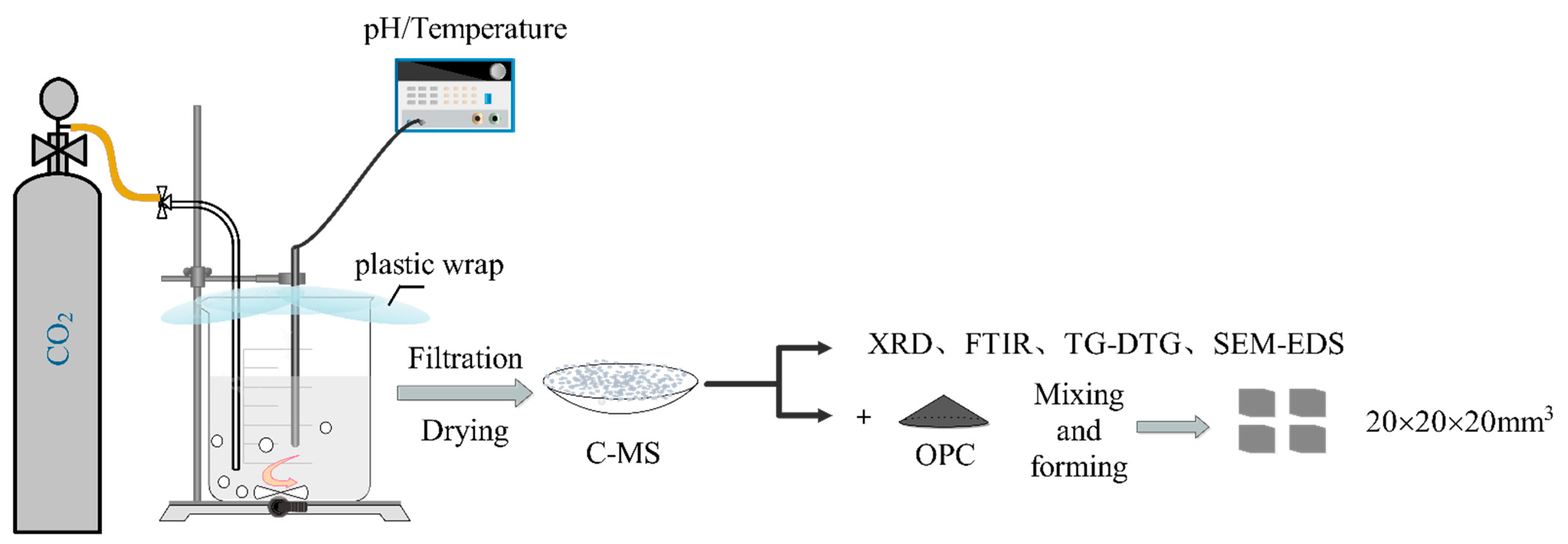
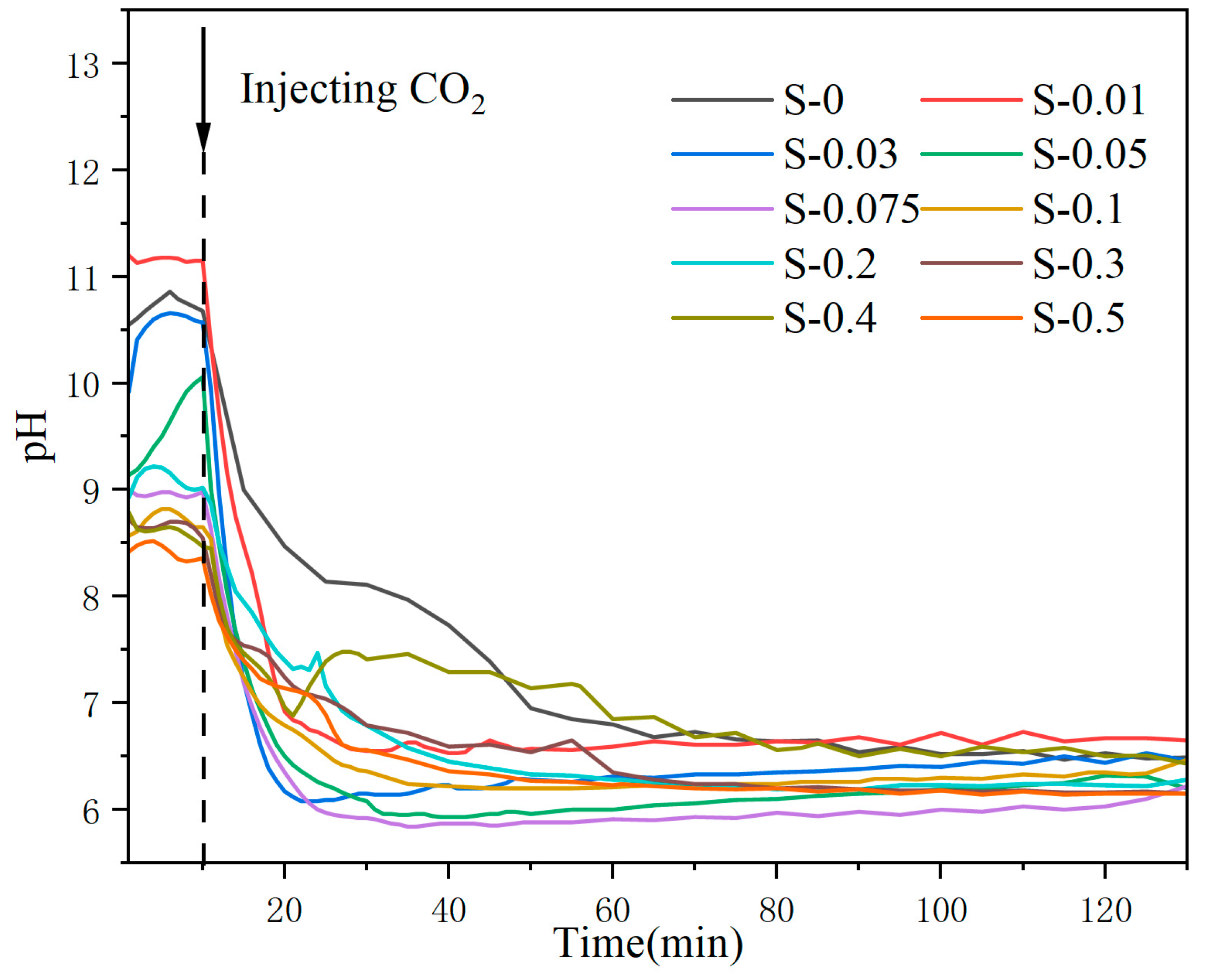
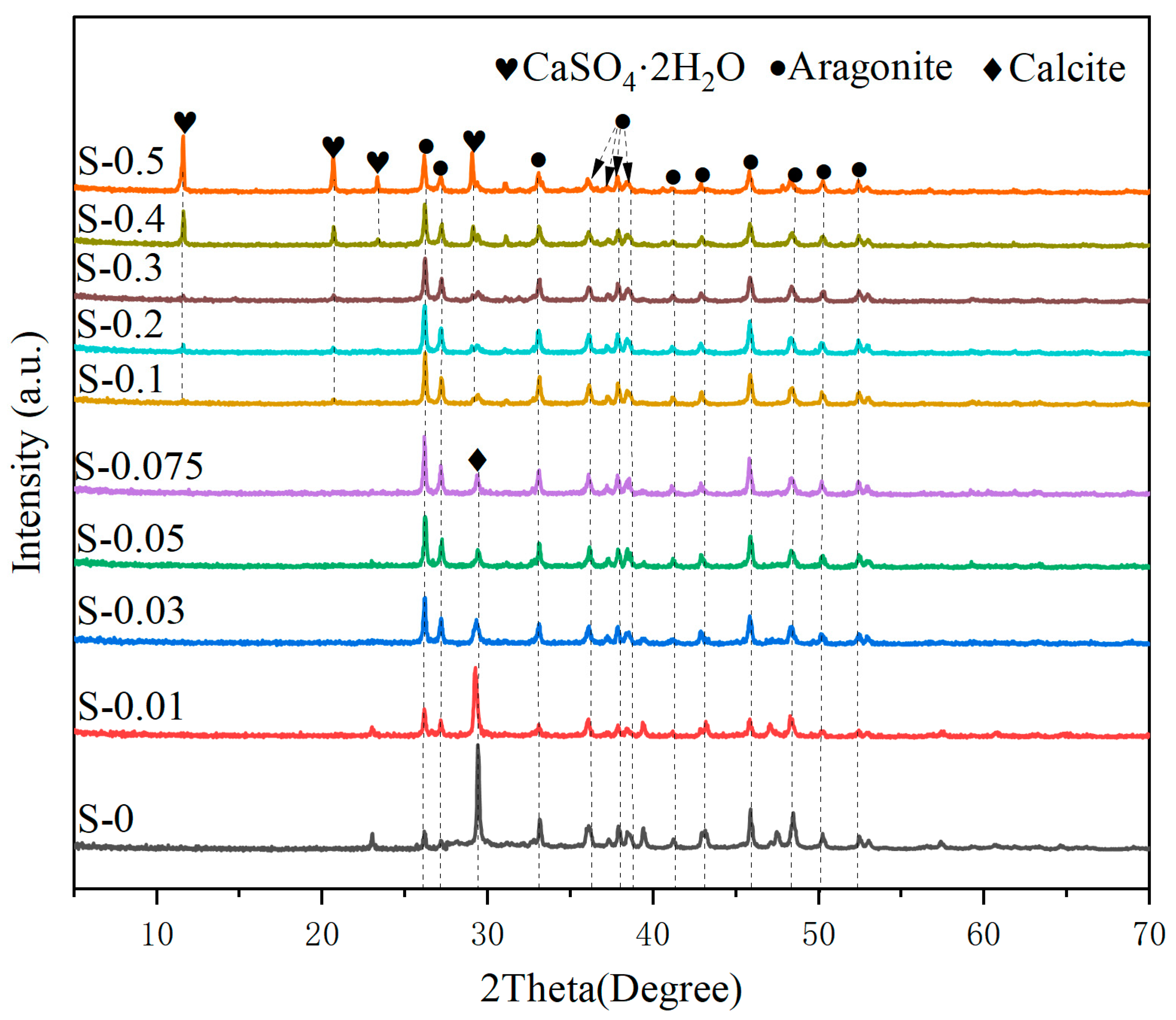


| Oxide | SiO2 | Al2O3 | CaO | Fe2O3 | MgO | Others |
|---|---|---|---|---|---|---|
| MS | 29.88 | 1.06 | 50.98 | 3.52 | 11.27 | 3.29 |
| OPC | 23.17 | 5.37 | 61.86 | 3.32 | 2.78 | 3.5 |
| Concentration (mol/L) | Calcite (%) | Aragonite (%) | CaSO4·2H2O (%) |
|---|---|---|---|
| 0 | 52.1% | 47.9% | 0 |
| 0.01 | 55% | 45% | 0 |
| 0.03 | 23.9% | 76.1% | 0 |
| 0.05 | 14.4% | 85.6% | 0 |
| 0.075 | 15.4% | 84.6% | 0 |
| 0.1 | 0 | 95.8% | 4.2% |
| 0.2 | 0 | 94.2% | 5.8% |
| 0.3 | 0 | 94.6% | 5.4% |
| 0.4 | 0 | 77.7% | 22.3% |
| 0.5 | 0 | 64.9% | 35.1% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ye, J.; Liu, S.; Fang, J.; Zhang, H.; Zhu, J.; Guan, X. Synthesis of Aragonite Whiskers by Co-Carbonation of Waste Magnesia Slag and Magnesium Sulfate: Enhancing Microstructure and Mechanical Properties of Portland Cement Paste. Buildings 2023, 13, 2888. https://doi.org/10.3390/buildings13112888
Ye J, Liu S, Fang J, Zhang H, Zhu J, Guan X. Synthesis of Aragonite Whiskers by Co-Carbonation of Waste Magnesia Slag and Magnesium Sulfate: Enhancing Microstructure and Mechanical Properties of Portland Cement Paste. Buildings. 2023; 13(11):2888. https://doi.org/10.3390/buildings13112888
Chicago/Turabian StyleYe, Junhao, Songhui Liu, Jingrui Fang, Haibo Zhang, Jianping Zhu, and Xuemao Guan. 2023. "Synthesis of Aragonite Whiskers by Co-Carbonation of Waste Magnesia Slag and Magnesium Sulfate: Enhancing Microstructure and Mechanical Properties of Portland Cement Paste" Buildings 13, no. 11: 2888. https://doi.org/10.3390/buildings13112888
APA StyleYe, J., Liu, S., Fang, J., Zhang, H., Zhu, J., & Guan, X. (2023). Synthesis of Aragonite Whiskers by Co-Carbonation of Waste Magnesia Slag and Magnesium Sulfate: Enhancing Microstructure and Mechanical Properties of Portland Cement Paste. Buildings, 13(11), 2888. https://doi.org/10.3390/buildings13112888







