Measurement of Water Retention Ratio in Rust Layer by Electrical Resistance †
Abstract
:1. Introduction
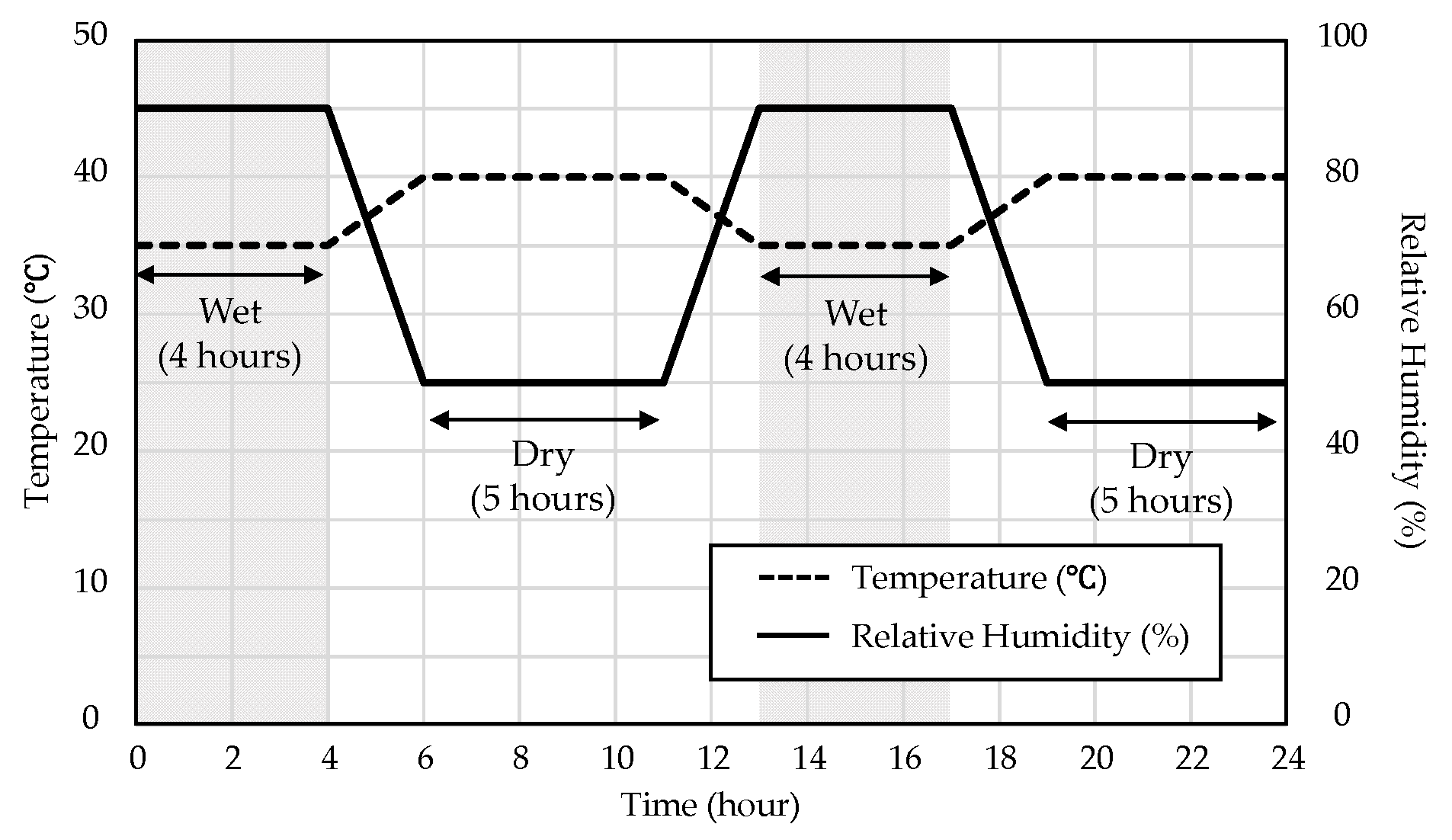
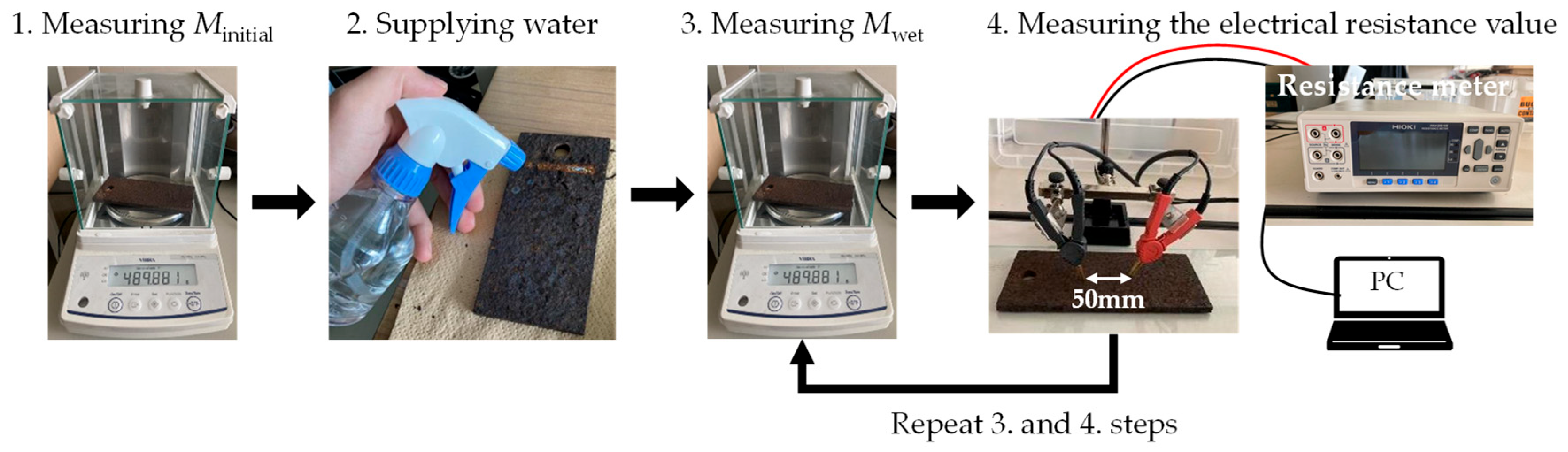
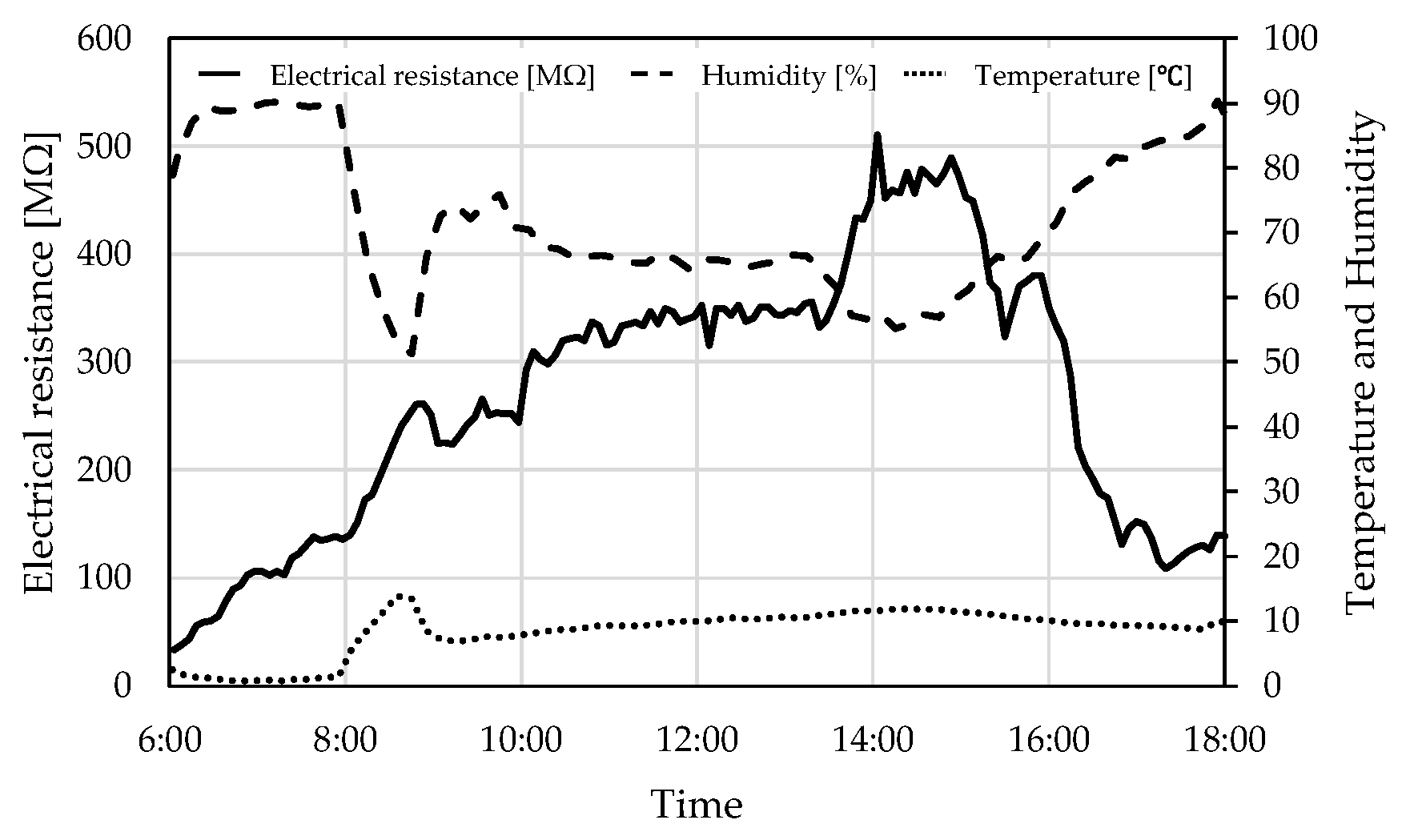
2. Materials and Measuring Methods
3. Electrical Resistance and Retained Water
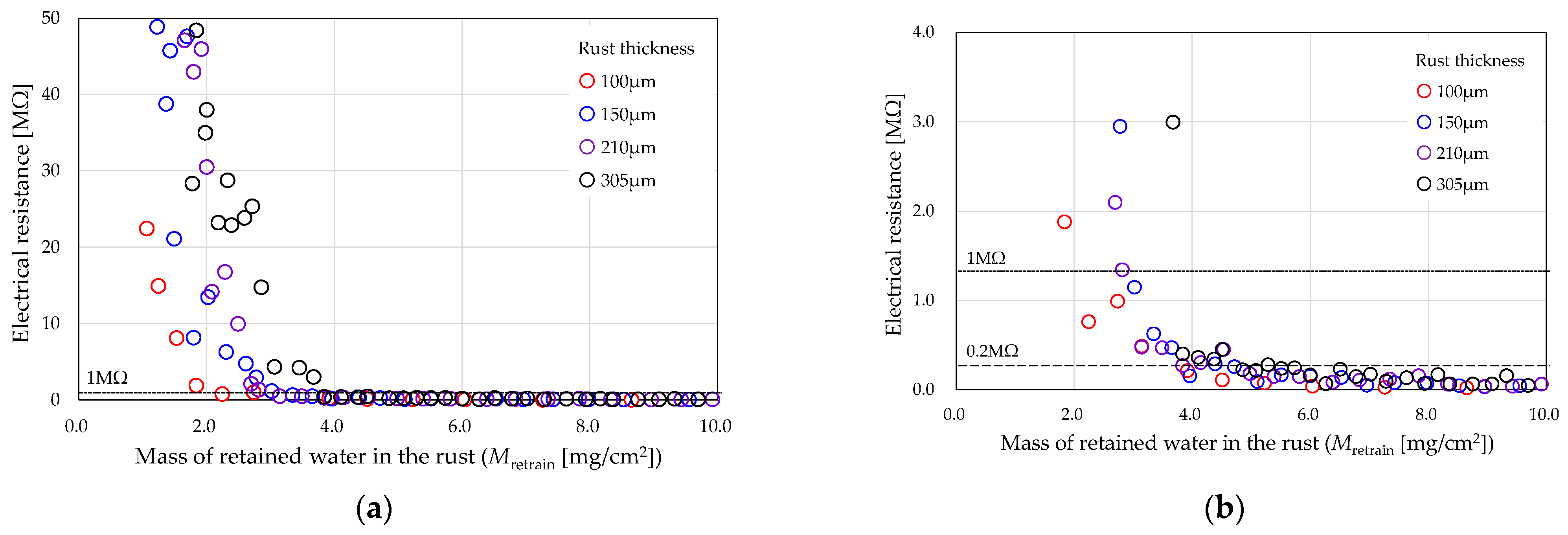
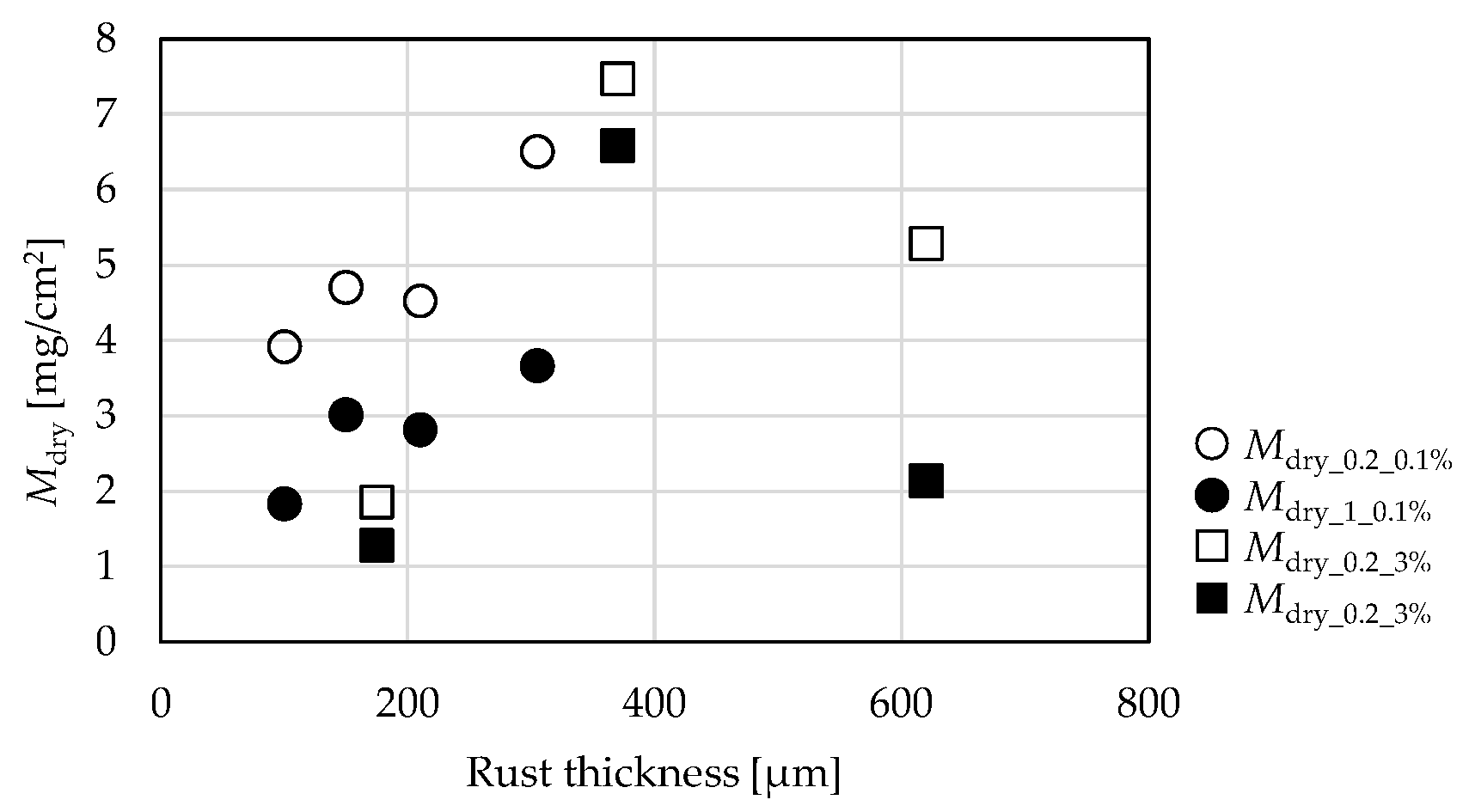
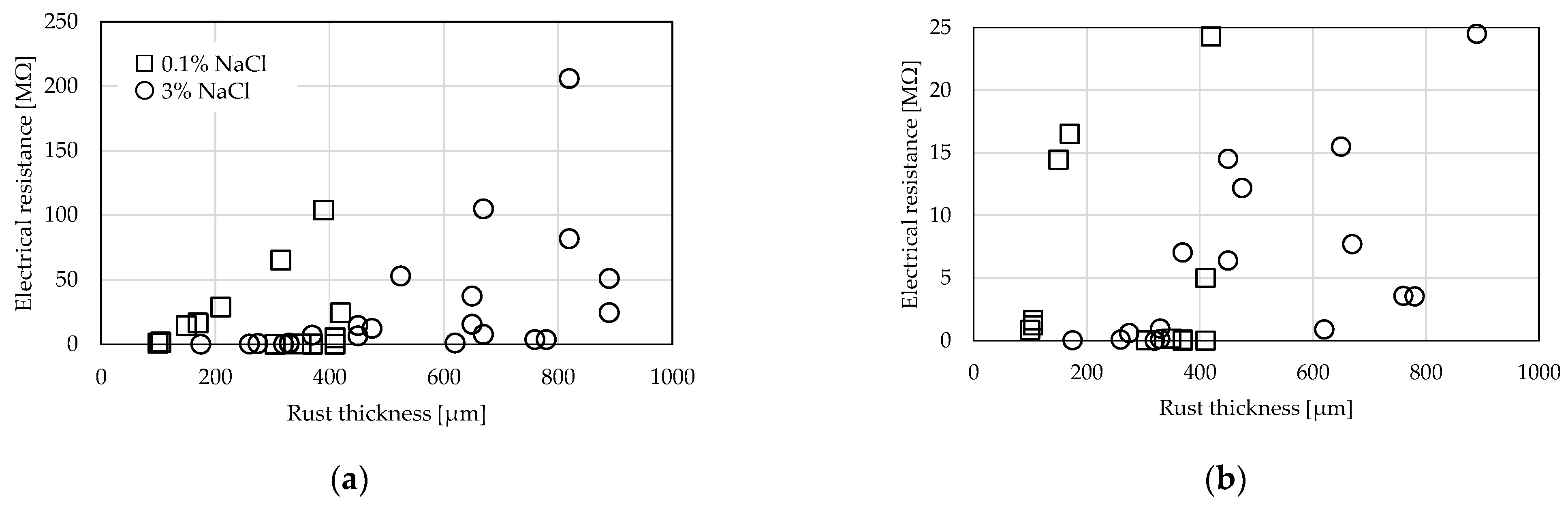
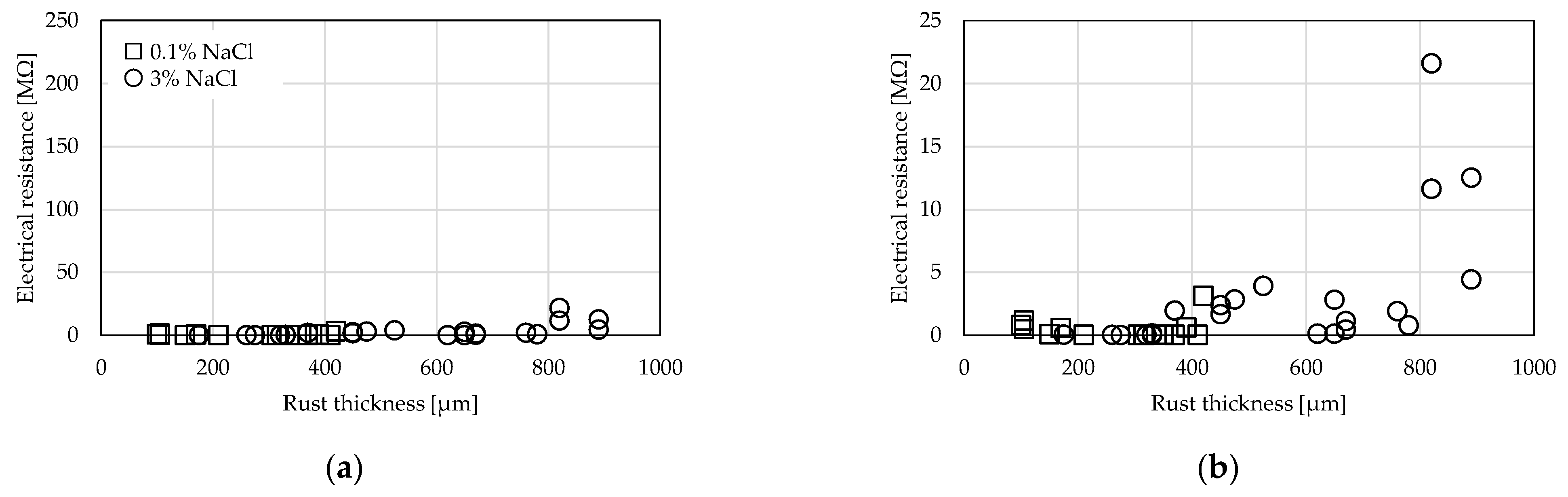
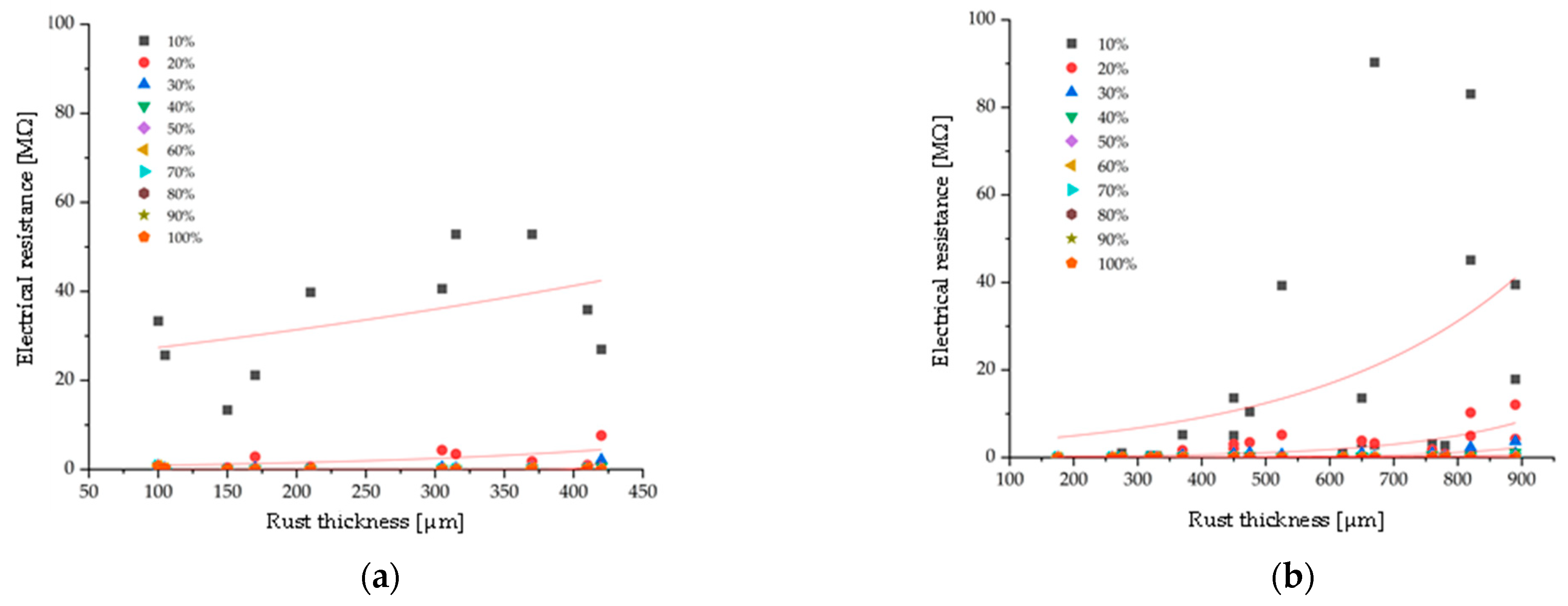
3.1. Comparison by Rust Thickness
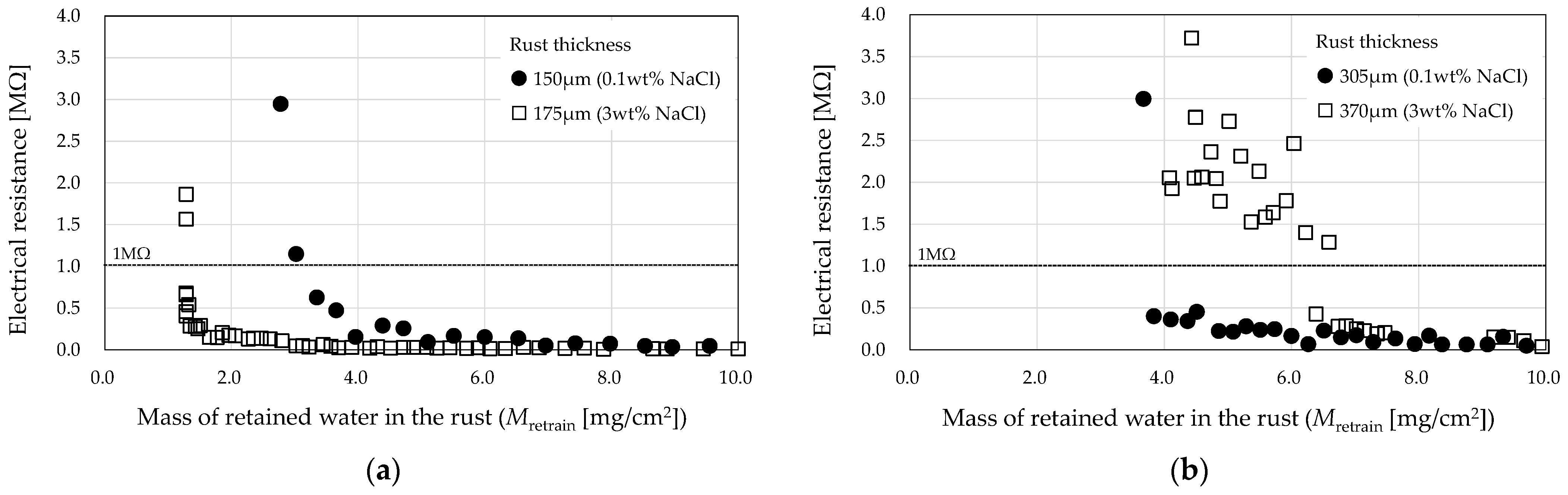
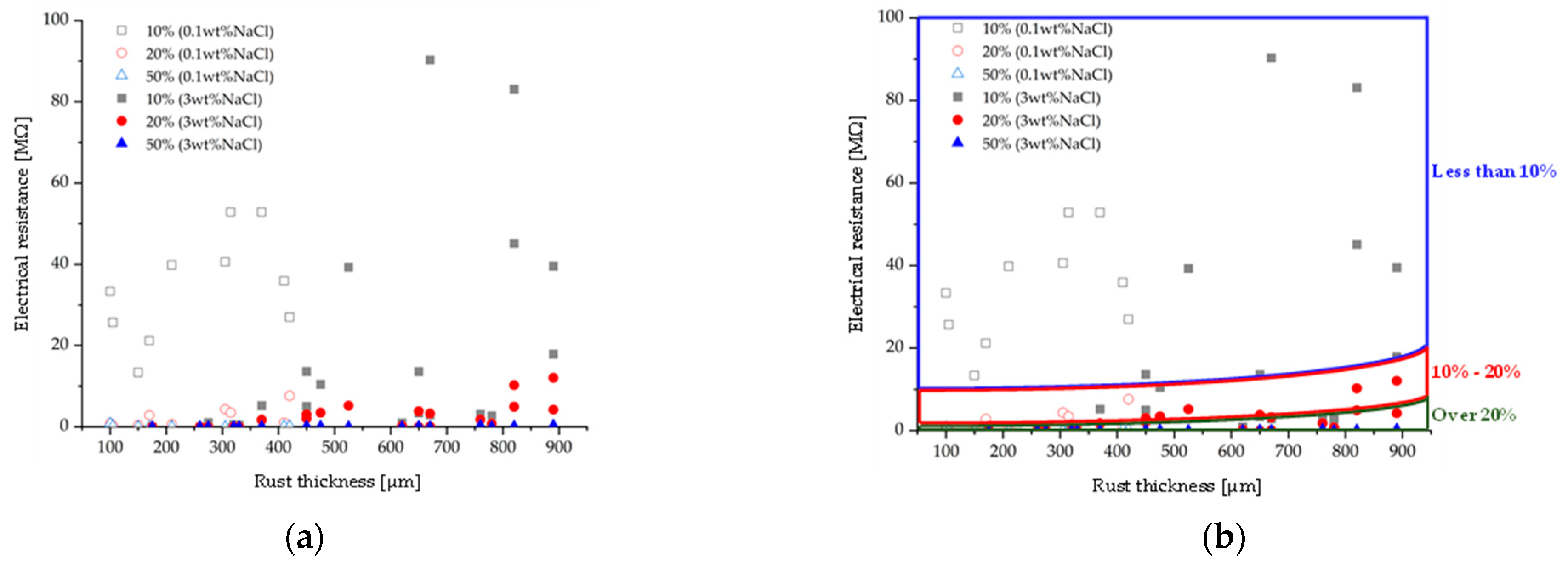
3.2. Comparison by Amount of Supplied NaCl
4. Prediction of Water Retention Ratio by Electrical Resistance and Rust Thickness
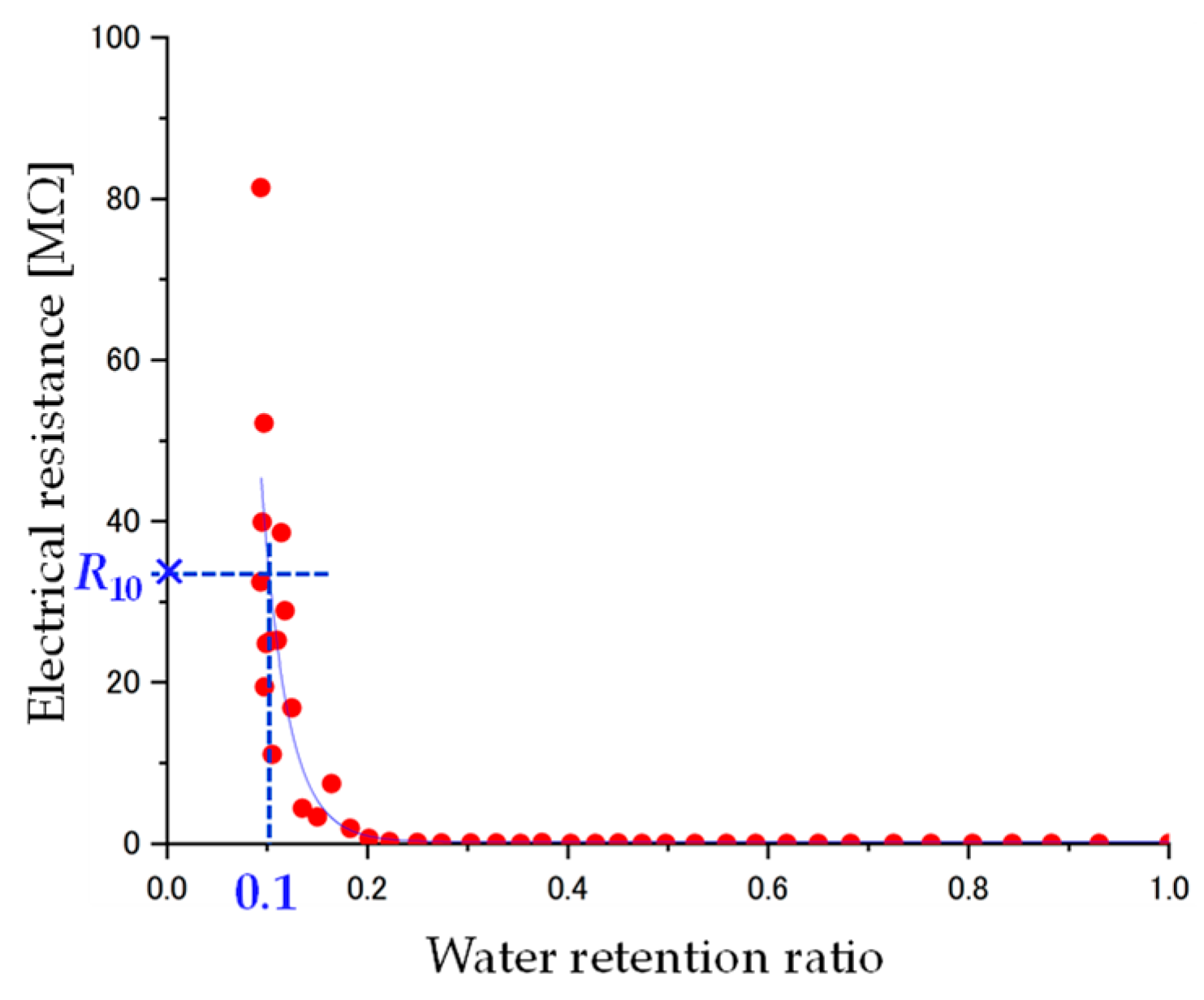
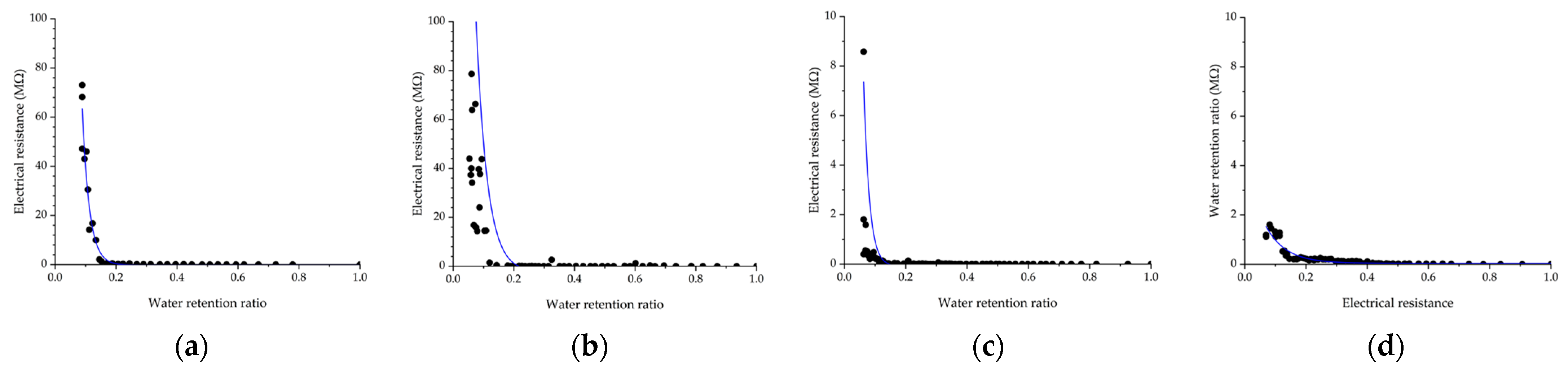
4.1. Water Retention Ratio and Electrical Resistance
4.2. Electrical Resistance in Each Water Retention Ratio
4.3. Prediction of Water Retention Ratio
5. Discussion
6. Conclusions
- (1)
- Based on the relationship between electrical resistance and the mass of retained water in the rust layer, it was found that electrical resistance increases as the retained water decreases.
- (2)
- It was also shown that, for a comparable amount of water retention, the electrical resistance decreases as the amount of NaCl supplied to the specimen increases.
- (3)
- The water retention ratio defined in this research was calculated by dividing the mass of the water-retained specimen by the mass of the full water-retained specimen. It was found that the electrical resistance decreases as the amount of NaCl supplied to the specimen increases, for the same water retention ratio. This trend aligns with conclusion (2).
- (4)
- Based on these measurement results, a water retention ratio prediction method for measuring electrical resistance and rust thickness is suggested. By plotting the electrical resistance and rust thickness to the suggested diagram, the approximate water retention ratio is predictable.
- (5)
- This diagram, however, still has a small number of specimen results, so its prediction accuracy is only indicative of a general trend. To improve its accuracy, more data will be collected.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vit, K.; Viktor, U.; Katerina, K. Development and failures of corrosion layers on typical surfaces of weathering steel bridges. Eng. Fail. Anal. 2016, 69, 147–160. [Google Scholar] [CrossRef]
- Agnieszka, K.; Leszek, K.; Izabela, K.; Damian, W.; Katarzyna, Z.; Urszula, P.; Tomasz, W.; Katarzyna, B. Corrosion assessment of a weathering steel bridge structure after 30 years of service. Materials 2021, 14, 3788. [Google Scholar] [CrossRef]
- Shuichi, H.; Takayuki, K.; Hideaki, M.; Masato, Y. Taxonomy for protective ability of rust layer using its composition formed on weathering steel bridge. Corros. Sci. 2007, 49, 1131–1142. [Google Scholar] [CrossRef]
- ISO-9223; Corrosion of Metals and Alloys—Corrosivity of Atmospheres—Classification, Determination and Estimation. ISO: Geneva, Switzerland, 2012.
- Shinohara, T.; Motoda, S.; Oshikawa, W. Evaluation of Corrosivity of Atmosphere by ACM Type Corrosion Sensor. Zairyo-to-Kankyo 2005, 54, 375–382. (In Japanese) [Google Scholar] [CrossRef]
- Pei, Z.; Xiao, K.; Chen, L.; Li, Q.; Wu, J.; Ma, L.; Li, X. Investigation of Corrosion Behaviors on an Fe/Cu-Type ACM Sensor under Various Environments. Metals 2020, 10, 905. [Google Scholar] [CrossRef]
- Ahn, J.-H.; Jeong, Y.-S.; Kim, I.-T.; Jeon, S.-H.; Park, C.-H. A Method for Estimating Time-Dependent Corrosion Depth of Carbon and Weathering Steel Using an Atmospheric Corrosion Monitor Sensor. Sensors 2019, 19, 1416. [Google Scholar] [CrossRef] [PubMed]
- Onda, T.; Nozoe, Y.; Nakajima, R.; Iwasaki, E.; Miura, M. Investigation of time of wetness determination of corroded steel using change in DC current resistance. In Proceedings of the Conference of Kanto Branch of Japan Society of Civil Engineers, Niigata, Japan, 13 March 2014; p. I-68. (In Japanese). [Google Scholar]
- Romain, R.; Stéphane, G.; Julien, G.; Ioannis, I.; Stéphanie, B. Reinforced concrete structures: A review of corrosion mechanisms and advances in electrical methods for corrosion monitoring. Constr. Build. Mater. 2021, 269, 121240. [Google Scholar]
- Tadeja, K.; Viljem, K.; Andrej, K.; Vili, M.; Branko, B.; Andraž, L. Development of an Electrical Resistance Sensor from High Strength Steel for Automotive Applications. Sensors 2019, 19, 1956. [Google Scholar]
- Wang, P.; Wang, p.; Li, Q.; Cheng, X.; Cai, J.; Zhang, D.; Li, X. Study of rust layer evolution in Q345 weathering steel utilizing electric resistance probes. Corros. Sci. 2023, 225, 111595. [Google Scholar] [CrossRef]
- Rina, H.; Ryotaro, Y.; Toshihiko, A. Evaluation of water retention of rust layer based on electrical resistance measurement. In Proceedings of the Sixth Australasia and South-East Asia Structural Engineering and Construction Conference (ASEA SEC 6), Sydney, Australia, 29 November 2022; Volume 9, p. STR09. [Google Scholar]
- Miki, C.; Ichikawa, A.; Ukai, M.; Takemura, M.; Nakayama, T.; Kihira, H. Proposal for Weathering Alloy Index and Accelerated Corrosion Test Method to Select Weathering Steels. J. Jpn. Soc. Civ. Eng. 2003, 2003, 271–281. (In Japanese) [Google Scholar] [CrossRef] [PubMed]




| Rust Thickness (μm) | 100–200 | 200–300 | 300–400 | 400–500 | 500–600 | 600–700 | 700–800 | 800–900 | Total Number of Specimens |
|---|---|---|---|---|---|---|---|---|---|
| 0.1 wt% NaCl | 5 | 1 | 6 | 3 | - | - | - | - | 15 |
| 3 wt% NaCl | 1 | 2 | 4 | 3 | 1 | 5 | 3 | 4 | 23 |
| Tap Water | 0.1 wt% NaCl | 1 wt% NaCl | 3 wt% NaCl | |
|---|---|---|---|---|
| Conductivity [μS/m] | 20 | 200 | 1700 | 4600 |
| Temperature [°C] | 24.1 | 24.1 | 24.1 | 23.8 |
| Rust Thickness (μm) | 100 | 105 | 150 | 170 | 210 | 305 | 315 | 350 | 370 | 390 | 410 | 420 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| COD | 0.95 | 0.93 | 0.90 | 0.88 | 0.95 | 0.82 | 0.31 | 0.83 | 0.76 | 0.82 | 0.60 | 0.83 |
| Rust Thickness (μm) | 175 | 260 | 275 | 320 | 330 | 370 | 450 | 475 | 525 | 620 | 650 | 670 | 760 | 780 | 820 | 890 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| COD | 0.66 | 0.84 | 0.19 | 0.57 | 0.54 | 0.85 | 0.59 | 0.53 | 0.35 | 0.87 | 0.83 | 0.84 | 0.62 | 0.41 | 0.83 | 0.54 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hasuike, R.; Yoneyama, R.; Aso, T. Measurement of Water Retention Ratio in Rust Layer by Electrical Resistance. Buildings 2023, 13, 2921. https://doi.org/10.3390/buildings13122921
Hasuike R, Yoneyama R, Aso T. Measurement of Water Retention Ratio in Rust Layer by Electrical Resistance. Buildings. 2023; 13(12):2921. https://doi.org/10.3390/buildings13122921
Chicago/Turabian StyleHasuike, Rina, Ryotaro Yoneyama, and Toshihiko Aso. 2023. "Measurement of Water Retention Ratio in Rust Layer by Electrical Resistance" Buildings 13, no. 12: 2921. https://doi.org/10.3390/buildings13122921
APA StyleHasuike, R., Yoneyama, R., & Aso, T. (2023). Measurement of Water Retention Ratio in Rust Layer by Electrical Resistance. Buildings, 13(12), 2921. https://doi.org/10.3390/buildings13122921








