Durability Deterioration of Geopolymer Stabilized Soft Soil under Sodium Sulfate and Magnesium Sulfate Attack: A Comparative Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Specimen Preparation
- (1)
- The dry soil in the geotechnical drums was poured into an appropriate amount of tap water and mixed evenly to prepare the remodeled soil. It was then sealed for 24 h before use so that the water could diffuse evenly into the soil and come into complete contact with the soil particles. The moisture content of the remodeled soil was adjusted to 50.2% to match the in situ soil as closely as possible;
- (2)
- The appropriate amount of NaOH fragments and Na2SiO3 solution were weighed together and poured into a magnetic stirrer. They were then stirred uniformly for the preparation of the composite alkali activator solution, which was left in the room for 24 h to come to room temperature in order to eliminate the effect of temperature on the test results;
- (3)
- The geopolymer precursor materials (slag and fly ash) were weighed and poured into the net slurry mixer for 3 min at a slow speed. Next, the alkali activator solution and distilled water were, in turn, poured into the mixer for 2 min at a slow speed, and then 3 min at a fast speed to prepare the geopolymer slurry;
- (4)
- The geopolymer slurry and the remodeled soil were added together into the mixing drum and mixed well to form the SF-GSSS slurry, which was then poured into the standard triplex mold (70.7 × 70.7 × 70.7 mm) three times. In addition, the surface of the sample was scraped smooth with a scraper. The air bubbles in the slurry were discharged by vibrating for more than 2 min during each pouring;
- (5)
- The samples were completely wrapped with plastic film and put into a standard curing box (temperature 20 ± 2 °C, humidity greater than 95%) for 1 day and then demolded, after which the samples were rewrapped and continuously cured until the set age.
2.3. Test Methods
3. Results and Discussion
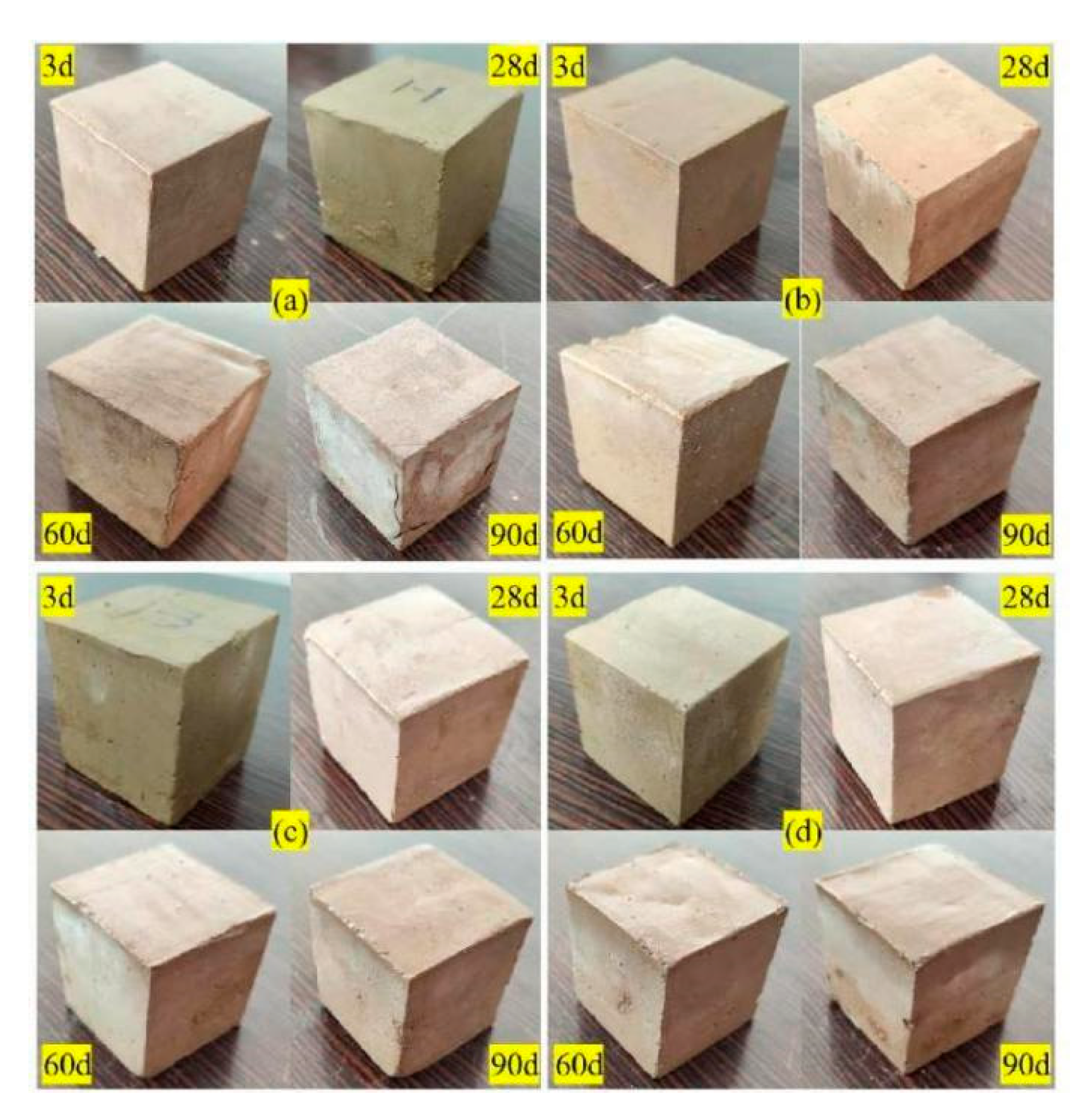
3.1. Visual Observation
3.2. Unconfined Compressive Strength
3.2.1. The UCS before Sulfate Attack
3.2.2. The UCS after Immersion in Na2SO4 Solution
3.2.3. The UCS after Immersion in MgSO4 Solution
3.3. Mass Change
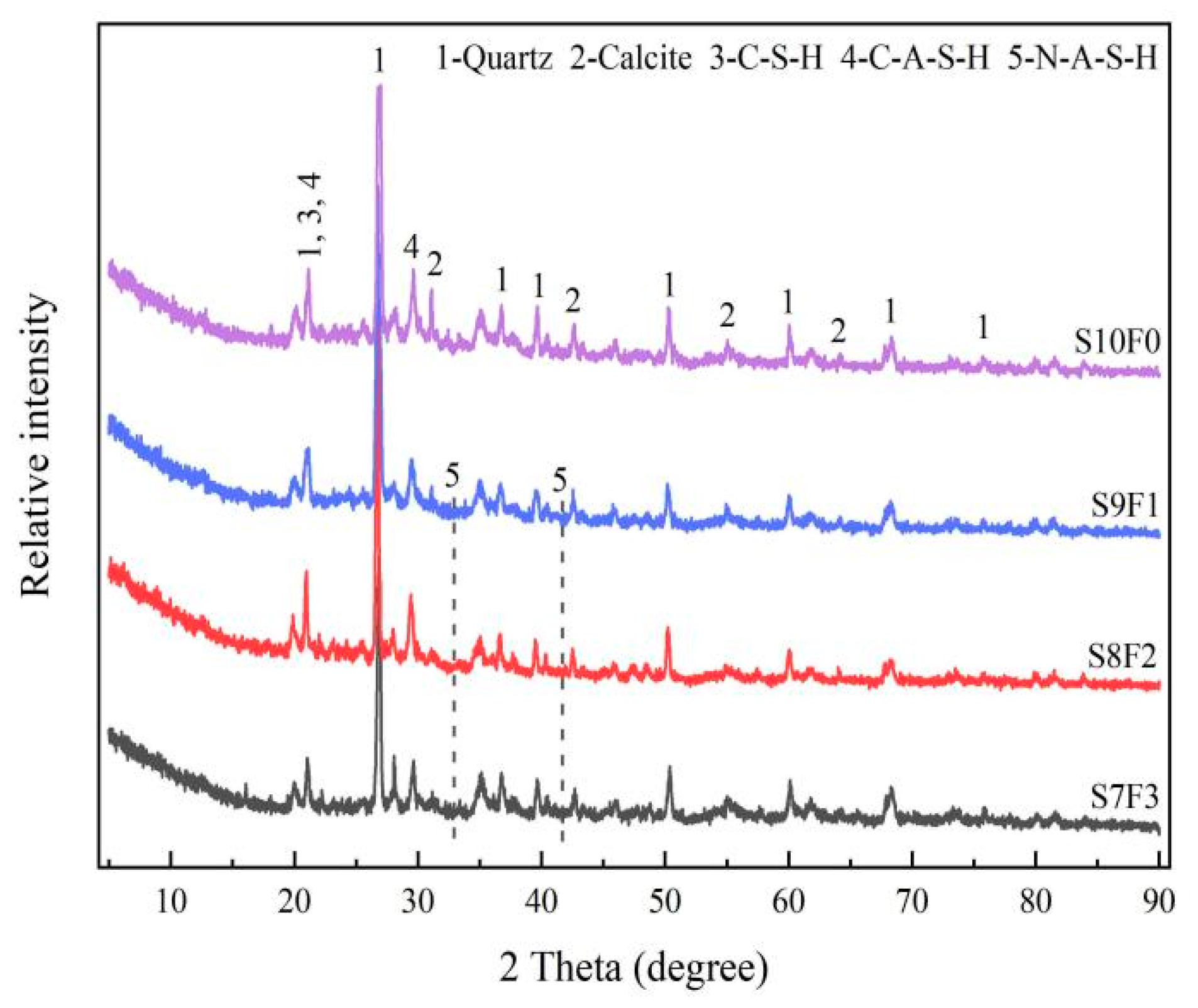
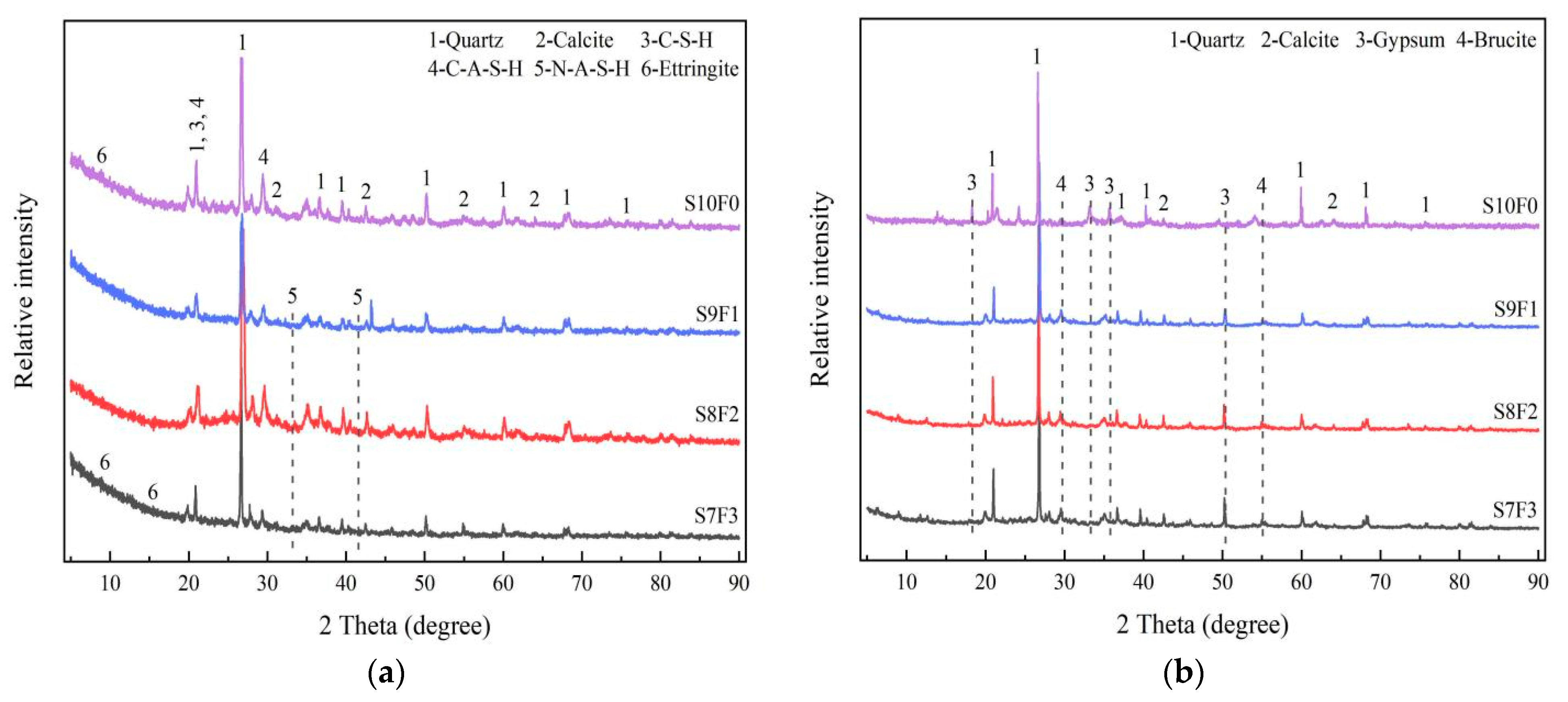
3.4. XRD Analysis
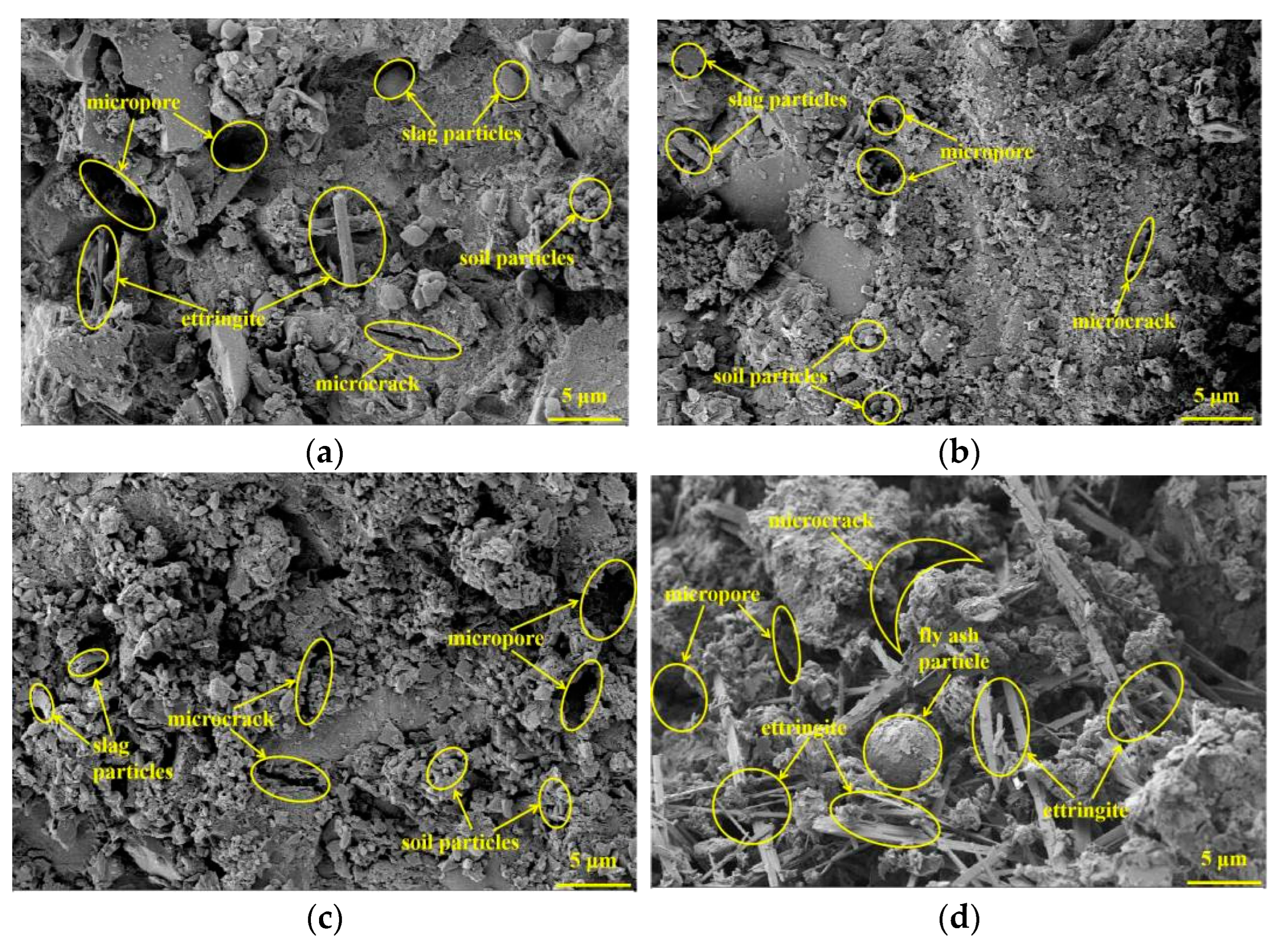
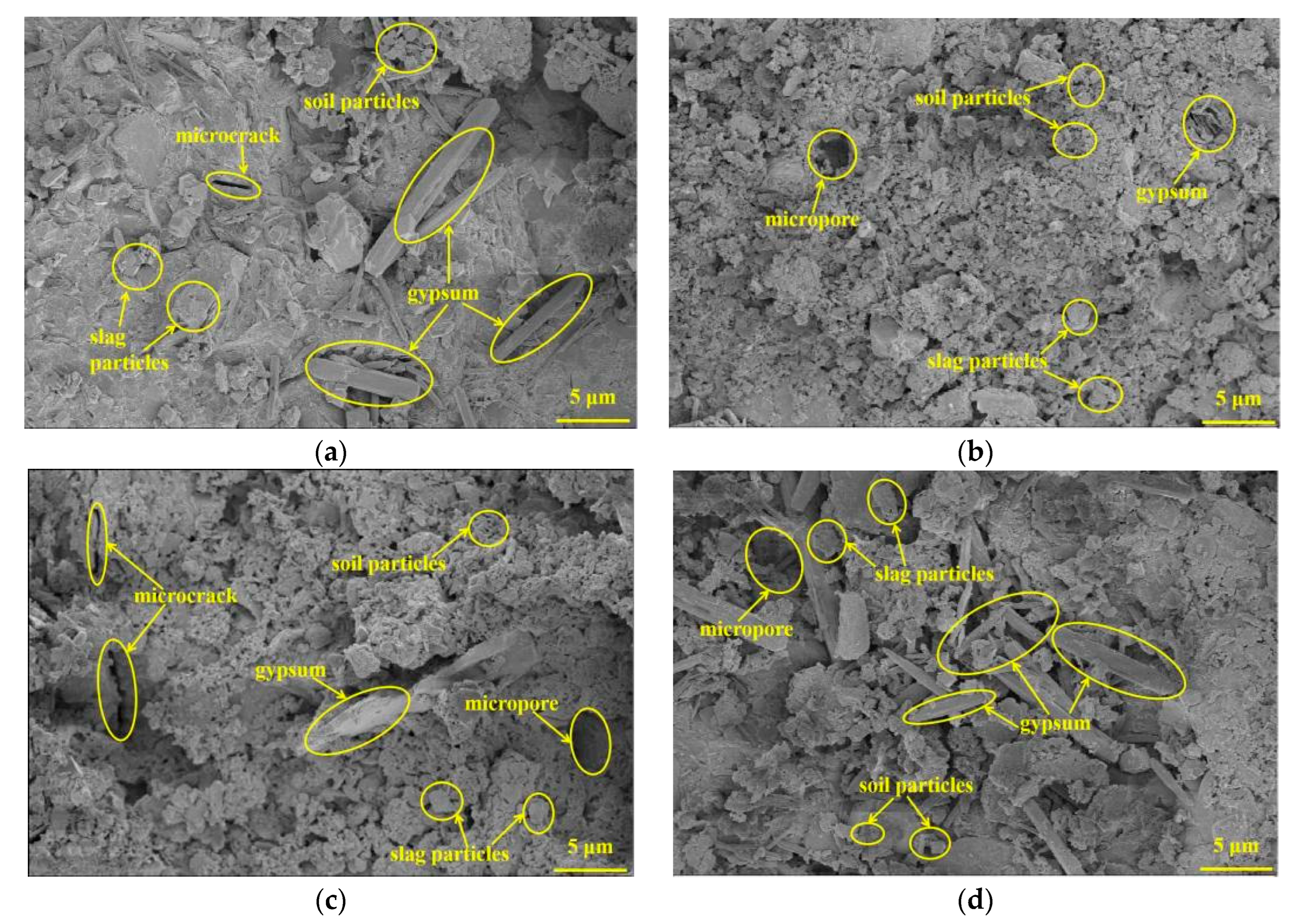
3.5. SEM Analysis
4. Mechanism Analysis of Sulfate Attack
5. Conclusions
- (1)
- The surface of SF-GSSS attacked by a Na2SO4 solution showed no obvious deterioration, while the surface of SF-GSSS attacked by a MgSO4 solution showed serious physical deterioration, such as swelling, cracking, and the shedding of soil particles;
- (2)
- The mass of SF-GSSS increased with the extension of the immersion period, whether immersed in a Na2SO4 or MgSO4 solution. At the same immersion age, the degree of mass growth of SF-GSSS immersed in a Na2SO4 solution was much lower than that of SF-GSSS immersed in a MgSO4 solution;
- (3)
- The UCS of SF-GSSS immersed in a Na2SO4 solution gradually decreased during the immersion period, while the UCS of SF-GSSS under the attack effect of a MgSO4 solution gradually decreased until 28 days of the immersion age, after which a significant decrease in strength occurred. In addition, the strength loss rate, throughout the immersion cycle, of SF-GSSS immersed in a MgSO4 solution was greater than when it was immersed in a Na2SO4 solution;
- (4)
- There is an important relationship between the slag/fly ash ratio and the resistance of SF-GSSS to sulfate attack. When exposed to Na2SO4 and MgSO4 solutions for 90 days, the strength loss rate of S9F1 was the lowest, which were 13.38% and 20.64%, and the strength loss rate of S7F3 was the highest, which were 20.64% and 85.26%, respectively;
- (5)
- The results of the XRD and SEM analysis indicated that the attack deterioration of the Na2SO4 solution mainly included the formation of microcracks and the expansion and cracking action of ettringite, while the MgSO4 solution mainly consisted of a weak cementation effect, as well as the swelling and cracking action of gypsum.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Cristelo, N.; Glendinning, S.; Fernandes, L.; Pinto, A.T. Effects of alkaline-activated fly ash and Portland cement on soft soil stabilisation. Acta Geotech. 2013, 8, 395–405. [Google Scholar] [CrossRef]
- Chompoorat, T.; Thepumong, T.; Khamplod, A.; Likitlersuang, S. Improving mechanical properties and shrinkage cracking characteristics of soft clay in deep soil mixing. Constr. Build. Mater. 2022, 316, 125858. [Google Scholar] [CrossRef]
- Zhang, G.; Ding, Z.; Wang, Y.; Fu, G.; Wang, Y.; Xie, C.; Zhang, Y.; Zhao, X.; Lu, X.; Wang, X. Performance Prediction of Cement Stabilized Soil Incorporating Solid Waste and Propylene Fiber. Materials 2022, 15, 4250. [Google Scholar] [CrossRef] [PubMed]
- Firoozi, A.A.; Guney Olgun, C.; Firoozi, A.A.; Baghini, M.S. Fundamentals of soil stabilization. Int. J. Geo-Eng. 2017, 8, 26. [Google Scholar] [CrossRef]
- Zhang, G.; Ding, Z.; Zhang, R.; Chen, C.; Fu, G.; Luo, X.; Wang, Y.; Zhang, C. Combined utilization of construction and demolition waste and propylene fiber in cement-stabilized soil. Buildings 2022, 12, 350. [Google Scholar] [CrossRef]
- Luo, Z.; Zhang, B.; Zou, J.; Luo, B. Sulfate erosion resistance of slag-fly ash based geopolymer stabilized soft soil under semi-immersion condition. Case Stud. Constr. Mat. 2022, 17, e01506. [Google Scholar] [CrossRef]
- Maddalena, R.; Roberts, J.J.; Hamilton, A. Can Portland cement be replaced by low-carbon alternative materials? A study on the thermal properties and carbon emissions of innovative cements. J. Clean. Prod. 2018, 186, 933–942. [Google Scholar] [CrossRef]
- Davidovits, J. Geopolymers: Inorganic polymeric new materials. J. Therm. Anal. Calorim. 1991, 37, 1633–1656. [Google Scholar] [CrossRef]
- Qaidi, S.M.A.; Tayeh, B.A.; Ahmed, H.U.; Emad, W. A review of the sustainable utilisation of red mud and fly ash for the production of geopolymer composites. Constr. Build. Mater. 2022, 350, 128892. [Google Scholar] [CrossRef]
- Ozturk, M.; Arslan, G. Shear Behavior of Granulated Blast Furnace Slag-Based Geopolymer-Reinforced Concrete Beams. Buildings 2022, 12, 2053. [Google Scholar] [CrossRef]
- Sruthi, S.; Gayathri, V. Synthesis and Evaluation of Eco-Friendly, Ambient-Cured, Geopolymer-Based Bricks Using Industrial By-Products. Buildings 2023, 13, 510. [Google Scholar] [CrossRef]
- Singh, N.B.; Middendorf, B. Geopolymers as an alternative to Portland cement: An overview. Constr. Build. Mater. 2020, 237, 117455. [Google Scholar] [CrossRef]
- Amer, A.A.; El-Hoseny, S. Properties and performance of metakaolin pozzolanic cement pastes. J. Therm. Anal. Calorim. 2017, 129, 33–44. [Google Scholar] [CrossRef]
- Tan, J.; Cai, J.; Huang, L.; Yang, Q.; Mao, M.; Li, J. Feasibility of using microwave curing to enhance the compressive strength of mixed recycled aggregate powder based geopolymer. Constr. Build. Mater. 2020, 262, 120897. [Google Scholar] [CrossRef]
- Sahoo, S.; Singh, S.P. Strength and durability properties of expansive soil treated with geopolymer and conventional stabilizers. Constr. Build. Mater. 2022, 328, 127078. [Google Scholar] [CrossRef]
- Ghadir, P.; Zamanian, M.; Mahbubi-Motlagh, N.; Saberian, M.; Li, J.; Ranjbar, N. Shear strength and life cycle assessment of volcanic ash-based geopolymer and cement stabilized soil: A comparative study. Transp. Geotech. 2021, 31, 100639. [Google Scholar] [CrossRef]
- Zhou, H.; Wang, X.; Wu, Y.; Zhang, X. Mechanical properties and micro-mechanisms of marine soft soil stabilized by different calcium content precursors based geopolymers. Constr. Build. Mater. 2021, 305, 124722. [Google Scholar] [CrossRef]
- Samantasinghar, S.; Singh, S.P. Strength and Durability of Granular Soil Stabilized with FA-GGBS Geopolymer. J. Mater. Civil. Eng. 2021, 33, 06021003. [Google Scholar] [CrossRef]
- Bilondi, M.P.; Toufigh, M.M.; Toufigh, V. Experimental investigation of using a recycled glass powder-based geopolymer to improve the mechanical behavior of clay soils. Constr. Build. Mater. 2018, 170, 302–313. [Google Scholar] [CrossRef]
- Luo, Y.; Meng, J.; Wang, D.; Jiao, L.; Xue, G. Experimental study on mechanical properties and microstructure of metakaolin based geopolymer stabilized silty clay. Constr. Build. Mater. 2022, 316, 125662. [Google Scholar] [CrossRef]
- Rollings, R.S.; Burkes, J.P.; Rollings, M.P. Sulfate attack on cement-stabilized sand. J. Geotech. Geoenviron. 1999, 125, 364–372. [Google Scholar] [CrossRef]
- Rajasekaran, G. Sulphate attack and ettringite formation in the lime and cement stabilized marine clays. Ocean Eng. 2005, 32, 1133–1159. [Google Scholar] [CrossRef]
- Puppala, A.J.; Intharasombat, N.; Vempati, R.K. Experimental studies on ettringite-induced heaving in soils. J. Geotech. Geoenviron. 2005, 131, 325–337. [Google Scholar] [CrossRef]
- Han, P.; Wang, S.; Chen, F.Y.; Bai, X. Mechanism of cement-stabilized soil polluted by magnesium sulfate. J. Cent. South Univ. 2015, 22, 1869–1877. [Google Scholar] [CrossRef]
- Bonen, D.; Cohen, M.D. Magnesium sulfate attack on portland cement paste-I. Microstructural analysis. Cem. Concr. Res. 1992, 22, 169–180. [Google Scholar] [CrossRef]
- Singh, B.; Ishwarya, G.; Gupta, M.; Bhattacharyya, S.K. Geopolymer concrete: A review of some recent developments. Constr. Build. Mater. 2015, 85, 78–90. [Google Scholar] [CrossRef]
- Vikas, G.; Rao, T.D. Setting time, workability and strength properties of alkali activated fly Ash and slag based geopolymer concrete activated with high silica modulus water glass. IJST-Trans. Civ. Eng. 2021, 45, 1483–1492. [Google Scholar] [CrossRef]
- Wang, S.D.; Scrivener, K.L.; Pratt, P.L. Factors affecting the strength of alkali-activated slag. Cem. Concr. Res. 1994, 24, 1033–1043. [Google Scholar] [CrossRef]
- Chew, S.H.; Kamruzzaman, A.H.M.; Lee, F.H. Physicochemical and engineering behavior of cement treated clays. J. Geotech. Geoenviron. 2004, 130, 696–706. [Google Scholar] [CrossRef]
- Luo, Z.; Luo, B.; Zhao, Y.; Li, X.; Su, Y.; Huang, H.; Wang, Q. Experimental Investigation of Unconfined Compression Strength and Microstructure Characteristics of Slag and Fly Ash-Based Geopolymer Stabilized Riverside Soft Soil. Buildings 2022, 14, 307. [Google Scholar] [CrossRef] [PubMed]
- Kamon, M.; Nontananandh, S.; Katsumi, T. Utilization of stainless-steel slag by cement hardening. Soils Found. 1993, 33, 118–129. [Google Scholar] [CrossRef] [PubMed]
- Sukmak, P.; Silva, P.D.; Horpibulsuk, S.; Chindaprasirt, P. Sulfate resistance of clay-portland cement and clay high-calcium fly ash geopolymer. J. Mater. Civil. Eng. 2015, 27, 04014158. [Google Scholar] [CrossRef]
- Jiang, N.J.; Du, Y.J.; Liu, K. Durability of lightweight alkali-activated ground granulated blast furnace slag (GGBS) stabilized clayey soils subjected to sulfate attack. Appl. Clay Sci. 2018, 161, 70–75. [Google Scholar] [CrossRef]
- Rashad, A.M.; Bai, Y.; Basheer, P.A.M.; Collier, N.C.; Milestone, N.B. Chemical and mechanical stability of sodium sulfate activated slag after exposure to elevated temperature. Cem. Concr. Res. 2012, 42, 333–343. [Google Scholar] [CrossRef]
- Bernal, S.A.; Provis, J.L. Durability of alkali-activated materials: Progress and perspectives. J. Am. Ceram. Soc. 2014, 97, 997–1008. [Google Scholar] [CrossRef]
- Bakharev, T. Durability of geopolymer materials in sodium and magnesium sulfate solutions. Cem. Concr. Res. 2005, 35, 1233–1246. [Google Scholar] [CrossRef]
- Wu, J.; Zheng, X.; Yang, A.; Li, Y. Experimental study on compressive strength of slag-fly ash based geopolymer stabilized silty clay. Rock Soil Mech. 2021, 42, 647–655. (In Chinese) [Google Scholar]
- Park, K.S.; Ni, Z.; Côté, A.P.; Yaghi, O.M. Exceptional chemical and thermal stability of zeolitic imidazolate frameworks. Proc. Natl. Acad. Sci. USA 2006, 103, 10186–10191. [Google Scholar] [CrossRef]
- Ismail, I.; Bernal, S.A.; Provis, J.L.; Hamdan, S.; Deventer, J.S.J.V. Microstructural changes in alkali activated fly ash/slag geopolymers with sulfate exposure. Mater. Struct. 2013, 46, 361–373. [Google Scholar] [CrossRef]
- Dener, M.; Karatas, M.; Mohabbi, M. Sulfate resistance of alkali-activated slag/Portland cement mortar produced with lightweight pumice aggregate. Constr. Build. Mater. 2021, 304, 124671. [Google Scholar] [CrossRef]
- Ye, H.; Chen, Z.; Huang, L. Mechanism of sulfate attack on alkali-activated slag: The role of activator composition. Cem. Concr. Res. 2019, 125, 105868. [Google Scholar] [CrossRef]
- Bonen, D.; Cohen, M.D. Magnesium sulfate attack on portland cement paste—II. Chemical and mineralogical analyses. Cem. Concr. Res. 1992, 22, 707–718. [Google Scholar] [CrossRef]
- Zhang, J.; Shi, C.; Zhang, Z.; Hu, X. Reaction mechanism of sulfate attack on alkali-activated slag/fly ash cements. Constr. Build. Mater. 2022, 318, 126052. [Google Scholar] [CrossRef]
- Džunuzović, N.; Komljenović, M.; Nikolić, V.; Ivanović, T. External sulfate attack on alkali-activated fly ash-blast furnace slag composite. Constr. Build. Mater. 2017, 157, 737–747. [Google Scholar] [CrossRef]
- Alcamand, H.A.; Borges, P.H.R.; Silva, F.A.; Trindade, A.C.C. The effect of matrix composition and calcium content on the sulfate durability of metakaolin and metakaolin/slag alkali-activated mortars. Ceram. Int. 2018, 44, 5037–5044. [Google Scholar] [CrossRef]
- Gong, K.; White, C.E. Nanoscale chemical degradation mechanisms of sulfate attack in alkali-activated slag. J. Phys. Chem. 2018, 122, 5992–6004. [Google Scholar] [CrossRef]
- Yao, J.; Qiu, H.; He, H.; Chen, X. Application of a Soft Soil Stabilized by Composite Geopolymer. J. Perform. Constr. Fac. 2021, 35, 04021018. [Google Scholar] [CrossRef]
- Kaur, M.; Singh, J.; Kaur, M. Synthesis of fly ash based geopolymer mortar considering different concentrations and combinations of alkaline activator solution. Ceram. Int. 2018, 44, 1534–1537. [Google Scholar] [CrossRef]
- Mithun, B.M.; Narasimhan, M.C. Performance of alkali activated slag concrete mixes incorporating copper slag as fine aggregate. J. Clean. Prod. 2016, 112, 837–844. [Google Scholar] [CrossRef]








| Soil Sample | Natural Moisture Content (%) | Specific Gravity | Liquid Limit (%) | Plastic Limit (%) | Compression Modulus (MPa) | Void Ration | Wet Density (g·cm−3) |
|---|---|---|---|---|---|---|---|
| Soft soil | 50.2 | 2.55 | 49.9 | 25.7 | 3.85 | 1.45 | 1.49 |
| CaO | Al2O3 | SiO2 | MgO | Fe2O3 | SO3 | K2O | TiO2 | |
|---|---|---|---|---|---|---|---|---|
| Slag | 35.25 | 16.75 | 34.55 | 5.06 | 1.55 | 1.26 | - | - |
| Fly ash | 2.34 | 34.72 | 53.10 | 0.88 | 2.55 | 0.37 | 1.78 | 1.27 |
| Mix Codes | Precursors Content (wt%) | Slag: Fly Ash | Slag Content (wt%) | Fly Ash Content (wt%) | Alkali Activator | Water/ Precursors | Curing Age (Days) | |
|---|---|---|---|---|---|---|---|---|
| Modulus | Content (wt%) | |||||||
| S10F0 | 25 | 10:0 | 25 | 0 | 1.2 | 40 | 0.4 | 28 |
| S9F1 | 25 | 9:1 | 22.5 | 2.5 | 1.2 | 40 | 0.4 | 28 |
| S8F2 | 25 | 8:2 | 20 | 5 | 1.2 | 40 | 0.4 | 28 |
| S7F3 | 25 | 7:3 | 17.5 | 7.5 | 1.2 | 40 | 0.4 | 28 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yi, X.; Wang, G.; Zhang, B.; Zhang, G.; Liu, Y.; Luo, Z. Durability Deterioration of Geopolymer Stabilized Soft Soil under Sodium Sulfate and Magnesium Sulfate Attack: A Comparative Study. Buildings 2023, 13, 1075. https://doi.org/10.3390/buildings13041075
Yi X, Wang G, Zhang B, Zhang G, Liu Y, Luo Z. Durability Deterioration of Geopolymer Stabilized Soft Soil under Sodium Sulfate and Magnesium Sulfate Attack: A Comparative Study. Buildings. 2023; 13(4):1075. https://doi.org/10.3390/buildings13041075
Chicago/Turabian StyleYi, Xinxiang, Guanci Wang, Benben Zhang, Genbao Zhang, Yuming Liu, and Zhengdong Luo. 2023. "Durability Deterioration of Geopolymer Stabilized Soft Soil under Sodium Sulfate and Magnesium Sulfate Attack: A Comparative Study" Buildings 13, no. 4: 1075. https://doi.org/10.3390/buildings13041075
APA StyleYi, X., Wang, G., Zhang, B., Zhang, G., Liu, Y., & Luo, Z. (2023). Durability Deterioration of Geopolymer Stabilized Soft Soil under Sodium Sulfate and Magnesium Sulfate Attack: A Comparative Study. Buildings, 13(4), 1075. https://doi.org/10.3390/buildings13041075







