The Influence of the Prestressing Level of the Fully Threaded Anchor Bar on the Corrosion Rate
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material
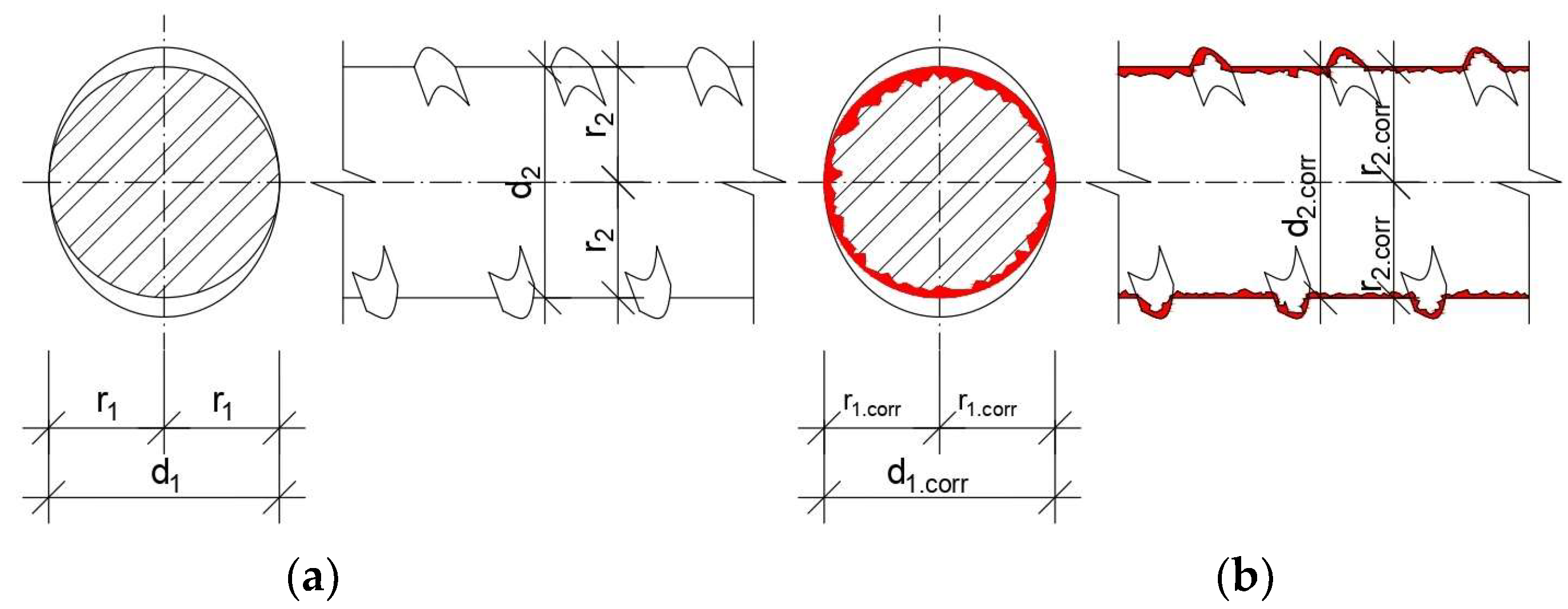
2.2. Calibration of Effective Uniform Corrosion
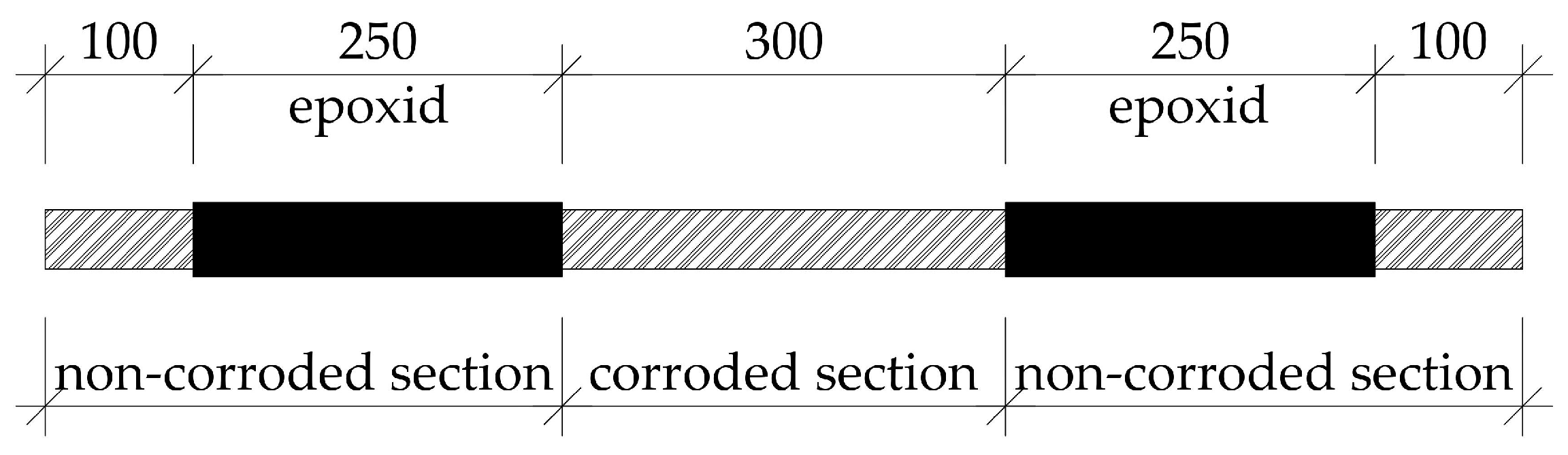
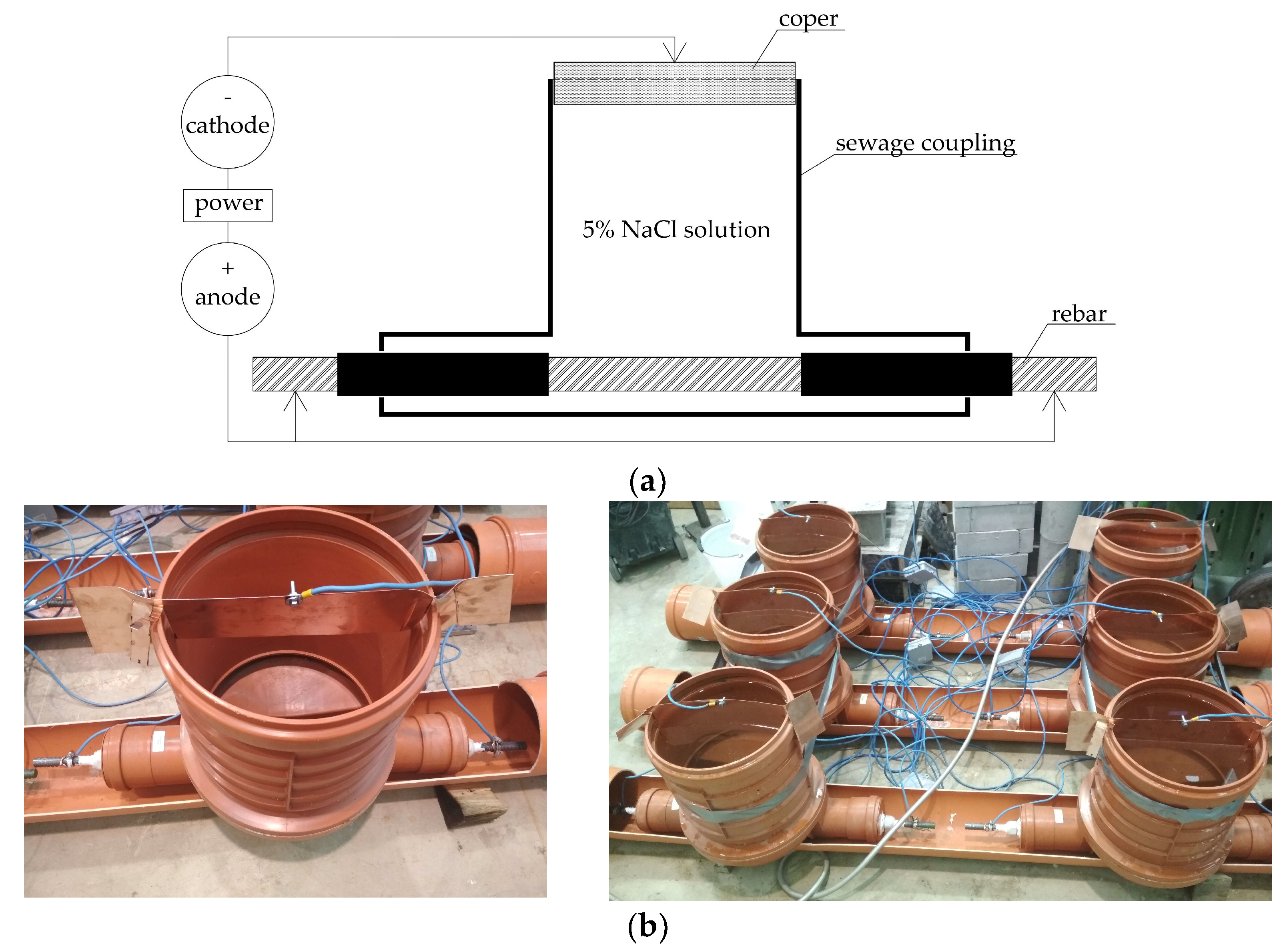
2.3. Sample Preparation and Electrical Current Connection
2.4. Theoretical Calculation Using Faraday’s Law
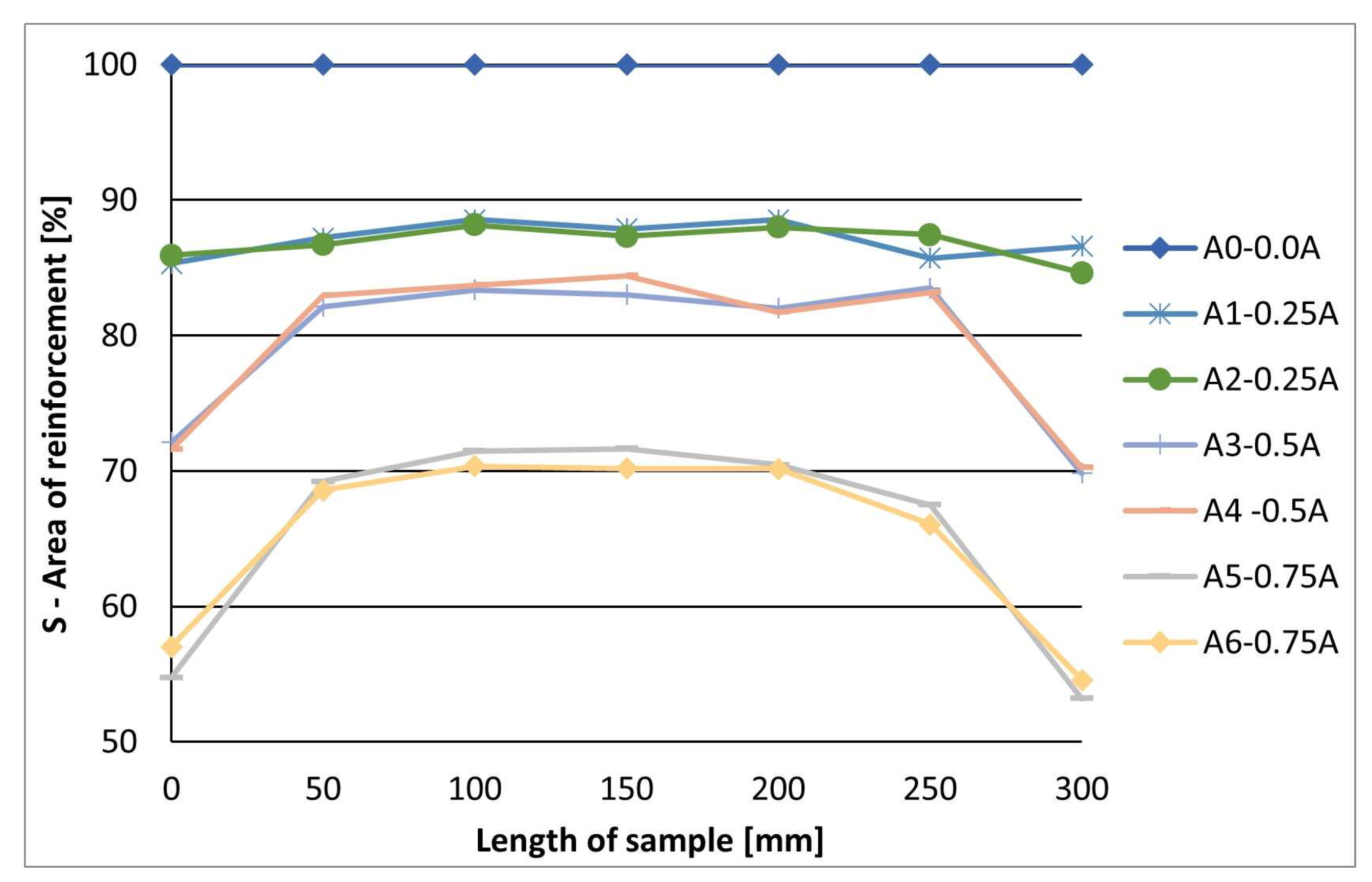
2.5. Influence of Electric Current Value
2.6. Influence of Prestressing Level on Corrosion
2.7. Evaluation Method Using Weight Loss
2.8. Summary of All Experimental Samples
3. Discussion
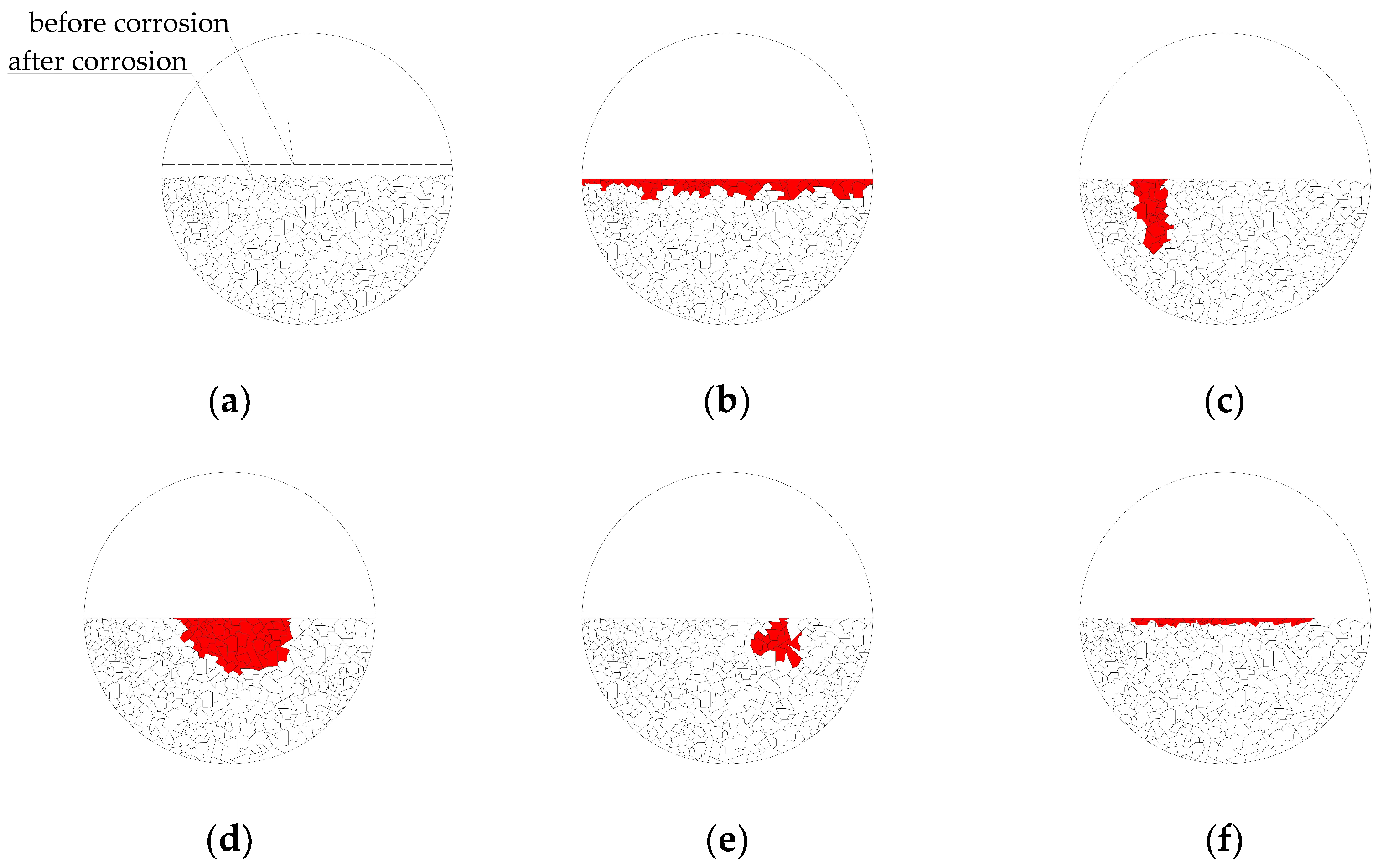
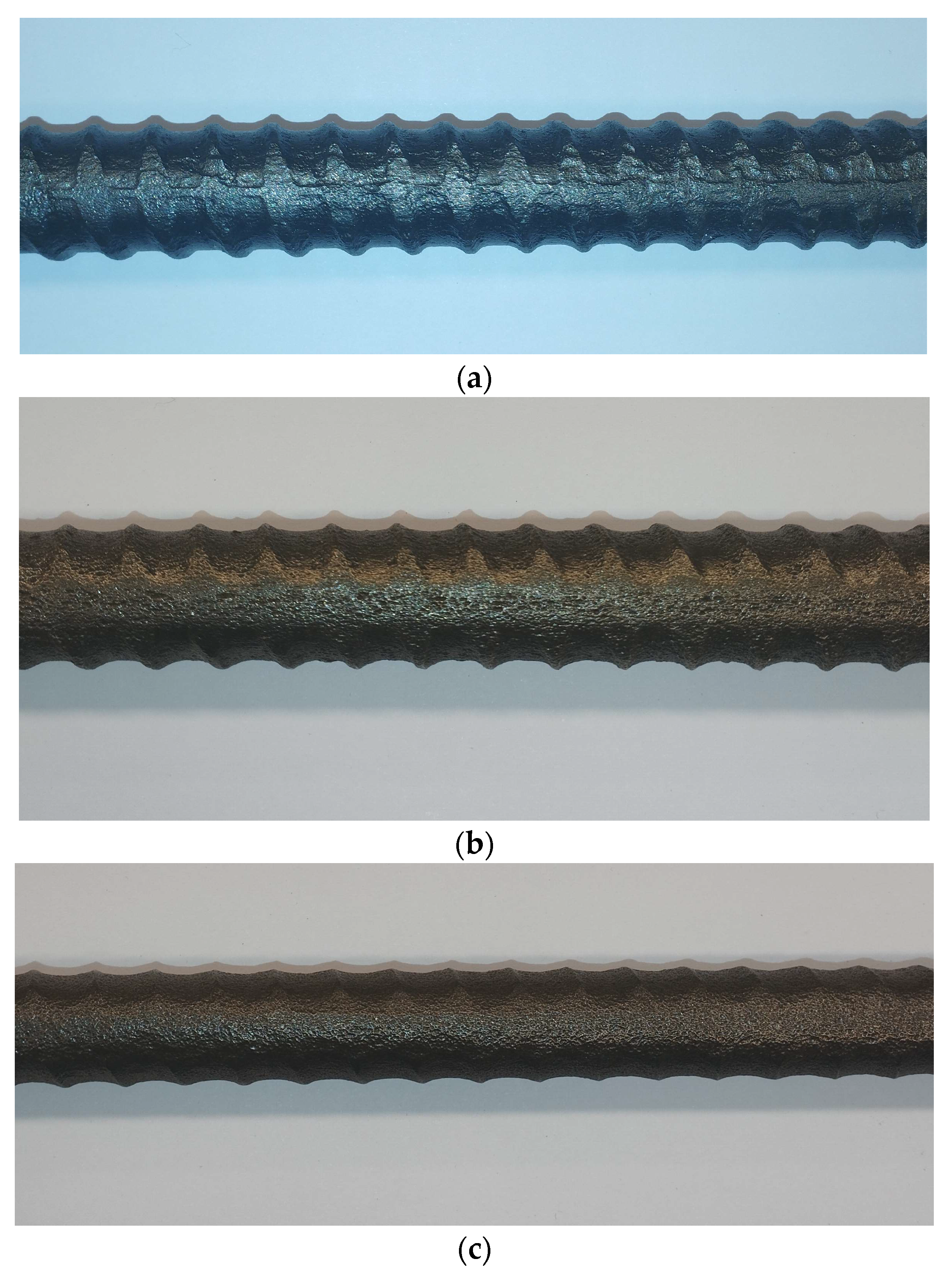
3.1. Corrosion Morphology
3.2. Influence of Electric Current Value
3.3. Electric Current Efficiency
3.4. Effect of Prestressing Level on Corrosion
4. Conclusions
- The distance of the copper (as a cathode) from the sample has a significant effect on the course and outcomes of the experiment. If the copper is very close, the sample cannot corrode evenly, but primary corrosion occurs in the vicinity of the copper. In addition, for uniform corrosion of the sample, it has a better effect if the sample is placed horizontally during the experiment.
- Experimental results have shown that there is no obvious difference in the sample morphology corrosion during the same time of corrosion exposure and the same value of electric current at the different levels of prestressing.
- The electric current value significantly affects the corrosion of prestressing bars. The optimal electric current value for the selected sample’s diameter is 0.25 A, which does not create significant pitting corrosion at the beginning and end of the corrosion area of the sample. This pitting corrosion could lead to the premature failure or rupture of the sample.
- The efficiency of the electric current was slightly lower than the theoretical calculation using Faraday’s Law. This could have been caused by the loss of current in competing reactions, so only a part of the external current oxidizes the anode to form corrosion products. Although Faraday’s Law does not apply exactly, it is possible to predict a rough estimate of the corrosion degree with known input data.
- Experimental, as well as the theoretical results of corrosion at different time dependencies, show that the degree of corrosion increased with the prolongation of the corrosion test time, and the degree of corrosion with time had a linear progression.
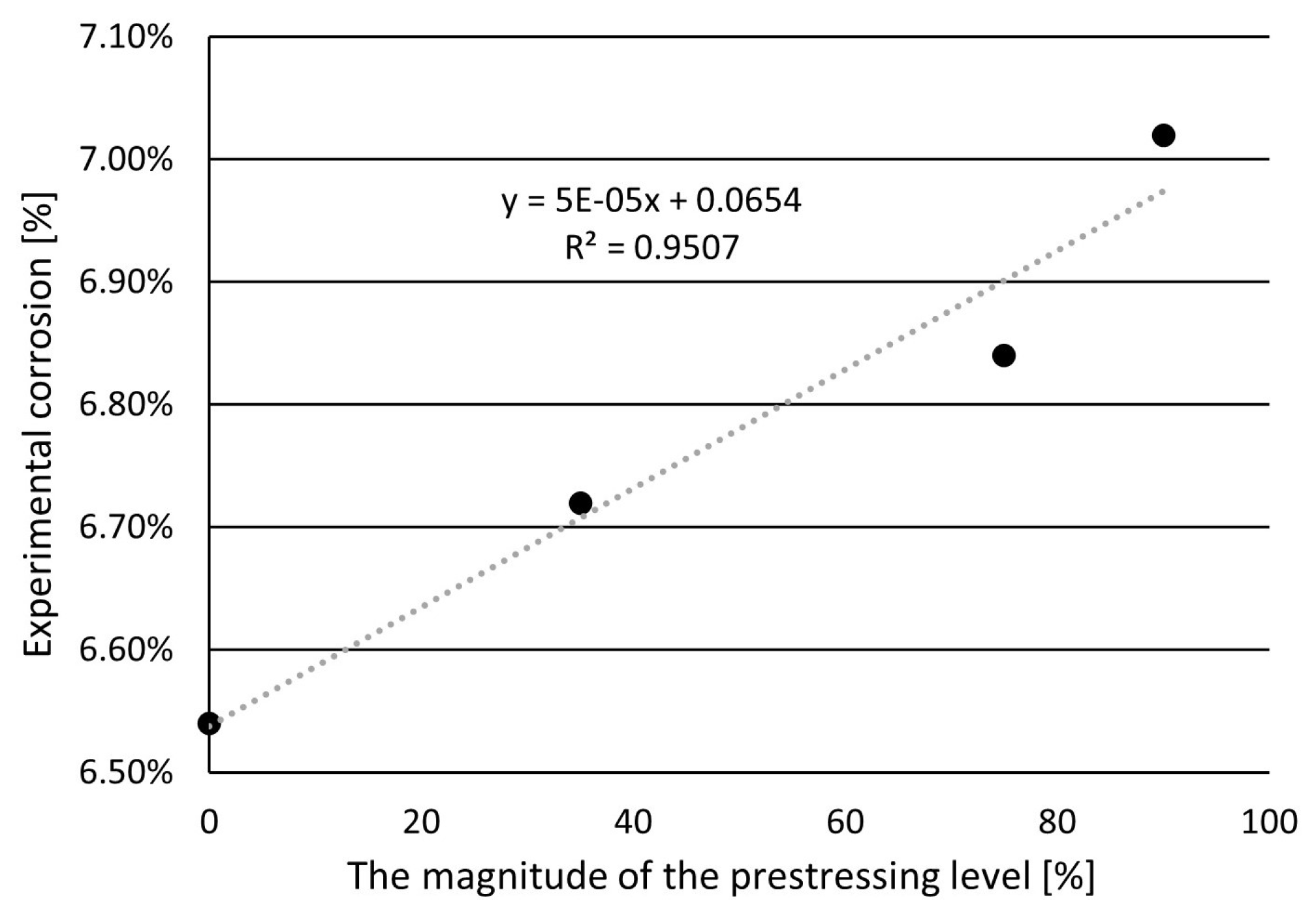
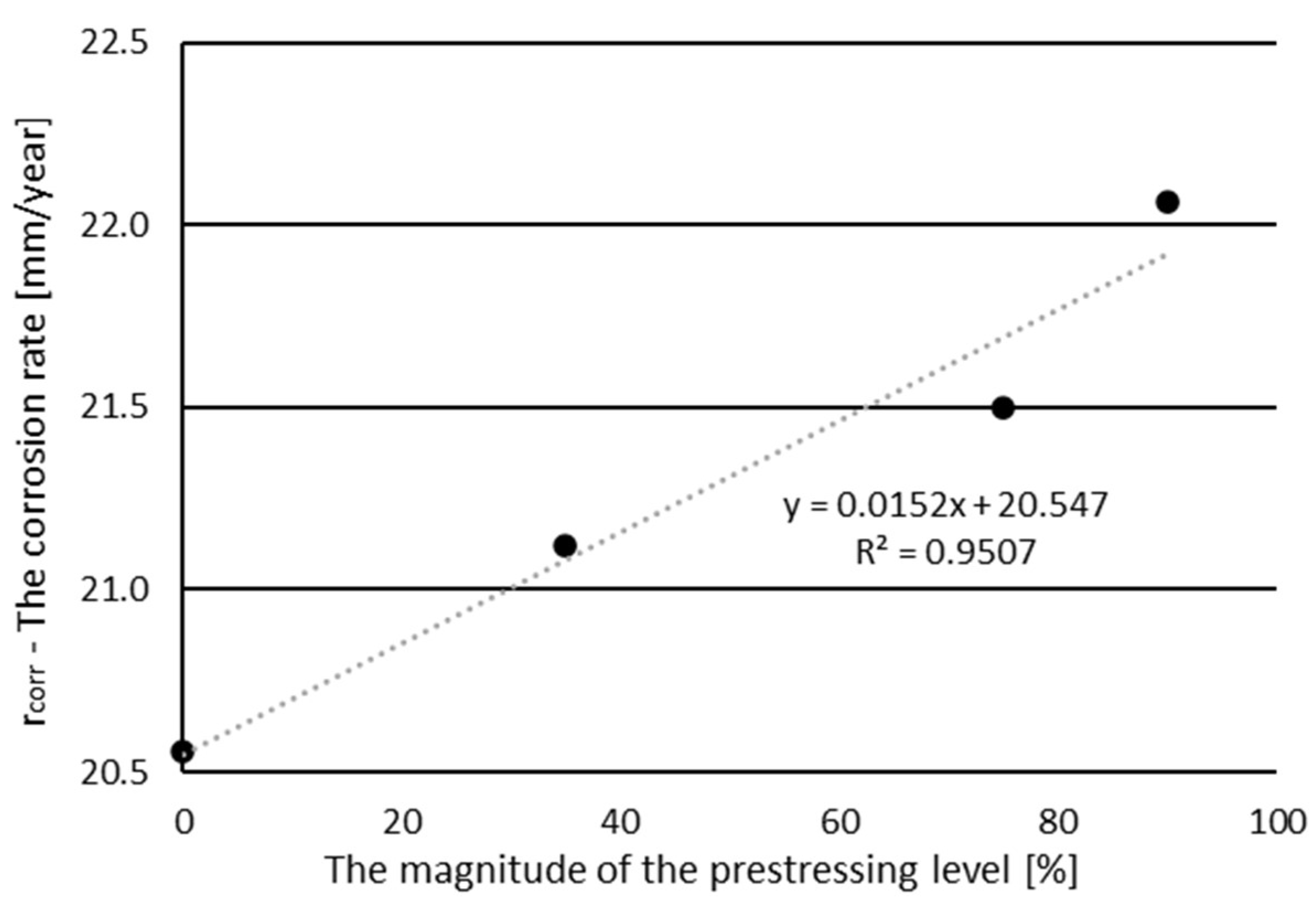
- The prestressing level affected the corrosion degree of the prestressing steel bar. The higher the level of prestressing, the higher the degree of corrosion. The degree of corrosion of the sample at the maximum selected stresses of 90% of measured tensile strength increased by 7.3% compared to the unstressed state.
- The prestressing force during the electrochemical test decreased. This may have been caused by the corrosion of the sample and the reduction of the surface area of the prestressing steel bar, resulting in a decrease in the stresses of the prestressing steel bar.
- The results obtained during the experimental campaign are affected by the coating and quality of the chemical composition. If the coating and chemical composition of the CKT bar differs, the results can significantly vary. In this basic experiment, which is the crucial base for future detailed analysis, the specimens with the same coating and chemical composition were studied. In the future, it is important to enlarge the number of experimental specimens and observe the corrosion of different types of prestressing steel, material properties, and diameters. Moreover, the study would benefit from the observation of the corrosion of prestressing steel embedded in the surrounding concrete with different classes and compositions. This way, the study would describe the real behavior of the corrosion losses in more detail.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ozbolt, J.; Oršanic, F.; Balabanic, G.; Kušter, M. Modelling damage in concrete caused by corrosion of reinforcement: Coupled 3D FE model. Int. J. Fract. 2012, 178, 233–244. [Google Scholar] [CrossRef]
- Moravcik, M.; Bujnakova, P.; Bahleda, F. Failure and damage of a first-generation precast prestressed bridge in Slovakia. Struct. Concr. 2020, 16, 2353–2362. [Google Scholar] [CrossRef]
- Tu, B.; Dong, Y.; Fang, Z. Time-Dependent Reliability and Redundancy of Corbared Prestressed Concrete Bridges at Material Component, and System Levels. J. Bridge Eng. 2019, 24, 04019085. [Google Scholar] [CrossRef]
- Kralovanec, J.; Bahleda, F.; Moravcik, M. State of Prestressed Analysis of 62-Year-Old Bridge. Materials 2022, 15, 3583. [Google Scholar] [CrossRef]
- Abdelaziz, M.M.; Gomaa, M.; El-Ghazaly, A.H. Progressive collapse analysis of prestressed concrete girder bridges using improved applied element method. Adv. Bridge Eng. 2022, 3, 24. [Google Scholar] [CrossRef]
- Kralovanec, J.; Moravcik, M.; Jost, J. Analysis of prestressed in precast prestressed concrete beams. Civ. Environ. Eng. 2021, 17, 184–191. [Google Scholar] [CrossRef]
- Bobalo, T.; Blikharskyy, Y.; Kopiika, N.; Volynets, M. Serviceability of RC Beams Reinforced with High Strength Rebar’s and Steel Plate. In Proceedings of the Advances in Resource-Saving Technologies and Materials in Civil and Environmental Engineering (CEE 2019), Lviv, Ukraine, 11–13 September 2019; Lecture Notes in Civil Engineering. Springer International Publishing: Berlin/Heidelberg, Germany, 2020; Volume 47, pp. 25–33. [Google Scholar] [CrossRef]
- Vereecken, E.; Botte, W.; Lombaert, G.; Caspeele, R. Assessment of corroded prestressed and posttensioned concrete structures: A review. Struct. Concr. 2021, 22, 2556–2580. [Google Scholar] [CrossRef]
- Huang, G.; Wu, B.; Shen, Y.; Wang, L.; Li, G. Comparative experiment of steel bar corrosion at concrete construction joints. Front. Mater. 2022, 9, 1094696. [Google Scholar] [CrossRef]
- Chen, M.; Qian, H. Effects of natural minor corrosion levels on mechanical property and bond anchorage behavior of CRB600H steel bars. Constr. Build. Mater. 2022, 340, 127660. [Google Scholar] [CrossRef]
- Vizhi, I.M.; Kalirajan, K.; Selvaraj, S. Study of Carbon Steel Corrosion in 1.0N HCL Medium by Mimusops Elengi Leaves Extract by Electro Chemical Studies. J. Adv. Sci. Res. 2019, 10 (Suppl. S2), 324–327. [Google Scholar]
- Bejinariu, C.; Munteanu, C.; Florea, D.C.; Istrate, B.; Cimpoesu, N.; Alexandru, A.; Sandu, V.A. Elektro-chemical Corrosion of a Cast Iron Protected with a Al2O3 Ceramic Layer. Rev. Chim. 2018, 69, 3586–3589. [Google Scholar] [CrossRef]
- Wu, X.; Li, L.; Li, H.; Li, B.; Ling, Z. Effect of strain level on corrosion of stainless steel bar. Constr. Build. Mater. 2018, 163, 189–199. [Google Scholar] [CrossRef]
- Li, J.; Miki, T.; Yang, Q.; Mao, M. Experimental Study on Prestressed Strength of Corbared Prestressed Concrete Steel Strands. J. Adv. Concr. Technol. 2022, 20, 550–563. [Google Scholar] [CrossRef]
- Hui, L.; Wu, X. Effect of Tension Strain Level on Reinforcement Corrosion. J. Mater. Civ. Eng. 2018, 30, 04017311. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, X.; Zhang, J.; Yi, J.; Liu, Y. Simplified Model for Corrosion-Induced Bond Degradation between Steel Strand and Concrete. J. Mater. Civ. Eng. 2017, 24, 04016257. [Google Scholar] [CrossRef]
- Kucharikova, L.; Liptakova, T.; Tillova, E.; Bonek, M.; Medvecka, D. Corrosion Behaviour Correlation of the Secondary Aluminium Casts in Natural Atmosphere and Laboratory Conditions. Arch. Metall. Mater. 2020, 65, 1455–1462. [Google Scholar]
- Faten, Y.T.; Mohammed, A.M.; Hayder, M.O. Numerical Analysis of Corrosion Reinforcements in Fibrous Concrete Beams. Civ. Environ. Eng. 2021, 17, 259–269. [Google Scholar] [CrossRef]
- Koteš, P.; Strieška, M.; Bahleda, F.; Bujňaková, P. Prediction of RC Bridge Member Resistance Decreasing in Time under Various Conditions in Slovakia. Materials 2020, 13, 1125. [Google Scholar] [CrossRef] [Green Version]
- Blikharskyy, Y.; Selejdak, J.; Kopiika, N. Specifics of corrosion processes in thermally strengthened rebar. Case Stud. Constr. Mater. 2021, 15, e00646. [Google Scholar] [CrossRef]
- Tian, Y.; Zhang, G.; Ye, H.; Zeng, Q.; Zhang, Z.; Tian, Z.; Jin, X.; Jin, N.; Chen, Z.; Wang, J. Corrosion of steel rebar in concrete induced by chloride ions under natural environments. Constr. Build. Mater. 2023, 369, 130504. [Google Scholar] [CrossRef]
- Joch, R.; Pilc, J.; Daniš, I.; Drbúl, M.; Krajčoviech, S. Analysis of Surface roughness in turning process using rotating tool with chip breaker for specific shape of automotive transmission shafts. Transp. Res. Procedia 2019, 40, 295–301. [Google Scholar] [CrossRef]
- Kralovanec, J.; Moravcik, M. Experimental measurements in the field of prestressing force monitoring. Civ. Environ. Eng. 2022, 18, 104–110. [Google Scholar] [CrossRef]
- Ivosevic, S.; Kovac, N.; Vastag, G.; Majeric, P.; Rudof, R.J. The Analyses of the Rate of Pitting Corrosion of a NiTi Rod in a Natural Marine Environment. Marit. Transp. Sci. Spec. Ed. 2022, 4, 87–99. [Google Scholar] [CrossRef]
- Kralovanec, J.; Bahleda, F.; Prokop, J.; Moravcik, M.; Neslusan, M. Verification of Actual Prestressed in Existing Pre-Tensioned Members. Appl. Sci. 2021, 11, 5971. [Google Scholar] [CrossRef]
- Krajci, Ľ.; Jerga, J. Assessment of Steel Reinforcement Corrosion State by Parameters of Potentiodynamic Diagrams. Civ. Environ. Eng. 2015, 11, 95–102. [Google Scholar] [CrossRef] [Green Version]
- Dasar, A.; Patah, D.; Hamada, H.; Yamamoto, D.; Sagawa, Y. Life performance of 40-year-old RC Beams with different concrete covers and bar diameters in natural corrosion environments. Structures 2022, 46, 2031–2046. [Google Scholar] [CrossRef]
- Strieška, M.; Koteš, P. Corrosion map of zinc in Slovakia. Pollack Periodica 2018, 13, 129–136. [Google Scholar] [CrossRef]
- Jabir, O.Y.; Oleiwi, H.M.; Sultan, A.A. Reliability of Non—Destructive Technologies in Corrosion Detection of Reinforced Concrete Structures. Civ. Environ. Eng. 2022, 18, 137–147. [Google Scholar] [CrossRef]
- Jančula, M.; Jošt, J.; Gocál, J. Influence of aggressive environmental actions on bridge structures. Transp. Res. Procedia 2021, 55, 1229–1235. [Google Scholar] [CrossRef]
- Gandel, R.; Jerabek, J.; Marcalikova, Z. Reinforced Concrete Beams Without Shear Reinforcement Using Fiber Reinforced Concrete and Alkali-Activated Material. Civ. Environ. Eng. 2023, 19, 348–356. [Google Scholar] [CrossRef]
- Du, Y.G.; Clark, L.A.; Chan, A.H.C. Residual capacity of corroded reinforcing bars. Mag. Concr. Res. 2005, 57, 135–147. [Google Scholar] [CrossRef]
- Bacharz, K.; Raczkiewicz, W.; Bacharz, M.; Grzmil, W. Manufacturing Errors of Concrete Cover as a Reason of Reinforcement Corrosion in a Precast Element—Case Study. Coatings 2019, 9, 702. [Google Scholar] [CrossRef] [Green Version]
- Al-Sulaimani, G.J.; Kaleemullah, M.; Basunbul, I.A. Rasheeduzzafar, Influence of corrosion and cracking on bond behavior and strength of reinforced concrete members. ACI Struct. J. 1990, 87, 220–231. [Google Scholar] [CrossRef]
- Maaddawy, T.E. Long-term performance of corrosion-damaged reinforced concrete beams. ACI Struct. J. 2005, 102, 649–656. [Google Scholar] [CrossRef]
- Nossoni, G.; Harichandran, R.S. Harichandran, Electrochemistry of Accelerated Corrosion Testing Using an Impressed Current. In Proceedings of the Transportation Research Board 90th Annual Meeting, Washington, DC, USA, 23–27 January 2011. [Google Scholar]
- STN 03 8137; Corrosion Prevention. Metals, Alloys and Metal Coatings. Metallographic Examination of Corrosion Stress. Slovak Technical Standard. Slovak Office of Standards, Metrology and Testing: Bratislava, Slovakia, 1989.
- Hadzima, B.; Liptáková, T. Fundamentals of Electrochemical Corrosion of Metals; EDIS—The Publisher of UNIZA: Žilina, Slovakia, 2008. (In Slovak) [Google Scholar]
- Dziková, J.; Kajánek, D.; Nový, F.; Pastorek, F. Corrosion of Metal Materials; EDIS—The Publisher of UNIZA: Žilina, Slovakia, 2021. (In Slovak) [Google Scholar]
- STN EN 1537; Execution of Special Geotechnical Works. Ground Anchors. Slovak Technical Standard. Slovak Office of Standards, Metrology and Testing: Bratislava, Slovakia, 2013.
- Gago, F.; Muzik, J.; Bulko, R. The slope stability solution using meshless local Petrov-Galerkin method. Transp. Res. Procedia 2019, 40, 686–693. [Google Scholar] [CrossRef]
- Blikharskyy, Y.; Selejdak, J.; Kopiika, N. Corrosion Fatigue Damages of Rebars under Loading in Time. Materials 2021, 14, 3416. [Google Scholar] [CrossRef] [PubMed]
- Drusa, M.; Mihalik, J.; Muzik, J.; Gago, F. The Role of Geotechnical Monitoring at Design of Foundation Structures and Their Verification—Part 2. Civ. Environ. Eng. 2021, 17, 681–689. [Google Scholar] [CrossRef]
- Nossoni, G.; Harichandran, R. Current Efficiency in Accelerated Corrosion Testing of Concrete. Corrosion 2012, 68, 801–809. [Google Scholar] [CrossRef]
- Li, H.; Qiu, T.; Li, Z.; Lin, F.; Zhang, Q. Analytical Model for Ionic Current Dominated Corrosion of Nanoelectrodes in Metallized Films: Frequency and Electric Stress. J. Chem. Phys. 2023, 158, 114702. [Google Scholar] [CrossRef]
- Wang, X.; Cao, G.; Wang, B.; Xing, Y.; Lu, M.; Qiao, L.; Zhang, L. Effect of electric-arc-induced ablation on corrosion behavior of pipeline steel under high-voltage direct current interference. Anti-Corros. Methods Mater. 2022, 69, 481–489. [Google Scholar] [CrossRef]





| C | Mn | Si | Pmax | Smax |
|---|---|---|---|---|
| 0.70 | 0.70 | 0.30 | 0.035 | 0.035 |
| Sample No. | Time (Days) | Electric Current (A) | Tensile Strength (kN) |
|---|---|---|---|
| 1 | 15 | 2.00 | 0 |
| 2 | 15 | 2.00 | 0 |
| 3 | 15 | 3.00 | 0 |
| 4 | 15 | 3.00 | 0 |
| 5 | 15 | 4.00 | 0 |
| 6 | 15 | 4.00 | 0 |
| 7 | 10 | 3.00 | 0 |
| 8 | 10 | 3.00 | 0 |
| 9 | 10 | 3.00 | 0 |
| 10 | 10 | 3.00 | 0 |
| 11 | 10 | 3.00 | 0 |
| 12 | 10 | 3.00 | 0 |
| 13 | 10 | 1.50 | 0 |
| 14 | 10 | 1.50 | 0 |
| 15 | 20 | 2.00 | 0 |
| 16 | 20 | 2.00 | 0 |
| 17 | 30 | 2.50 | 0 |
| 18 | 30 | 2.50 | 0 |
| 19 | 10 | 0.25 | 0 |
| 20 | 10 | 0.25 | 0 |
| 21 | 10 | 0.50 | 0 |
| 22 | 10 | 0.50 | 0 |
| 23 | 10 | 0.75 | 0 |
| 24 | 10 | 0.75 | 0 |
| 25 | 20 | 0.25 | 0 |
| 26 | 20 | 0.25 | 0 |
| 27 | 20 | 0.50 | 0 |
| 28 | 20 | 0.50 | 0 |
| 29 | 20 | 0.75 | 0 |
| 30 | 20 | 0.75 | 0 |
| 31 | 30 | 0.25 | 0 |
| 32 | 30 | 0.25 | 0 |
| 33 | 30 | 0.50 | 0 |
| 34 | 30 | 0.50 | 0 |
| 35 | 30 | 0.75 | 0 |
| 36 | 30 | 0.75 | 0 |
| 37 | 20 | 0.25 | 85 |
| 38 | 20 | 0.25 | 85 |
| 39 | 20 | 0.25 | 170 |
| 40 | 20 | 0.25 | 170 |
| 41 | 20 | 0.25 | 210 |
| 42 | 20 | 0.25 | 210 |
| 43 | 20 | 0.25 | 85 |
| 44 | 20 | 0.25 | 85 |
| 45 | 20 | 0.25 | 170 |
| 46 | 20 | 0.25 | 170 |
| 47 | 20 | 0.25 | 210 |
| 48 | 20 | 0.25 | 210 |
| Electric Current Value (A) | Time (Days) | Theorical Corrosion (%) | Experimental Corrosion (%) | Electric Current Efficiency (%) |
|---|---|---|---|---|
| 10 | 3.50 | 3.22 | 92.00 | |
| 0.25 | 20 | 7.00 | 6.54 | 93.42 |
| 30 | 10.50 | 9.78 | 93.14 | |
| 10 | 7.00 | 6.52 | 93.14 | |
| 0.5 | 20 | 14.00 | 13.37 | 95.50 |
| 30 | 21.00 | 20.07 | 95.57 | |
| 10 | 10.50 | 10.12 | 96.38 | |
| 0.75 | 20 | 21.00 | 20.25 | 96.43 |
| 30 | 31.50 | 29.74 | 94.41 |
| Prestressing Level (%) | Time (Days) | Theorical Corrosion (%) | Experimental Corrosion (%) | Electric Current Efficiency (%) |
|---|---|---|---|---|
| 0 | 20 | 7.00 | 6.54 | 93.42 |
| 35 | 20 | 7.00 | 6.72 | 96.00 |
| 75 | 20 | 7.00 | 6.84 | 97.71 |
| 90 | 20 | 7.00 | 7.02 | 100.29 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zahuranec, M.; Koteš, P.; Kraľovanec, J. The Influence of the Prestressing Level of the Fully Threaded Anchor Bar on the Corrosion Rate. Buildings 2023, 13, 1592. https://doi.org/10.3390/buildings13071592
Zahuranec M, Koteš P, Kraľovanec J. The Influence of the Prestressing Level of the Fully Threaded Anchor Bar on the Corrosion Rate. Buildings. 2023; 13(7):1592. https://doi.org/10.3390/buildings13071592
Chicago/Turabian StyleZahuranec, Michal, Peter Koteš, and Jakub Kraľovanec. 2023. "The Influence of the Prestressing Level of the Fully Threaded Anchor Bar on the Corrosion Rate" Buildings 13, no. 7: 1592. https://doi.org/10.3390/buildings13071592
APA StyleZahuranec, M., Koteš, P., & Kraľovanec, J. (2023). The Influence of the Prestressing Level of the Fully Threaded Anchor Bar on the Corrosion Rate. Buildings, 13(7), 1592. https://doi.org/10.3390/buildings13071592








