Abstract
CO2 emission limits introduced by the European Union are encouraging works on new-generation materials with reduced clinker content. Currently, fumed silica from hard coal combustion is used in cement and concrete technology in Europe and Poland. Its wide application depends mainly on its chemical and phase composition, especially the reactivity of pozzolanic acids and its high fineness similar to cement Many authors studied the influence of fly ashes from hard coal combustion, in accordance with PN-EN 450-1 and 450-2, on the properties of concrete, including the course of the carbonation process. There are no studies in the literature involving ashes from sewage sludge. The objective of the research is to assess the course of carbonation of concrete produced on the basis of fly ash from the thermal transformation of sewage sludge over time and to describe this phenomenon in a mathematical form. An additional objective was to analyze the physicochemical composition of sludge ash in accordance with the requirements of EN 450-1, ASTM-C618-03. In addition, this study also demonstrated the possibility of producing fly ash-modified standard concrete through the thermal treatment of sewage sludge. The average compressive strengths of Krakow gray concrete after curing for 28, 56, 90, and 365 days were 50.1 MPa, 50.6 MPa, 50.8 MPa, and 61.9 MPa, respectively. On the one hand, the additives introduced in the concrete mixture accelerate the carbonation process by shifting the carbonation front deep into the concrete and, on the other hand, create a denser microstructure In all cases, the largest increase in carbonation depth was observed up to the 56th day of the study, while the smallest increase was observed between 90 and 180 days. The diffusivity decreases and the rate of carbonation is reduced. The determined regression coefficients of hyperbolic models indicate the proper adjustment of the adopted hyperbolic model to the results of laboratory tests under accelerated carbonation conditions (R = 0.85–0.99), regardless of the content of fly ash from sewage sludge in ordinary concrete samples.
1. Introduction
Sustainable development of construction poses more and more new challenges for science that will allow the shaping of an energy-efficient and friendly environment, but also durable material, technological and design solutions. It is essential that in addition to the strength characteristics of concrete, including above all compressive strength, an important feature of concrete elements is their durability. It affects the operating time of a given structure and determines the scope and frequency of repairs that should be carried out to maintain the functionality of buildings. It is worth adding here that durability does not mean that concrete is resistant to various types of influences or has unlimited service life [1]. It is conditioned by many factors related to the quality and type of raw materials used, the method of building the concrete mix itself, the conditions and method of care for “immature, young” concrete, or the properties of concrete. Looking at concrete from the side of its microstructure, its durability will be shaped by the nature of the porosity of the cements used, their quantity, admixture properties, proportions of binder hydration products, and the water-cement (binder) coefficient [2]. Insufficient durability is manifested by the destruction of concrete, which can be caused by internal or external factors. These various interactions may be mechanical, physical, or chemical. Physical causes include the influence of high temperatures, alternating freezing and defrosting of concrete, while chemical causes include alkaline-carbonate and alkaline-silicate reactions. External chemical aggression manifests itself primarily in the interaction of aggressive ions-sulfates, chlorides, or carbon dioxide, as well as many gases and liquids of industrial or natural origin. It is worth emphasizing here that the destruction of concrete and thus its durability is rarely caused by one separate cause. It is often difficult to attribute concrete damage to some particular factor, however, the quality of concrete almost always starts this process [3].
In this paper, the sensitivity of concrete to the addition of carbonation is considered to be one of the most important factors affecting the durability of structures. Carbonation occurs in any unprotected concrete structure because the structure is partially or fully submerged in an atmosphere containing a sufficient concentration of carbon dioxide to initiate the process. Carbonation does not directly affect the durability of concrete, but it does pose a threat to the reinforcement, causing it to become dull and begin to corrode.
Carbonation is the transformation of the physicochemical properties of concrete (mainly mortar) under the influence of long-term exposure to carbon dioxide. Carbon dioxide is always present in the indoor atmosphere and atmosphere of buildings. In the atmosphere, its content is usually 0.03%, but it can be as high as 0.3% in highly urbanized areas, industrial areas, or traffic arteries. The mechanism that causes carbonization is the reaction of carbon dioxide in the atmosphere with calcium hydroxide, one of the hydration products of cement. The reaction products are water and calcium carbonate. Lime and hydrated aluminosilicates contained in the C-S-H phase can also be carbonized, but at high CO2 concentrations, their importance for concrete properties is very important [3]. The carbonization process is as follows [4,5]:
Carbonation occurs gradually from the outside of the concrete, which is directly exposed to carbon dioxide. This is happening at a decreasing pace, as CO2 diffuses through pores containing an already treasury surface concrete zone. In concrete fully saturated with water, carbon dioxide diffuses very slowly, which slows down the carbonation course. In water-unsaturated and damp concrete, the course of carbonation depends on the reaction between the diffusion rate of Ca2+ ions and carbon dioxide. The characterization product crystallizes on the surface of portlandite to form a protective layer if the diffusion of carbon dioxide is faster than Ca2+. However, this layer is not tight enough, so the process is delayed [6]. If Ca2+ ions diffuse faster than CO2, carbonate crystallization itself takes place in the capillary pore space and on the C-S-H surface, reducing the porosity of the concrete. The presented mechanism is visible in the case of concretes made on the basis of Portland cement [7], metallurgical cement [8,9,10], and with the addition of fly ash [11,12].
The phenomena of ion transport in concrete are related to its structure, i.e., its porosity, shape, and size of pores, and connections of the pore system as well as their transmission. The carbonation process depends on the characteristics of the pore systems found in concrete [13,14]. This characteristic, due to the filling of carbonation products of voids, changes over time. As a result, the size of the diameter of individual pores is reduced and the continuity of pore systems is reduced. According to the literature, as a result of filling the pores with reaction products in the treasury area, the porosity of concrete decreases [6].
Thus, in concrete, which is not exposed to the surface of another building material, the carbonation process is carried out constantly [3,15,16]. The speed of carbonation over time is determined by internal and external factors. It depends on the type of concrete, the value of the w/c ratio, the amount of binder, the method of compacting the mix, concrete care, and environmental conditions during concrete operation. External factors include CO2 concentration, air temperature, and humidity. The rate of carbonation of concrete increases with increasing concentration of carbon dioxide (approximately proportional to the element from this concentration), primarily at high values of the water-cement index. CO2 transport takes place through a system of pores contained in hardened cement grout [2]. The increase in temperature accelerates the carbonation process, causing an increase in the rate of carbon dioxide diffusion, while very low humidity limits this process, as well as the saturation of concrete with water. The carbonation process under such conditions occurs only on the surface of portlandite grains, and the resulting calcium carbonate forms a barrier layer on its surface. In addition, the product of carbonation is also water, which promotes the dissolution of calcium hydroxide and thus enables slow carbonation at low humidity. In contrast, in concrete saturated with water, carbonation is hindered by the slow diffusion of carbon dioxide in the aquatic environment. The most intense course of carbonation occurs when the relative humidity of the air is 50–70% [17]. In completely dry concrete, the process is difficult because, for pre-dissolution of hydroxide, a certain minimum humidity is needed. As a result of carbonation, water is released (the product of the reaction), and the resulting environment promotes the dissolution of hydroxide.
Among the technological factors, in addition to the influence of the quality of workmanship, moisture care of concrete is important for the course of the carbonation process. Care treatments directly affect the concrete layer. Numerous scientific publications conducted for various types of concrete indicate that the longer the moisture care of concrete is carried out, the lower the susceptibility of concrete to carbonation [18,19,20]. The care treatment is important in the case of concretes produced on the basis of mineral additives—fly ash or cements with additives.
Among the internal factors, the progress of carbonation is determined by the tightness of concrete, which is associated with the water-cement indicator. Concretes with low w/c are less susceptible to carbonation because they are characterized by a lower CO2 diffusion coefficient, which is a characteristic determining the course of the carbonation process. This is primarily due to the tightness of the concrete. In sealed concrete, where the number of pores is limited, carbon dioxide diffusion is difficult. The slowdown in the course of carbonation is also determined by a greater degree of cement fragmentation. A faster hydration process of cement with a larger specific surface area delays CO2 penetration [17,21,22].
Currently used on a large scale by-products of coal combustion—fly ash, blast furnace slag, and silica dust in concrete technology can improve selected technical properties of concrete and are beneficial for ecological reasons and economic [23,24,25,26,27]. Partially replacing cement clinker with additives can lead to a reduction in the carbon footprint of concrete—an important aspect of a sustainable environment. For fly ash used as a type II additive for concrete according to EN 206 and as the main component of ordinary cements, various requirements apply according to EN 197-1. In the European Union countries, in light of the requirements of EN 450-1, the content of reactive CaO above 10% by mass excludes the use of ashes as a substitute in concrete. Such a restriction does not apply to the use of ash as a component of cement [28,29,30,31,32].
The assessment of the carbonation of concretes produced with type II additives (fly ash, micro silica, natural humors, or blast furnace slag) should take into account their impact on the formation of the microstructure of hardened cement grout, their pozzolanic activity, and w/c values [2,17]. The influence of mineral additives on the course of carbonation consists of two opposite effects:
- -
- Increasing the depth of carbon dioxide penetration—the Ca(OH)2 content is low in concrete with additives, which results in a higher difference in CO2 concentrations in pores and intensifies diffusion,
- -
- Inhibitory—associated with the sealing of the concrete structure by ash and additional products of the pozzolanic reaction.
The study on the influence of various types of ashes compliant with EN 450-1 and EN 450-2 on the properties of concrete, including the course of the carbonation process, was carried out by many authors [32,33,34,35,36,37]. Fly ashes used as a replacement for part of the binder-cement cause an increase in the tendency to carbonation, but this is related to the type of ash and the value of the w/c ratio. A larger amount of ash introduced into concrete increases the susceptibility to carbonation but also increases the tightness of concrete [38,39]. However, the use of ash for concrete may be beneficial when de-icing salts or chlorides from seawater poses a threat [38]. The carbonation resistance of fly ash containing fly ash depends on its microstructure. Due to pozzolanic reactions, some of the fly ash combines with Ca(OH)2, resulting in a decrease in Ca(OH)2 in the concrete. It should be emphasized that the results of many authors are difficult to directly compare due to differences in concrete formulations and curing conditions, types of ash used, and their relationship to cement quality [40,41,42]. The method of introducing fly ash into the concrete mix is important—whether the additive is introduced as an increase in the amount of binder or as a replacement for part of the cement. In the first case, the most important is the role of the compaction effect, in the second case, the effect of calcium hydroxide deficiency strongly affects the progress of carbonation [43]. Bier’s research points to the double opposite effects of fly ash on the carbonation process. The carbonation rate is higher when the amount of Ca(OH)2 in the cement grout is lower. In this way, the presence of ash in concrete can increase the carbonation zone and accelerate its pace. On the other hand, the forming thicker, hardened fly ash paste reduces the diffusivity and speed of carbonation limitation [44]. The results of the research [4,45] do not agree with the dominant effect—braking or accelerating, in the case of carbonation of concrete with lime ash. The authors concluded that proper maturation of concrete is the most important for the proper course of pozzolanic reactions and beneficial for obtaining the effect of compacting the microstructure [4]. The presented research results [46] indicate that the replacement of lime ash in the amount of 10–20% of the cement mass does not affect the course of electrochemical processes on the steel surface and does not worsen the protective properties of concrete. However, there are also studies that clearly indicate an increase in the dynamics of the progress of the carbonation of concrete with lime ash [43,47]. The dynamics of the development of carbonation change with increasing ash content.
Regardless of the method of introducing ash into concrete—as a substitute for cement or aggregate, many authors indicate that the carbonation of concretes with lime ash is less susceptible to the progress of carbonation than silica ash [36,43]. In addition, no fundamental differences were found when comparing concretes with ash from fluidized bed combustion with traditional ashes [48].
Wrapped steel with hydrated cement grout undergoes passivation, quickly producing a passivation layer that adheres strongly to the steel and protects it against reactions with water and oxygen.
The carbonation of concrete according to the authors’ findings [49,50,51,52,53,54,55] can be described by the hyperbolic function of its depth in time (the inverse of the square root of time), which has an asymptotic value parallel to the timeline. For the purposes of the analysis presented in the article, the resistance of concrete to carbonation was selected as a determinant of concrete durability. The model of hyperbolic carbonation adopted by Woyciechowski is expressed by the general formula:
The general carbonation model can be expressed in extended form by introducing additional variables and then the depth of carbonation is expressed as a function of three variables: service time t, water-cement index w/c, and initial care time tec.
where:
h is the depth of carbonation, mm,
w/c—water-cement ratio,
t—exposure time, days,
a, b, c—characteristic factors of the model.
The presented hyperbolic model allows the determination of the maximum depth of carbon dioxide saturation (limits of the hyperbolic model).
The main objective of the study was to assess the course of carbonation of concrete produced on the basis of fly ash from the thermal processing of sewage sludge over time and to describe this phenomenon in a mathematical form. An additional objective was to analyze the physic-chemical composition of ash in accordance with the requirements of EN 450-1, ASTM-C618-03. The aim of the analysis was to determine the influence of fly ash properties on the compressive strength of concrete produced with its participation, where other by-products from hard coal combustion are used [32,56].
2. Materials and Methods
In order to assess the physic-chemical properties, fly ash from sewage sludge was tested. For experimental research, separate batches of material were collected during the period of continuous operation of sludge from the sewage treatment plant in Krakow in three periods. The parties were assembled in May 2020, 2021 and 2022. This frequency of sampling made it possible to assess the variability of the properties of a given material. Fly ash was stored in sealed plastic containers. For laboratory tests, a concrete mix of C20/25 class S3 was designed according to EN 206 + A2: 2021-08 using the calculation and experimental method according to Bukowski [30,57]. Portland cement CEM I 42.5R, fine aggregate with a grain size of 0–2 mm and coarse aggregate with a grain size of 2–16 mm, water, and an addition—fly ash from the thermal transformation of sewage sludge was used to prepare the mixture.
In order to determine the influence of fly ash on the progress of the depth of carbonation of ordinary concrete, samples were prepared:
• P—with the addition of fly ash from the thermal treatment of sewage sludge in an amount of 2.5% to 20%.
In the research program, variable w/c values were assumed. The composition of prepared concrete townspeople per 1 m3 is presented in Table 1. During the measurements, cement was replaced with fly ash from sludge—the amount of cement decreased, and the amount of water remained constant.

Table 1.
Proportions of concrete mix by weight.
The chemical composition of fly ash was carried out on the Epsilon 3 spectrometer (Panalytical) by the method of energy dispersion X-ray fluorescence (XRF). The test was determined in the measurement range of Na-Am elements on an apparatus equipped with a 9W X-ray tube, 50 kV, 1 mA, 4096 channel spectrum analyzer, 6 measurement filters (Cu-500, Cu-300, Ti, Al-50, Al-200, Ag) and a high-resolution semiconductor SDD detector (Be window, 50 μm thick) cooled by a Peltier cell. The analysis of the grain size distribution of the material was based on the phenomenon of laser diffraction using the Mastersizer 3000 analyzer (Malvern Instruments, Malvern, England). Grain sizes with equivalent diameters ranging from 0.1 μm to 1000 μm were analyzed. The measurement was carried out in a dispersing liquid (demineralized water) in the presence of an ultrasonic probe in order to break up larger aggregates of the tested samples. Compressive strength tests were carried out in accordance with the guidelines contained in EN 12390-3:2011 [58] in the hydraulic machine H011 Matest (Italy). The samples were tested after a 28- and 56-day concrete maturation period For laboratory tests, a concrete mix of concrete class C20/25 with consistency S3 was designed according to PN-EN 206 + A2:2021-08 using the calculation and experimental method according to Bukowski.
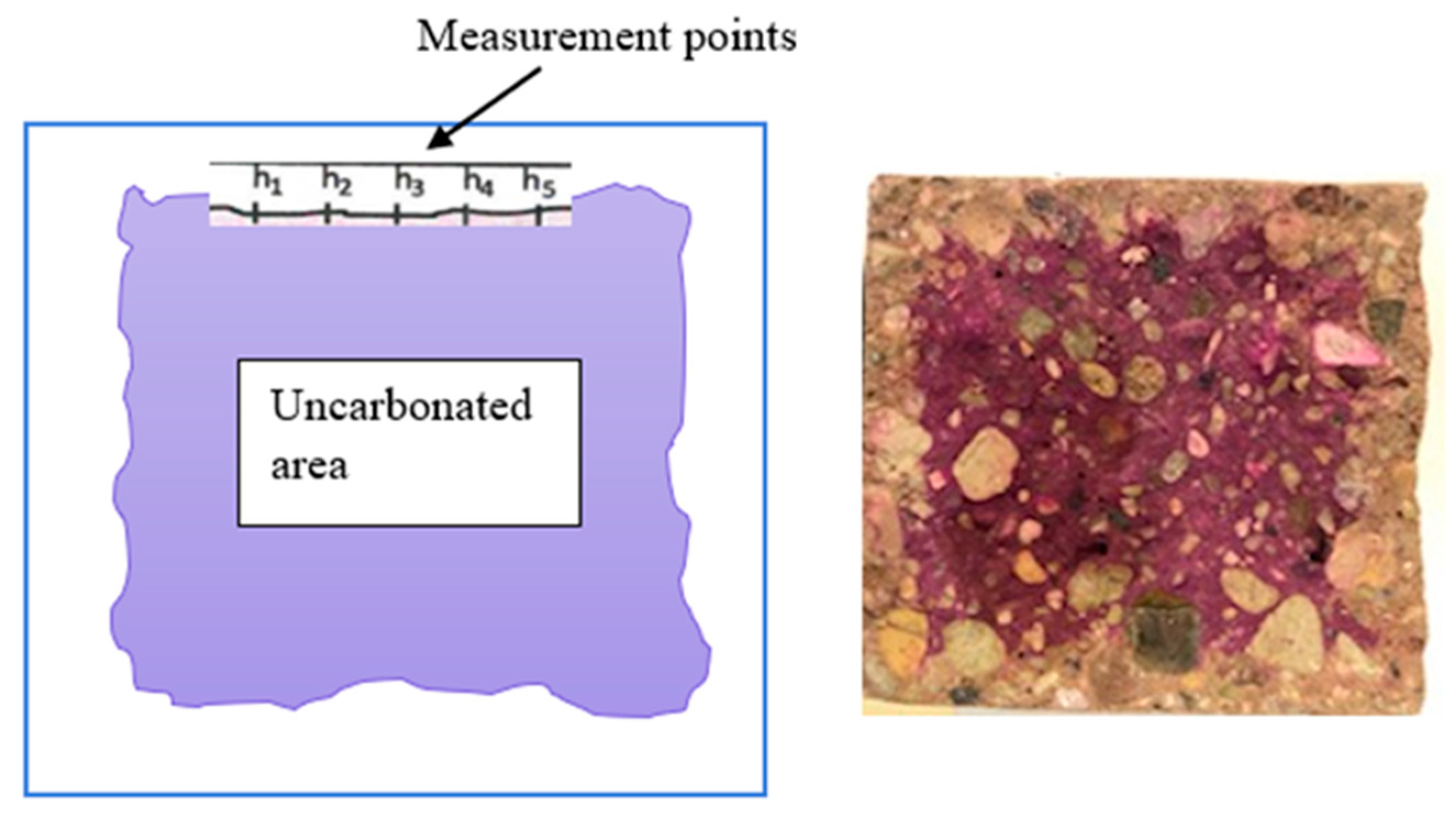
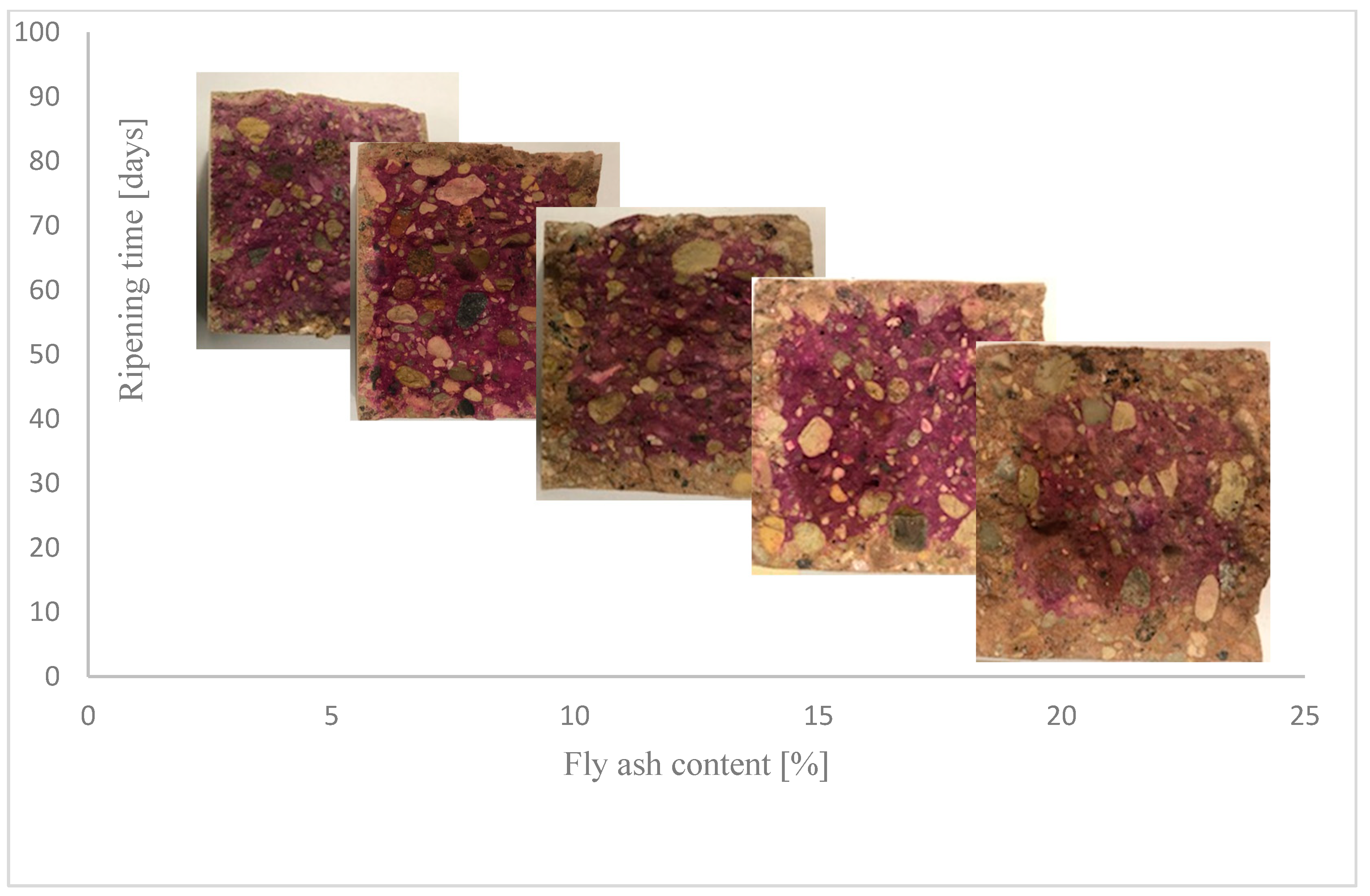
The accelerated test of resistance to carbonation of concrete was carried out according to the procedure given in EN 12390-12:2010 “Testing hardened concrete—Part 12: Determination of the potential carbonation resistance of concrete: Accelerated carbonation method”. The test was carried out on samples measuring 500 × 100 × 100 [mm] [59]. The test samples matured in water for 28 days, then for 14 days the mass stabilized under conditions of relative humidity of 60 ± 10% and temperature of 21 ± 2 °C. The measurement of the depth of the carbonation front took place after 56, 70, and 90 days from the concentration of samples in the carbonation chamber at CO2 = 4%, temperature t = 20 °C +/− 2 °C, and relative humidity RH = 50–60%. To measure the carbonation front, made on the exposed, fresh surface of the sample, a phenolphthalein solution with a ratio of 1 g phenolphthalein to 70 g of ethyl alcohol was used, then diluted in 30 g of distilled water. The depth of carbonation is measured by applying a phenolphthalein index to the surface of the fresh break of the sample. Cubic samples had to be broken in half, perpendicular to the unparaffin-protected surfaces. The depth of carbonation is the average depth measured at 5 points on all 4 sides with an accuracy of 1 mm (Figure 1).
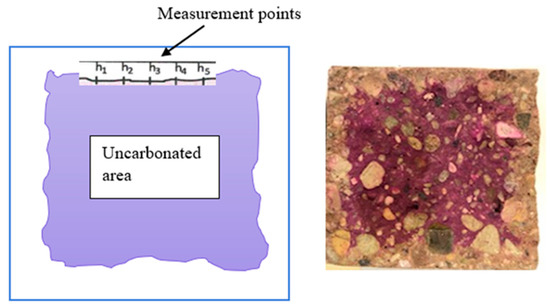
Figure 1.
The method of reading the depth of carbonation.
3. Results
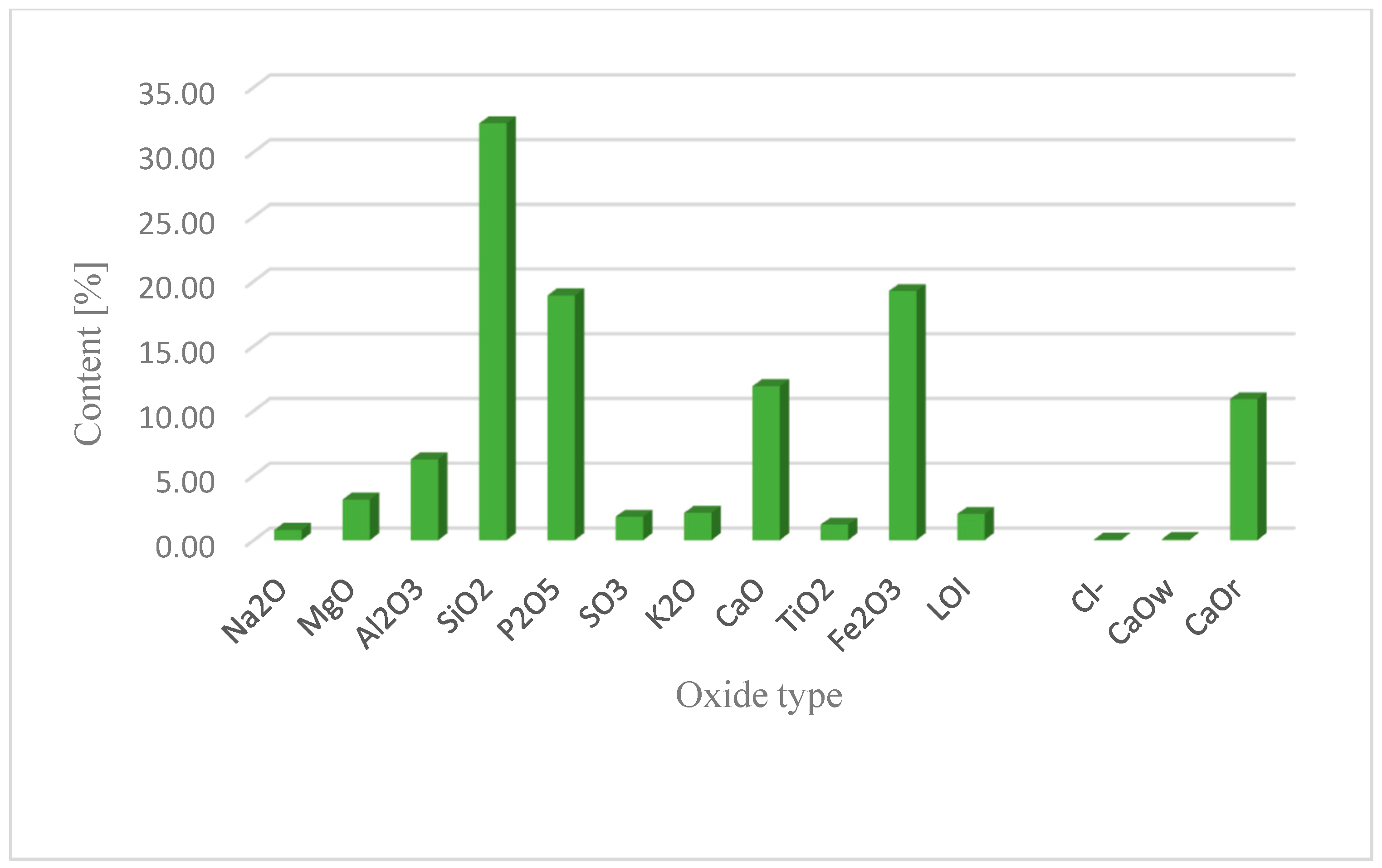
This section may be divided into subheadings. It should provide a concise and precise description of the experimental results, their interpretation, as well as the experimental conclusions that can be drawn. Figure 2 shows the results of investigations of the chemical composition of individual batches of fly ash from sewage sludge. The results for limit values for single results were related to the requirements of EN 450-1: 2012 and ASTM-C618-03 [32,56].
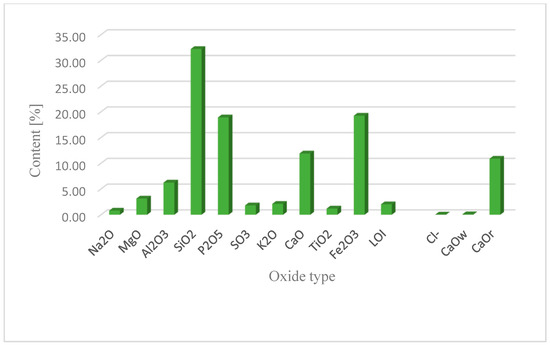
Figure 2.
Chemical composition of fly ash from municipal sewage sludge combustion.
Based on the results, it was found that the ash samples met the requirements of EN 450-1:2012 [32] regarding CaO and reactive CaO content. The variability of CaO content in fly ash was low at −0.08% by mass (limit value is −1.6%) and reactive at −10.89% by mass (limit value less than 11% by mass). A higher active calcium oxide content affects the hydraulic properties of the ash and the formation of the C-S-H phase. In addition, the content of active silica in sewage sludge ash does not meet the standard requirement (the lower limit is 25% by weight)—15.24%. The result shows a volatility of 88%. The total SiO2 content was −32.21% by mass, with a deviation of 58%. Considering that the basic oxides for making cement clinker are: CaO, SiO2, Al2O3, and Fe2O3, there is no requirement for the total content of the three oxides (SiO2, Al2O3, Fe2O3). Examination of ash samples from the thermal treatment of sewage sludge showed a dispersion of −57.71% by weight. Considering ASTM-C618-03, the ash content of Krakow WWTP meets the requirements for the sum of oxides (SiO2, Al2O3, Fe2O3 ≥ 50%) [56].
Concrete based on the studied fly ash is less likely to undergo volume changes during curing due to the lower content of MgO released in the free form [60,61]. Meet the total alkali content (single result limit ≤ 5%). The tested ash is characterized by a small dispersion of results −0.80% by weight. The proportion of chloride is 0.04% by weight. When looking at the SO3 content, an observed result of 1.82% corresponds to a variability of 41%. The samples complied with the requirements of EN 450-1 and ASTM-C618-03 [32,56].
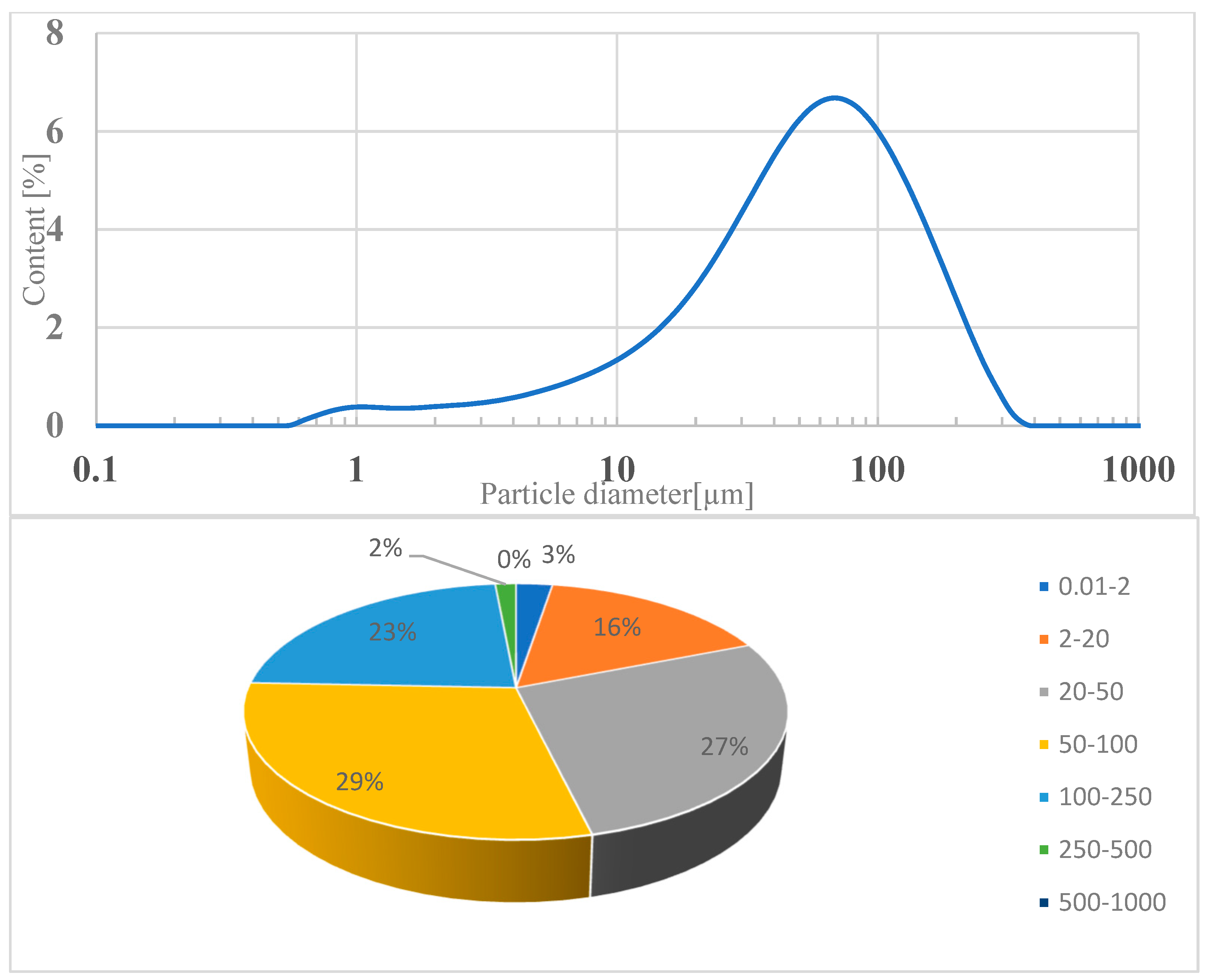
The loss on ignition (indicating the amount of unburned carbon in the fly ash sample) is significantly lower compared to quartz ash currently used in concrete technology. This is due to the combustion technology of the fluidized bed furnace and the higher combustion temperature of more than 850 °C. The loss on ignition of ash in sewage sludge was 0.7% by weight. According to the standard, these values correspond to class A. The slight variation in the obtained results indicates the stability of the municipal sludge incineration technology. A characteristic feature of the ash from the thermal treatment of sewage sludge is a high phosphate content, which exceeds the limit for individual results (less than 5.5% by weight) by three to five times. The phosphate content was −18.91% by weight. This is due to the removal of phosphorus from municipal wastewater and its accumulation in sewage sludge. The particle size distribution of the tested fly ash showed a unimodal distribution with a maximum value of 65 μm. Particles with a diameter of 2–250 μm accounted for more than 91% of the volume. The dominant grain fractions in this range are 20–50 μm (27.25%), 50–100 μm (29.39%), and 100–250 μm (22.89%)—Figure 3. The specific density of ash was—2.780 kg/dm3.
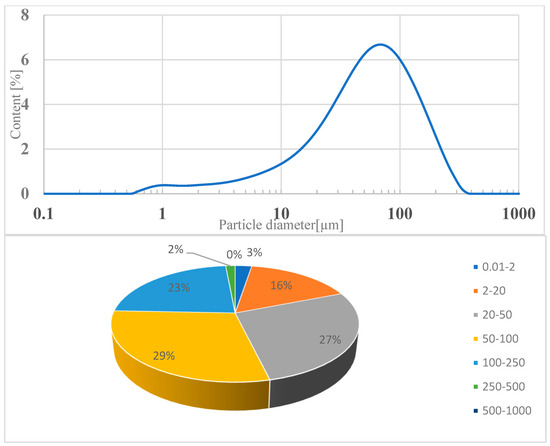
Figure 3.
Volume distribution of individual particle fractions in fly ash from Krakow.
A characteristic feature of fly ash from sewage sludge is the irregularity of grains with a strongly developed surface showing high porosity. Chemical analysis in the micro area showed a diverse elemental composition. Grains with chemical composition dominate aluminum, phosphorus, silicon, and iron. The mineral composition is dominated by anhydrite and quartz as well as phosphates in the form of apatite and fluorapatite. These phases are the main carriers of P2O5 occurring in increased amounts compared to the content known from conventional ashes. According to ASTM C379-65T [61], the dripping activity of fly ash is determined by its SiO2 and Al2O3 content. The total content of SiO2 and Al2O3. More than 20% indicated that the test samples had pozzolanic properties [62,63,64,65]. The total content of active silica and aluminum in the fly ash of Krakow WWTP was 21.49. The findings also determined that the pozzolanic activity of the fly ash after 28 and 90 days of maturation after thermal treatment of sewage sludge did not meet the requirements of EN 450-1:2012 [32]. The activity rate was 71.7% after 28 days and 84.1% after 90 days. After 180 days of maturation, the activity rate exceeds the required value (85%). However, it should be noted that the standard refers to quartz ash. During the same period, the pozzolanic activity of sludge incineration ash was lower than that of coal combustion fly ash. However, they have reached the required indicator values (85%), which allows them to be classified as active mineral additives.
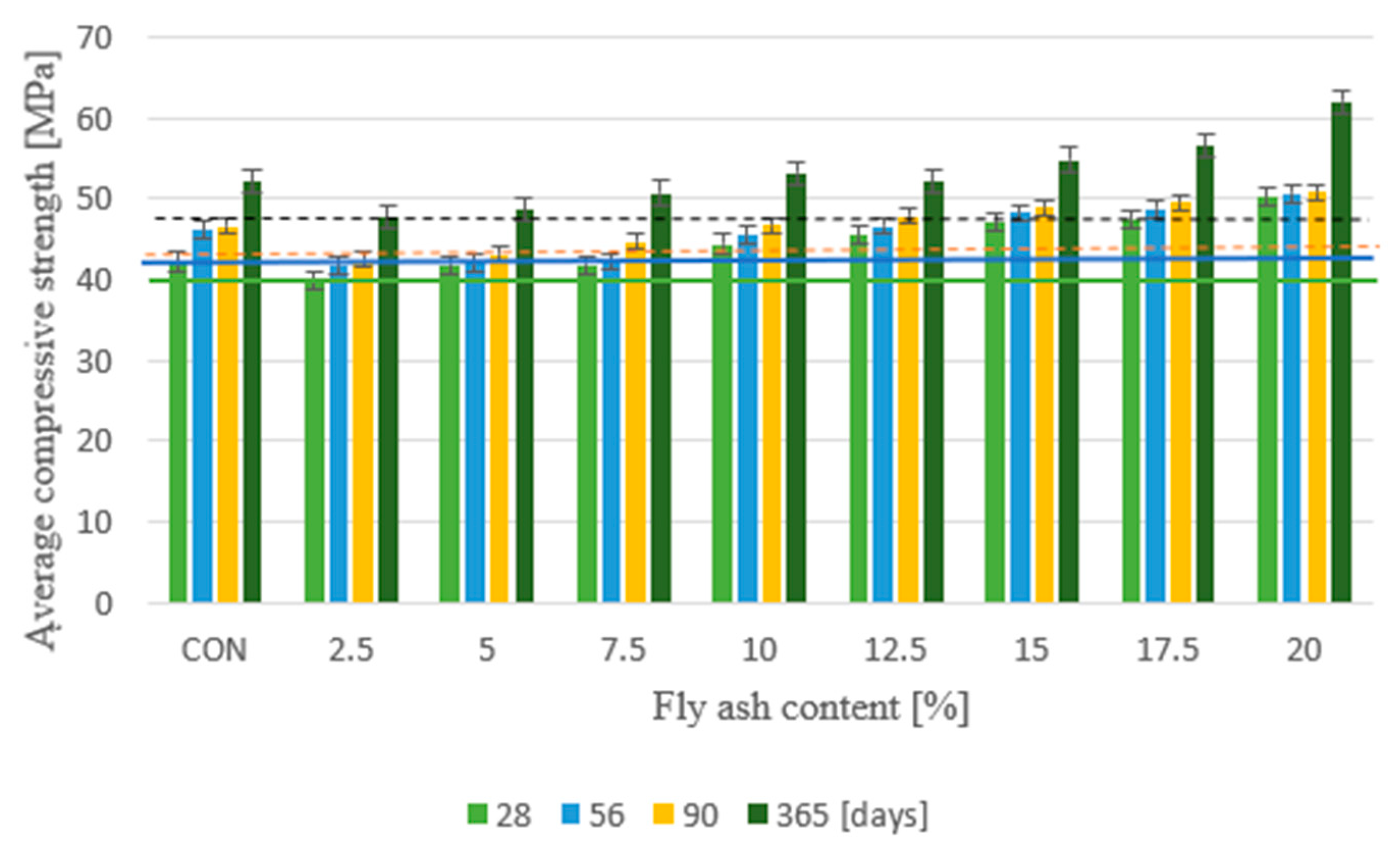
After aging for 28 days, the concrete with sewage sludge heat-treated fly ash replacing 20% cement had the highest compressive strength of 50.1 MPa, while the concrete with cement instead of cement had the lowest strength of 39.7 MPa. The ash content is 2.5%. Compared with the reference concrete, the strength increased by 18.7%, and the concrete decreased by 5.7%. The highest compressive strength was 50.6 MPa after 56 days, 50.8 MPa after 90 days, and 61.9 MPa after 365 days. The concrete that replaced 20% cement with ash also met this requirement, and the minimum strength after 56 days was 41.6 MPa. 42.5 MPa, equal to 47.7 MPa after 365 days Concrete with 2.5% fly ash content. Replacing cement with fly ash after thermal treatment of sewage sludge increased the compressive strength by more than 10% compared to the control concrete without addition—Figure 4. Taking into account the physico-chemical composition and pozzolanic properties of the fly ashes used from the thermal transformation of sewage sludge from various sewage treatment plants, it was noted that a higher concentration of SiO2, Al2O3, and Fe2O3 (Kraków—57.71%) and lower P2O5, CaO (Kraków—30.81%) has a positive effect on the increase in compressive strength of the produced concretes. In addition, it was observed that the compressive strength of concrete with different shares of ash from sewage sludge from the sewage treatment plant in Krakow increased in all periods. According to the information presented in the works, the optimal amount of fly ash from the thermal processing of sewage sludge in cement composites ranges from 5% to 20%.
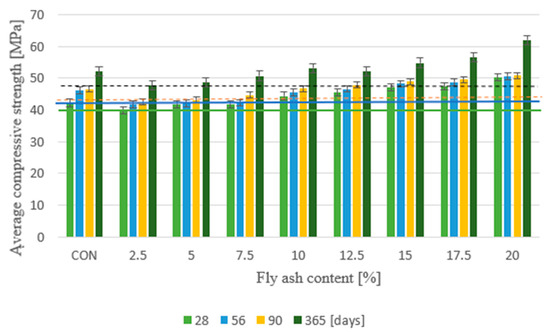
Figure 4.
Average compressive strength.
The Course of Carbonation Adopted as the Basis for Modelling
Figure 5 shows a graphical comparison of the treasury areas (grey) and non-treasury areas (purple). During the measurement of the depth of carbonation, each time a fragment of it was chipped off from the sample and the range of carbonation was examined at the fresh turn of concrete with phenolphthalein reagent. The results of the depth of saturation of the CO2 samples with the phenolphthalein test were calculated as arithmetic means of 10 measurements on each of the three samples in the series (five on each of the two lateral surfaces of a single sample) [63].
Figure 5.
Carbonation course marked by phenolphthalein index.
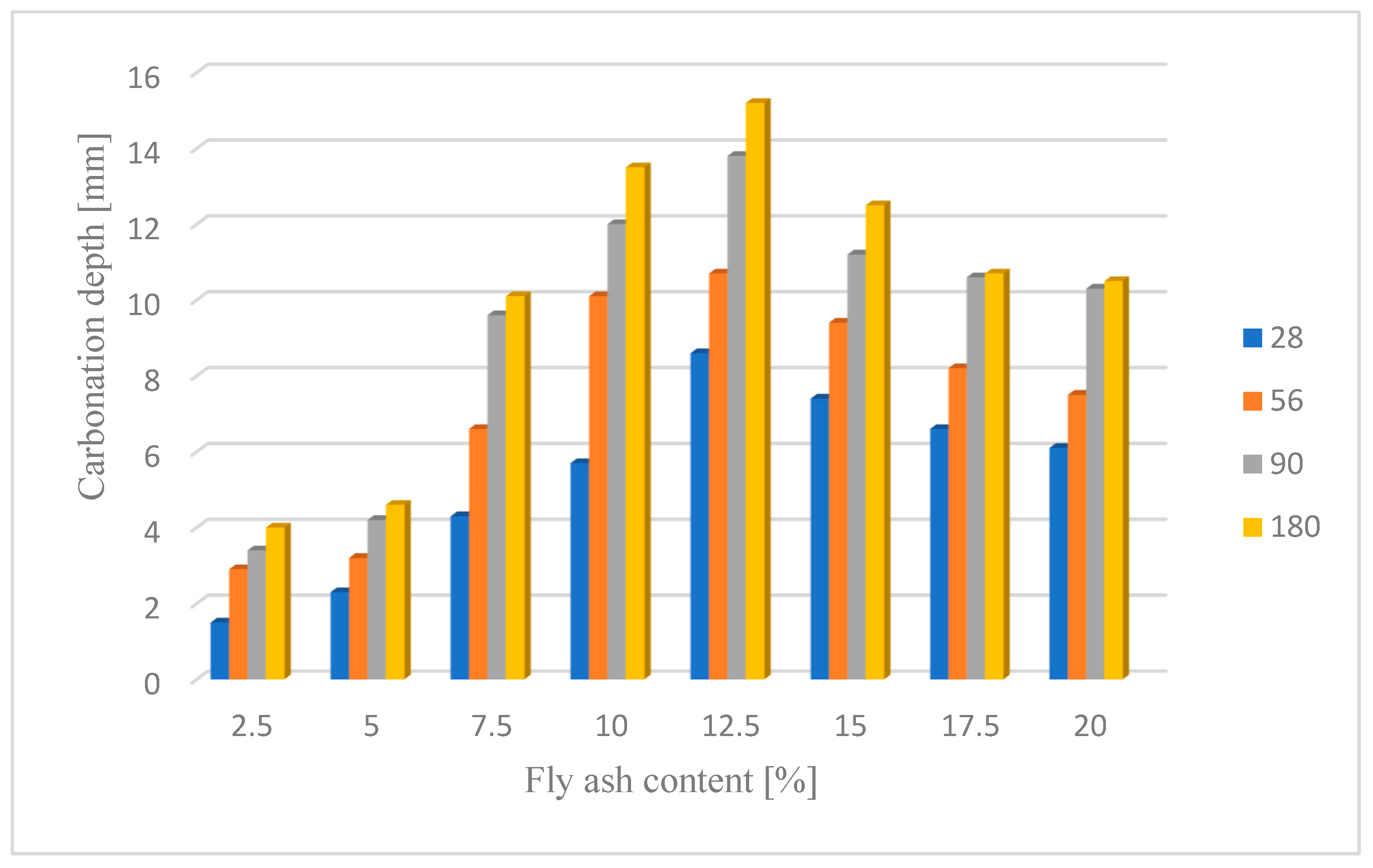
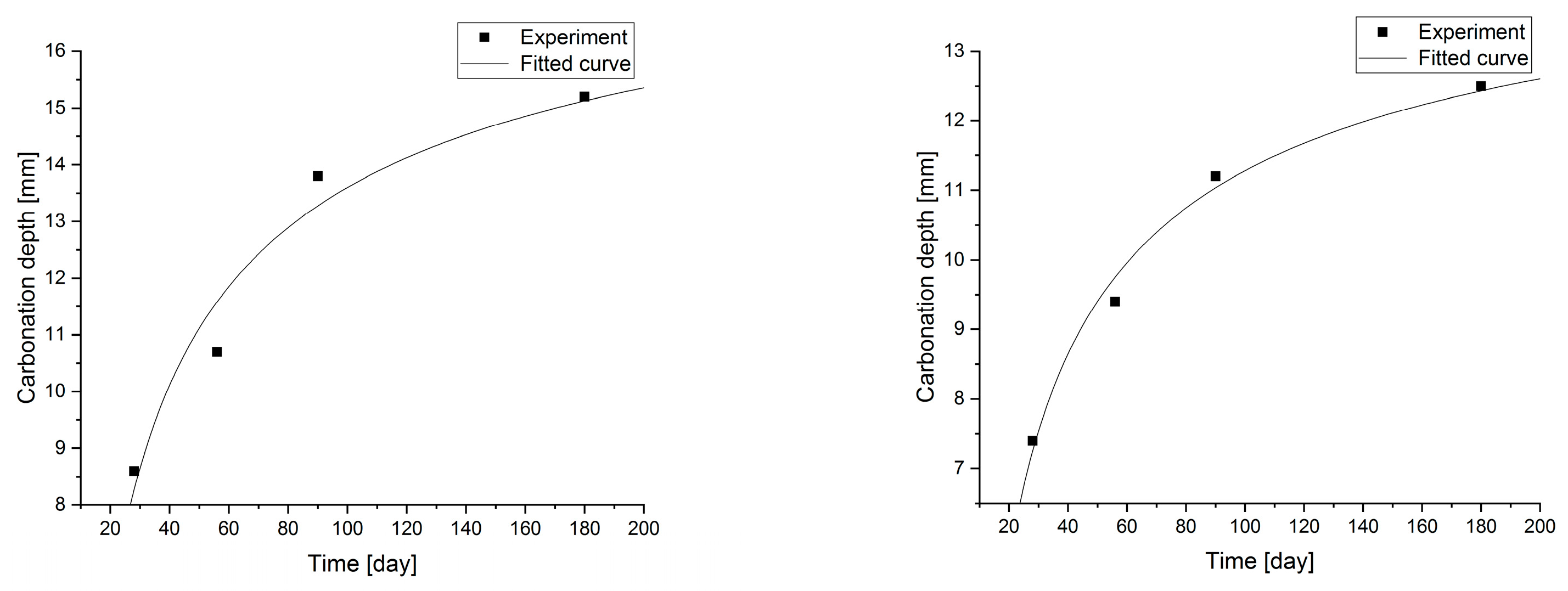
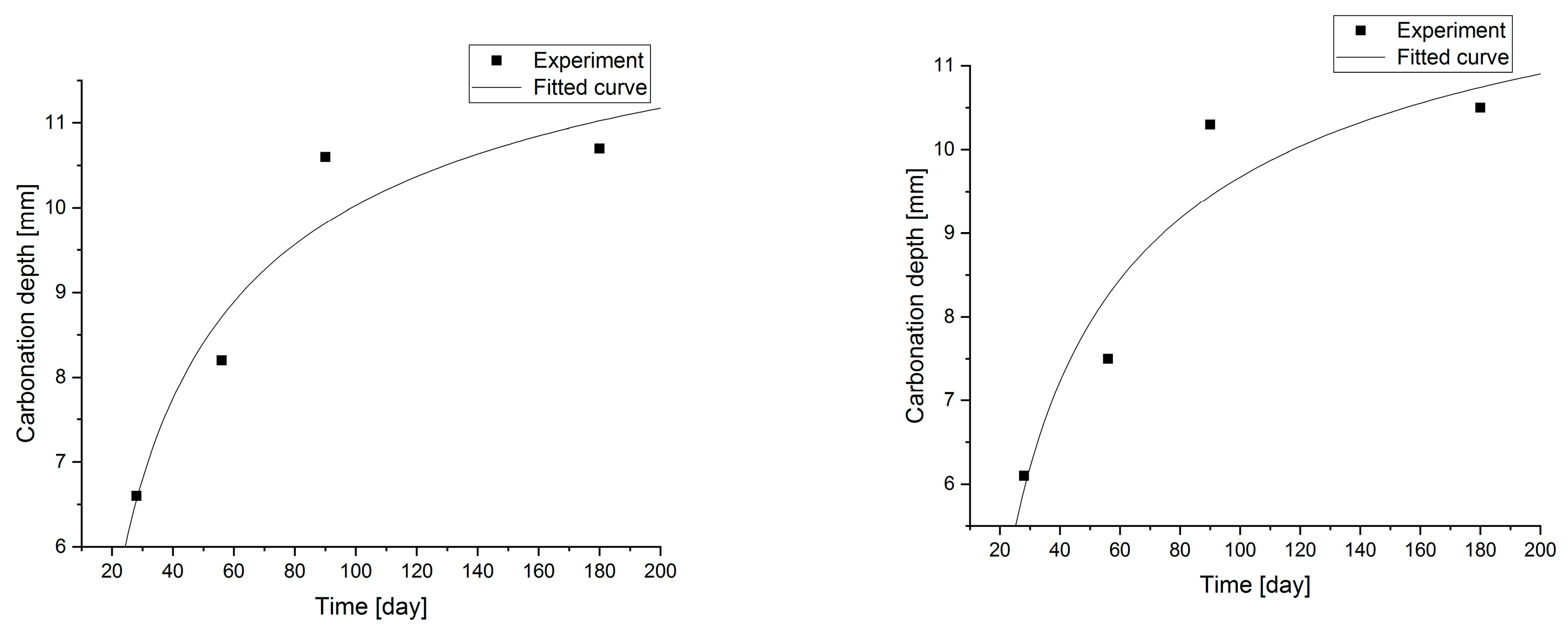
Changes in the depth of carbonation over time for concretes with different fly ash content from thermal treatment of sewage sludge are shown in Figure 6. Different dynamics of increasing the depth of carbonation can be observed. In all cases, the largest increase in the depth of carbonation was observed up to day 56 of the study, while the smallest increase was between days 90 and 180. In the case of concretes with a content of 2.5% and 10.0% ashes (ratio p/c = 0.026 and p/c = 0.111), there was the fastest increase in the depth of carbonation in the period up to 56 days of 93% and 77% respectively. In addition, it was observed that the carbonation depth for concretes with the addition of ash in an amount of up to 12.5% increased to 15.2 mm, while after increasing the content of the additive, the depth of carbonation began to decrease. The content of carbon-dioxide calcium hydroxide leaven in the hardened leaven is lower (additive introduced as a substitute for cement), thus the carbonation depth is greater.
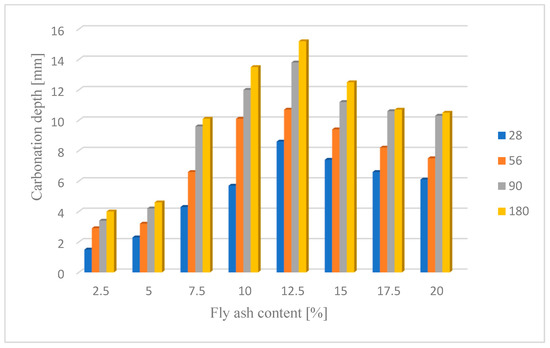
Figure 6.
Depth of carbonation after different operating time.
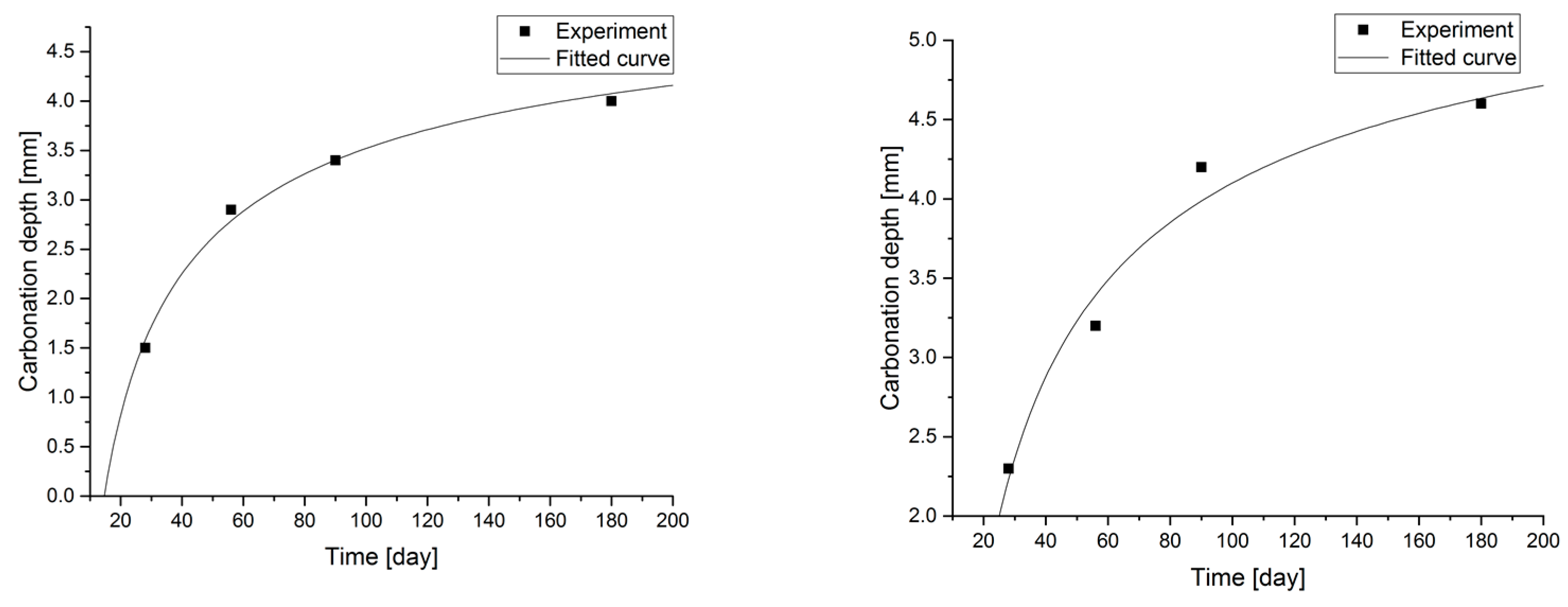
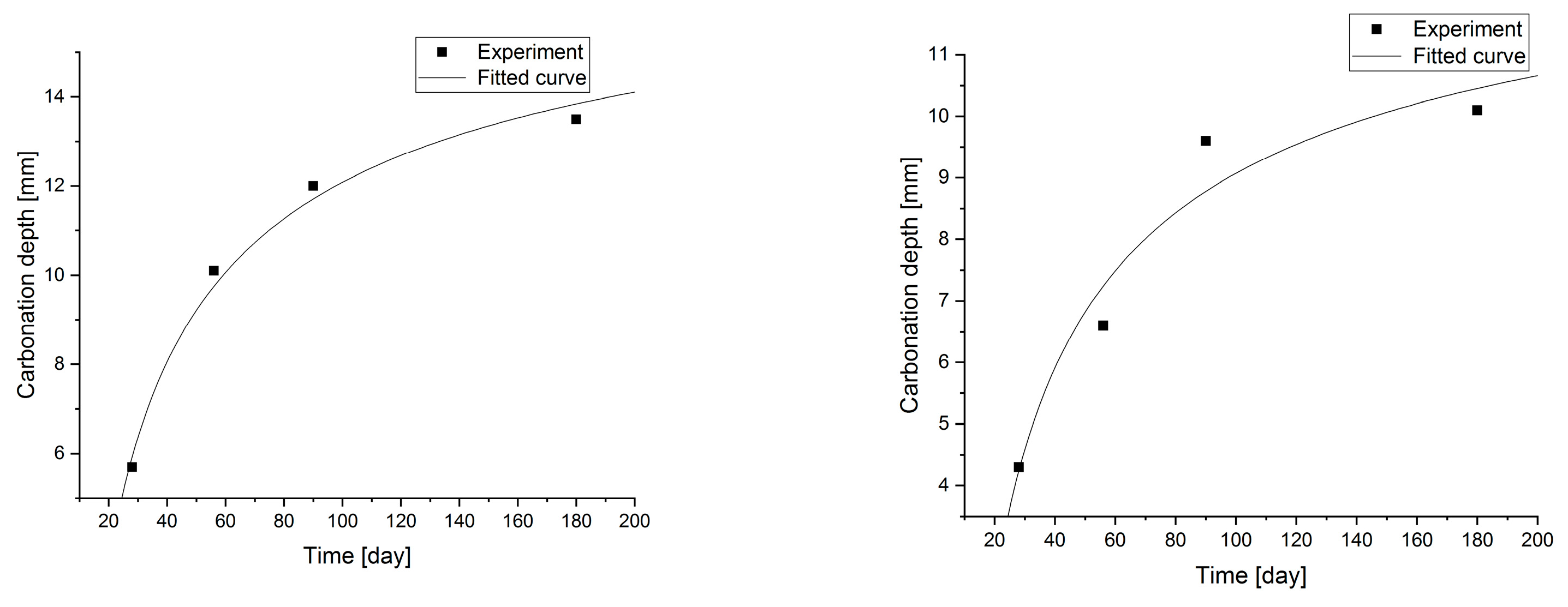
Statistical methods (Statistica 10) for carbonization model analysis using carbonation depth studies up to 180 days. The models are presented graphically (Figure 7, Figure 8, Figure 9 and Figure 10) and are listed in Table 2 together with correlation coefficients and carbonation depth maxima (asymptotes of the model).
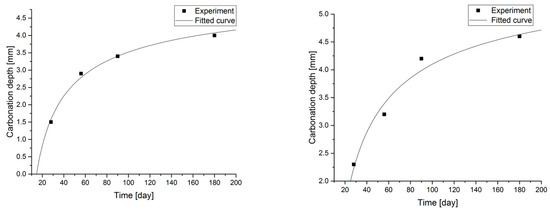
Figure 7.
Carbonation models determined under accelerated conditions for concretes with a content of 2.5 and 5.0% fly ash.
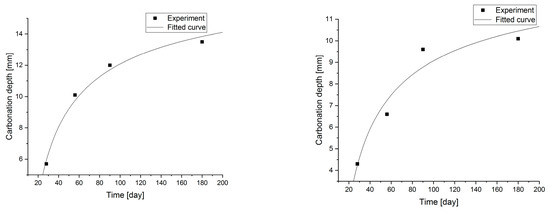
Figure 8.
Carbonation models determined under accelerated conditions for concretes with a content of 7.5 and 10.0% fly ash.
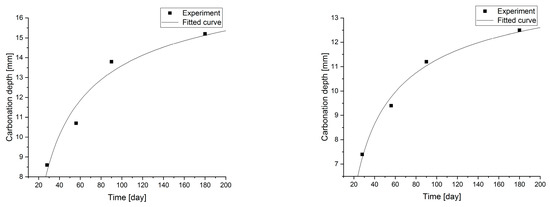
Figure 9.
Carbonation models determined under accelerated conditions for concretes with a content of 2.5 and 5.0% fly ash.
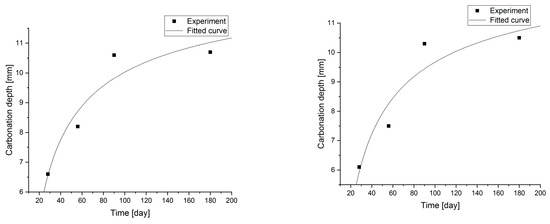
Figure 10.
Carbonation models determined under accelerated conditions for concretes with fly ash content.

Table 2.
List of carbonation models under accelerated conditions for concretes with different fly ash content.
4. Discussion
According to the literature, the presence of chlorides and sulfates can negatively affect the properties of concrete and lead to its corrosion. Of all ions, chloride ions penetrate the cement matrix the fastest. A chloride attack can cause the pH of the concrete to drop and form swelling compounds that can lead to the cracking of the concrete and corrosion of the reinforcement. The chloride content in concrete also affects the matrix form (C-S-H phase) of hydrated calcium silicate [65]. SO3 content exceeding 3wt% will lead to sulfate corrosion of concrete. However, studies by Garcés have shown that sulfates contained in fly ash after thermal treatment of sewage sludge do not react with cement [66,67]. It is believed that the presence of phosphate ions may affect the slow build-up of strength in concrete containing co-fired ash due to the delay in the cement hydration process [67,68].
According to De Noirfontaine, when such ash is used to produce cement, too high a phosphorus content reduces the quality of the clinker and decomposes lite (C3S) into belite (C2S), and lime (CaO) [68]. Phosphorus contained in fly ash retards the hydration process of the binder [32,69]. The results presented in other works [70] also confirmed that the pozzolanic activity of fly ash after thermal treatment of sewage sludge reaches the standard value (85%) after a longer maturation period. The presence of ash, which accounts for 25% of the weight of cement, affects the delay of the setting process of the mortar and the slower development of the compressive strength of the concrete compared to composites made of Portland cement alone. However, the required strength of structural concrete can be achieved by extending the curing time [70,71,72,73,74,75,76]. The determined regression coefficients of the hyperbolic model showed that the hyperbolic model used was a reasonable fit to the laboratory test results (R = 0.85–0.99) obtained under accelerated carbonation conditions, regardless of the fly ash content in the sewage sludge in the normal concrete samples. From day 28 to day 56, a sharp increase in carbonation depth was observed. The further development of the phenomenon has slowed down and the measured value after 180 days has approached the asymptotic value. According to the literature [4], the carbonation depth values obtained under accelerated carbonization conditions are generally greater than those obtained for the same concrete under natural carbonation conditions. The additive introduced into the concrete mix, on the one hand, accelerates the carbonation process by moving the carbonation front deep into the concrete, and on the other hand, a more compact microstructure is created. There is a decrease in diffusivity and a reduction in the rate of carbonation [3,43].
5. Conclusions
Based on the conducted research, the following conclusions were formulated:
1. Thermally treated sewage sludge ash showed a different chemical composition compared to silica ash. Silica, calcium, phosphorus, and aluminum oxides were the most abundant in these sewages sludge ash. The total content of silicon dioxide (SiO2), alumina (Al2O3), and iron oxide (Fe2O3) in fly ash from the thermal treatment of sewage sludge does not meet the requirements contained in EN 450-1 + A1:2012. However, there are no provisions for the chemical and physical properties of ashes obtained from the combustion of sewage sludge, limiting their potential use in concrete technology.
2. The activity of fly ash from the thermal treatment of sewage sludge does not meet the requirements of EN 450-1:2012 after 28 and 90 days of ripening. The activity rate after 28 days of ripening for Krakow ash was 71.7%, while after 90 days it was 84.1%. Thus, innovative ashes from sludge combustion showed less pozzolanic activity than fly ash from coal combustion in the same period. However, they have reached the required indicator values (85%), which allows them to be classified as active mineral additives.
3. Concrete containing more than 10% sewage sludge fly ash instead of cement was characterized by higher compressive strength than the control concrete without the additive. The mean compressive strengths of Krakow ash-bearing concrete after curing for 28, 56, 90, and 365 days were 50.1 MPa, 50.6 MPa, 50.8 MPa, and 61.9 MPa, respectively. As a result, the strength properties of the concrete were able to achieve a strength of 25 MPa above the minimum value set during design.
4. According to information provided by other authors, their own research indicates that the optimal amount of fly ash from the thermal treatment of sewage sludge in concrete is 5–15%.
5. Taking into account the physico-chemical composition and pozzolanic properties of the fly ashes used from the thermal transformation of sewage sludge from various sewage treatment plants, it was noted that a higher concentration of SiO2, Al2O3, and Fe2O3 (Kraków—57.71%) and lower P2O5, CaO (Kraków—30.81%) has a positive effect on the increase in compressive strength of the produced concretes.
6. The degree of matching of the obtained material models using fly ash from Krakow for experimental data determined by the coefficient of determination is above 0.82, which is a satisfactory result.
7. The additive introduced into the concrete mix on the one hand accelerates the carbonation process by moving the carbonation front deep into the concrete. Diffusivity is reduced and the rate of carbonation is reduced.
Author Contributions
Conceptualization, M.Ż.; methodology, G.R.; validation, G.R. and K.P.; formal analysis, G.R. and K.R; investigation, B.Ż. and J.A.; resources, G.R. and M.Ż.; writing—original draft preparation, M.Ż. and M.Ż.; writing—review and editing, G.R. and K.R.; visualization, G.R. and M.Ż. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Data are not publicly available. The data may be made available upon request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Masters, L.W.; Brandt, E. Systematic methodology for service life. Prediction of building materials and components. Mater. Struct. 1988, 22, 285–392. [Google Scholar] [CrossRef]
- Deja, J. (Ed.) Concrete Technologies and Test Methods; Association of Cement Producers: Kraków, Poland, 2020. [Google Scholar]
- Neville, A.M. Concrete Properties; Association of Cement Producers: Kraków, Poland, 2012. [Google Scholar]
- Woyciechowski, P. Model of Concrete Carbonation; Publishing House of the Warsaw University of Technology: Warszawa, Poland, 2013. [Google Scholar]
- Majumdar, A.J.; Stuck, M.S. Microstructure of glass fibre reinforced supersulphated cement. Cem. Concr. Res. 1981, 11, 781–788. [Google Scholar] [CrossRef]
- Bakhareva, T.; Sanjayana, J.G.; Cheng, Y. Resistance of alkali-activated slag concrete to carbonation. Cem. Concr. Res. 2001, 31, 1277–1283. [Google Scholar] [CrossRef]
- Lagerblad, B. Carbon Dioxide Uptake during Concrete Life Cycle—State-of-the-Art; Swedish Cement and Concrete Research Institute—CBI: Stockholm, Sweden, 2005. [Google Scholar]
- Bernal, S.A.; de Gutierrez, R.M.; Provis, J.L. Carbonation of alkali-activated GBFS/MK concretes. In Proceedings of the International Congress on Durability of Concrete, Trondheim, Norway, 18–21 June 2012. [Google Scholar]
- Boos, P.; Giergiczny, Z. Testing the frost resistance of concrete with different cement types-experience from laboratory and practice. Archit. Civ. Eng. Environ. 2010, 2, 41–52. [Google Scholar]
- Deja, J. Corrosion durability of binders with different content of granulated blast furnace slag. Cem.-Lime-Concr. 2007, 12, 280–283. [Google Scholar]
- Du, J.; Jin, Z.; Jiang, J. Experimental Study of Fly Ash Concrete Carbonization. Coal Ash China 2005, 17, 9–11. [Google Scholar]
- Jackiewicz-Rek, W.; Woyciechowski, P. Influence of ash content on the course of carbonation of aerated concretes. In Proceedings of the 6th Conference Material Issues in Civil Engineering, MATBUD, Kraków, Poland, 20 April 2011; pp. 175–184. [Google Scholar]
- Johannesson, B.; Utgenannt, P. Microstructural changes caused by carbonation of cement mortar. Cem. Concr. Res. 2001, 31, 925–931. [Google Scholar] [CrossRef]
- Thiery, M.; Dangla, P.; Villain, G.; Platret, G.; Massieu, E.; Druon, M.; Baroghel-Bouny, V. Modeling the atmospheric carbonation of cementitious materials. Bull. Lab. Ponts Chaussees 2004, 252–253, 153–188. [Google Scholar]
- Fiertak, M.; Małolepszy, J. Concrete as a composite material subject to the influence of environmental factors. In Proceedings of the Scientific and Technical Symposium: Durability of Concrete and Its Technological, Material and Environmental Conditions, Kraków, Poland, 20 April 2004. [Google Scholar]
- Czarnecki, L.; Woyciechowski, P. Methods of Assessing the Course of Concrete Carbonation—Materials of the 2nd Scientific and Technical Symposium Concrete Durability; Górażdż Cement SA: Kraków, Poland, 2008; pp. 97–119. [Google Scholar]
- Kurdowski, W. Chemistry of Cement and Concrete; Polski Cement, Wydawnictwo Naukowe PWN: Kraków, Poland, 2010. [Google Scholar]
- Ahmaruzzaman, M. A review on the utilization of fly ash. Prog. Energy Combust. Sci. 2010, 36, 1349–1359. [Google Scholar] [CrossRef]
- Łagosz, A.; Deja, J. Evaluation of the impact of curing conditions and w/c ratio on the stiffness of the carbonation process of cement mortars. Cem.-Lime-Concr. 2011, 4, 207–216. [Google Scholar]
- Piasta, J.; Rawicz, Z.; Piasta, W. Carbonation of concrete in a reinforced concrete slab. In Proceedings of the Conference Proceedings of Days of Concrete, Wisła, Poland, 9–11 October 2008; pp. 277–286. [Google Scholar]
- Hainer, S.; Proske, T.; Graubner, C.A. Carbonation of cement reduced concretes. In Darmstadt Concrete—Annual Journal on Concrete and Concrete Structures 24; Darmstadt Concrete: Darmstadt, Germany, 2009. [Google Scholar]
- Kuosa, H.; Ferreira, M.R.; Holt, E. Concrete durability based on coupled deterioration by frost, carbonation and chloride. In Proceedings of the International Congress on Durability of Concrete, ICDC, Trondheim, Norway, 18–21 June 2012. article PP2. [Google Scholar]
- Bouikni, A.; Swamy, R.N.; Bali, A. Durability properties of concrete containing 50% and 65% slag. Constr. Build. Mater. 2009, 23, 2836–2845. [Google Scholar] [CrossRef]
- Dong, B.; Qiu, Q.; Xiang, J.; Huang, C.; Sun, H.; Xing, F.; Liu, W. Electrochemical impedance interpretation of the carbonation behavior for fly ash–slag–cement materials. Constr. Build. Mater. 2015, 93, 933–942. [Google Scholar] [CrossRef]
- Gao, Y.; Cheng, L.; Gao, Z.; Guo, S. Effects of different mineral admixtures on carbonation resistance of lightweight aggregate concrete. Constr. Build. Mater. 2013, 43, 506–510. [Google Scholar] [CrossRef]
- Khunthongkeaw, J.; Tangtermsiriku, S.; Leelawat, T. A study on carbonation depth prediction for fly ash concrete. Constr. Build. Mater. 2006, 20, 744–753. [Google Scholar] [CrossRef]
- Özbay, E.; Erdemir, M.; Durmuş, H.I. Utilization and efficiency of ground granulated blast furnace slag on concrete properties—A review. Constr. Build. Mater. 2016, 105, 423–434. [Google Scholar] [CrossRef]
- Kim, H.K.; Lee, H.K. Coal bottom ash in field of civil engineering: A review of advanced applications and environmental considerations. KSCE J. Civ. Eng. 2015, 19, 1802–1818. [Google Scholar] [CrossRef]
- Gonen, T.; Yazicioglu, S.; Demirel, B. The influence of freezing-thawing cycles on the capillary water absorption and porosity of concrete with mineral admixture. KSCE J. Civ. Eng. 2015, 19, 667. [Google Scholar] [CrossRef]
- EN 206+A2:2021-08; Concrete—Requirements, Properties, Production and Compliance. EN 197-1:2012 Cement—Part 1: Composition, Requirements and Conformity Criteria for Common Cements. Polish Standardization Committee: Warszawa, Poland, 2012.
- EN 450-1:2012; Fly Ash for Concrete. Part 1: Definitions, Specifications and Compliance Criteria. Polish Standardization Committee: Warszawa, Poland, 2012.
- EN 450-2:2006; Fly Ash for Concrete. Part 2. Compliance Assessment. Polish Standardization Committee: Warszawa, Poland, 2006.
- Arioz, O. Effects of elevated temperatures on properties of concrete. Fire Saf. J. 2007, 42, 516–522. [Google Scholar] [CrossRef]
- Brandt, A.M. Cement based composites: Materials, mechanical properties and performance. Taylor Fr. Group 2009, 535. [Google Scholar] [CrossRef]
- Jóźwiak-Niedźwiedzka, D. Influence of blended cements on the concrete resistance to carbonation. In Proceedings of the Brittle Matrix Composites 10; Woodhead Publishing: Warsaw, Poland, 2012; pp. 125–134. [Google Scholar]
- Shi, H.-S.; Xu, B.-W.; Zhou, X.-C. Influence of mineral admixtures on compressive strength, gas permeability and carbonation of high performance concrete. Constr. Build. Mater. 2009, 23, 1980–1985. [Google Scholar] [CrossRef]
- Jiang, L.; Liu, Z.; Ye, Y. Durability of concrete incorporating large volumes of low-quality fly ash. Cem. Concr. Res. 2004, 34, 1467–1469. [Google Scholar] [CrossRef]
- Hossain, K.M.A.; Lachemi, M. Development of model for the prediction of carbonation in pozzolanic concrete. In Proceedings of the Third International Conference on Construction Materials: Performance, Innovations and Structural Implications, University of British Columbia, Vancouver, BC, Canada, 22–24 August 2005. [Google Scholar]
- Kurda, R.; de Brito, J.; Silvestre, J.D. Carbonation of concrete made with high amount of fly ash and recycled concrete aggregates for utilization of CO2. J. CO2 Util. 2019, 29, 12–19. [Google Scholar] [CrossRef]
- Ghorbani, S.; Sharifi, S.; Ghorbani, S.; Tam, V.W.Y.; De Brito, J.; Kurda, R. Effect of crushed concrete waste’s maximum size as partial replacement of natural coarse aggregate on the mechanical and durability properties of concrete. Resour. Conserv. Recycl. 2019, 149, 664–673. [Google Scholar] [CrossRef]
- Carević, V.; Ignjatović, I.; Dragaš, J. Model for practical carbonation depth prediction for high volume fly ash concrete and recycled aggregate concrete. Constr. Build. Mater. 2019, 213, 194–208. [Google Scholar] [CrossRef]
- Papadakis, V.G. Effect of supplementary cementing materials on concrete resistance against carbonation and chloride ingress. Cem. Concr. Res. 2000, 30, 291–299. [Google Scholar] [CrossRef]
- Bier, T.A. Influence of type of cement and curing on carbonation progress and pore structure of hydrated cement paste. In Proceedings of the Materials Research Society Symposium, Erlangen, Germany, 27–29 April 1987; pp. 123–134. [Google Scholar]
- Dąbrowski, M.; Glinicki, M.A.; Gibas, K.; Jóźwiak-Niedźwiedzka, D. Effects of calcareous fly ash in blended cements on chloride ions migration and strength of air entrained concrete. Constr. Build. Mater. 2016, 126, 1044–1053. [Google Scholar] [CrossRef]
- Bary, B.; Sellier, A. Coupled moisture-carbon dioxide-calcium transfer model for carbonation of concrete. Cem. Concr. Res. 2004, 34, 1859–1872. [Google Scholar] [CrossRef]
- Woliński, P.; Woyciechowski, P.P.; Jaworska, B.; Adamczewski, G.; Tokarski, D.; Grudniewski, T.; Chodyka, M.; Nitychoruk, A. The influence of the mineral additives on the carbonation of cement composites. MATEC Web Conf. 2018, 196, 04062. [Google Scholar] [CrossRef]
- Glinicki, M.A.; Ładyżyński, K. Activated fly ash from fluidized bed boilers—A new additive to concrete. In Proceedings of the 18th Conference—Concrete and Prefabrication, Popowo, Poland, 10–11 April 2002. [Google Scholar]
- Czarnecki, L.; Woyciechowski, P. Concrete carbonation as a limited process and its relevance to CO2 sequestration. ACI Mater. J. 2012, 109, 275–282. [Google Scholar]
- Papadakis, V.G.; Fardis, M.N.; Vayenas, C.G. Effect of composition, environmental factors and cement-lime mortar coating on concrete carbonation. Mater. Struct. 1992, 25, 293–304. [Google Scholar] [CrossRef]
- Parrott, L.J.; Killoch, D.C. Carbonation in 36 years old, in-situ concrete. Cem. Concr. Res. 1989, 19, 649–656. [Google Scholar] [CrossRef]
- Bakker, R.F.M. Initiation period. In Corrosion of Steel in Concrete: Report of the Technical Committee 60-CSC RILEM; Schiessl, P., Ed.; Chapman and Hall: London, UK, 1988; pp. 22–54. [Google Scholar]
- Hergenröder, M. Zur Statistichen Instandhaltungsplanung Für Bestehende Betonbauwerke bei Karbonatisierung des Betons und Möglicher der Bewerhung. Ph.D. Thesis, Technische Universität, München, Germany, 1992. [Google Scholar]
- Nilsson, L.O. Interaction between microclimate and concrete—A perquisite for deterioration. Constr. Build. Mater. 1997, 10, 301–308. [Google Scholar] [CrossRef]
- Czarnecki, L.; Woyciechowski, P. Modelling of concrete carbonation; is it a process unlimited in time and restricted in space? Bull. Pol. Acad. Sci. Tech. Sci. 2015, 63, 43–54. [Google Scholar] [CrossRef]
- Czarnecki, L.; Woyciechowski, P.; Adamczewski, G. Risk of concrete carbonation with mineral industrial by-products. KSCE J. Civ. Engieering 2018, 22, 755–764. [Google Scholar] [CrossRef]
- ASTM C618-03; Standard Specification for Coal Fly Ash and Raw or Calcined Natural Pozzolan Use in Concrete. U.S. Department of Energy Office of Scientific and Technical Information: Washington, DC, USA, 1998.
- Jamroży, Z. Concrete and Its Technologies; Scientific Publishing House PWN: Warsow, Poland, 2015. [Google Scholar]
- EN 12390-3:2011; Concrete Testing. Part 3: Compressive Strength of Specimens for Strength Tests. Polish Standardization Committee: Warszawa, Poland, 2011.
- EN 12390-12:2010; Testing Hardened Concrete—Part 12: Determination of the Potential Carbonation Resistance of Concrete: Accelerated Carbonation Method. Polish Standardization Committee: Warszawa, Poland, 2010.
- Pachowski, J. Fly Ashes and Their Application in Road Construction; Communication and Connectivity: Warsaw, Poland, 1976. [Google Scholar]
- ASTM C379-65T; Specification for Fly Ash for Use as a Pozzolanic Material with Lime. American Society for Testing and Material: Washington, DC, USA, 1965.
- Bastion, S. Structural Concretes with Fly Ash; Arkady: Warszawa, Ploand, 1980. [Google Scholar]
- Tkaczewska, E. Properties of cements containing various grain fractions of siliceous fly ashes. Roads Bridges 2008, 4, 47–80. [Google Scholar]
- Szarek, Ł.; Wojtowska, M. Properties of fly ash from thermal treatment of municipal sewage sludge in terms of EN 450-1. Arch. Environ. Prot. 2018, 44, 63–69. [Google Scholar] [CrossRef]
- Eglinton, M.S. Concrete and Its Chemical Behavior; Thomas Telford: London, UK, 1987. [Google Scholar]
- Garcés, P.; Carrión, M.P.; García-Alcocel, E.; Payá, J.; Monzó, J.; Borrachero, M.V. Mechanical and physical properties of cement blended with sewage sludge ash. Waste Manag. 2008, 28, 2495–2502. [Google Scholar] [CrossRef] [PubMed]
- Małolepszy, J.; Tkaczewska, E. Influence of fly ashes from coal and biomass co-combustion on hydration process and cement properties. In Proceedings of the Materials of the 4th Scientific and Technical Conference “Concrete Days—Tradition and Modernity”, Wisła, Poland, 9–11 October 2006; pp. 591–601. [Google Scholar]
- De Noirfontaine, M.N.; Tusseau-Nenez, S.; Signes-Frehel, M.; Gasecki, G.; Girod-Labianca, C. Efect of phosphorus on tricalcium silicate T1: From synthesis to structural characterization. J. Am. Ceram. Soc. 2009, 92, 2337–2344. [Google Scholar] [CrossRef]
- Tarko, B.; Gorazda, K.; Wzorek, Z.; Nowak, A.K.; Kowalski, Z.; Kulczycka, J.; Henclik, A. Recovery of phosphorus from industrial sewage sludge ashes. Chem. Ind. 2014, 93, 1041–1044. [Google Scholar]
- Rutkowska, G.; Fronczyk, J.; Wichowski, P. Research on the Possibility of Using Fly Ashes from Combustion of Municipal Sewage Sludge on Properties of Ordinary Concretes. Annu. Set Environ. Prot. 2018, 20, 1113–1128. [Google Scholar]
- Kosior-Kazberuk, M. New mineral additives for concrete. Constr. Environ. Eng. 2011, 29, 47–55. [Google Scholar]
- Rutkowska, G.; Fronczyk, J.; Filipczuk, S. Influence of fly ash properties from thermal conversion of sewage sludge on the parameters of ordinary concrete. Acta Sci. Pol. Archit. 2020, 19, 43–54. [Google Scholar] [CrossRef]
- Rutkowska, G.; Wichowski, P.; Franus, M.; Mendryk, M.; Fronczyk, J. Modification of Ordinary Concrete Using Fly Ash from Combustion of Municipal Sewage Sludge. Materials 2020, 13, 487. [Google Scholar] [CrossRef] [PubMed]
- Rutkowska, G.; Wichowski, P.; Fronczyk, J.; Franus, M.; Chalecki, M. Use of fly ashes from municipal sewage sludge combustion in production of ash concretes. Constr. Build. Mater. 2018, 188, 874–883. [Google Scholar] [CrossRef]
- Fontes, C.M.A.; Barbosa, M.C.; Filho, R.D.T.; Goncalves, J.P. Potentiality of sewage sludge ash as mineral additive in cement mortar and high performance concrete. In Proceedings of the Conference: Use of Recycled Materials in Buildings and Structures (RILEM Publications), Barcelona, Spain, 8–11 November 2004; pp. 797–806. [Google Scholar]
- Yen, C.L.; Tseng, D.H.; Lin, T.T. Characterization of eco-cement paste produced from waste sludges. Chemosphere 2011, 84, 220–226. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).