The Influence of GGBFS as an Additive Replacement on the Kinetics of Cement Hydration and the Mechanical Properties of Cement Mortars
Abstract
1. Introduction
2. Materials and Methods
2.1. Cement
2.2. Ground-Granulated Blast Furnace Slag
2.3. The Setting Time and the Heat of Hydration of OPC in the System of Cement Pastes and Their Mixtures Prepared with GGBFS as a Replacement Additive
2.4. Developing the Model of Cement Hydration
2.5. Preparation Cement Mortars
3. Results and Discussion
3.1. Influence of the Addition of GGBFS on Setting Time
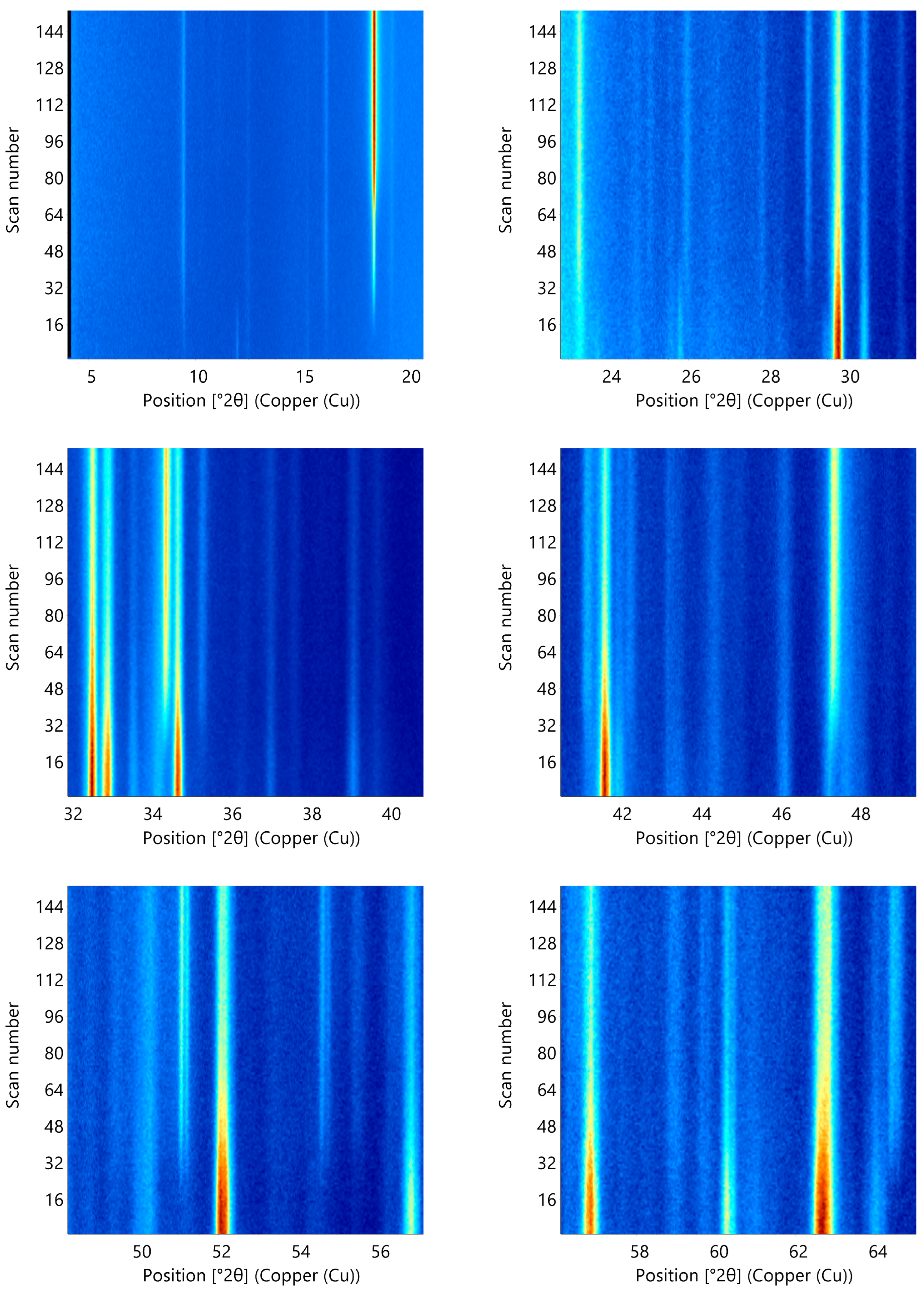
3.2. Structure Changes during Cement Hydration Followed by Using In Situ X-ray Diffraction
3.3. Developed Microstructure of Cement Composite Determined by SEM
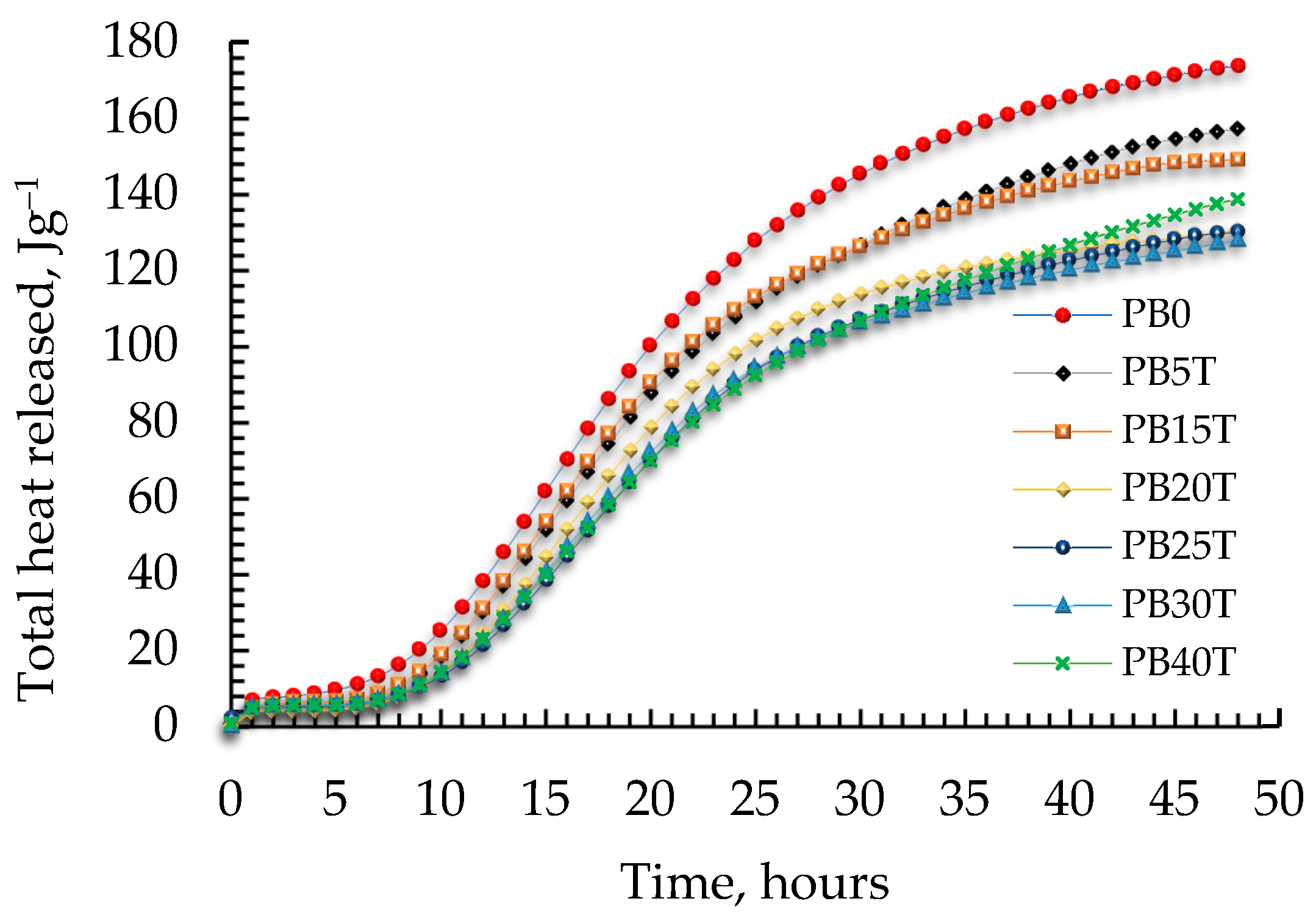
3.4. The Heat Released during Hydration in the System of Cement Paste
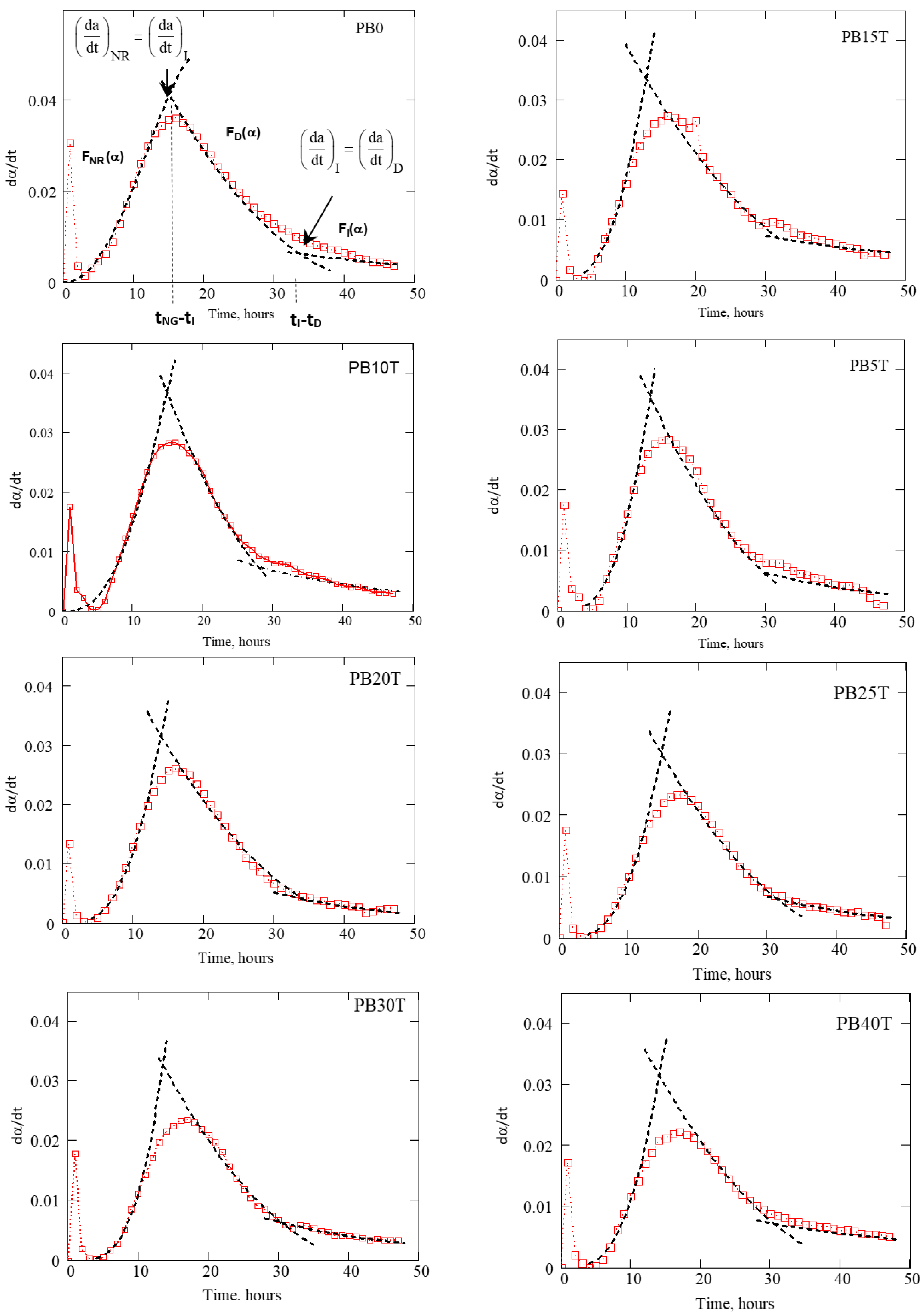
3.5. Kinetics Analysis of the Hydration of Cement
3.6. Mechanical Properties of Cement Mortars
4. Conclusions
- The delayed setting of cement with the replacement additive GGBFS in amounts of 5–15 wt.% and the accelerated setting of cement with the addition of 20–40 wt.% GGBFS originates from the physical role of GGBFS as a nucleation site.
- The total heat of hydration released during the 48 h shows that the value of total heat released is reduced up to 26.36% by increasing the amount of replacement additive GGBFS up to 40 wt.%.
- Calorimetric measurements can be used for the kinetic analysis. The calculated value of the exponent n is in the range 3.083–4.798, suggesting three-dimensional growth of hydration products as well as hetero nucleation and growth in polydisperse systems.
- Shortening the transition time (), the time when the process of nucleation and growth as the fastest process of cement hydration limits the overall process of hydration, confirms the role of GGBFS as an accelerator of cement.
- The developed mechanical properties show that the replacement additive GGBFS slightly decreases the mechanical properties in the early phase of hydration until it contributes to increase the mechanical properties in the later phase of cement hydration. This influence is due not only to the physical role, but also to the pozzolanic reactivity of GGBFS.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| α | Degree of hydration |
| BFS | Blast furnace slag |
| Cp | Heat capacity |
| C3S | Alite |
| C2S | Belite |
| C3A | Tricalcium aluminate |
| C4AF | Tetracalcium aluminoferrite |
| D | Diffusion |
| DMC | Differential microcalorimetry |
| FA | Fly ash |
| GBFS | Granulated blast furnace slag |
| GGBFS | Ground granulated blast furnace slag |
| I | Interaction at the phase boundary |
| Nucleation and growth constant | |
| Interaction at the phase boundary constant | |
| Diffusion constant | |
| NG | Nucleation and growth |
| OPC | Ordinary Portland cement |
| PXRD | Powder X-ray diffraction |
| Q(t) | Heat released in time |
| Qmax | Total released heat of hydration after 90 days |
| SEI | Secondary electron image |
| SEM/EDS | Scanning electron microscopy/energy-dispersive spectroscopy |
| SF | Silica fume |
| Transition time | |
| Transition time | |
| XRF | X-ray fluorescence |
References
- Garside, M. Global Cement Production 1995–2022. Available online: https://www.statista.com/statistics/1087115/global-cement-production-volume/ (accessed on 12 June 2023).
- Chen, C.; Xu, R.; Tong, D.; Qin, X.; Cheng, J.; Liu, J.; Zheng, B.; Yan, L.; Zhang, Q. A Striking Growth of CO2 emissions from the Global Cement Industry Driven by New Facilities in Emerging Countries. Environ. Res. Lett. 2022, 17, 044007. [Google Scholar] [CrossRef]
- Ueki, Y. History and Utilization of Portland Blast Furnace Slag Cement. Nippon. Steel Sumitomo Met. Tech. Rep. 2015, 109, 109–113. [Google Scholar]
- Tao, J.; Wei, X. Effect of Ground Granulated Blast-Furnace Slag on the Hydration and Properties of Cement Paste. Adv. Cem. Res. 2019, 31, 251–260. [Google Scholar] [CrossRef]
- Norrarat, P.; Tangchirapat, W.; Songpiriyakij, S.; Jaturapitakkul, C. Evaluation of Strengths from Cement Hydration and Slag Reaction of Mortars Containing High Volume of Ground River Sand and GGBF Slag. Adv. Civ. Eng. 2019, 2019, 4892015. [Google Scholar] [CrossRef]
- Paris, J.M.; Roessler, J.G.; Ferraro, C.C.; Deford, H.D.; Townsend, T.G. A Review of Waste Products Utilized as Supplements to Portland Cement in Concrete. J. Clean. Prod. 2016, 121, 1–18. [Google Scholar] [CrossRef]
- Jozić, D.; Zelić, J. The Effect of Fly Ash on Cement Hydration in Aqueous Suspensions. Ceramics–Silikáty 2006, 50, 98–105. [Google Scholar]
- Shariq, M.; Prasad, J.; Masood, A. Effect of GGBFS on Time Dependent Compressive Strength of Concrete. Constr. Build. Mater. 2010, 24, 1469–1478. [Google Scholar] [CrossRef]
- Zelić, J.; Rušić, D.; Krstulović, R. A Mathematical Model for Prediction of Compressive Strength in Cement-Silica Fume Blends. Cem. Concr. Res. 2004, 34, 2319–2328. [Google Scholar] [CrossRef]
- Jozić, D. A Study on the Effect of Fly Ash from the Thermo-Electric Power Plant on the Physical and Chemical Properties and on the Behaviour of the Cement Composite. Ph.D. Thesis, University of Split, Faculty of Chemistry and Technology, Split, Croatia, 2007. [Google Scholar]
- Crossin, E. The Greenhouse Gas Implications of Using Ground Granulated Blast Furnace Slag as a Cement Substitute. J. Clean. Prod. 2015, 95, 101–108. [Google Scholar] [CrossRef]
- Gruskovnjak, A. Hydration Mechanisms of Activated Blast Furnace Slag. Ph.D. Thesis, University Bern, Bern, Switzerland, 2006; pp. 1–123. [Google Scholar]
- Václavík, V.; Dirner, V.; Dvorský, T.; Daxner, J. The Use of Blast Furnace Slag. Metalurgija 2012, 51, 461–464. [Google Scholar]
- Tsakiridis, P.E.; Papadimitriou, G.D.; Tsivilis, S.; Koroneos, C. Utilization of Steel Slag for Portland Cement Clinker Production. J. Hazard. Mater. 2008, 152, 805–811. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Wang, Z.; Liu, Y.; Hu, W.; Ni, W. On the Use of Blast Furnace Slag and Steel Slag in the Preparation of Green Artificial Reef Concrete. Constr. Build. Mater. 2016, 112, 241–246. [Google Scholar] [CrossRef]
- Divsholi, B.S.; Lim, D.T.Y.; Teng, S. Evaluation of heat of hydration for high performance concrete incorporating normal and ultra fine ground granulated blast furnace slag. In Proceedings of the 37th Conference on Our World in Concrete & Structures, Singapore, 29–31 August 2012; pp. 1–13. [Google Scholar]
- Ogawa, S.; Hyodo, H.; Hirao, H.; Yamada, K.; Matsui, A.; Hooton, D. Sulfate resistance improvement of blended cement based on ground granulated blast furnace slag. In Proceedings of the 3rd ACF International Conference—ACF/VCA 2008, Ho Chi Minh City, Vietnam, 11–13 November 2008; pp. 499–506. [Google Scholar]
- Fang, K.; Wang, D.; Gu, Y. Utilization of Gasification Coarse Slag Powder as Cement Partial Replacement: Hydration Kinetics Characteristics, Microstructure and Hardening Properties. Materials 2023, 16, 1922. [Google Scholar] [CrossRef]
- Ballim, Y.; Graham, P.C. The Effects of Supplementary Cementing Materials in Modifying the Heat of Hydration of Concrete. Mater. Struct. 2008, 42, 803–811. [Google Scholar] [CrossRef]
- Yalçınkaya, Ç.; Çopuroğlu, O. Hydration Heat, Strength and Microstructure Characteristics of UHPC Containing Blast Furnace Slag. J. Build. Eng. 2021, 34, 101915–101935. [Google Scholar] [CrossRef]
- Thomas, J.J.; Biernacki, J.J.; Bullard, J.W.; Bishnoi, S.; Dolado, J.S.; Scherer, G.W.; Luttge, A. Modeling and Simulation of Cement Hydration Kinetics and Microstructure Development Cement and Concrete Research Modeling and Simulation of Cement Hydration Kinetics and Microstructure Development. Cem. Concr. Res. 2011, 41, 1257–1278. [Google Scholar] [CrossRef]
- Ahmad, J.; Kontoleon, K.J.; Majdi, A.; Naqash, M.T.; Deifalla, A.F.; Kahla, N.B.; Isleem, H.F.; Qaidi, S.M.A. A Comprehensive Review on the Ground Granulated Blast Furnace Slag (GGBS) in Concrete Production. Sustainability 2022, 14, 8783. [Google Scholar] [CrossRef]
- Sandhu, A.R.; Rind, T.A.; Kalhoro, S.A.; Lohano, R.; Laghari, F.H. Effect on the compressive strength of mortars using ground granulated blast furnace slag as a partial replacement of cement. J. Appl. Eng. Sci. 2019, 9, 183–186. [Google Scholar] [CrossRef]
- Jeffery, J.W. The Crystal Structure of Tricalcium Silicate. Acta Crystallogr. 1952, 5, 26–35. [Google Scholar] [CrossRef]
- Izadifar, M.; Ukrainczyk, N.; Salah Uddin, K.M.; Middendorf, B.; Koenders, E. Dissolution of β-C2S Cement Clinker: Part 2 Atomistic Kinetic Monte Carlo (KMC) Upscaling Approach. Materials 2022, 15, 6716. [Google Scholar] [CrossRef]
- Mishra, R.K.; Fernández-Carrasco, L.; Flatt, R.J.; Heinz, H. A Force Field for Tricalcium Aluminate to Characterize Surface Properties, Initial Hydration, and Organically Modified Interfaces in Atomic Resolution. Dalt. Trans. 2014, 43, 10602–10616. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Ren, Q.; He, J.; Jiang, S.; Cheng, X.; Yu, Y.; Huang, S.; Zhang, C.; Zhou, M. New Understanding of Early Hydration of C4AF under Surface Vitrification. Powder Technol. 2021, 377, 372–378. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhang, Z. Effect of Fineness on the Pozzolanic Reaction Kinetics of Slag in Composite Binders: Experiment and Modelling. Constr. Build. Mater. 2021, 273, 121695. [Google Scholar] [CrossRef]
- Jozić, D.; Zelić, J.; Janjatović, I. Influence of the Coarse Fly Ash on the Mechanical Properties of the Cement Mortars. Ceram.–Silikáty 2010, 54, 144–151. [Google Scholar]
- Brachaczek, W.; Chleboś, A.; Kupczak, M.; Spisak, S.; Stybak, M.; Żyrek, K. Influence of the Addition of Ground Granulated Blast Furnace Slag, Fly Silica Ash and Limestone on Selected Properties of Cement Mortars. Mater. Proc. 2023, 13, 32. [Google Scholar] [CrossRef]
- Krstulović, R.; Dabić, P. A Conceptual Model of the Cement Hydration Process. Cem. Concr. Res. 2000, 30, 693–698. [Google Scholar] [CrossRef]
- Dabić, P.; Krstulović, R.; Rušić, D. New Approach in Mathematical Modelling of Cement Hydration Development. Cem. Concr. Res. 2000, 30, 1017–1021. [Google Scholar] [CrossRef]
- Bezjak, A. Nuclei Growth Model in Kinetic Analysis of Cement Hydration. Cem. Concr. Res. 1986, 16, 605–609. [Google Scholar] [CrossRef]
- Zelic, J.; Rusic, D.; Veza, D.; Krstulovic, R. The Role of Silica Fume in the Kinetics and Mechanisms during the Early Stage of Cement Hydration. Cem. Concr. Res. 2000, 30, 1655–1662. [Google Scholar] [CrossRef]
- Bezjak, A.; Jelenic, I. On the Determination of Rate Constants for Hydration. Cem. Concr. Res. 1980, 10, 553–563. [Google Scholar] [CrossRef]
- Wei, Z.; Zhang, Q.; Li, X. Crystallization Kinetics of α-Hemihydrate Gypsum Prepared by Hydrothermal Method in Atmospheric Salt Solution Medium. Crystals 2021, 11, 843. [Google Scholar] [CrossRef]
- Gutzow, I.; Todorova, S.; Jordanov, N. Kinetics of Chemical Reactions and Phase Transitions at Changing Temperature: General Reconsiderations and a New Approach. Bulg. Chem. Commun. 2010, 42, 79–102. [Google Scholar]
- Berodier, E.; Scrivener, K. Understanding the Filler Effect on the Nucleation and Growth of C-S-H. J. Am. Ceram. Soc. 2014, 97, 3764–3773. [Google Scholar] [CrossRef]
- de Matos, P.R.; Andrade Neto, J.S.; Jansen, D.; De la Torre, A.G.; Kirchheim, A.P.; Campos, C.E.M. In-Situ Laboratory X-ray Diffraction Applied to Assess Cement Hydration. Cem. Concr. Res. 2022, 162, 106988. [Google Scholar] [CrossRef]
- Neubauer, J. Refined Ettringite (Ca6Al(SO4)3(OH)12·26H2O) Structure for Quantitative X-Ray Diffraction Analysis. Powder Diffr. 2006, 1, 4–11. [Google Scholar] [CrossRef]
- Munjal, P.; Hau, K.K.; Aheng Ahun Hon, A. Journal of Building Engineering Ef Fe Ct of GGBS and Curing Conditions on Strength and Microstructure Properties of Oil Well Cement Slurry. J. Build. Eng. 2021, 40, 102331. [Google Scholar] [CrossRef]
- Kozlova, I.; Samchenko, S.; Zemskova, O. Physico-Chemical Substantiation of Obtaining an Effective Cement Composite with Ultrafine GGBS Admixture. Buildings 2023, 13, 925. [Google Scholar] [CrossRef]
- Ahmad, J.; Martínez-García, R.; Szelag, M.; de-Prado-Gil, J.; Marzouki, R.; Alqurashi, M.; Hussein, E.E. Effects of Steel Fibers (SF) and Ground Granulated Blast Furnace Slag (GGBS) on Recycled Aggregate Concrete. Materials 2021, 14, 7497. [Google Scholar] [CrossRef]
- Escalante-Garcıa, J.I.; Sharp, J.H. Effect of Temperature on the Hydration of the Main Clinker. Cem. Concr. Res. 1998, 28, 1259–1274. [Google Scholar] [CrossRef]







| Chemical and Mineralogical Composition | |
|---|---|
| wt.% | |
| SiO2 | 19.68 |
| Al2O3 | 4.92 |
| Fe2O3 | 3.12 |
| CaO | 63.86 |
| MgO | 2.09 |
| SO3 | 3.14 |
| Na2O | 0.20 |
| K2O | 1.40 |
| Loss of ignition (LOI), % | 0.18 |
| Physical Characteristics | |
| Specific mass, kg/m3 | 3.13 |
| Specific surface area, Blain, m2/kg | 368.9 |
| Consistency, % | 26 |
| Start setting | 2 h 20 min |
| End setting | 4 h 0 min |
| wt.% | |
|---|---|
| SiO2 | 38.95 |
| Al2O3 | 9.79 |
| Fe2O3 | 0.68 |
| CaO | 39.83 |
| MgO | 4.60 |
| SO3 | 2.24 |
| Na2O | 0.33 |
| K2O | 0.86 |
| Loss of ignition (LOI), % | 0.77 |
| Physical characteristic | |
| Specific mass, kg/m3 | 2.8 |
| Specific surface area, Blain, m2/kg | 398.56 |
| Map Sum Spectrum | wt.% | Map Sum Spectrum | wt.% |
|---|---|---|---|
| O | 36.3 | Mg | 1.6 |
| Ca | 20.2 | Zn | 1.2 |
| Fe | 15.7 | K | 0.8 |
| Si | 14.0 | S | 0.5 |
| Al | 3.9 | P | 0.4 |
| Cu | 2.9 | Ti | 0.2 |
| Mn | 2.3 | Total | 100 |
| Specimen | Sand, g | Cement, g | GGBFS, g | Master Glenium ACE 430, g | Consistency, mm | Water, mL | w/c | w/(GGBFS + c) |
|---|---|---|---|---|---|---|---|---|
| PB0 | 1350 | 450 | 0 | 0 | 207 | 225 | 0.500 | 0.500 |
| PB0S | 1350 | 450 | 0 | 3.9 | 200 | 175 | 0.389 | 0.389 |
| PBT5S | 1350 | 427.5 | 22.5 | 3.9 | 190 | 175 | 0.409 | 0.389 |
| PBT15S | 1350 | 382.5 | 67.5 | 3.9 | 195 | 175 | 0.458 | 0.389 |
| PBT20S | 1350 | 360 | 90 | 3.9 | 210 | 175 | 0.486 | 0.389 |
| PBT25S | 1350 | 337.5 | 112.5 | 3.9 | 190 | 165 | 0.489 | 0.367 |
| PBT30S | 1350 | 315 | 135 | 3.9 | 182 | 167 | 0.530 | 0.371 |
| PBT40S | 1350 | 270 | 180 | 3.9 | 200 | 165 | 0.611 | 0.367 |
| Specimens | Total Heat Release during the First 48 h, J/g |
|---|---|
| PB0 | 173.84 |
| PB5T | 149.10 |
| PB15T | 147.28 |
| PB20T | 138.69 |
| PB25T | 130.69 |
| PB30T | 130.16 |
| PB40T | 128.01 |
| Specimen | Kinetic Parameters | |||||
|---|---|---|---|---|---|---|
| n | ||||||
| PB0 | 3.083 | 0.043 | 15.068 | 0.022 | 30.609 | 0.0053 |
| PB5T | 3.930 | 0.044 | 12.701 | 0.024 | 29.083 | 0.0083 |
| PB10T | 4.473 | 0.048 | 12.101 | 0.022 | 27.76 | 0.0083 |
| PB15T | 3.764 | 0.044 | 12.408 | 0.021 | 31.76 | 0.0032 |
| PB20T | 4.157 | 0.043 | 13.232 | 0.020 | 34.252 | 0.0029 |
| PB25T | 4.234 | 0.043 | 13.989 | 0.022 | 31.30 | 0.0068 |
| PB30T | 4.798 | 0.046 | 12.986 | 0.023 | 30.324 | 0.0079 |
| PB40T | 4.266 | 0.043 | 13.354 | 0.022 | 30.483 | 0.0026 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jozić, D.; Ljubičić, B.; Petrović, A.; Čović, A.; Juradin, S. The Influence of GGBFS as an Additive Replacement on the Kinetics of Cement Hydration and the Mechanical Properties of Cement Mortars. Buildings 2023, 13, 1960. https://doi.org/10.3390/buildings13081960
Jozić D, Ljubičić B, Petrović A, Čović A, Juradin S. The Influence of GGBFS as an Additive Replacement on the Kinetics of Cement Hydration and the Mechanical Properties of Cement Mortars. Buildings. 2023; 13(8):1960. https://doi.org/10.3390/buildings13081960
Chicago/Turabian StyleJozić, Dražan, Branimir Ljubičić, Andrija Petrović, Anđela Čović, and Sandra Juradin. 2023. "The Influence of GGBFS as an Additive Replacement on the Kinetics of Cement Hydration and the Mechanical Properties of Cement Mortars" Buildings 13, no. 8: 1960. https://doi.org/10.3390/buildings13081960
APA StyleJozić, D., Ljubičić, B., Petrović, A., Čović, A., & Juradin, S. (2023). The Influence of GGBFS as an Additive Replacement on the Kinetics of Cement Hydration and the Mechanical Properties of Cement Mortars. Buildings, 13(8), 1960. https://doi.org/10.3390/buildings13081960








