Effect of Graphene Oxide as a Nanomaterial on the Durability Behaviors of Engineered Cementitious Composites by Applying RSM Modelling and Optimization
Abstract
:1. Introduction
2. Research Significance
3. Materials and Methods
3.1. Materials
3.2. Mix Proportions of GO-ECC
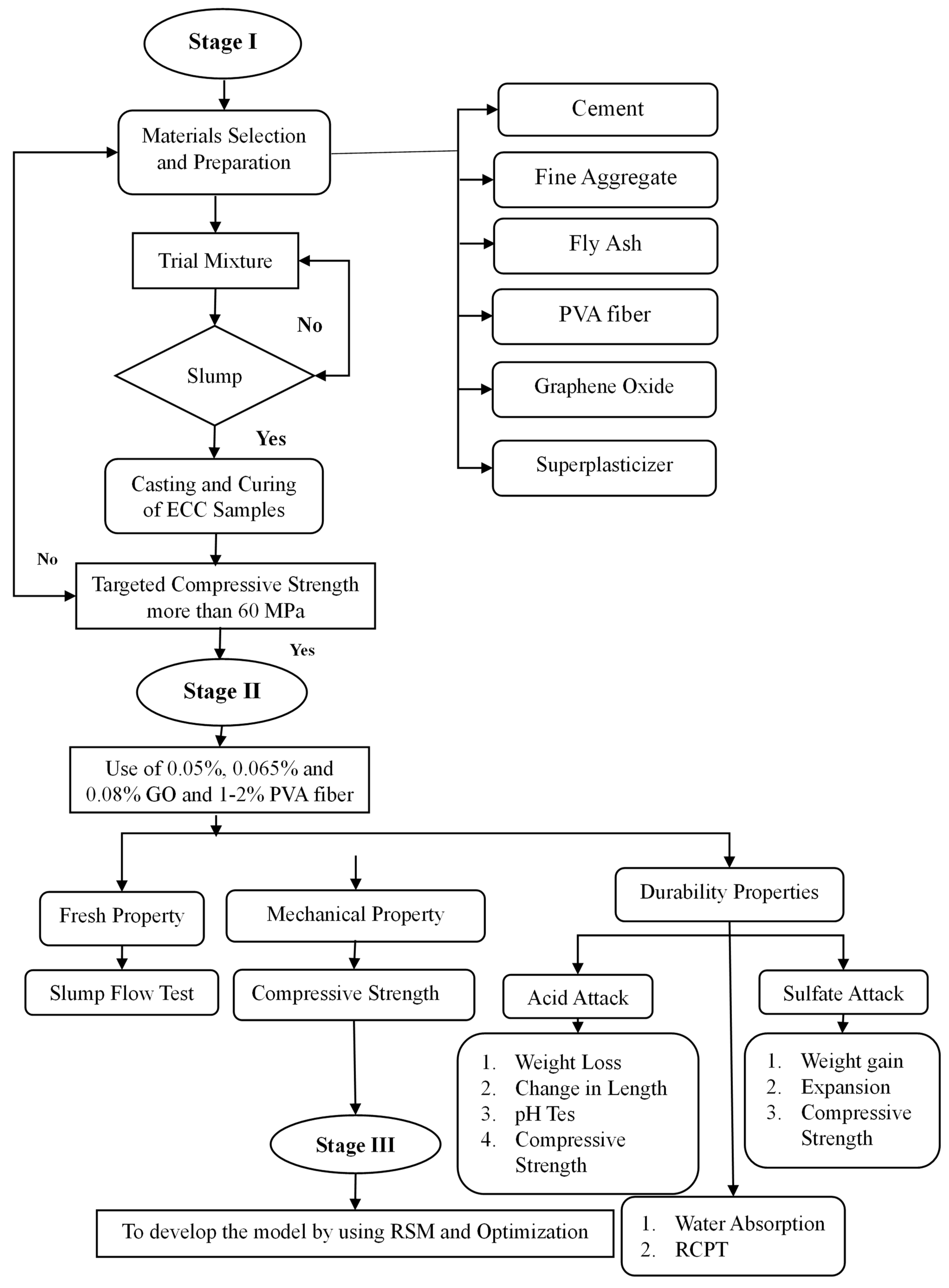
3.3. Sample Preparation and Testing Methods
4. Results and Discussion
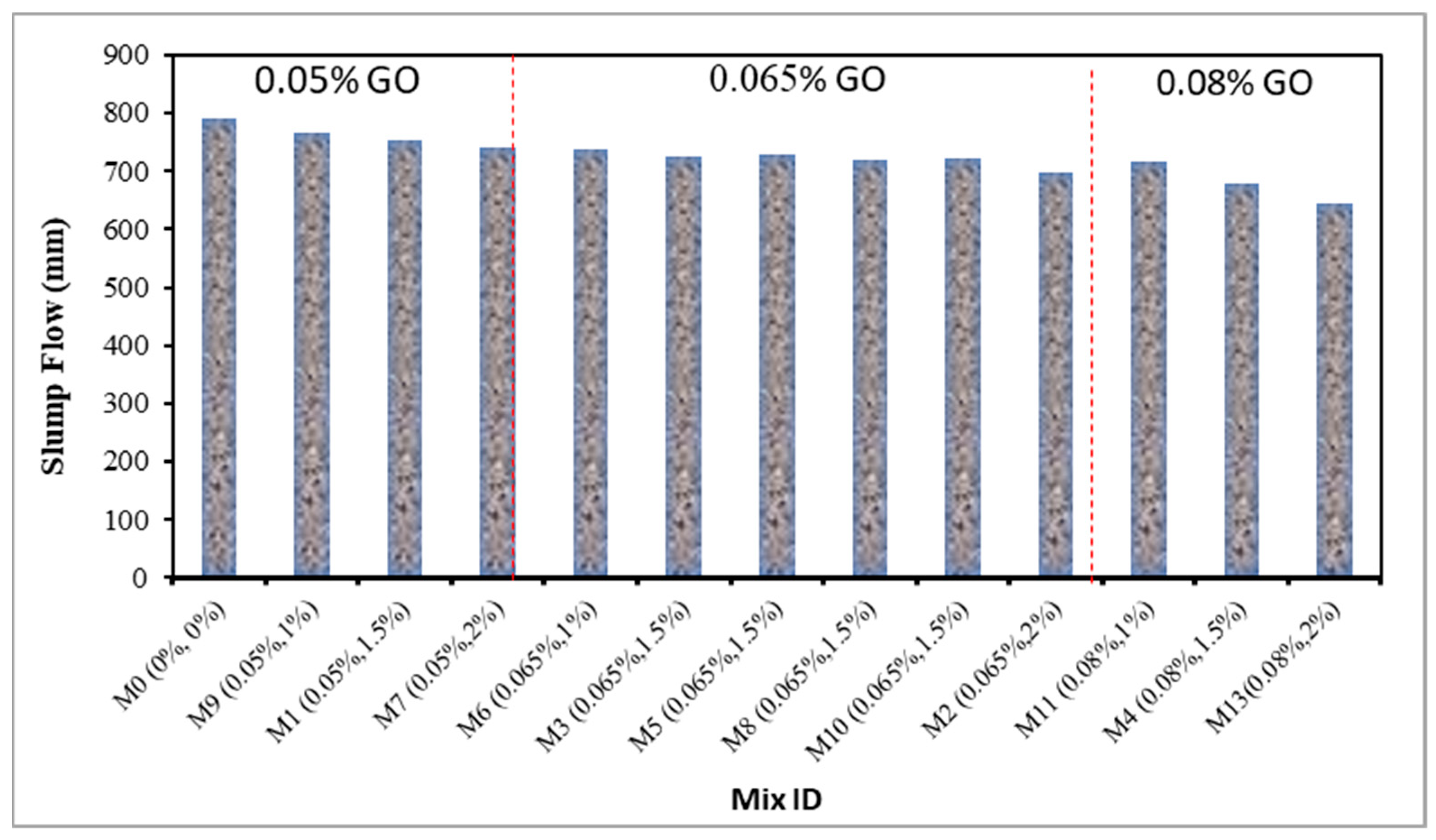
4.1. Slump Flow of Fresh GO-ECC
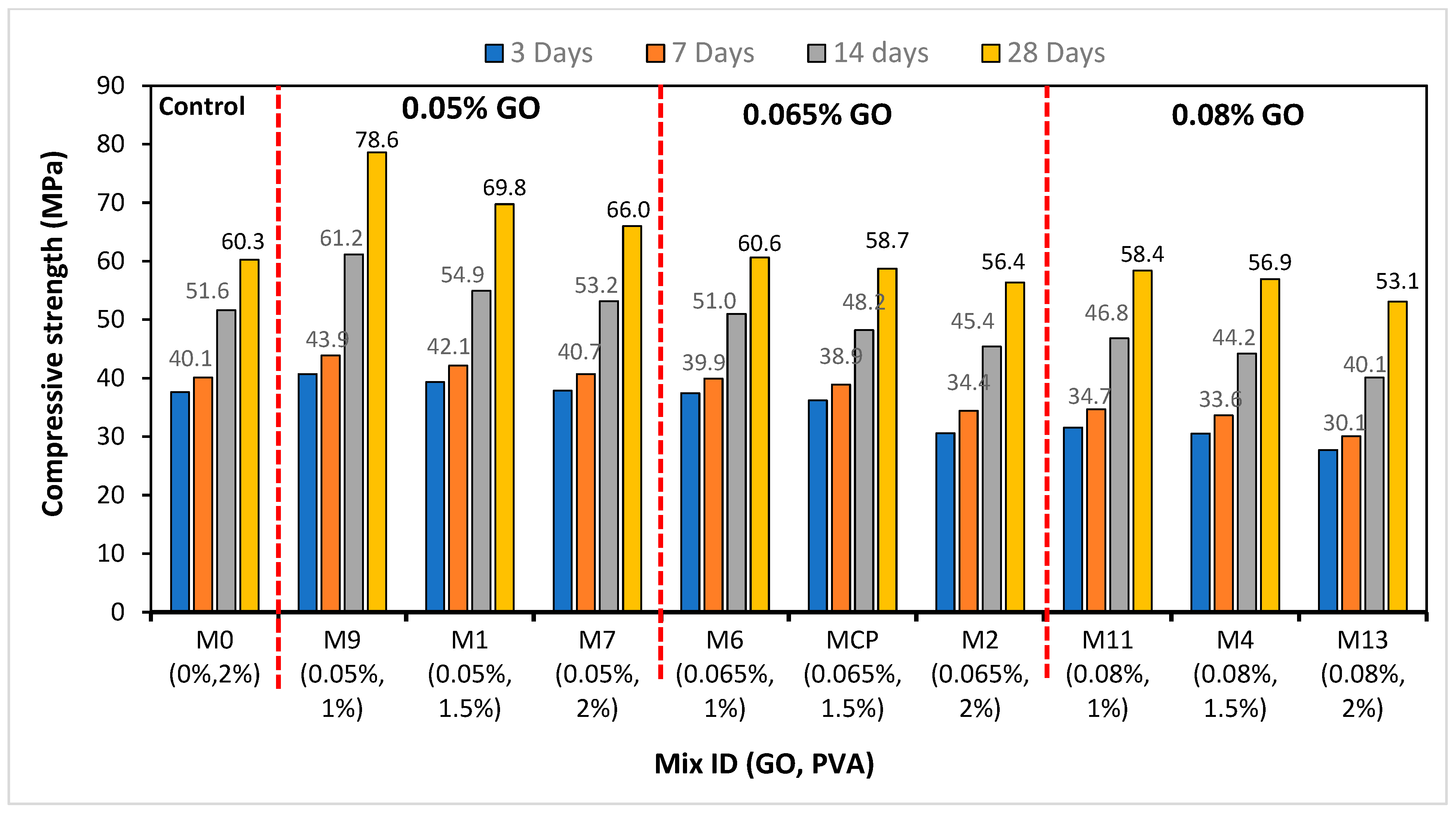
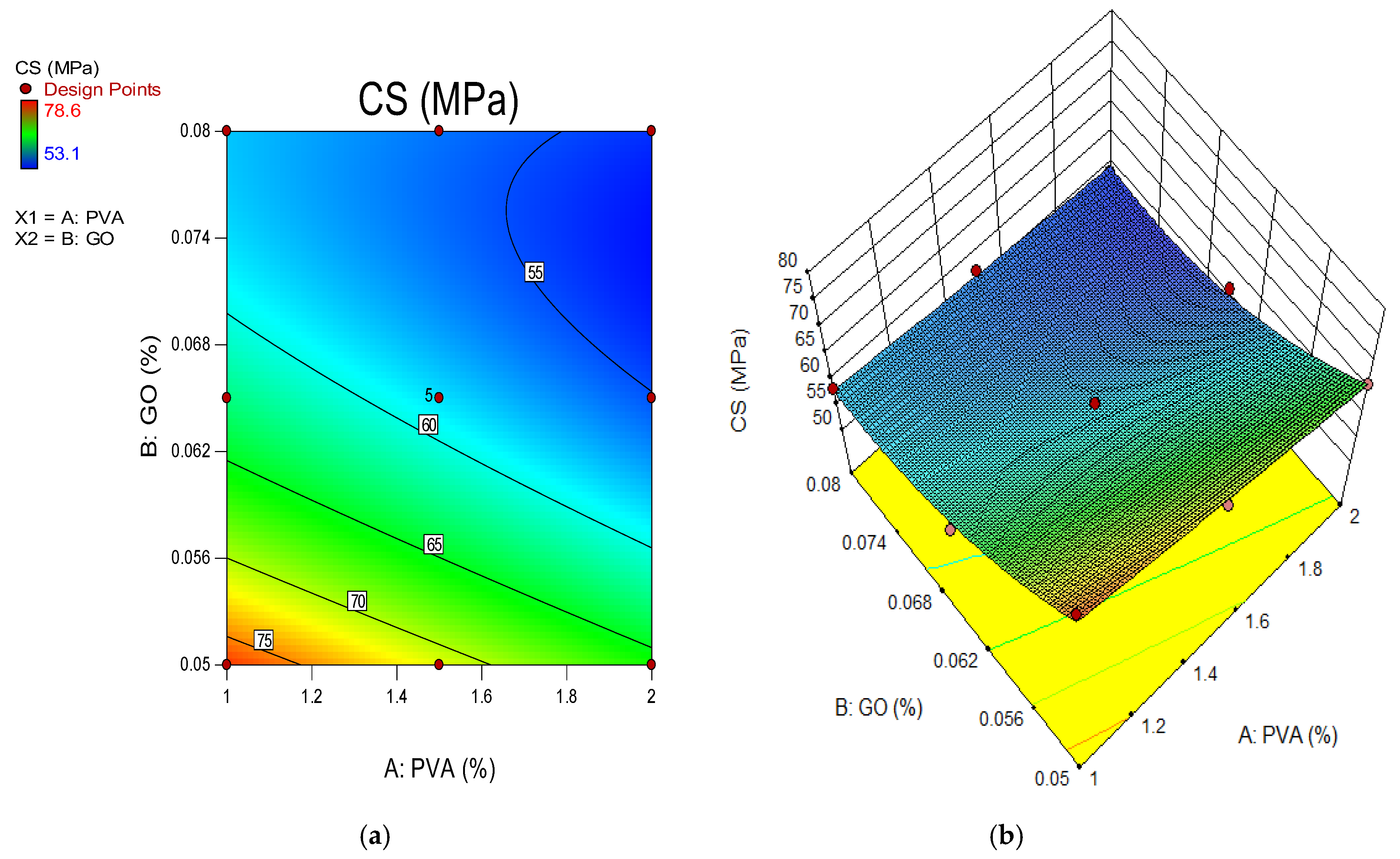
4.2. Compressive Strength of GO-ECC
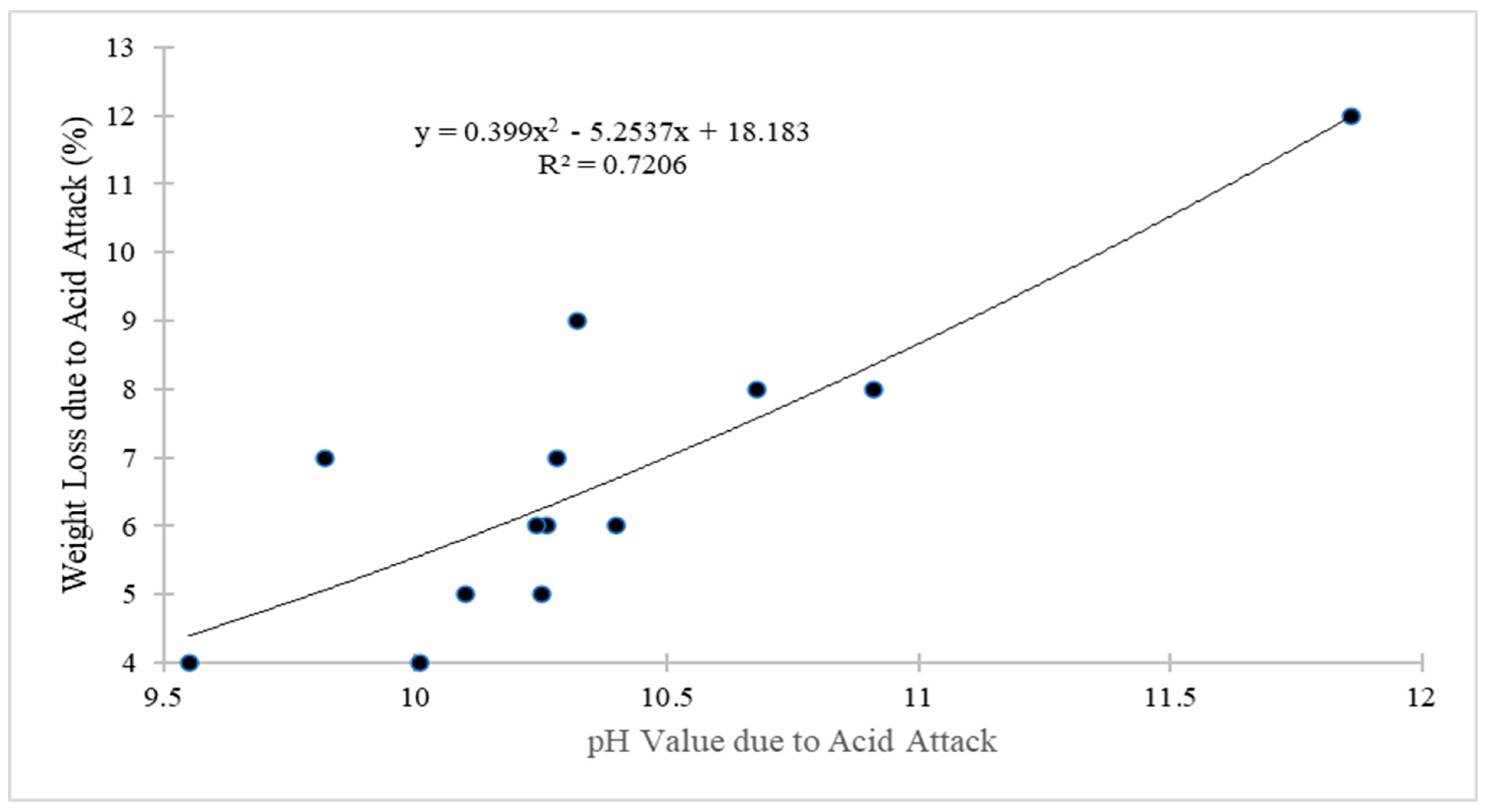
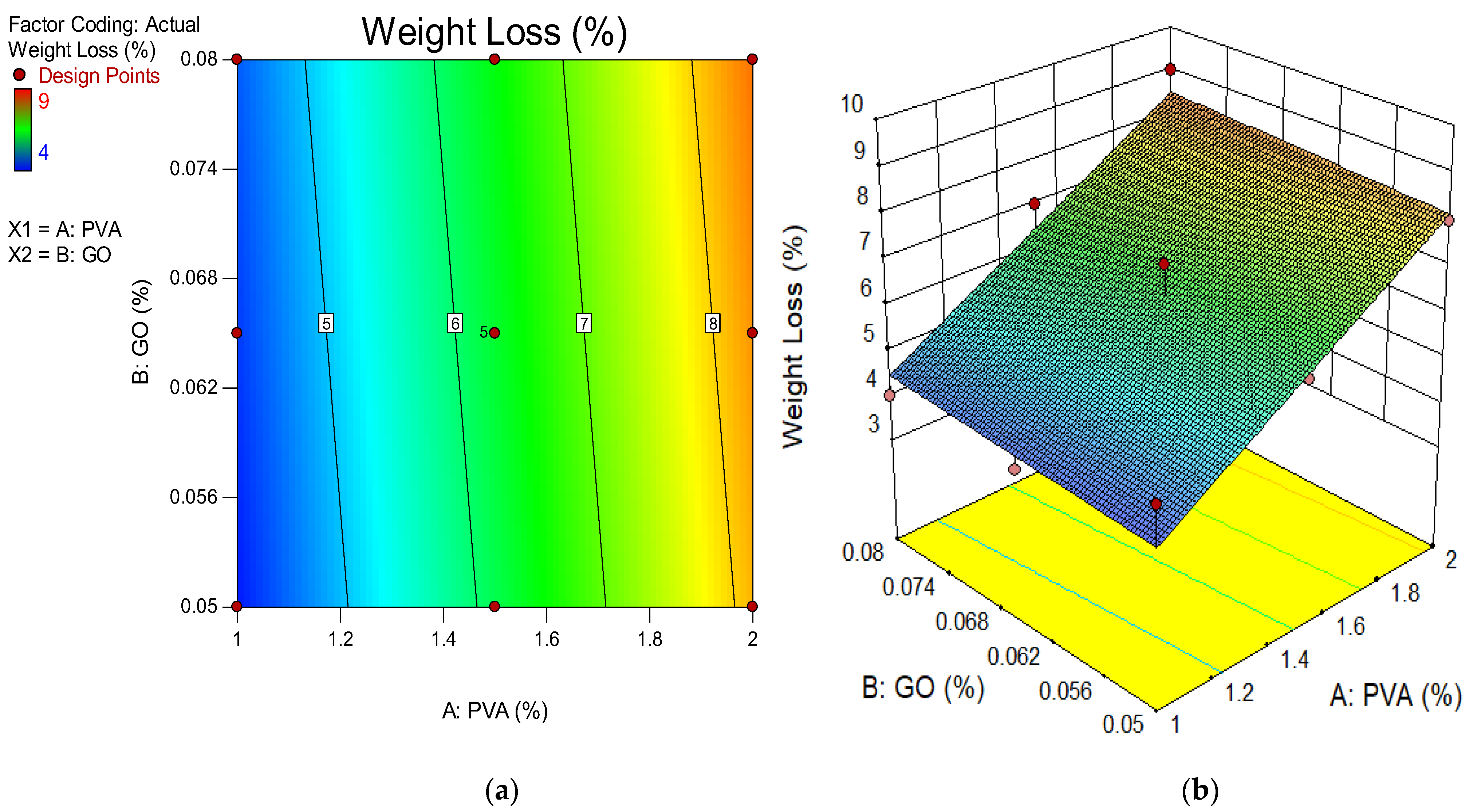
4.3. Weight Loss Due Acid Attack
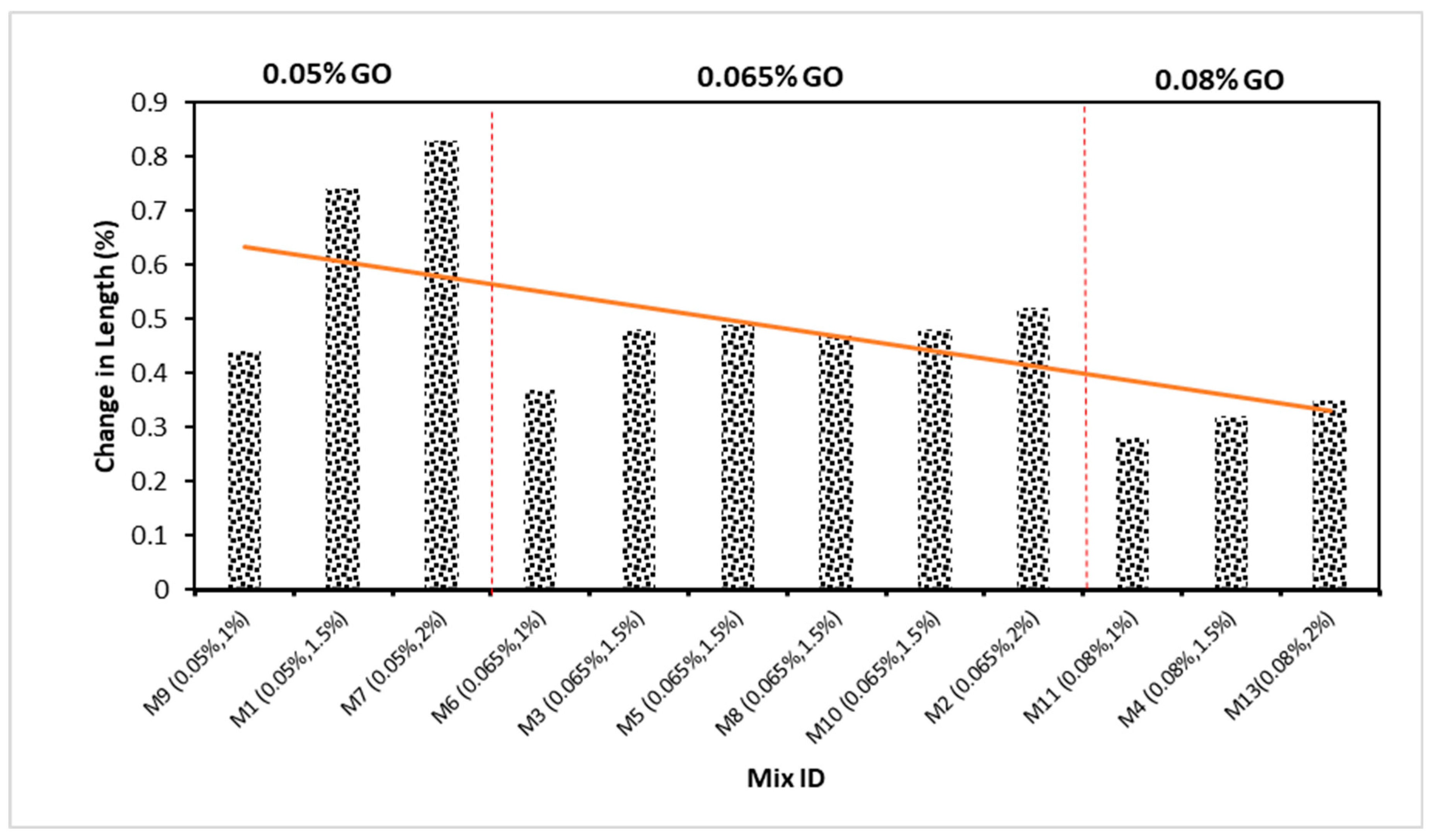
4.4. Change in Length Due to Acid Attack
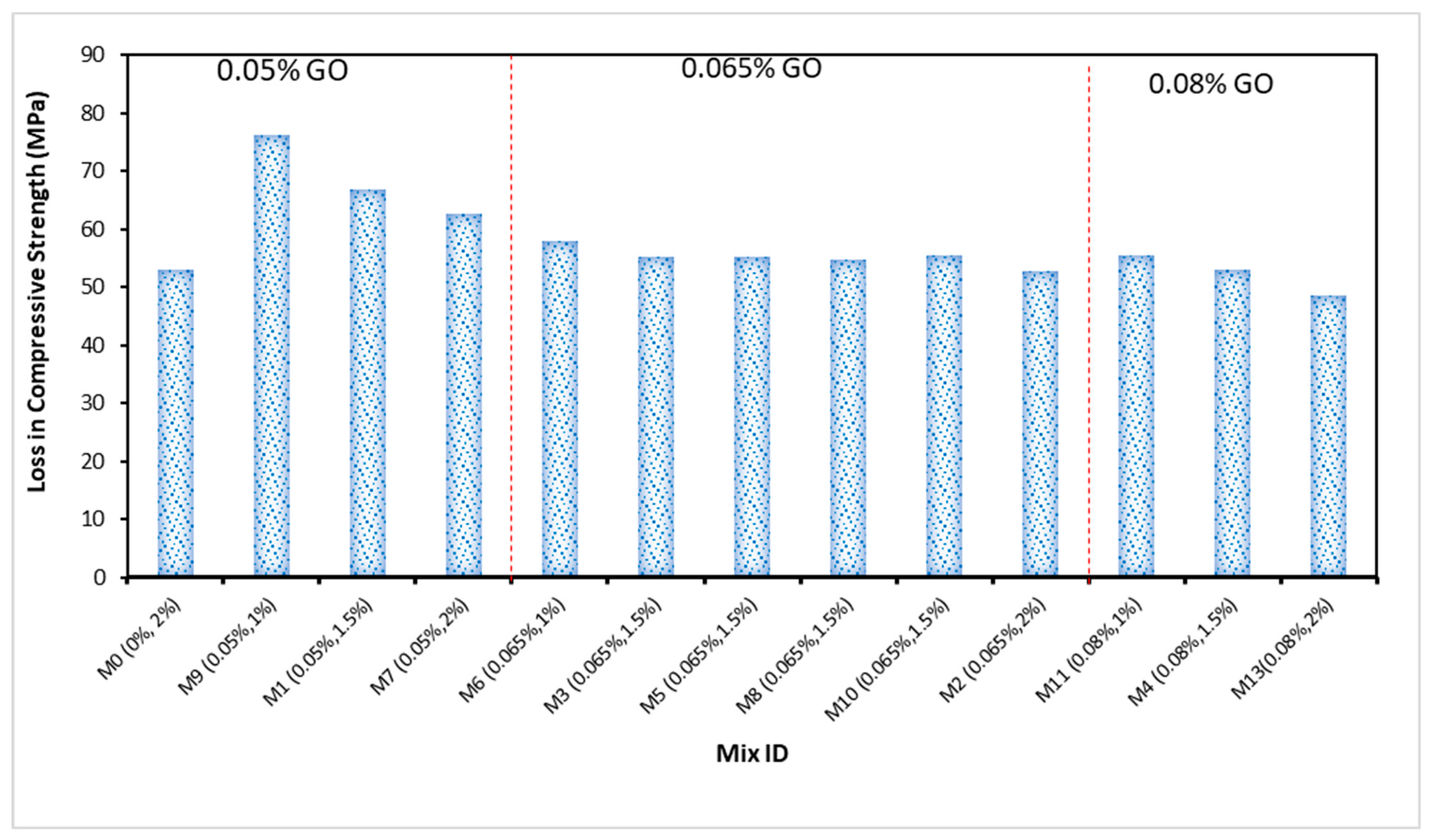
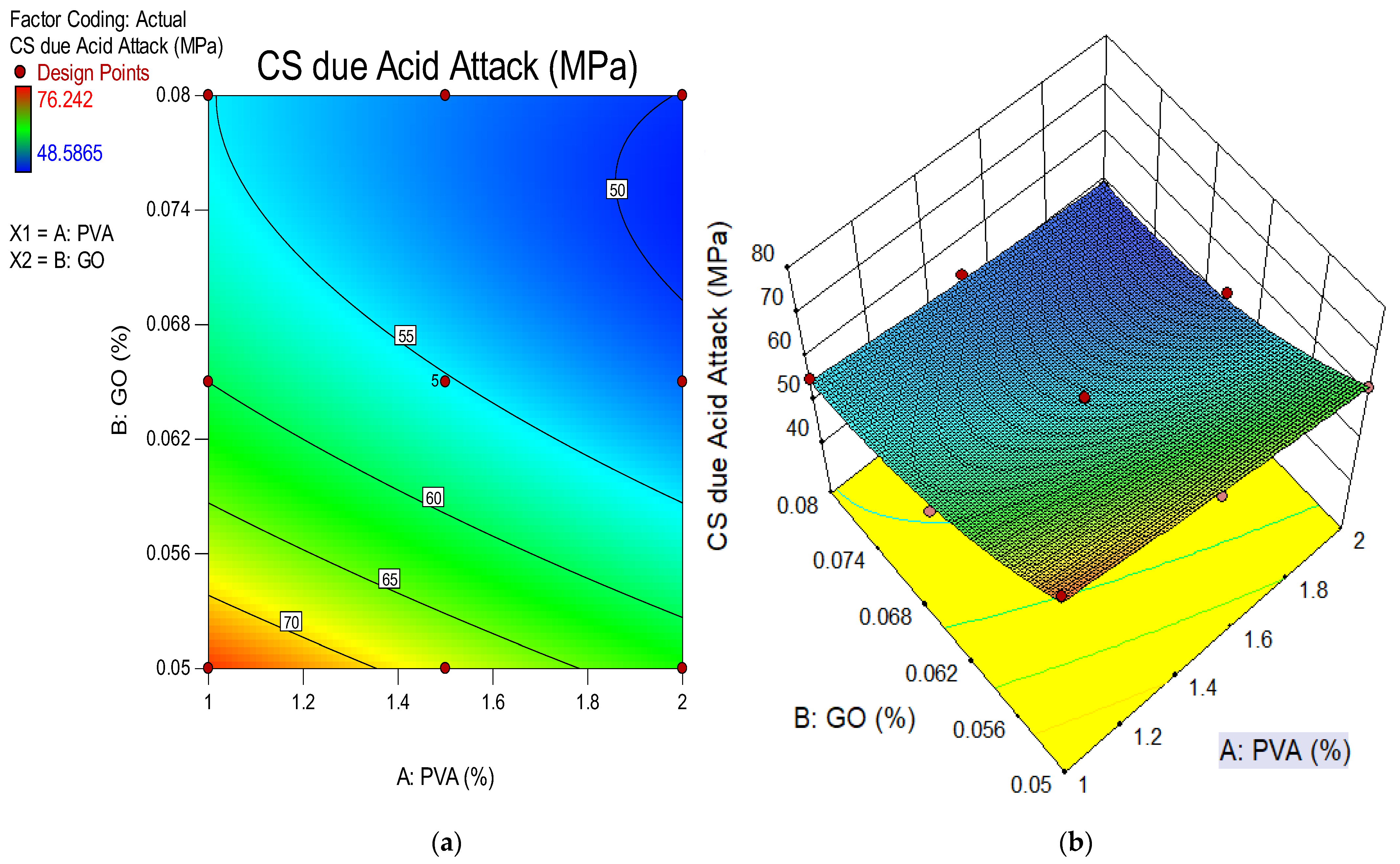
4.5. Compressive Strength Due to Acid Attack
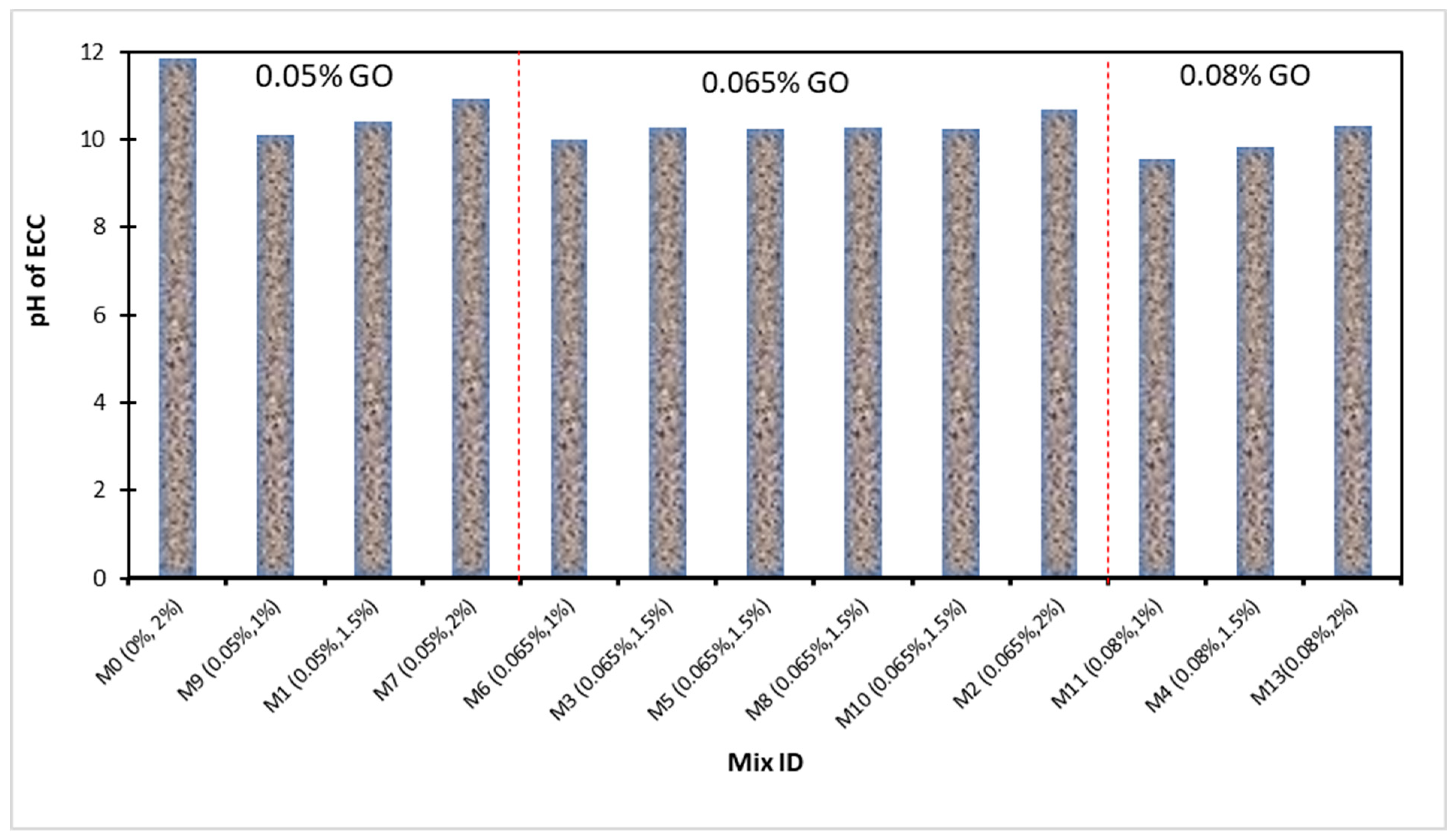
4.6. pH Test
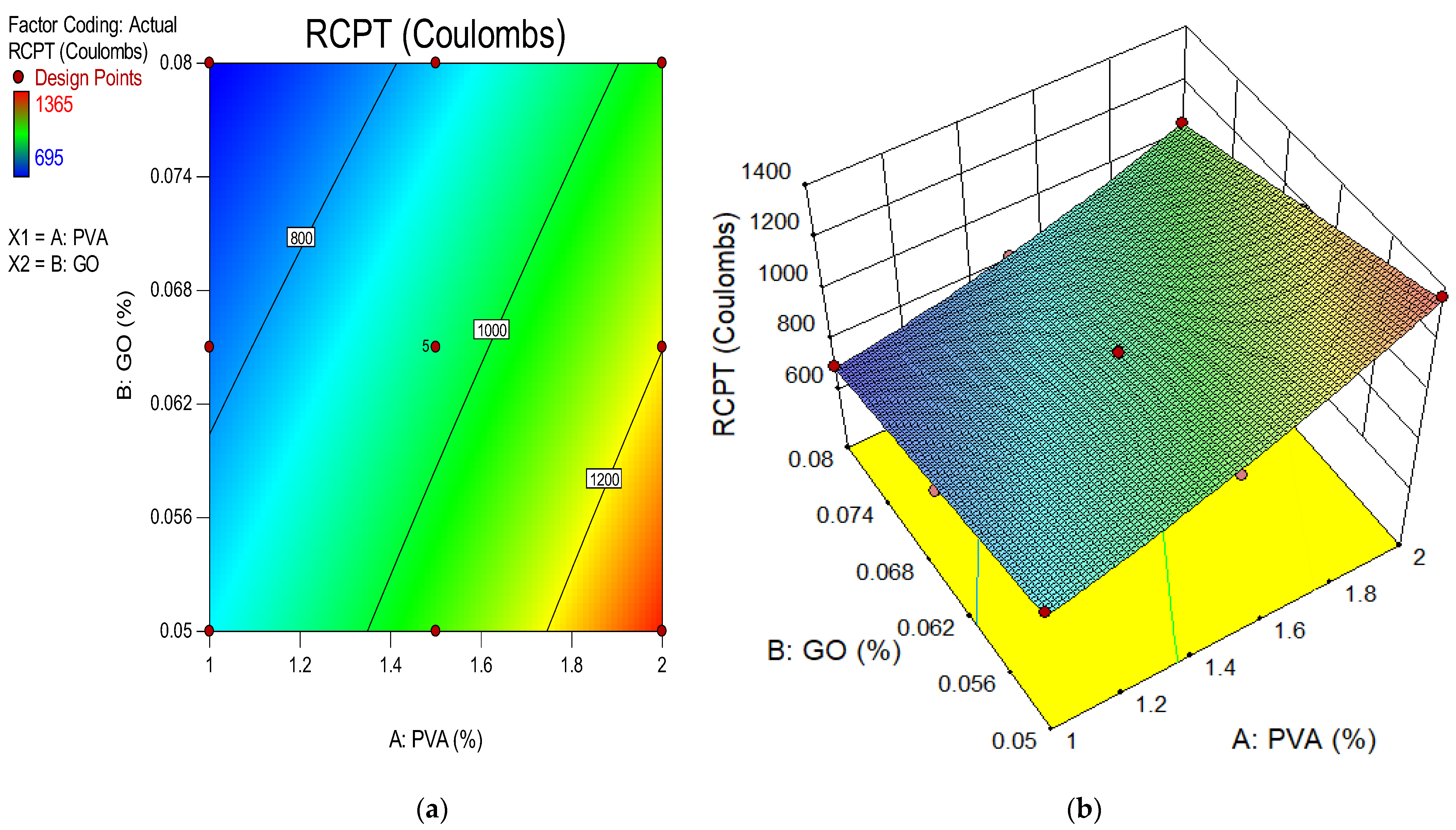
4.7. Rapid Chloride Penetration Test (RCPT)
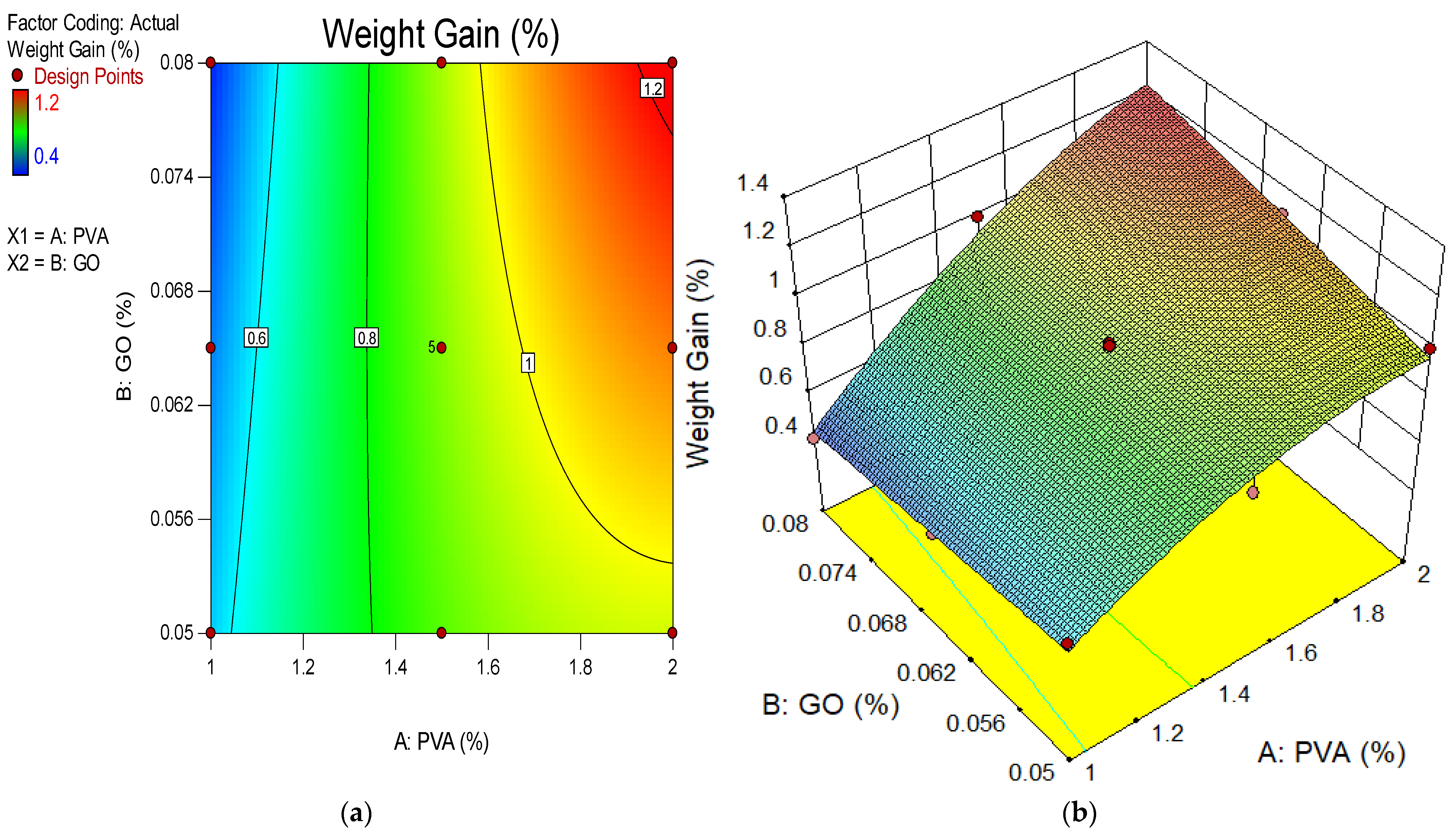
4.8. Weight Gain Due to Sulphate Attack
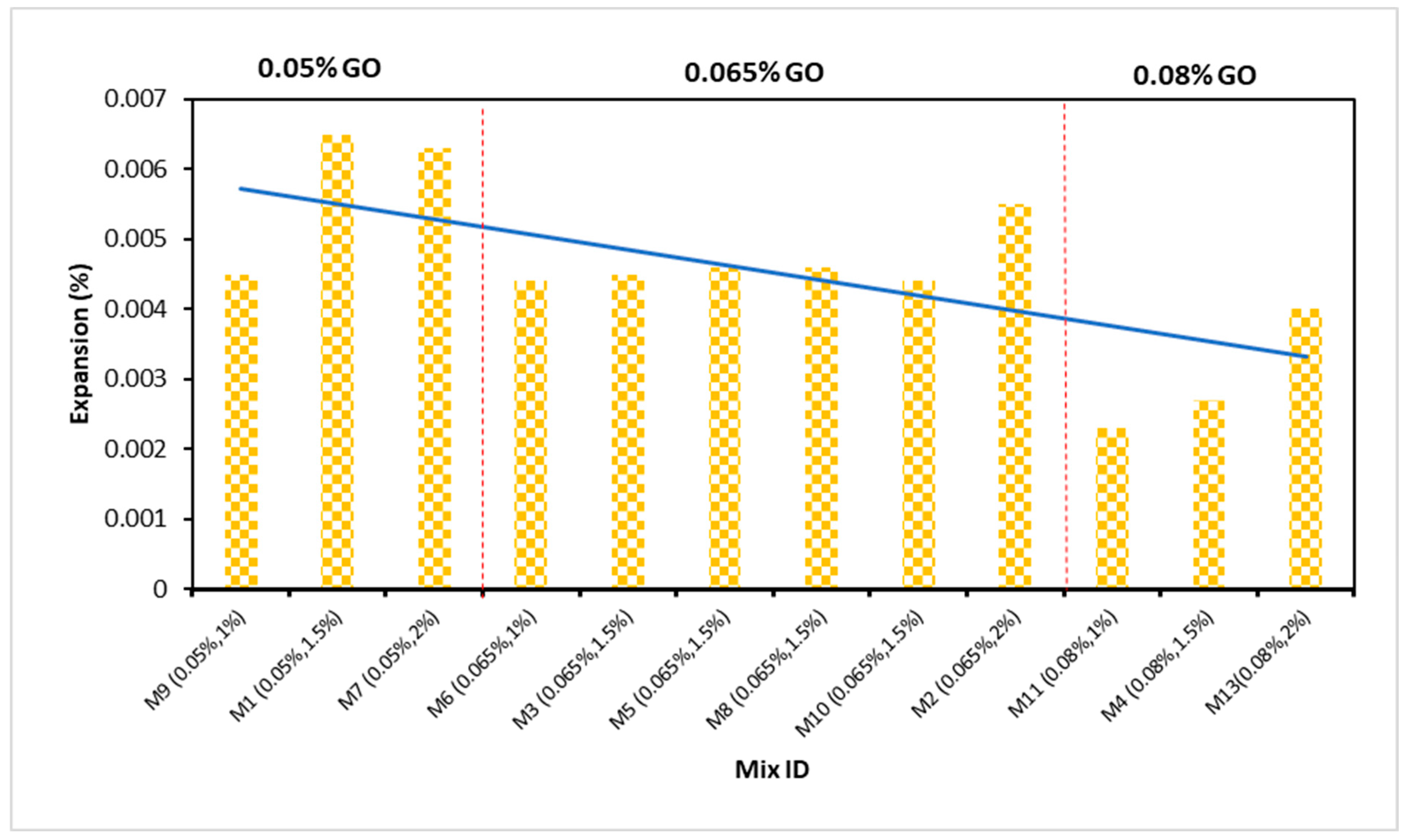
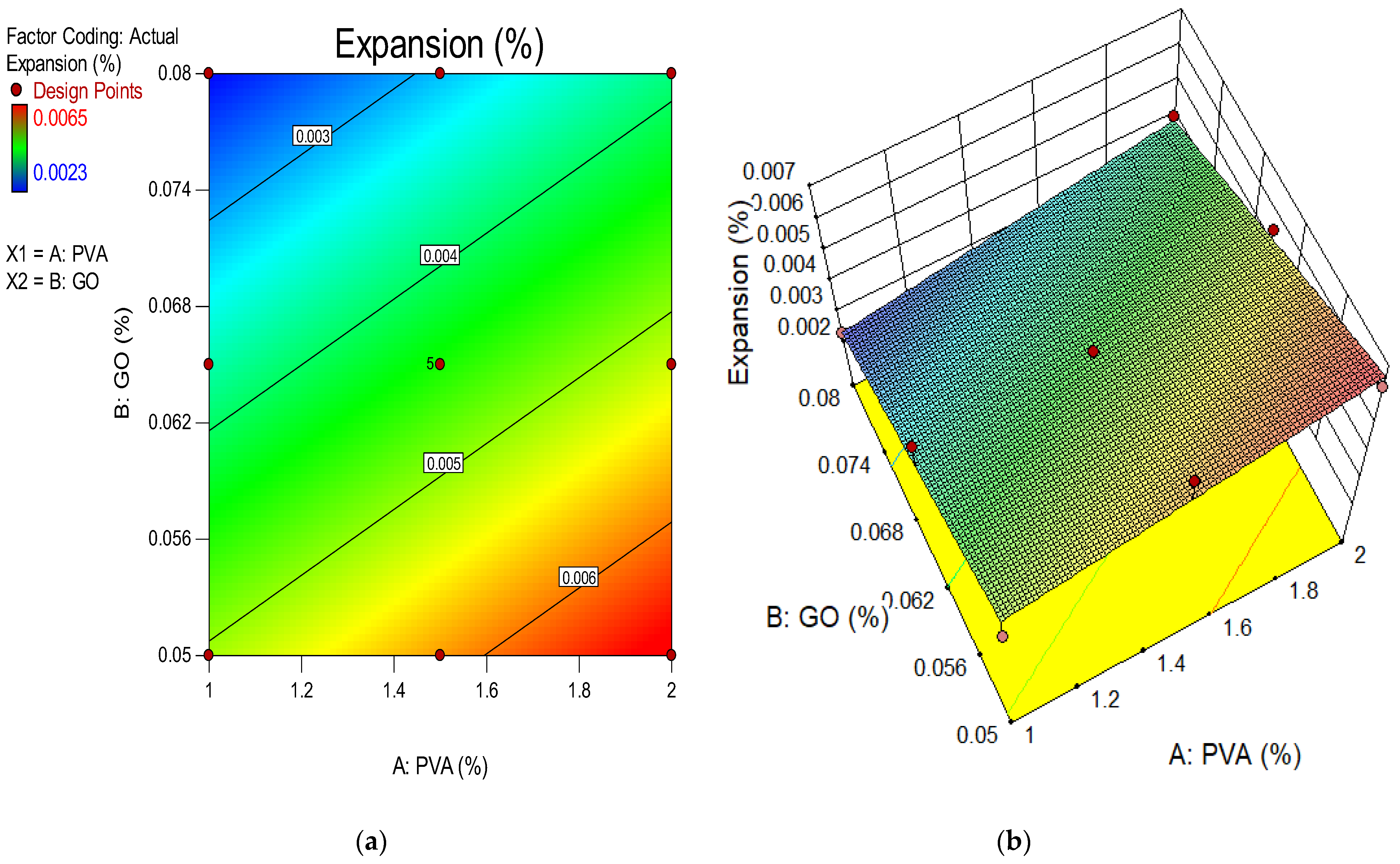
4.9. Expansion Due to Sulphate Attack
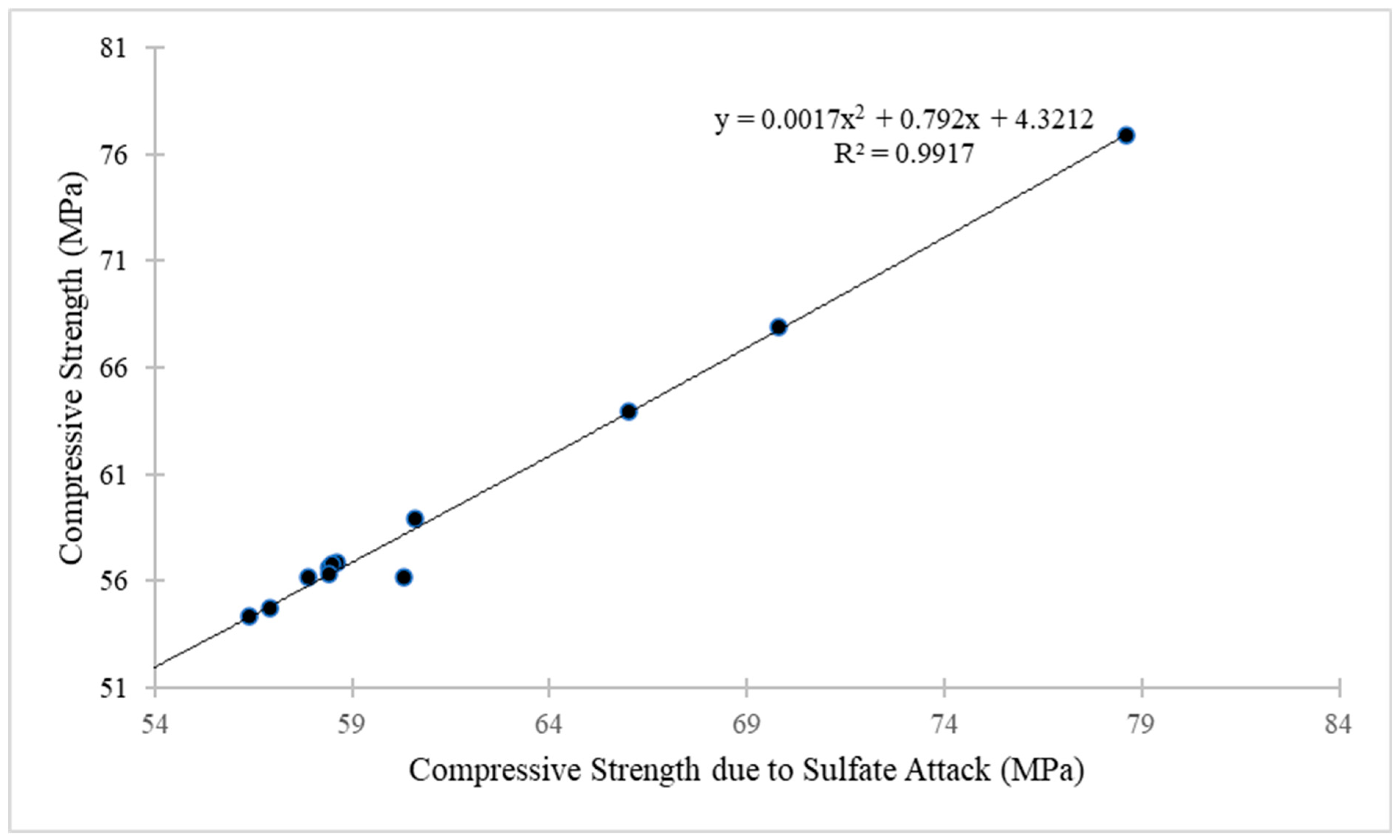
4.10. Compressive Strength of GO-ECC Due to Sulphate Attack
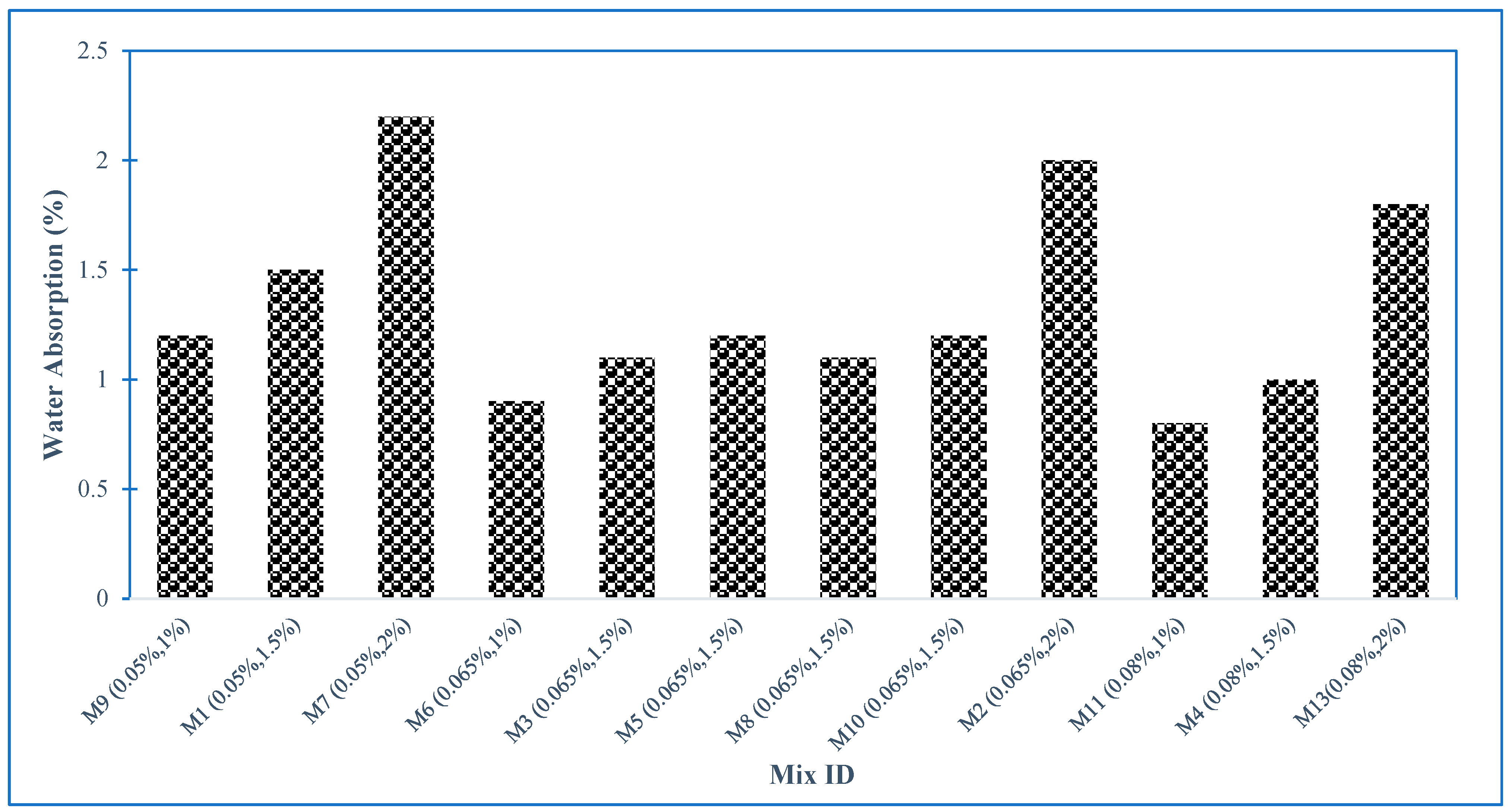
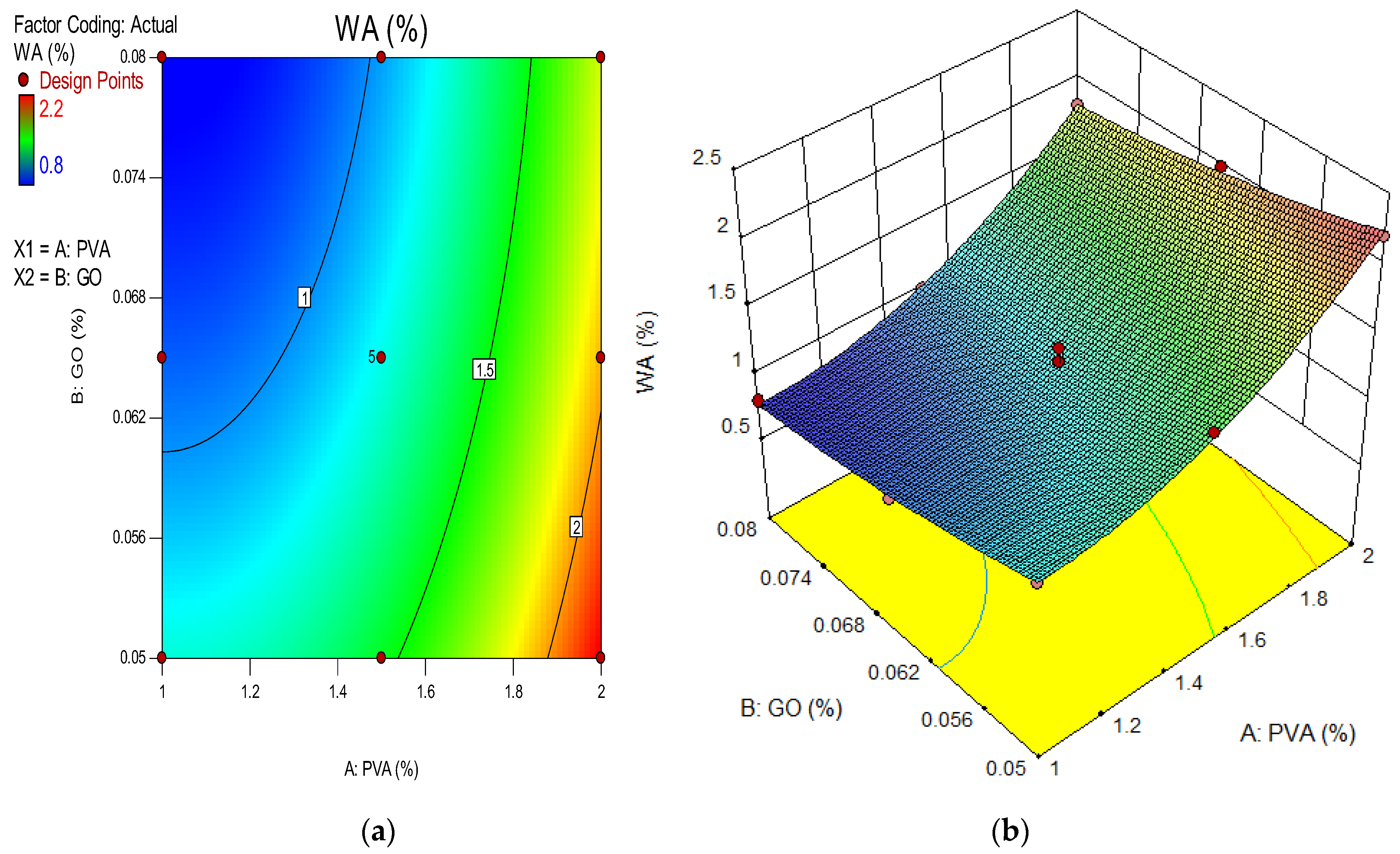
4.11. Water Absorption of GO-ECC
5. Predictive Model Developments Using Response Surface Methodology (RSM) and Optimization
5.1. Analysis of Variance (ANOVA)
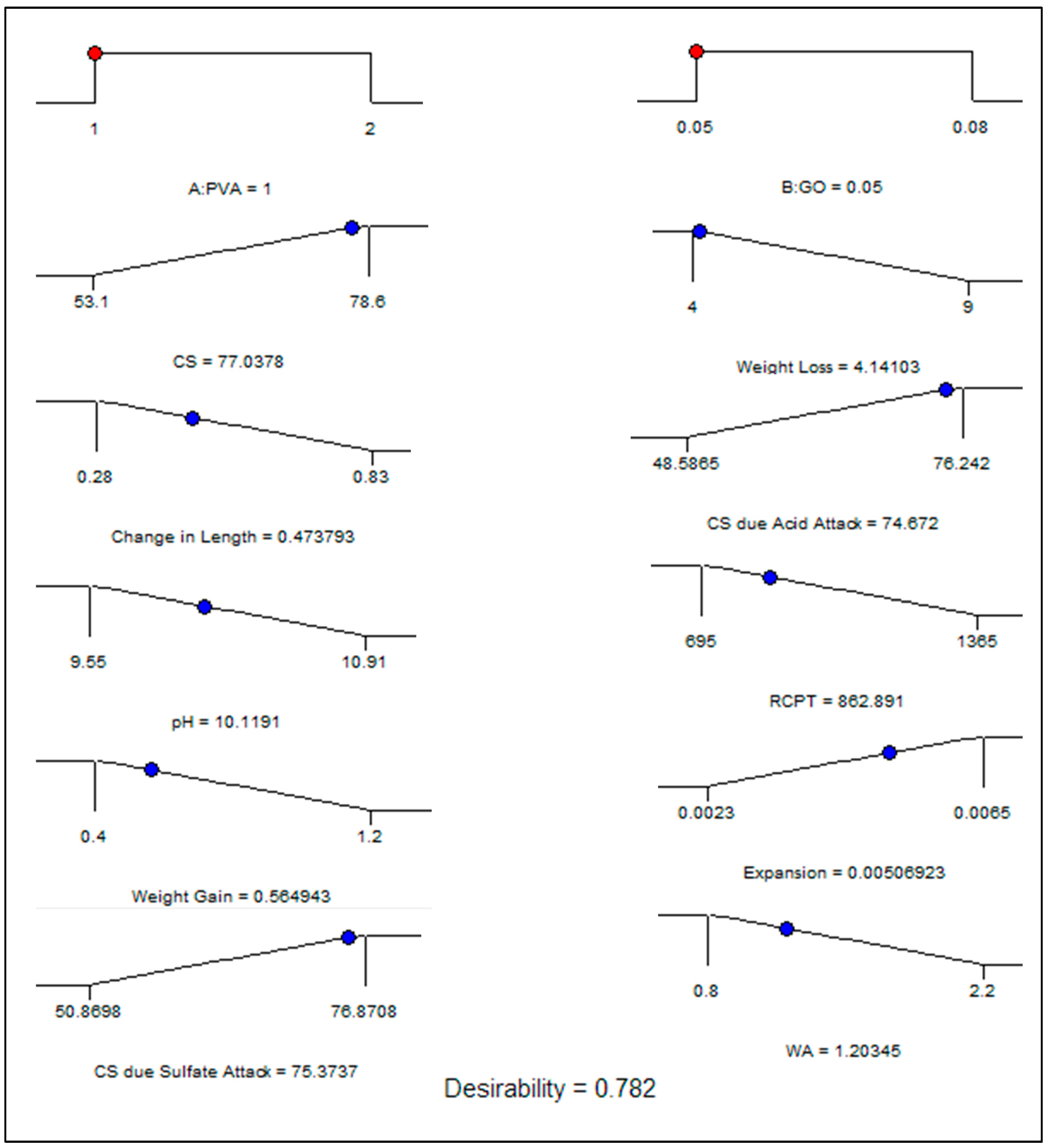
5.2. Optimization
6. Conclusions
- The optimum slump flow is recorded as 792 mm at control mixture while the minimum slump flow was 645 mm at 0.08% of GO along with 25 of the PVA fiber in the ECC. The slump flow of fresh ECC mixture was reduced as the concentration of the GO as a nanoscale particle increased;
- The optimum compressive strength of the ECC accumulation with 1% of PVA fiber was shown to be 0.05% of GO while the minimum strength of the ECC accumulation with 1% of PVA as fiber was recorded as 0.08% of GO as a nanoparticle in ECC. This indicates that the addition of 0.05% GO by the weight of PC in the ECC provides the best strength, and with additional accumulations of GO in ECC, the strength starts to reduce in every curing period;
- The weight loss and change in length of ECC were shown to decrease when the concentration of GO as a nanoscale particle increased for 28 days. The finding showed that the accumulation of more GO in ECC resulted in more durable ECC. By assessing mixtures with the same 1% PVA concentration but differing GO levels, the higher GO level generated superior weight loss outcomes. The GO in the mixture also helped to improve the ECC’s durability whenever exposed to extreme conditions such as acidic environments;
- The maximum pH value was recorded as 11.86 in the control mixture, while the minimum pH value was noted as 9.55 at 0.08% of GO as a nanoscale particle, along with 1% of PVA fiber in ECC. The use of PVA fibers in the production of ECC increased the pH value, but the all-pH values were lower than that of the control mixture. When the quantity of GO increased, the pH value decreased;
- The weight gain and expansion of ECC due to sulfate attack was reduced when the concentration of GO as a nanomaterial increased in the ECC. The resistance impact response to a sulfate attack is attributable to the pozzolanic properties and filling influence of GO as nanoparticles, which occupy the pore spaces and further prevent sulfate ion infiltration. Their depleting efficiency may be due to the aggregation of nanomaterials at high concentrations;
- The water absorption of the ECC mixture was reduced when the concentration of GO as nanomaterials increased in the ECC mixture, after 28 days. This reduction in water absorption is due to the fineness of GO particles which seals the micropores left by other components of the ECC mixture;
- The optimum RCPT was observed to be 1650 Coulombs for the control mixture while the minimum RCPT was shown to be 695 Coulombs at 0.08% of GO along with 1% of PVA fiber in ECC for 28 days. Higher resistance against chloride ion penetration was achieved with the use of GO as nanoparticles. The chloride permeability values reduced with the addition of GO as nanoparticles. All mixes were classified as being in the very low chloride-permeability range except for the control mixture, M1, M7, M2 and M13, which were classified in the low permeability group;
- RSM models were designed to predict the compressive strength, water absorption, acid resistance, and sulfate resistance at 28 days, depending on the proportions of the PVA fibers and GO as nanomaterials in the production of ECC. Each model adjusted R-squared to the predicted R-squared difference was less than 0.2, and had at least a 95% confidence level.
7. Limitations
- Developing a suitable mixing strategy for efficient GO dispersion in large-scale field applications might result in high GO sheet doses and unsatisfactory performance;
- The usage of GO as a nanoscale particle in the construction industry is problematic due to its significantly higher cost when compared to conventional concrete.
8. Future Recommendations:
- The characteristics of the mixtures developed for this research were evaluated after 28 days of curing. Therefore, it is important to research how GO affects the long-term durability behavior and time-dependent deformations such as fatigue and creep behavior of ECC;
- It is important to look at how GO affects concrete and the steel reinforced ECC’s structural performance;
- Additionally, it is possible to conduct research on the chemical connections and behavior of GO mixed with other additives, including millet husk ash, wheat straw ash, silica fume, and other nanoparticles;
- The utilization of 0.05% GO as a nanoparticle in an ECC mixture reinforced with 1% of PVA fiber by volume fraction has the potential to reduce the difficulties associated with the large-scale application of ECC as well as the harmful impacts of sulfate attacks and acid attacks. Therefore, it is recommended for practical applications in the construction industry.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ECC | Engineered Cementitious Composites |
| GO | Graphene Oxide |
| RSM | Response Surface Methodology |
| CCD | Central composite design |
| PVA Fiber | Polyvinyl Alcohol fiber |
| HPFRCC | of high-performance fiber-reinforced cementitious composite |
| MPa | Mega Pascal |
| PC | Portland cement |
| FG | functional groups |
| FA | Fly Ash |
| XRF | X-ray fluorescence spectroscopy |
| SP | Superplasticizer |
| RCPT | rapid chloride permeability test |
| H2SO4 | sulfuric acid |
| Na2SO4 | sodium sulphate |
| NaCl | sodium chloride |
| NaOH | sodium hydroxide |
| DC | Direct Current |
| MK | Metakaolin |
| C-S-H | calcium-silicate-hydrates |
| ASTM | American Society for Testing and Materials |
| BS | British Standard |
| DOE | Design of Experiment |
| FCCD | face-cantered central composite design |
| CS | Compressive Strength |
| ANOVA | Analysis of variance |
| WA | Water Absorption |
References
- An, C.; Duan, M.; Toledo Filho, R.D.; Estefen, S.F. Collapse of sandwich pipes with PVA fiber reinforced cementitious composites core under external pressure. Ocean Eng. 2014, 82, 1–13. [Google Scholar] [CrossRef]
- Sahmaran, M.; Li, M.; Li, V.C. Transport Properties of Engineered Cementitious Composites under Chloride Exposure. ACI Mater. J. 2007, 104, 604–611. [Google Scholar]
- Khed, V.C.; Mohammed, B.S.; Liew, M.S.; Zawawi, N.A.W.A. Development of response surface models for self-compacting hybrid fibre reinforced rubberized cementitious composite. Constr. Build. Mater. 2020, 232, 117191. [Google Scholar] [CrossRef]
- Mohammed, B.S.; Khed, V.C.; Liew, M.S. Optimization of hybrid fibres in engineered cementitious composites. Constr. Build. Mater. 2018, 190, 24–37. [Google Scholar] [CrossRef]
- Zhang, Z.; Ma, H.; Qian, S. Investigation on Properties of ECC Incorporating Crumb Rubber of Different Sizes. J. Adv. Concr. Technol. 2015, 13, 241–251. [Google Scholar] [CrossRef]
- Singh, M.; Saini, B.; Chalak, H. Performance and composition analysis of engineered cementitious composite (ECC)—A review. J. Build. Eng. 2019, 26, 100851. [Google Scholar] [CrossRef]
- Wille, K.; Naaman, A.E.; El-Tawil, S.; Parra-Montesinos, G.J. Ultra-high performance concrete and fiber reinforced concrete: Achieving strength and ductility without heat curing. Mater. Struct. Constr. 2012, 45, 309–324. [Google Scholar] [CrossRef]
- Li, V.C. Engineered Cementitious Composite (Ecc): Material, Structural, and Durability Performance, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2008; pp. 1001–1048. [Google Scholar]
- Zheng, Q.; Han, B.; Cui, X.; Yu, X.; Ou, J. Graphene-Engineered Cementitious Composites: Small Makes a Big Impact. Nanomater. Nanotechnol. 2017, 7, 1847980417742304. [Google Scholar] [CrossRef]
- Bassuoni, M.; Nehdi, M. Durability of self-consolidating concrete to sulfate attack under combined cyclic environments and flexural loading. Cem. Concr. Res. 2009, 39, 206–226. [Google Scholar] [CrossRef]
- Grengg, C.; Mittermayr, F.; Baldermann, A.; Böttcher, M.; Leis, A.; Koraimann, G.; Grunert, P.; Dietzel, M. Microbiologically induced concrete corrosion: A case study from a combined sewer network. Cem. Concr. Res. 2015, 77, 16–25. [Google Scholar] [CrossRef]
- Diab, A.M.; Elyamany, H.E.; Abd Elmoaty, A.E.M.; Shalan, A.H. Prediction of concrete compressive strength due to long term sulfate attack using neural network. Alex. Eng. J. 2014, 53, 627–642. [Google Scholar] [CrossRef]
- Arel, H.Ş.; Thomas, B.S. The effects of nano- and micro-particle additives on the durability and mechanical properties of mortars exposed to internal and external sulfate attacks. Results Phys. 2017, 7, 843–851. [Google Scholar] [CrossRef]
- Mehta, P. Mechanism of sulfate attack on portland cement concrete—Another look. Cem. Concr. Res. 1983, 13, 401–406. [Google Scholar] [CrossRef]
- Tchamba, A.; Sofack, J.; Yongue, R.; Melo, U. Formulation of calcium dialuminate (CaO·2Al2O3) refractory cement from local bauxite. J. Asian Ceram. Soc. 2015, 3, 164–172. [Google Scholar] [CrossRef]
- Harrison, W.H. Durability of Concrete in Acidic Soils and Waters. Concrete 1987, 21, 18–24. [Google Scholar]
- Soutsos, M. Concrete Durability: A Practical Guide to the Design of Durable Concrete Structures; Thomas Telford Ltd.: London, UK, 2009; p. 314. [Google Scholar]
- Gaze, M.; Crammond, N. The formation of thaumasite in a cement: Lime: Sand mortar exposed to cold magnesium and potassium sulfate solutions. Cem. Concr. Compos. 2000, 22, 209–222. [Google Scholar] [CrossRef]
- Stanton, T.E. Expansion of Concrete through Reaction between Cement and Aggregate. Trans. Am. Soc. Civ. Eng. 1942, 107, 54–84. [Google Scholar] [CrossRef]
- Diab, A.M.; Elyamany, H.E.; Elmoaty, A.E.M.A.; Sreh, M.M. Effect of nanomaterials additives on performance of concrete resistance against magnesium sulfate and acids. Constr. Build. Mater. 2019, 210, 210–231. [Google Scholar] [CrossRef]
- Diab, A.M.; Awad, A.E.M.; Elyamany, H.E.; Elmoaty, A.E.M.A. Guidelines in compressive strength assessment of concrete modified with silica fume due to magnesium sulfate attack. Constr. Build. Mater. 2012, 36, 311–318. [Google Scholar] [CrossRef]
- Yang, D.-Y.; Luo, J.-J. The damage of concrete under flexural loading and salt solution. Constr. Build. Mater. 2012, 36, 129–134. [Google Scholar] [CrossRef]
- Essawy, A.A.; El Aleem, S.A. Physico-mechanical properties, potent adsorptive and photocatalytic efficacies of sulfate resisting cement blends containing micro silica and nano-TiO2. Constr. Build. Mater. 2014, 52, 1–8. [Google Scholar] [CrossRef]
- Atahan, H.N.; Arslan, K.M. Improved durability of cement mortars exposed to external sulfate attack: The role of nano & micro additives. Sustain. Cities Soc. 2016, 22, 40–48. [Google Scholar] [CrossRef]
- Pavlík, V.; Unčík, S. The rate of corrosion of hardened cement pastes and mortars with additive of silica fume in acids. Cem. Concr. Res. 1997, 27, 1731–1745. [Google Scholar] [CrossRef]
- Dreyer, D.R.; Park, S.; Bielawski, C.W.; Ruoff, R.S. The chemistry of graphene oxide. Chem. Soc. Rev. 2010, 39, 228–240. [Google Scholar] [CrossRef]
- Compton, O.C.; Nguyen, S. Graphene Oxide, Highly Reduced Graphene Oxide, and Graphene: Versatile Building Blocks for Carbon-Based Materials. Small 2010, 6, 711–723. [Google Scholar] [CrossRef]
- McAllister, M.J.; Li, J.-L.; Adamson, D.H.; Schniepp, H.C.; Abdala, A.A.; Liu, J.; Herrera-Alonso, M.; Milius, D.L.; Car, R.; Prud’Homme, R.K.; et al. Single Sheet Functionalized Graphene by Oxidation and Thermal Expansion of Graphite. Chem. Mater. 2007, 19, 4396–4404. [Google Scholar] [CrossRef]
- Yang, H.; Cui, H.; Tang, W.; Li, Z.; Han, N.; Xing, F. A critical review on research progress of graphene/cement based composites. Compos. Part A Appl. Sci. Manuf. 2017, 102, 273–296. [Google Scholar] [CrossRef]
- Han, B.; Sun, S.; Ding, S.; Zhang, L.; Yu, X.; Ou, J. Review of nanocarbon-engineered multifunctional cementitious composites. Compos. Part A Appl. Sci. Manuf. 2015, 70, 69–81. [Google Scholar] [CrossRef]
- Sharma, S.; Kothiyal, N.C. Influence of graphene oxide as dispersed phase in cement mortar matrix in defining the crystal patterns of cement hydrates and its effect on mechanical, microstructural and crystallization properties. RSC Adv. 2015, 5, 52642–52657. [Google Scholar] [CrossRef]
- Tong, T.; Fan, Z.; Liu, Q.; Wang, S.; Tan, S.; Yu, Q. Investigation of the effects of graphene and graphene oxide nanoplatelets on the micro- and macro-properties of cementitious materials. Constr. Build. Mater. 2016, 106, 102–114. [Google Scholar] [CrossRef]
- Al-Dahawi, A.; Yıldırım, G.; Öztürk, O.; Şahmaran, M. Assessment of self-sensing capability of Engineered Cementitious Composites within the elastic and plastic ranges of cyclic flexural loading. Constr. Build. Mater. 2017, 145, 1–10. [Google Scholar] [CrossRef]
- Lin, C.; Wei, W.; Hu, Y.H. Catalytic behavior of graphene oxide for cement hydration process. J. Phys. Chem. Solids 2016, 89, 128–133. [Google Scholar] [CrossRef]
- Devi, S.C.; Khan, R.A. Influence of graphene oxide on sulfate attack and carbonation of concrete containing recycled concrete aggregate. Constr. Build. Mater. 2020, 250, 118883. [Google Scholar] [CrossRef]
- Zhu, Y.; Murali, S.; Cai, W.; Li, X.; Suk, J.W.; Potts, J.R.; Ruoff, R.S. Graphene and Graphene Oxide: Synthesis, Properties, and Applications. Adv. Mater. 2010, 22, 3906–3924. [Google Scholar] [CrossRef]
- Dikin, D.A.; Stankovich, S.; Zimney, E.J.; Piner, R.D.; Dommett, G.H.B.; Evmenenko, G.; Nguyen, S.T.; Ruoff, R.S. Preparation and characterization of graphene oxide paper. Nature 2007, 448, 457–460. [Google Scholar] [CrossRef]
- Mohammed, A.; Sanjayan, J.; Duan, W.; Nazari, A. Incorporating graphene oxide in cement composites: A study of transport properties. Constr. Build. Mater. 2015, 84, 341–347. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, J.; Lu, C.-X.; Liu, B.-W.; Zhang, K.; Li, C.-Z. Influence of graphene oxide additions on the microstructure and mechanical strength of cement. Carbon 2015, 95, 1083–1084. [Google Scholar] [CrossRef]
- Zhao, L.; Guo, X.; Song, L.; Song, Y.; Dai, G.; Liu, J. An intensive review on the role of graphene oxide in cement-based materials. Constr. Build. Mater. 2020, 241, 117939. [Google Scholar] [CrossRef]
- Pan, Z.; He, L.; Qiu, L.; Korayem, A.H.; Li, G.; Zhu, J.W.; Collins, F.; Li, D.; Duan, W.H.; Wang, M.C. Mechanical properties and microstructure of a graphene oxide—Cement composite. Cem. Concr. Compos. 2015, 58, 140–147. [Google Scholar] [CrossRef]
- Fan, D.; Lue, L.; Yang, S. Molecular dynamics study of interfacial stress transfer in graphene-oxide cementitious composites. Comput. Mater. Sci. 2017, 139, 56–64. [Google Scholar] [CrossRef]
- Lee, S.-J.; Jeong, S.-H.; Kim, D.-U.; Won, J.-P. Graphene oxide as an additive to enhance the strength of cementitious composites. Compos. Struct. 2020, 242, 112154. [Google Scholar] [CrossRef]
- Chopra, P.; Kumar, R.; Kumar, M. Artificial Neural Networks for the Prediction of Compressive Strength of Concrete. Int. J. Appl. Sci. Eng. 2015, 13, 187–204. [Google Scholar]
- Zhang, L.; Sojobi, A.; Kodur, V.; Liew, K. Effective utilization and recycling of mixed recycled aggregates for a greener environment. J. Clean. Prod. 2019, 236, 117600. [Google Scholar] [CrossRef]
- Alsanusi, S.; Bentaher, L. Prediction of Compressive Strength of Concrete from Early Age Test Result Using Design of Experiments (RSM). Int. J. Civ. Environ. Struct. Constr. Archit. Eng. 2015, 9, 1522–1526. [Google Scholar]
- Qurishee, M.A.; Iqbal, I.T.; Islam, M.S.; Islam, M.M. Use of Slag As Coarse Aggregate and Its Effect on Mechanical Properties of Concrete. In Proceedings of the 3rd International Conference on Advances in Civil Engineering, CUET, Chittagong, Bangladesh, 21–23 December 2016. [Google Scholar]
- Alyamac, K.E.; Ghafari, E.; Ince, R. Development of eco-efficient self-compacting concrete with waste marble powder using the response surface method. J. Clean. Prod. 2017, 144, 192–202. [Google Scholar] [CrossRef]
- Şimşek, B.; Uygunoğlu, T.; Korucu, H.; Kocakerim, M.M. Analysis of the effects of dioctyl terephthalate obtained from polyethylene terephthalate wastes on concrete mortar: A response surface methodology based desirability function approach application. J. Clean. Prod. 2018, 170, 437–445. [Google Scholar] [CrossRef]
- Tyagi, M.; Rana, A.; Kumari, S.; Jagadevan, S. Adsorptive removal of cyanide from coke oven wastewater onto zero-valent iron: Optimization through response surface methodology, isotherm and kinetic studies. J. Clean. Prod. 2018, 178, 398–407. [Google Scholar] [CrossRef]
- Desai, K.M.; Survase, S.A.; Saudagar, P.S.; Lele, S.; Singhal, R.S. Comparison of artificial neural network (ANN) and response surface methodology (RSM) in fermentation media optimization: Case study of fermentative production of scleroglucan. Biochem. Eng. J. 2008, 41, 266–273. [Google Scholar] [CrossRef]
- Mehri, E.; Maryam, N.; Ghasem, N.; Ali Asghar, G. Modeling and Optimization of Ethanol Fermentation Using Saccharomyces Cerevisiae: Response Surface Methodology and Artificial Neural Network. Chem. Ind. Chem. Eng. Q. 2013, 19, 241–252. [Google Scholar]
- Bheel, N.; Benjeddou, O.; Almujibah, H.R.; Abbasi, S.A.; Sohu, S.; Ahmad, M.; Sabri, M.M.S. Effect of calcined clay and marble dust powder as cementitious material on the mechanical properties and embodied carbon of high strength concrete by using RSM-based modelling. Heliyon 2023, 9, e15029. [Google Scholar] [CrossRef]
- Awolusi, T.; Oke, O.; Akinkurolere, O.; Sojobi, A. Application of response surface methodology: Predicting and optimizing the properties of concrete containing steel fibre extracted from waste tires with limestone powder as filler. Case Stud. Constr. Mater. 2019, 10, e00212. [Google Scholar] [CrossRef]
- ASTM C150; Standard Specification for Portland Cement. ASTM International: West Conshohocken, PA, USA, 2015.
- ASTM C618-15; Standard Specification for Coal Fly Ash and Raw or Calcined Natural Pozzolan for Use in Concrete. ASTM International: West Conshohocken, PA, USA, 2015.
- Zhang, Z.; Yuvaraj, A.; Di, J.; Qian, S. Matrix design of light weight, high strength, high ductility ECC. Constr. Build. Mater. 2019, 210, 188–197. [Google Scholar] [CrossRef]
- Mohammed, B.S.; Achara, B.E.; Liew, M.S.; Alaloul, W.; Khed, V.C. Effects of elevated temperature on the tensile properties of NS-modified self-consolidating engineered cementitious composites and property optimization using response surface methodology (RSM). Constr. Build. Mater. 2019, 206, 449–469. [Google Scholar] [CrossRef]
- BS EN 12390-3; Testing Harden Concrete. Compressive Strength of Test Specimens. BSI: London, UK, 2009.
- BS 1881: Part 122: 1983; Method for Determination of Water Absorption. British Standards Institute: London, UK; Department, C.T.S British Stand: London, UK, 1983.
- AASHTO (T277-15); Electrical Indication of Concrete’s Ability to Resist Chloride Ion Penetration. American Association of State and Highway Transportation Officials: Washington, DC, USA, 2019.
- ASTM C1202-19; Standard Test Method for Electrical Indication of Concrete’s Ability to Resist Chloride Ion Penetration. ASTM: West Conshohocken, PA, USA, 2019.
- Praveenkumar, T.R.; Vijayalakshmi, M.M.; Meddah, M.S. Strengths and durability performances of blended cement concrete with TiO2 nanoparticles and rice husk ash. Constr. Build. Mater. 2019, 217, 343–351. [Google Scholar] [CrossRef]
- Self-Compacting Concrete European Project Group. The European Guidelines for Self-Compacting Concrete: Specification, Production and Use. Eur. Guidel. Self Compact. Concr. 2005, 68. [Google Scholar]
- Arun, B.R.; Nagaraja, P.S.; Srishaila, J.M. An Effect of NaOH Molarity on Fly Ash—Metakaolin-Based Self-Compacting Geopolymer Concrete. Lect. Notes Civ. Eng. 2019, 25, 233–244. [Google Scholar] [CrossRef]
- Bheel, N.; Awoyera, P.; Tafsirojjaman, T.; Sor, N.H.; Sohu, S. Synergic effect of metakaolin and groundnut shell ash on the behavior of fly ash-based self-compacting geopolymer concrete. Constr. Build. Mater. 2021, 311, 125327. [Google Scholar] [CrossRef]
- Bheel, N.; Awoyera, P.; Shar, I.A.; Abbasi, S.A.; Khahro, S.H. Synergic effect of millet husk ash and wheat straw ash on the fresh and hardened properties of Metakaolin-based self-compacting geopolymer concrete. Case Stud. Constr. Mater. 2021, 15, e00729. [Google Scholar] [CrossRef]
- Kong, H.-J.; Bike, S.G.; Li, V.C. Development of a self-consolidating engineered cementitious composite employing electrosteric dispersion/stabilization. Cem. Concr. Compos. 2003, 25, 301–309. [Google Scholar] [CrossRef]
- Abdulkadir, I.; Mohammed, B.S.; Ali, M.O.A.; Liew, M.S. Effects of Graphene Oxide and Crumb Rubber on the Fresh Properties of Self-Compacting Engineered Cementitious Composite Using Response Surface Methodology. Materials 2022, 15, 2519. [Google Scholar] [CrossRef]
- Wei, Z.; Wang, Y.; Qi, M.; Bi, J.; Yang, S.; Yuan, X. The role of sucrose on enhancing properties of graphene oxide reinforced cement composites containing fly ash. Constr. Build. Mater. 2021, 293, 123507. [Google Scholar] [CrossRef]
- Lee, S.-J.; Jeong, S.-H.; Kim, D.-U.; Won, J.-P. Effects of graphene oxide on pore structure and mechanical properties of cementitious composites. Compos. Struct. 2020, 234, 111709. [Google Scholar] [CrossRef]
- Lv, S.; Ma, Y.; Qiu, C.; Sun, T.; Liu, J.; Zhou, Q. Effect of graphene oxide nanosheets of microstructure and mechanical properties of cement composites. Constr. Build. Mater. 2013, 49, 121–127. [Google Scholar] [CrossRef]
- Gong, K.; Pan, Z.; Korayem, A.H.; Qiu, L.; Li, D.; Collins, F.; Wang, C.M.; Duan, W.H. Reinforcing Effects of Graphene Oxide on Portland Cement Paste. J. Mater. Civ. Eng. 2015, 27, 1125. [Google Scholar] [CrossRef]
- Kang, D.; Seo, K.S.; Lee, H.; Chung, W. Experimental study on mechanical strength of GO-cement composites. Constr. Build. Mater. 2017, 131, 303–308. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, J.; Lu, C.-X.; Liu, B.-W.; Zhang, K.; Li, C.-Z. Influence of graphene oxide additions on the microstructure and mechanical strength of cement. New Carbon Mater. 2015, 30, 349–356. [Google Scholar] [CrossRef]
- Bheel, N.; Ali, M.O.A.; Kırgız, M.S.; Shafiq, N.; Gobinath, R. Effect of graphene oxide particle as nanomaterial in the production of engineered cementitious composites including superplasticizer, fly ash, and polyvinyl alcohol fiber. Mater. Today Proc. 2023. [Google Scholar] [CrossRef]
- Ramyar, K.; Inan, G. Sodium sulfate attack on plain and blended cements. Build. Environ. 2007, 42, 1368–1372. [Google Scholar] [CrossRef]
- Mohammed, B.S.; Yen, L.Y.; Haruna, S.; Huat, M.L.S.; Abdulkadir, I.; Al-Fakih, A.; Liew, M.S.; Zawawi, N.A.W.A. Effect of Elevated Temperature on the Compressive Strength and Durability Properties of Crumb Rubber Engineered Cementitious Composite. Materials 2020, 13, 3516. [Google Scholar] [CrossRef]
- Sabapathy, L.; Mohammed, B.S.; Al-Fakih, A.; Wahab, M.M.A.; Liew, M.S.; Amran, Y.H.M. Acid and Sulphate Attacks on a Rubberized Engineered Cementitious Composite Containing Graphene Oxide. Materials 2020, 13, 3125. [Google Scholar] [CrossRef]
- Gu, L.; Bennett, T.; Visintin, P. Sulphuric acid exposure of conventional concrete and alkali-activated concrete: Assessment of test methodologies. Constr. Build. Mater. 2018, 197, 681–692. [Google Scholar] [CrossRef]
- Chintalapudi, K.; Pannem, R.M.R. Enhanced chemical resistance to sulphuric acid attack by reinforcing Graphene Oxide in Ordinary and Portland Pozzolana cement mortars. Case Stud. Constr. Mater. 2022, 17, e01452. [Google Scholar] [CrossRef]
- Arif, M.; Gupta, V.; Choudhary, H.; Kumar, S.; Basu, P. Performance evaluation of cement concrete containing sandstone slurry. Constr. Build. Mater. 2018, 184, 432–439. [Google Scholar] [CrossRef]
- Aiken, T.A.; Kwasny, J.; Sha, W.; Soutsos, M.N. Effect of slag content and activator dosage on the resistance of fly ash geopolymer binders to sulfuric acid attack. Cem. Concr. Res. 2018, 111, 23–40. [Google Scholar] [CrossRef]
- Wan, X.-M.; Wittmann, F.H.; Zhao, T.-J.; Fan, H. Chloride content and pH value in the pore solution of concrete under carbonation. J. Zhejiang Univ. A 2013, 14, 71–78. [Google Scholar] [CrossRef]
- Kumari, K.; Preetha, R.; Ramachandran, D.; Vishwakarma, V.; George, R.P.; Sundaramurthy, C.; Mudali, U.K.; Pillai, C.S. Nanoparticles for enhancing mechanical properties of fly ash concrete. Mater. Today Proc. 2016, 3, 2387–2393. [Google Scholar] [CrossRef]
- Zeng, H.; Li, Y.; Zhang, J.; Chong, P.; Zhang, K. Effect of limestone powder and fly ash on the pH evolution coefficient of concrete in a sulfate-freeze—Thaw environment. J. Mater. Res. Technol. 2021, 16, 1889–1903. [Google Scholar] [CrossRef]
- Khotbehsara, M.M.; Miyandehi, B.M.; Naseri, F.; Ozbakkaloglu, T.; Jafari, F.; Mohseni, E. Effect of SnO2, ZrO2, and CaCO3 nanoparticles on water transport and durability properties of self-compacting mortar containing fly ash: Experimental observations and ANFIS predictions. Constr. Build. Mater. 2018, 158, 823–834. [Google Scholar] [CrossRef]
- Schwarz, N.; Neithalath, N. Influence of a fine glass powder on cement hydration: Comparison to fly ash and modeling the degree of hydration. Cem. Concr. Res. 2008, 38, 429–436. [Google Scholar] [CrossRef]
- Miyandehi, B.M.; Feizbakhsh, A.; Yazdi, M.A.; Liu, Q.-F.; Yang, J.; Alipour, P. Performance and properties of mortar mixed with nano-CuO and rice husk ash. Cem. Concr. Compos. 2016, 74, 225–235. [Google Scholar] [CrossRef]
- Luhar, S.; Luhar, I.; Nicolaides, D.; Gupta, R. Durability Performance Evaluation of Rubberized Geopolymer Concrete. Sustainability 2021, 13, 5969. [Google Scholar] [CrossRef]
- Rodríguez, A.; Santamaría-Vicario, I.; Calderón, V.; Junco, C.; García-Cuadrado, J. Study of the expansion of cement mortars manufactured with Ladle Furnace Slag LFS. Mater. Constr. 2019, 69, 183. [Google Scholar] [CrossRef]
- Sarwary, M.H.; Yıldırım, G.; Al-Dahawi, A.; Anıl, Ö.; Khiavi, K.A.; Toklu, K.; Şahmaran, M. Self-Sensing of Flexural Damage in Large-Scale Steel-Reinforced Mortar Beams. ACI Mater. J. 2019, 116, 51715581. [Google Scholar] [CrossRef]
- Anwar, A.; Mohammed, B.S.; Wahab, M.B.A.; Liew, M. Enhanced properties of cementitious composite tailored with graphene oxide nanomaterial—A review. Dev. Built Environ. 2019, 1, 100002. [Google Scholar] [CrossRef]
- Tittelboom, K.V.; De Belie, N.; Hooton, D. A Critical Review on Test Methods for Evaluating the Resistance of Concrete against Sulfate Attack. In Proceedings of the Chapter 4 in Performance of Cement-Based Materials in Aggressive Aqueous Environments, Rilem Proceedings, Toulouse, France, 3–5 June 2009; PRO 63. pp. 298–306. [Google Scholar]
- Hill, J.; Byars, E.; Sharp, J.; Lynsdale, C.; Cripps, J.; Zhou, Q. An experimental study of combined acid and sulfate attack of concrete. Cem. Concr. Compos. 2003, 25, 997–1003. [Google Scholar] [CrossRef]
- Honglei, C.; Zuquan, J.; Penggang, W.; Jianhong, W.; Jian, L. Comprehensive resistance of fair-faced concrete suffering from sulfate attack under marine environments. Constr. Build. Mater. 2021, 277, 122312. [Google Scholar] [CrossRef]
- Li, Y.; Yang, X.; Lou, P.; Wang, R.; Li, Y.; Si, Z. Sulfate attack resistance of recycled aggregate concrete with NaOH-solution-treated crumb rubber. Constr. Build. Mater. 2021, 287, 123044. [Google Scholar] [CrossRef]
- Behfarnia, K.; Farshadfar, O. The effects of pozzolanic binders and polypropylene fibers on durability of SCC to magnesium sulfate attack. Constr. Build. Mater. 2013, 38, 64–71. [Google Scholar] [CrossRef]
- Khan, M.H.; Zhu, H.; Sikandar, M.A.; Zamin, B.; Ahmad, M.; Sabri, M.M.S. Effects of Various Mineral Admixtures and Fibrillated Polypropylene Fibers on the Properties of Engineered Cementitious Composite (ECC) Based Mortars. Materials 2022, 15, 2880. [Google Scholar] [CrossRef]
- Mohammed, B.S.; Haruna, S.; Wahab, M.M.B.A.; Liew, M. Optimization and characterization of cast in-situ alkali-activated pastes by response surface methodology. Constr. Build. Mater. 2019, 225, 776–787. [Google Scholar] [CrossRef]
- Ghafari, E.; Costa, H.; Júlio, E. RSM-based model to predict the performance of self-compacting UHPC reinforced with hybrid steel micro-fibers. Constr. Build. Mater. 2014, 66, 375–383. [Google Scholar] [CrossRef]
- Mohammed, B.S. Evaluating the static and dynamic modulus of elasticity of roller compacted rubbercrete using response surface methodology. Int. J. Geomate 2018, 14, 42833. [Google Scholar] [CrossRef]
- Sadhukhan, B.; Mondal, N.K.; Chattoraj, S. Optimisation using central composite design (CCD) and the desirability function for sorption of methylene blue from aqueous solution onto Lemna major. Karbala Int. J. Mod. Sci. 2016, 2, 145–155. [Google Scholar] [CrossRef]
- Zahid, M.; Shafiq, N.; Isa, M.H.; Gil, L. Statistical modeling and mix design optimization of fly ash based engineered geopolymer composite using response surface methodology. J. Clean. Prod. 2018, 194, 483–498. [Google Scholar] [CrossRef]
- Mtarfi, N.; Rais, Z.; Taleb, M.; Kada, K. Effect of fly ash and grading agent on the properties of mortar using response surface methodology. J. Build. Eng. 2017, 9, 109–116. [Google Scholar] [CrossRef]
- Mermerdaş, K.; Algın, Z.; Oleiwi, S.M.; Nassani, D.E. Optimization of lightweight GGBFS and FA geopolymer mortars by response surface method. Constr. Build. Mater. 2017, 139, 159–171. [Google Scholar] [CrossRef]
- Murali, M.; Mohammed, B.S.; Abdulkadir, I.; Liew, M.S.; Alaloul, W.S. Utilization of Crumb Rubber and High-Volume Fly Ash in Concrete for Environmental Sustainability: RSM-Based Modeling and Optimization. Materials 2021, 14, 3322. [Google Scholar] [CrossRef] [PubMed]












| Materials | Compound (%) | Specific Gravity | Blaine Fineness (m2/kg) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| SiO2 | Al2O3 | Fe2O3 | MnO | CaO | MgO | Na2O | K2O | T2O | |||
| PC | 20.76 | 5.54 | 3.35 | - | 61.4 | 2.48 | 0.19 | 0.78 | - | 3.15 | 290 |
| FA | 57.01 | 20.96 | 4.15 | 0.033 | 9.79 | 1.75 | 2.23 | 1.53 | 0.68 | 2.38 | 325 |
| Materials | Compound (%) | Specific Gravity | Blaine Fineness (m2/Kg) | Loss on Ignition | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| SiO2 | Al2O3 | Fe2O3 | MnO | CaO | MgO | Na2O | K2O | T2O | ||||
| FA | 57.01 | 20.96 | 4.15 | 0.033 | 9.79 | 1.75 | 2.23 | 1.53 | 0.68 | 2.38 | 290 | 1.25 |
| Cement | 20.76 | 5.54 | 3.35 | - | 61.4 | 2.48 | 0.19 | 0.78 | - | 3.15 | 325 | 2.20 |
| Mix ID | Materials (%) | Quantity of Materials Used in ECC Mixture (kg/m3) | ||||
|---|---|---|---|---|---|---|
| GO | PVA | PC | Fly Ash | Sand | Water | |
| M0 | 0.00 | 2 | 583 | 700 | 467 | 385 |
| M1 | 0.05 | 1.5 | 583 | 700 | 467 | 385 |
| M2 | 0.065 | 2 | 583 | 700 | 467 | 385 |
| M3 | 0.065 | 1.5 | 583 | 700 | 467 | 385 |
| M4 | 0.08 | 1.5 | 583 | 700 | 467 | 385 |
| M5 | 0.065 | 1.5 | 583 | 700 | 467 | 385 |
| M6 | 0.065 | 1 | 583 | 700 | 467 | 385 |
| M7 | 0.05 | 2 | 583 | 700 | 467 | 385 |
| M8 | 0.065 | 1.5 | 583 | 700 | 467 | 385 |
| M9 | 0.05 | 1 | 583 | 700 | 467 | 385 |
| M10 | 0.065 | 1.5 | 583 | 700 | 467 | 385 |
| M11 | 0.08 | 1 | 583 | 700 | 467 | 385 |
| M12 | 0.065 | 1.5 | 583 | 700 | 467 | 385 |
| M13 | 0.08 | 2 | 583 | 700 | 467 | 385 |
| Response | Source | Sum of Squares | Df | Mean Square | F-Value | p-Value > F | Significance |
|---|---|---|---|---|---|---|---|
| Model | 531.85 | 5 | 106.37 | 59.08 | <0.0001 | Yes | |
| A-PVA | 81.40 | 1 | 81.40 | 45.21 | 0.0003 | Yes | |
| B-GO | 352.67 | 1 | 352.67 | 195.87 | <0.0001 | Yes | |
| AB | 13.32 | 1 | 13.32 | 7.40 | 0.0298 | No | |
| A2 | 0.098 | 1 | 0.098 | 0.054 | 0.8223 | No | |
| B2 | 70.11 | 1 | 70.11 | 38.94 | 0.0004 | Yes | |
| Compressive Strength | Residual | 12.60 | 7 | 1.80 | |||
| Lack of Fit | 12.57 | 3 | 4.19 | 538.66 | <0.0001 | Yes | |
| Pure Error | 0.031 | 4 | 7.780 × 10−³ | ||||
| Cor Total | 544.45 | 12 | |||||
| Model | 24.17 | 2 | 12.08 | 26.25 | 0.0001 | Yes | |
| A-PVA | 24.00 | 1 | 24.00 | 52.14 | <0.0001 | Yes | |
| B-GO | 0.17 | 1 | 0.17 | 0.36 | 0.5607 | No | |
| Weight Loss | Residual | 4.60 | 10 | 0.46 | |||
| Lack of Fit | 1.80 | 6 | 0.30 | 0.43 | 0.8303 | No | |
| Pure Error | 2.80 | 4 | 0.70 | ||||
| Cor Total | 28.77 | 12 | |||||
| Model | 0.28 | 5 | 0.056 | 64.96 | <0.0001 | Yes | |
| A-PVA | 0.062 | 1 | 0.062 | 71.40 | <0.0001 | Yes | |
| B-GO | 0.19 | 1 | 0.19 | 215.59 | <0.0001 | Yes | |
| AB | 0.026 | 1 | 0.026 | 29.47 | 0.0010 | Yes | |
| Change in Length | A2 | 5.255 × 10−³ | 1 | 5.255 × 10−³ | 6.05 | 0.0435 | Yes |
| B2 | 4.729 × 10−³ | 1 | 4.729 × 10−³ | 5.44 | 0.0524 | No | |
| Residual | 6.080 × 10−³ | 7 | 8.686 × 10−4 | ||||
| Lack of Fit | 5.800 × 10−³ | 3 | 1.933 × 10−³ | 27.62 | 0.0039 | Yes | |
| Pure Error | 2.800 × 10−4 | 4 | 7.000 × 10−5 | ||||
| Cor Total | 0.29 | 12 | |||||
| Model | 597.95 | 5 | 119.59 | 60.98 | <0.0001 | Yes | |
| A-PVA | 110.13 | 1 | 110.13 | 56.16 | 0.0001 | Yes | |
| B-GO | 392.44 | 1 | 392.44 | 200.11 | <0.0001 | Yes | |
| AB | 11.45 | 1 | 11.45 | 5.84 | 0.0463 | Yes | |
| Compressive Strength due to Acid Attack | A2 | 0.60 | 1 | 0.60 | 0.31 | 0.5977 | No |
| B2 | 66.35 | 1 | 66.35 | 33.83 | 0.0007 | Yes | |
| Residual | 13.73 | 7 | 1.96 | ||||
| Lack of Fit | 13.40 | 3 | 4.47 | 55.24 | 0.0010 | Yes | |
| Pure Error | 0.32 | 4 | 0.081 | ||||
| Cor Total | 611.68 | 12 | |||||
| Model | 1.39 | 5 | 0.28 | 362.31 | <0.0001 | Yes | |
| A-PVA | 0.84 | 1 | 0.84 | 1096.08 | <0.0001 | Yes | |
| B-GO | 0.49 | 1 | 0.49 | 640.52 | <0.0001 | Yes | |
| AB | 4.000 × 10−4 | 1 | 4.000 × 10−4 | 0.52 | 0.4944 | No | |
| pH Value | A2 | 0.029 | 1 | 0.029 | 37.51 | 0.0005 | Yes |
| B2 | 0.049 | 1 | 0.049 | 63.24 | <0.0001 | Yes | |
| Residual | 5.389 × 10−³ | 7 | 7.698 × 10−4 | ||||
| Lack of Fit | 4.989 × 10−³ | 3 | 1.663 × 10−³ | 16.63 | 0.0101 | Yes | |
| Pure Error | 4.000 × 10−4 | 4 | 1.000 × 10−4 | ||||
| Cor Total | 1.40 | 12 | |||||
| Model | 3.671 × 105 | 5 | 73,429.32 | 199.49 | <0.0001 | Yes | |
| A-PVA | 2.714 × 105 | 1 | 2.714 × 105 | 737.22 | <0.0001 | Yes | |
| B-GO | 86,880.67 | 1 | 86,880.67 | 236.03 | <0.0001 | Yes | |
| Rapid Chloride Penetration Test | AB | 4225.00 | 1 | 4225.00 | 11.48 | 0.0116 | Yes |
| A2 | 3816.37 | 1 | 3816.37 | 10.37 | 0.0147 | Yes | |
| B2 | 13.03 | 1 | 13.03 | 0.035 | 0.8561 | No | |
| Residual | 2576.63 | 7 | 368.09 | ||||
| Lack of Fit | 2563.83 | 3 | 854.61 | 267.07 | <0.0001 | Yes | |
| Pure Error | 12.80 | 4 | 3.20 | ||||
| Cor Total | 3.697 × 105 | 12 | |||||
| Model | 0.62 | 5 | 0.12 | 63.28 | <0.0001 | Yes | |
| A-PVA | 0.54 | 1 | 0.54 | 273.87 | <0.0001 | Yes | |
| B-GO | 6.667 × 10−³ | 1 | 6.667 × 10−³ | 3.38 | 0.1085 | No | |
| AB | 0.040 | 1 | 0.040 | 20.29 | 0.0028 | Yes | |
| Weight Gain | A2 | 0.031 | 1 | 0.031 | 15.49 | 0.0056 | Yes |
| B2 | 7.389 × 10−5 | 1 | 7.389 × 10−5 | 0.037 | 0.8520 | No | |
| Residual | 0.014 | 7 | 1.972 × 10−3 | ||||
| Lack of Fit | 0.013 | 3 | 4.467 × 10−3 | 44.67 | 0.0016 | Yes | |
| Pure Error | 4.000 × 10−4 | 4 | 1.000 × 10−4 | ||||
| Cor Total | 0.64 | 12 | |||||
| Model | 1.516 × 10−5 | 2 | 7.582 × 10−6 | 41.11 | <0.0001 | Yes | |
| A-PVA | 3.682 × 10−6 | 1 | 3.682 × 10−6 | 19.96 | 0.0012 | Yes | |
| B-GO | 1.148 × 10−5 | 1 | 1.148 × 10−5 | 62.25 | <0.0001 | Yes | |
| Expansion | Residual | 1.844 × 10−6 | 10 | 1.844 × 10−7 | |||
| Lack of Fit | 1.816 × 10−6 | 6 | 3.027 × 10−7 | 43.25 | 0.0014 | Yes | |
| Pure Error | 2.800 × 10−8 | 4 | 7.000 × 10−9 | ||||
| Cor Total | 1.701 × 10−5 | 12 | |||||
| Model | 552.25 | 5 | 110.45 | 63.26 | <0.0001 | Yes | |
| A-PVA | 87.78 | 1 | 87.78 | 50.27 | 0.0002 | Yes | |
| B-GO | 364.65 | 1 | 364.65 | 208.84 | <0.0001 | Yes | |
| AB | 13.85 | 1 | 13.85 | 7.93 | 0.0259 | Yes | |
| Compressive Strength due to Sulfate Attack | A2 | 0.31 | 1 | 0.31 | 0.18 | 0.6849 | No |
| B2 | 69.63 | 1 | 69.63 | 39.88 | 0.0004 | Yes | |
| Residual | 12.22 | 7 | 1.75 | ||||
| Lack of Fit | 11.93 | 3 | 3.98 | 54.90 | 0.0010 | Yes | |
| Pure Error | 0.29 | 4 | 0.072 | ||||
| Cor Total | 564.47 | 12 | |||||
| Model | 2.15 | 5 | 0.43 | 86.25 | <0.0001 | Yes | |
| A-PVA | 1.60 | 1 | 1.60 | 320.86 | <0.0001 | Yes | |
| B-GO | 0.28 | 1 | 0.28 | 56.43 | 0.0001 | Yes | |
| AB | 0.000 | 1 | 0.000 | 0.000 | 1.0000 | No | |
| A2 | 0.19 | 1 | 0.19 | 37.50 | 0.0005 | No | |
| Water Absorption | B2 | 0.010 | 1 | 0.010 | 2.01 | 0.1987 | No |
| Residual | 0.035 | 7 | 4.992 × 10−³ | ||||
| Lack of Fit | 6.943 × 10−³ | 3 | 2.314 × 10−³ | 0.33 | 0.8050 | No | |
| Pure Error | 0.028 | 4 | 7.000 × 10−³ | ||||
| Cor Total | 2.19 | 12 |
| Model Validation Constraints | CS | Weight Loss | Change in Length | CS Due Acid Attack | pH | RCPT | Weight Gain | Expansion | CS Due to Acid Attack | WA |
|---|---|---|---|---|---|---|---|---|---|---|
| Std. Dev. | 1.34 | 0.68 | 0.029 | 1.40 | 0.028 | 19.19 | 0.044 | 4.295 × 10−4 | 1.32 | 0.071 |
| Mean | 61.05 | 6.31 | 0.48 | 57.72 | 10.23 | 966.54 | 0.86 | 4.469 × 10−³ | 59.01 | 1.33 |
| C.V. % | 2.20 | 10.76 | 6.14 | 2.43 | 0.27 | 1.98 | 5.18 | 9.61 | 2.24 | 5.31 |
| PRESS | 126.00 | 7.82 | 0.058 | 135.93 | 0.051 | 18,660.17 | 0.14 | 3.852 × 10−6 | 120.84 | 0.11 |
| −2 Log Likelihood | 36.49 | 23.39 | −62.79 | 37.60 | −64.36 | 105.65 | −52.13 | −168.10 | 36.09 | −40.05 |
| R-Squared | 0.9769 | 0.8400 | 0.9789 | 0.9776 | 0.9962 | 0.9930 | 0.9784 | 0.8916 | 0.9783 | 0.9840 |
| Adj R-Squared | 0.9603 | 0.8080 | 0.9638 | 0.9615 | 0.9934 | 0.9881 | 0.9629 | 0.8699 | 0.9629 | 0.9726 |
| Pred R-Squared | 0.7686 | 0.7281 | 0.7976 | 0.7778 | 0.9637 | 0.9495 | 0.7854 | 0.7735 | 0.7859 | 0.9494 |
| Adeq Precision | 24.902 | 13.296 | 27.802 | 26.008 | 70.207 | 51.097 | 26.519 | 21.004 | 25.890 | 30.556 |
| BIC | 51.88 | 31.09 | −47.40 | 52.99 | −48.97 | 121.04 | −36.74 | −160.40 | 51.48 | −24.66 |
| AICc | 62.49 | 32.06 | −36.79 | 63.60 | −38.36 | 131.65 | −26.13 | −159.43 | 62.09 | −14.05 |
| Factors | Input Factors | Responses (Output Factors) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| GO (%) | PVA (%) | CS (MPa) | WA (%) | RCPT (Coulombs) | Sulfate Attack | Acid Attack | |||||||
| Wt. Gain (%) | Expansion (%) | CS (MPa) | Wt. Loss (%) | Change in Length (%) | CS | pH | |||||||
| Value | Min. | 0.05 | 1 | 53.1 | 0.80 | 695 | 0.40 | 0.0023 | 50.86 | 4 | 0.28 | 48.58 | 9.55 |
| Max. | 0.08 | 2 | 78.6 | 2.20 | 1365 | 1.20 | 0.0065 | 76.87 | 9 | 0.83 | 76.24 | 10.91 | |
| Goal | Range | Range | Max. | Min. | Min. | Min. | Max. | Max | Min. | Min. | Max. | Min. | |
| Optimization Results | 0.05 | 1 | 77.03 | 1.20 | 862.89 | 0.56 | 0.0050 | 75.37 | 4.14 | 0.47 | 74.67 | 10.11 | |
| Desirability | 78.2% (0.782) | ||||||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bheel, N.; Mohammed, B.S.; Liew, M.S.; Zawawi, N.A.W.A. Effect of Graphene Oxide as a Nanomaterial on the Durability Behaviors of Engineered Cementitious Composites by Applying RSM Modelling and Optimization. Buildings 2023, 13, 2026. https://doi.org/10.3390/buildings13082026
Bheel N, Mohammed BS, Liew MS, Zawawi NAWA. Effect of Graphene Oxide as a Nanomaterial on the Durability Behaviors of Engineered Cementitious Composites by Applying RSM Modelling and Optimization. Buildings. 2023; 13(8):2026. https://doi.org/10.3390/buildings13082026
Chicago/Turabian StyleBheel, Naraindas, Bashar S. Mohammed, M. S. Liew, and Noor Amila Wan Abdullah Zawawi. 2023. "Effect of Graphene Oxide as a Nanomaterial on the Durability Behaviors of Engineered Cementitious Composites by Applying RSM Modelling and Optimization" Buildings 13, no. 8: 2026. https://doi.org/10.3390/buildings13082026








