Formation of Cellular Concrete Structures Based on Waste Glass and Liquid Glass
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Research Methods
- V is the volume of the product, m3.
3. Results and Discussion
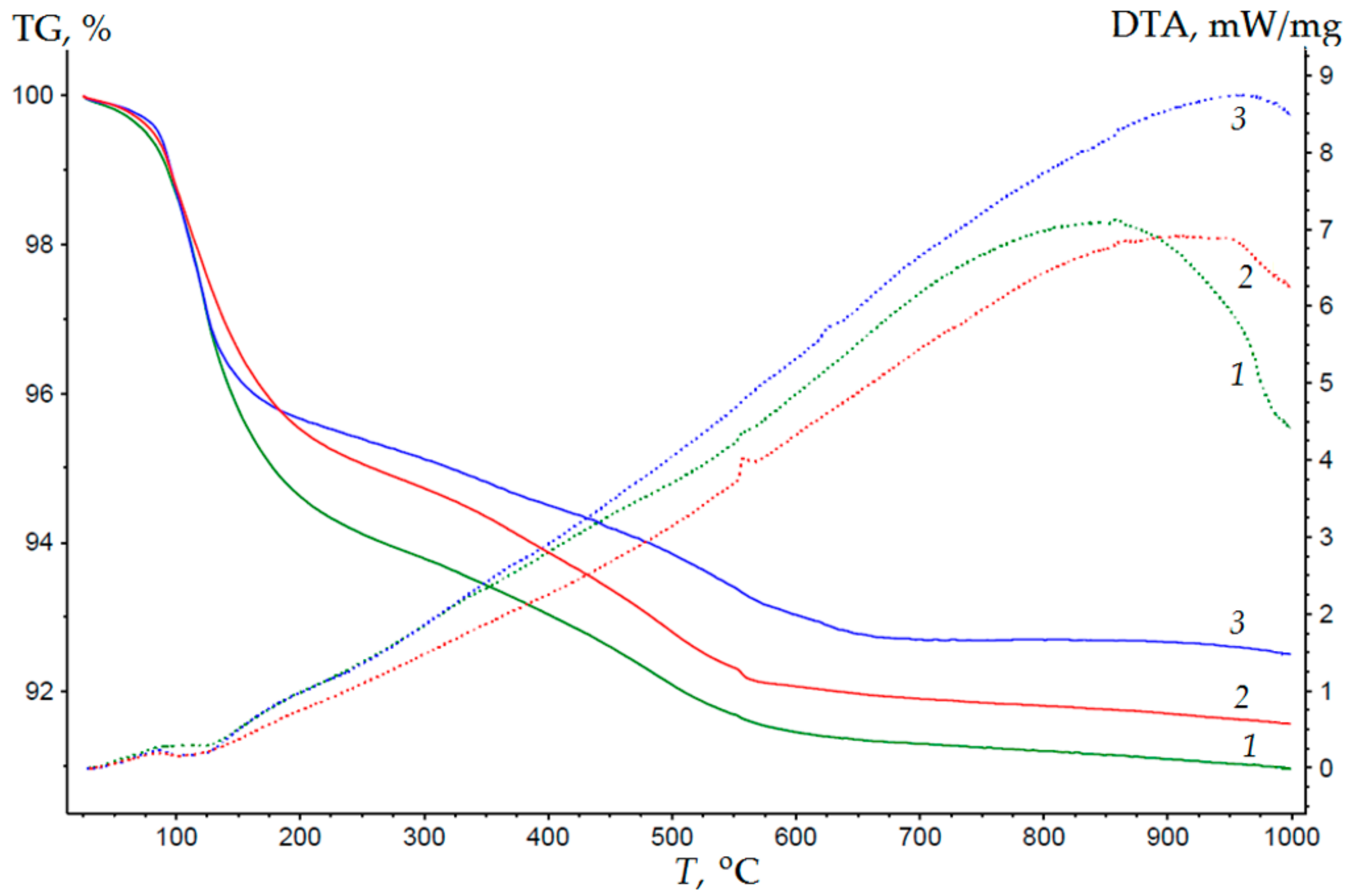
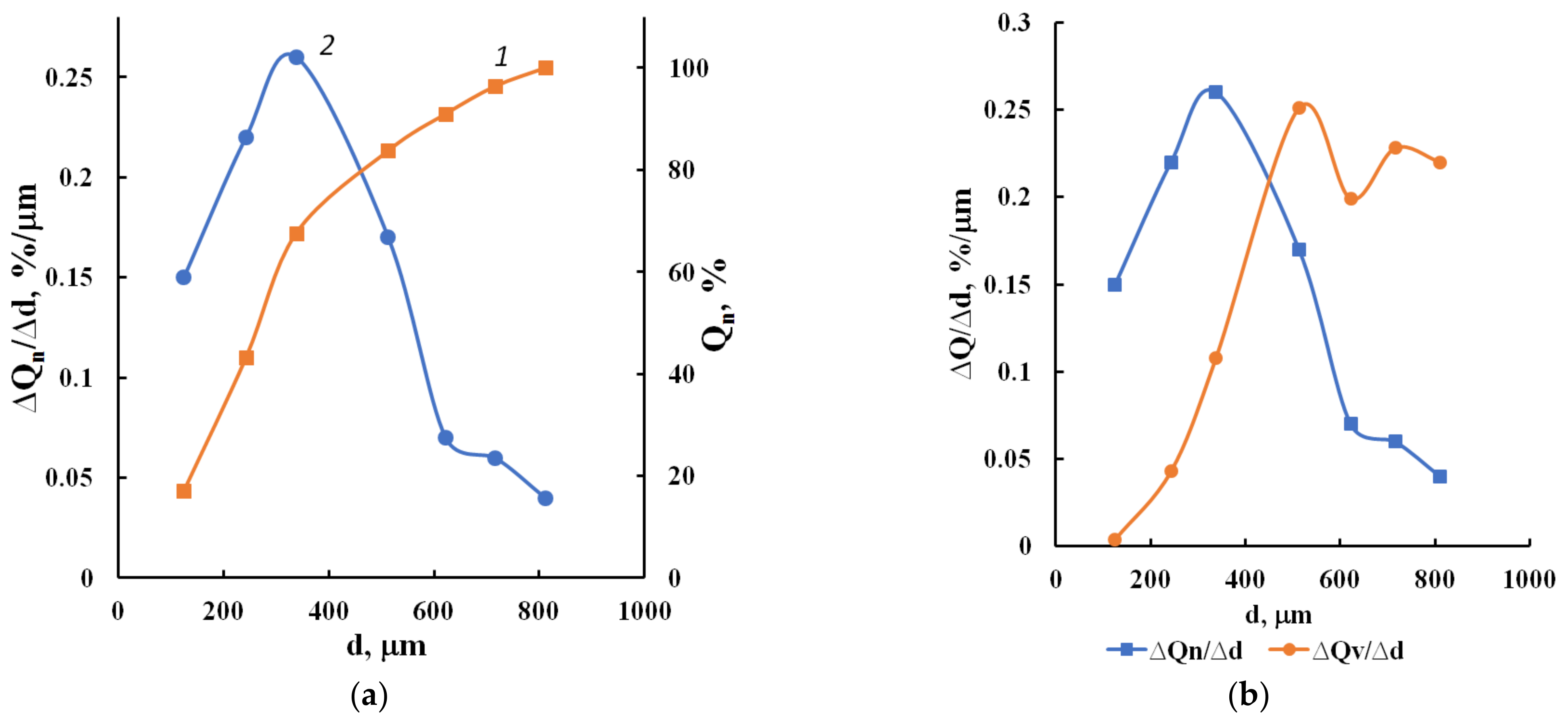
3.1. Composition, Morphology and Dispersion of Technical Glass Waste
3.2. Mechanisms of the Structure Formation of Cellular Concrete Based on Waste Glass and Liquid Glass
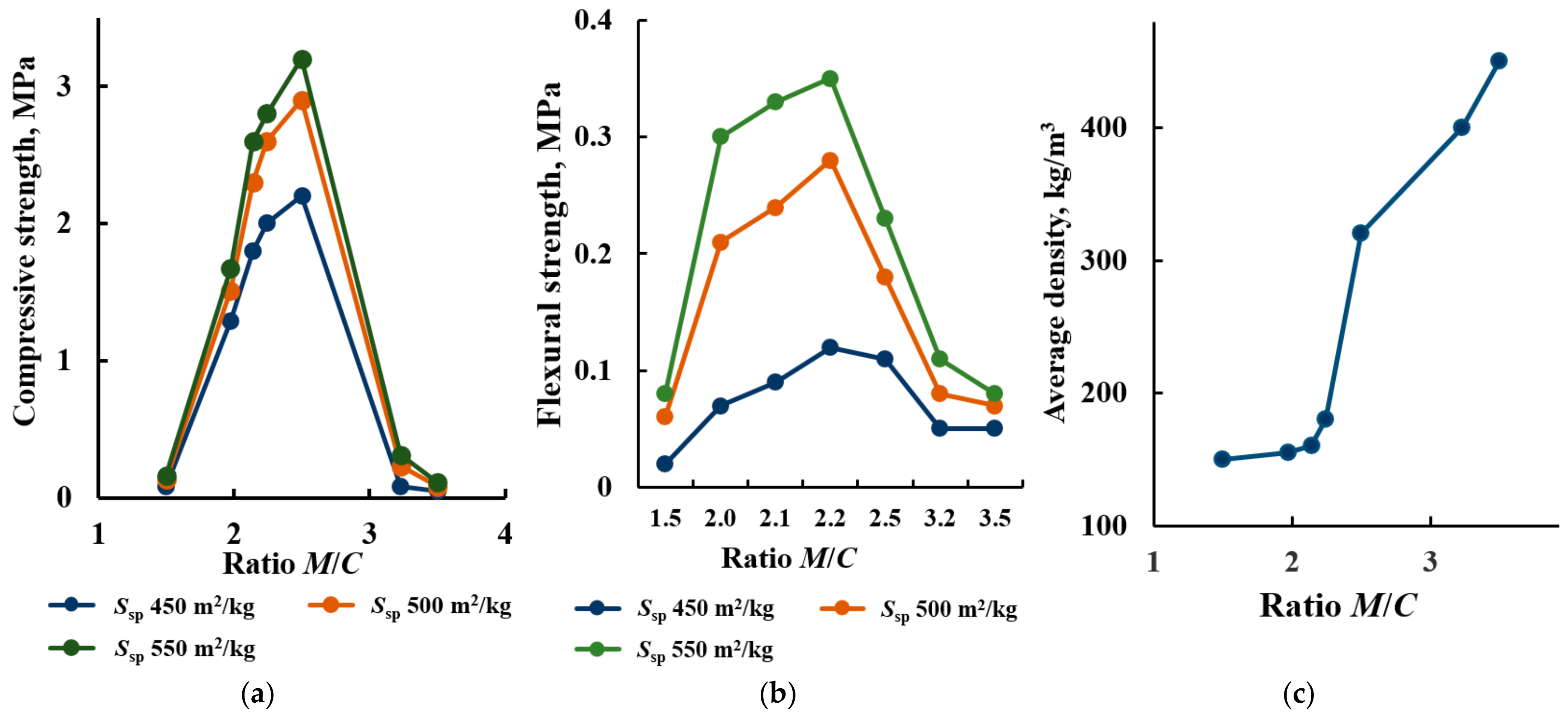
3.2.1. The Effect of the Dispersion of Glass Particles on the Strength of Glass-Filled Cellular Concrete
- Polycondensation of silicic acid:nSi(OH)4 → (OH)3SiO(Si(OH)2)n–2OSi(OH)3 + (n − 1)H2O;
- Formation of hydrosilicates during cement hydration:C3S + H2O → (0.8–1.0)CaO·SiO2·(1.0–1.5)H2O + 2Ca(OH)2;
- Formation of double silicates and silica gel:Na2O·2SiO2 + mH2O + Ca(OH)2 → Na2O·CaO·SiO2·nH2O + SiO2 + (m − n)H2O.
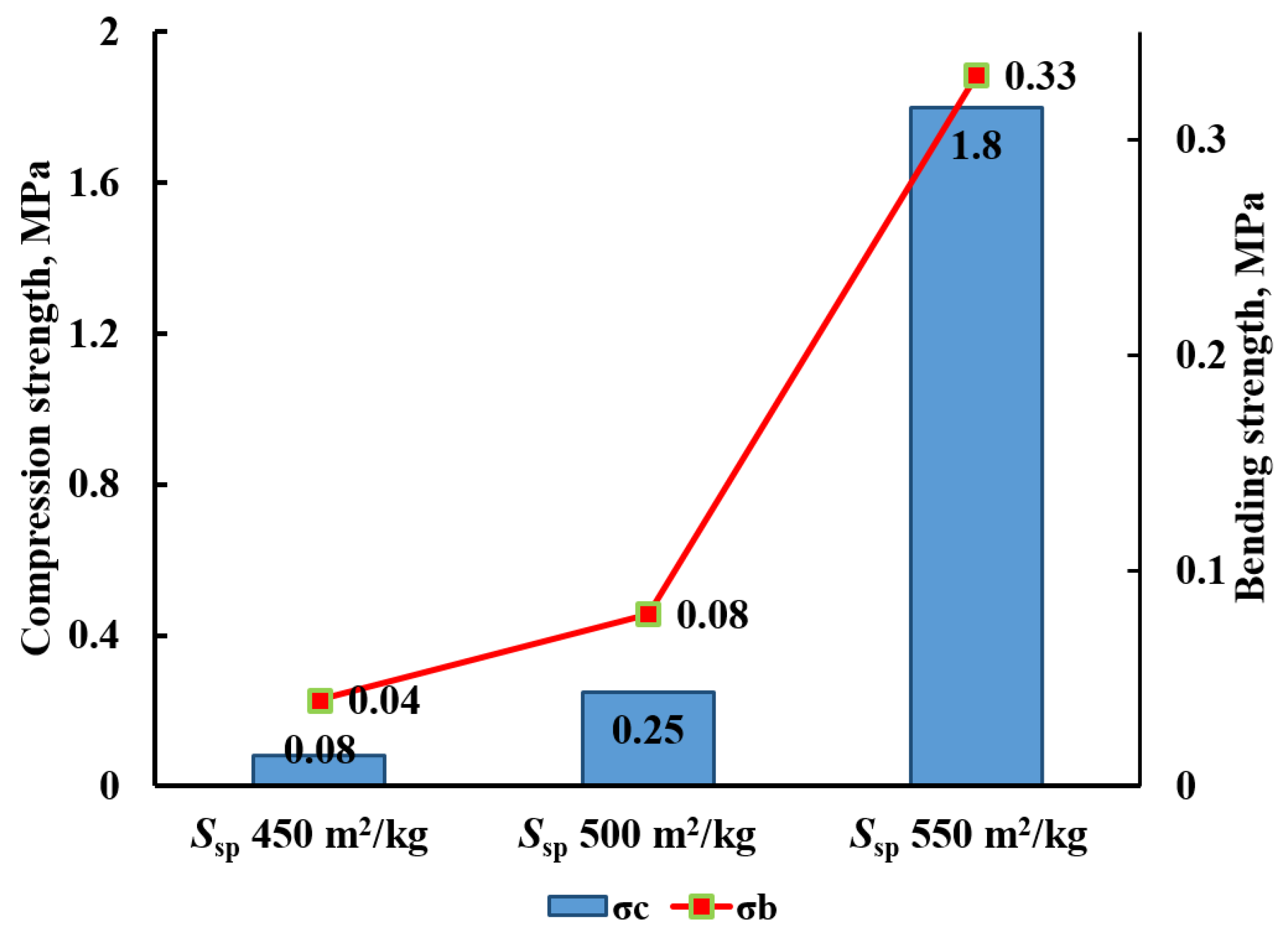
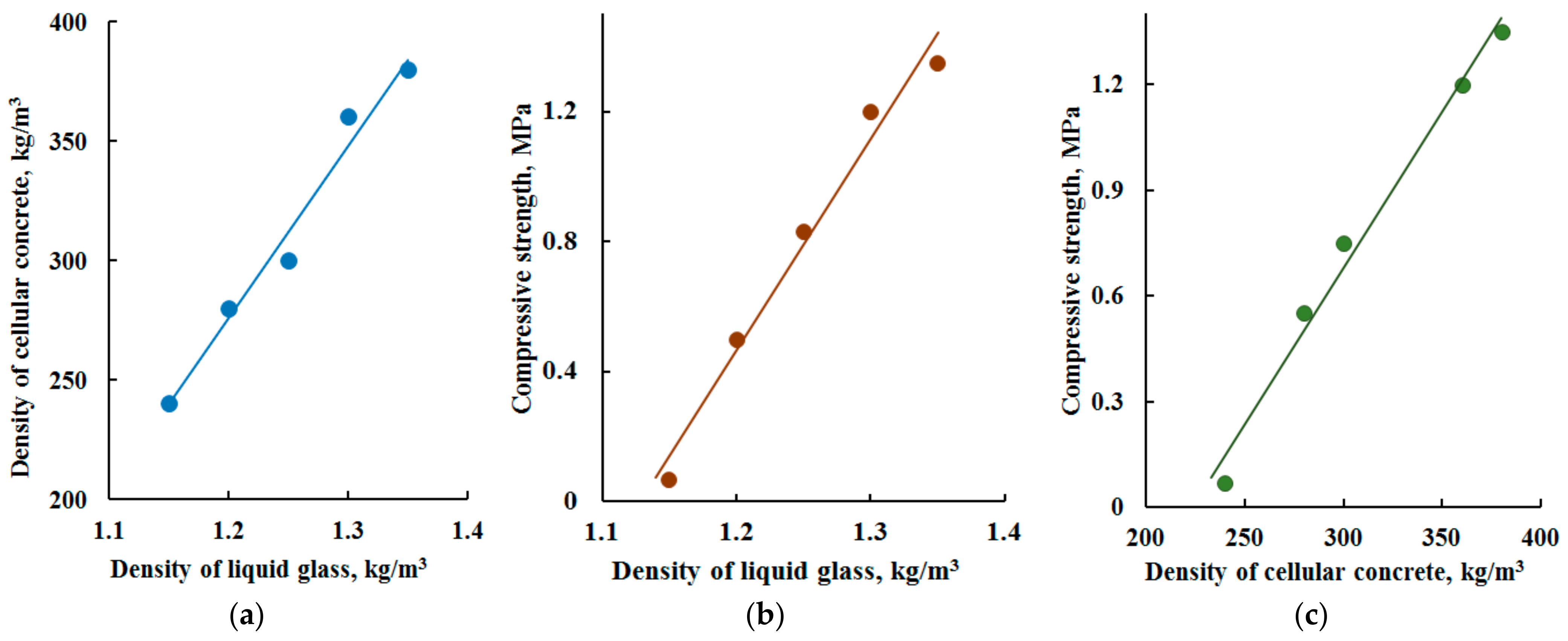
3.2.2. Influence of Liquid Glass Density on the Density and Strength of Cellular Concrete
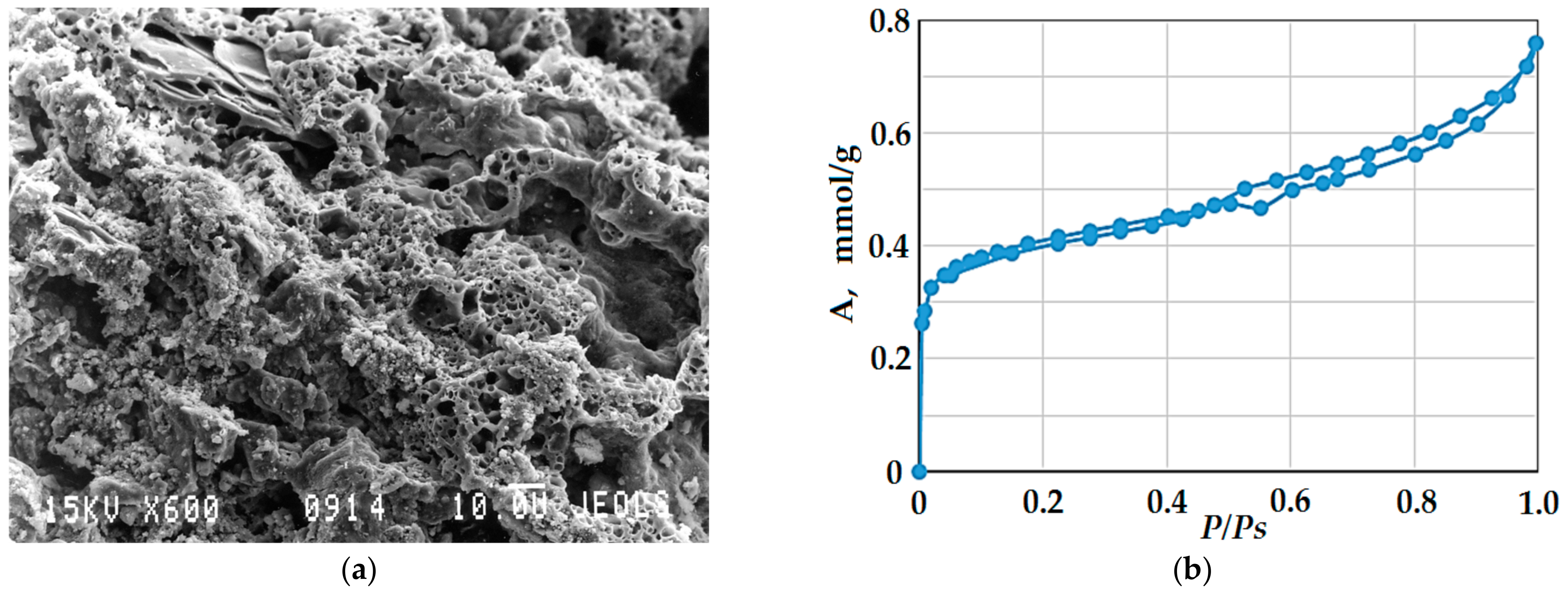
3.2.3. Formation of a Porous Structure
3.2.4. Water Absorption and Water Resistance
3.3. Application of Non-Autoclaved Ultra-Lightweight Cellular Concrete as a Building Material
4. Conclusions
- An ultra-lightweight glass-filled cellular concrete based on Portland cement, glass waste and liquid glass is proposed. A mixture of sodium hexafluorosilicate and hydroxide is used as a hardening activator, and aluminum powder serves as a gas-forming agent. Mixing of the initial components initiates a complex of hydrolytic and gas-forming exothermic reactions leading to heating (80–100 °C), foaming and subsequent solidification of the system to form a porous silicate stone for 20–40 min. The obtained material does not require additional heat treatment. By varying the ratio and dispersion of the components, a cellular material with the following characteristics can be obtained: an average density in the dry state of 150–320 kg/m3; a compressive strength and bending strength of 2.0 MPa and 0.38 MPa, respectively; a thermal conductivity coefficient of 0.05–0.09 W/(K·m); a maximum operating temperature of 800 °C. Optimal porosity and strength of the material are achieved by using a mixture of crushed cullet (modulus of fineness Fm = 0.945) with ground glass (Ssp = 450–550 m2/kg) with a mass ratio of ground/coarse equal to 1.97–2.24.
- The mechanism of formation of a durable porous structure of glass-filled cellular concrete consists of partial dissolution and subsequent joint solidification of the reaction layer at the ‘solution/glass particle’ interface due to the formation of a three-dimensional structural framework. The stabilization of the structure is provided by the reinforcing action of coarse glass particles and by the formation of insoluble compounds (silicates and aluminosilicates). The total porosity of the samples, depending on the density, reaches 68–85%, and the closed porosity is 54–76%, which causes low thermal conductivity of the samples, thereby determining high performance characteristics. The interpore walls have the structure of a solidified gel and are characterized by the presence of micropores, the size of which is 1.5–2 nm, and the specific volume of mesopores reaches 57 cm3/g. Despite the high water absorption (36–38 wt.%), the resulting porous material is characterized by high water resistance.
- Based on a comparison of the characteristics of the obtained material with known data for autoclaved and non-autoclaved lightweight cellular concretes, a conclusion was made about the possibility of using the ultra-lightweight glass-filled cellular concrete as a heat and sound insulation material, as well as a repairing composition. The cellular concrete blocks can be used for the infill masonry and for the construction of non-bearing internal walls. The proposed material has the following advantages: the energy efficiency of the production technology compared to autoclaved aerated concrete; the resource efficiency of the technology due to the use of a small proportion of cement (9–12%) and a large proportion of glass waste (38–47%); incombustibility of the material; environmental expediency due to the use of non-degradable glass waste.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Czarnecki, S.; Rudner, M. Recycling of materials from renovation and demolition of building structures in the spirit of sustainable material engineering. Buildings 2023, 13, 1842. [Google Scholar] [CrossRef]
- Roland, C.; Martin, G.; Mair, S. Sustainability and the circular economy. In Assessing Progress towards Sustainability: Frameworks, Tools and Case Studies; Elsevier: Oxford, UK, 2022; pp. 35–56. [Google Scholar]
- Adekomaya, O.; Majozi, T. Mitigating environmental impact of waste glass materials: Review of the existing reclamation options and future outlook. Environ. Sci. Pollut. Res. 2021, 28, 10488. [Google Scholar] [CrossRef] [PubMed]
- Bento, A.C.; Kubaski, E.T.; Sequinel, T.; Pianaro, S.A.; Varela, J.A.; Tebcherani, S.M. Glass foam of macroporosity using glass waste and sodium hydroxide as the foaming agent. Ceram. Int. 2013, 39, 2423. [Google Scholar] [CrossRef]
- Chong, F.; Jianwei, L.; Gao, Y.; Dagestani, A.A.; Liu, W.; Luo, X.; Baobao, Z.; Wu, H.; Huang, M.; Lifu, L.; et al. Recycling of waste glass as raw materials for the preparation of self-cleaning, light-weight and high-strength porous ceramics. J. Clean. Prod. 2021, 317, 128395. [Google Scholar]
- Andreola, F.; Lancellotti, I.; Taurino, R.; Leonelli, C.; Barbieri, L. Production of cement blocks and new ceramic materials with high content of glass waste. Key Eng. Mater. 2016, 663, 34. [Google Scholar] [CrossRef]
- Heriyanto; Pahlevani, F.; Sahajwalla, V. From waste glass to building materials—An innovative sustainable solution for waste glass. J. Clean. Prod. 2018, 191, 192. [Google Scholar] [CrossRef]
- Polley, C.; Cramer, S.M.; de la Cruz, R.V. Potential for using waste glass in Portland cement concrete. J. Mater. Civ. Eng. 1998, 10, 210. [Google Scholar] [CrossRef]
- Shao, Y.; Lefort, T.; Moras, S.; Rodriguez, D. Studies on concrete containing ground waste glass. Cem. Concr. Res. 2000, 30, 91. [Google Scholar] [CrossRef]
- Dyer, T.D.; Dhir, R.K. Chemical reactions of glass cullet used as cement component. J. Mater. Civ. Eng. 2001, 13, 412. [Google Scholar] [CrossRef]
- Topcu, I.B.; Canbaz, M. Properties of concrete containing waste glass. Cem. Concr. Res. 2004, 34, 267. [Google Scholar] [CrossRef]
- Corinaldesi, V.; Gnappi, G.; Moriconi, G.; Montenero, A. Reuse of ground waste glass as aggregate for mortars. Waste Manag. 2005, 25, 197. [Google Scholar] [CrossRef] [PubMed]
- Taha, B.; Nounu, G. Properties of concrete contains mixed colour waste recycled glass as sand and cement replacement. Constr. Build. Mater. 2008, 22, 713. [Google Scholar] [CrossRef]
- Patel, D.; Tiwari, R.P.; Shrivastava, R.; Yadav, R.K. Effective utilization of waste glass powder as the substitution of cement in making paste and mortar. Constr. Build. Mater. 2019, 199, 406. [Google Scholar] [CrossRef]
- Ahmad, J.; Zhou, Z.; Usanova, K.I.; Vatin, N.I.; El-Shorbagy, M.A. A Step towards concrete with partial substitution of waste glass (WG) in concrete: A review. Materials 2022, 15, 2525. [Google Scholar] [CrossRef] [PubMed]
- Younsi, A.; Mahi, M.A.; Hamami, A.E.A.; Belarbi, R.; Bastidas-Arteaga, E. High-volume recycled waste glass powder cement-based materials: Role of glass powder granularity. Buildings 2023, 13, 1783. [Google Scholar] [CrossRef]
- Rivera, J.F.; Cuarán-Cuarán, Z.I.; Vanegas-Bonilla, N.; Mejía de Gutiérrez, R. Novel use of waste glass powder: Production of geopolymeric tiles. Adv. Powder Technol. 2018, 29, 3448. [Google Scholar] [CrossRef]
- Henao Rios, L.M.; Hoyos Triviño, A.F.; Villaquirán-Caicedo, M.A.; Mejía de Gutiérrez, R. Effect of the use of waste glass (as precursor, and alkali activator) in the manufacture of geopolymer rendering mortars and architectural tiles. Constr. Build. Mater. 2023, 363, 129760. [Google Scholar] [CrossRef]
- Adesina, A. Durability and microstructural characteristics of alkali activated materials made with waste glass as precursor: A review. Clean. Mater. 2022, 6, 100134. [Google Scholar] [CrossRef]
- Tushar, Q.; Salehi, S.; Santos, J.; Zhang, G.; Bhuiyan, M.A.; Arashpour, M.; Giustozzi, F. Application of recycled crushed glass in road pavements and pipeline bedding: An integrated environmental evaluation using LCA. Sci. Total Environ. 2023, 881, 163488. [Google Scholar] [CrossRef]
- Abu Salem, Z.T.; Khedawi, T.S.; Baker, M.B.; Abendeh, R. Effect of waste glass on properties of asphalt concrete mixtures. Jordan J. Civ. Eng. 2017, 11, 117. [Google Scholar]
- Kalampokis, S.; Kalama, D.; Kesikidou, F.; Stefanidou, M.; Manthos, E. Assessment of waste glass incorporation in asphalt concrete for surface layer construction. Materials 2023, 16, 4938. [Google Scholar] [CrossRef] [PubMed]
- Burghardt, T.E.; Ettinger, K.; Köck, B.; Hauzenberger, C. Glass beads for road markings and other industrial usage: Crystallinity and hazardous elements. Case Stud. Constr. Mater. 2022, 17, e01213. [Google Scholar] [CrossRef]
- Cozzarini, L.; Marsich, L.; Ferluga, A.; Schmid, C. Life cycle analysis of a novel thermal insulator obtained from recycled glass waste. Dev. Built Environ. 2020, 3, 100014. [Google Scholar] [CrossRef]
- Cozzarini, L.; De Lorenzi, L.; Barago, N.; Sbaizero, O.; Bevilacqua, P. Expanded Glass for Thermal and Acoustic Insulation from Recycled Post-Consumer Glass and Textile Industry Process Waste. Materials 2023, 16, 1721. [Google Scholar] [CrossRef] [PubMed]
- König, J.; Petersen, R.R.; Yue, Y. Fabrication of highly insulating foam glass made from CRT panel glass. Ceram. Int. 2015, 41, 9793. [Google Scholar] [CrossRef]
- Hamada, H.M.; Shi, J.; Abed, F.; Humada, A.M.; Majdi, A. Recycling solid waste to produce eco-friendly foamed concrete: A comprehensive review of approaches. J. Environ. Chem. Eng. 2023, 11, 111353. [Google Scholar] [CrossRef]
- Das, A.; Mahanwar, P. A brief discussion on advances in polyurethane applications. Adv. Ind. Eng. Polym. Res. 2020, 3, 93. [Google Scholar] [CrossRef]
- Kumar, D.; Alam, M.; Zou, P.X.W.; Sanjayan, J.G.; Memon, R.A. Comparative analysis of building insulation material properties and performance. Renew. Sustain. Energy Rev. 2020, 131, 110038. [Google Scholar] [CrossRef]
- Schiavoni, S.; D’Alessandro, F.; Bianchi, F.; Asdrubali, F. Insulation materials for the building sector: A review and comparative analysis. Renew. Sustain. Energy Rev. 2016, 62, 988. [Google Scholar] [CrossRef]
- Yang, S.; Zhang, L. Research on Properties of Rock-mineral Wool as Thermal Insulation Material for Construction. Adv. Mater. Res. 2012, 450-451, 618. [Google Scholar] [CrossRef]
- Wongkeo, W.; Chaipanich, A. Compressive strength, microstructure and thermal analysis of autoclaved and air cured structural lightweight concrete made with coal bottom ash and silica fume. Mater. Sci. Eng. A 2010, 527, 3676. [Google Scholar] [CrossRef]
- Qu, X.; Zhao, X. Previous and present investigations on the components, microstructure and main properties of autoclaved aerated concrete—A review. Constr. Build. Mater. 2017, 135, 505. [Google Scholar] [CrossRef]
- Sulhadi; Susanto; Priyanto, A.; Fuadah, A.; Aji, M.P. Performance of Porous Composite from Waste Glass on Salt Purification Process. Procedia Eng. 2017, 170, 41. [Google Scholar] [CrossRef]
- Takei, T.; Ota, H.; Dong, Q.; Miura, A.; Yonesaki, Y.; Kumada, N.; Takahashi, H. Preparation of porous material from waste bottle glass by hydrothermal treatment. Ceram. Int. 2012, 38, 2153. [Google Scholar] [CrossRef]
- Esmaily, H.; Nuranian, H. Non-autoclaved high strength cellular concrete from alkali activated slag. Constr. Build. Mater. 2012, 26, 200. [Google Scholar] [CrossRef]
- Miryuk, O.A. Porous materials based on liquid glass. MOJ App. Bio. Biomech. 2018, 2, 170. [Google Scholar]
- Liu, Y.-L.; Liu, C.; Qian, L.-P.; Wang, A.-G.; Sun, D.-S.; Guo, D. Foaming processes and properties of geopolymer foam concrete: Effect of the activator. Constr. Build. Mater. 2023, 391, 131830. [Google Scholar] [CrossRef]
- Hribar, U.; Spreitzer, M.; König, J. Applicability of water glass for the transfer of the glass-foaming process from controlled to air atmosphere. J. Clean. Prod. 2021, 282, 125428. [Google Scholar] [CrossRef]
- EN 12390-3:2019; Testing Hardened Concrete—Part 3: Compressive Strength of Test Specimens. European Committee for Standardization: Brussels, Belgium, 2019.
- EN 12390-5:2019; Testing Hardened Concrete—Part 5: Flexural Strength of Test Specimens. European Committee for Standardization: Brussels, Belgium, 2019.
- Samchenko, S.V.; Aleksandrova, O.V.; Zaitseva, A.A. Aerated concrete based on cullet and liquid glass. Mater. Sci. Forum 2019, 974, 362. [Google Scholar] [CrossRef]
- Samchenko, S.V.; Zaitseva, A.A. Possibility of the use of ground glass break in the production of aerated concrete. Solid State Phenom. 2022, 334, 233. [Google Scholar] [CrossRef]
- Huang, Z.; Zhang, T.; Wen, Z. Proportioning and characterization of Portland cement-based ultra-lightweight foam concretes. Constr. Build. Mater. 2015, 79, 390. [Google Scholar] [CrossRef]
- Michelini, E.; Ferretti, D.; Miccoli, L.; Parisi, F. Autoclaved aerated concrete masonry for energy efficient buildings: State of the art and future developments. Constr. Build. Mater. 2023, 402, 132996. [Google Scholar] [CrossRef]
- Chaipanich, A.; Chindaprasirt, P. The properties and durability of autoclaved aerated concrete masonry blocks. In Eco-Efficient Masonry Bricks and Blocks: Design, Properties and Durability; Woodhead Publishing: Cambridge, UK, 2015; pp. 215–230. [Google Scholar]
- EN 771-4:2011+A1:2015; Specification for Masonry Units—Part 4: Autoclaved Aerated Concrete Masonry Units. European Committee for Standardization: Brussels, Belgium, 2015.
- ASTM C1691-21; Standard Specification for Unreinforced Autoclaved Aerated Concrete (AAC) Masonry Units. Annual Book of ASTM Standards, Volume 04.05. ASTM International: West Conshohocken, PA, USA, 2021.
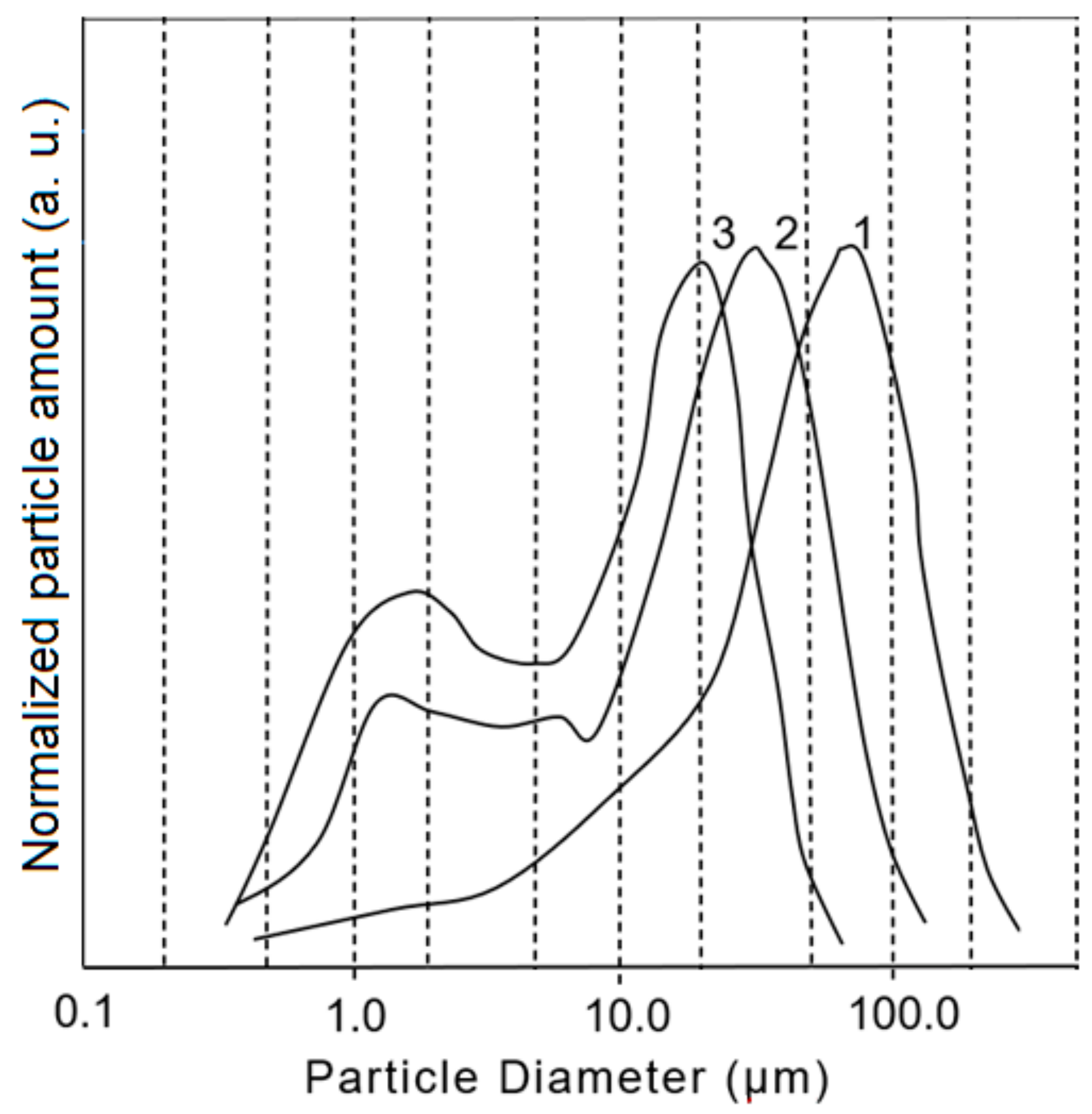
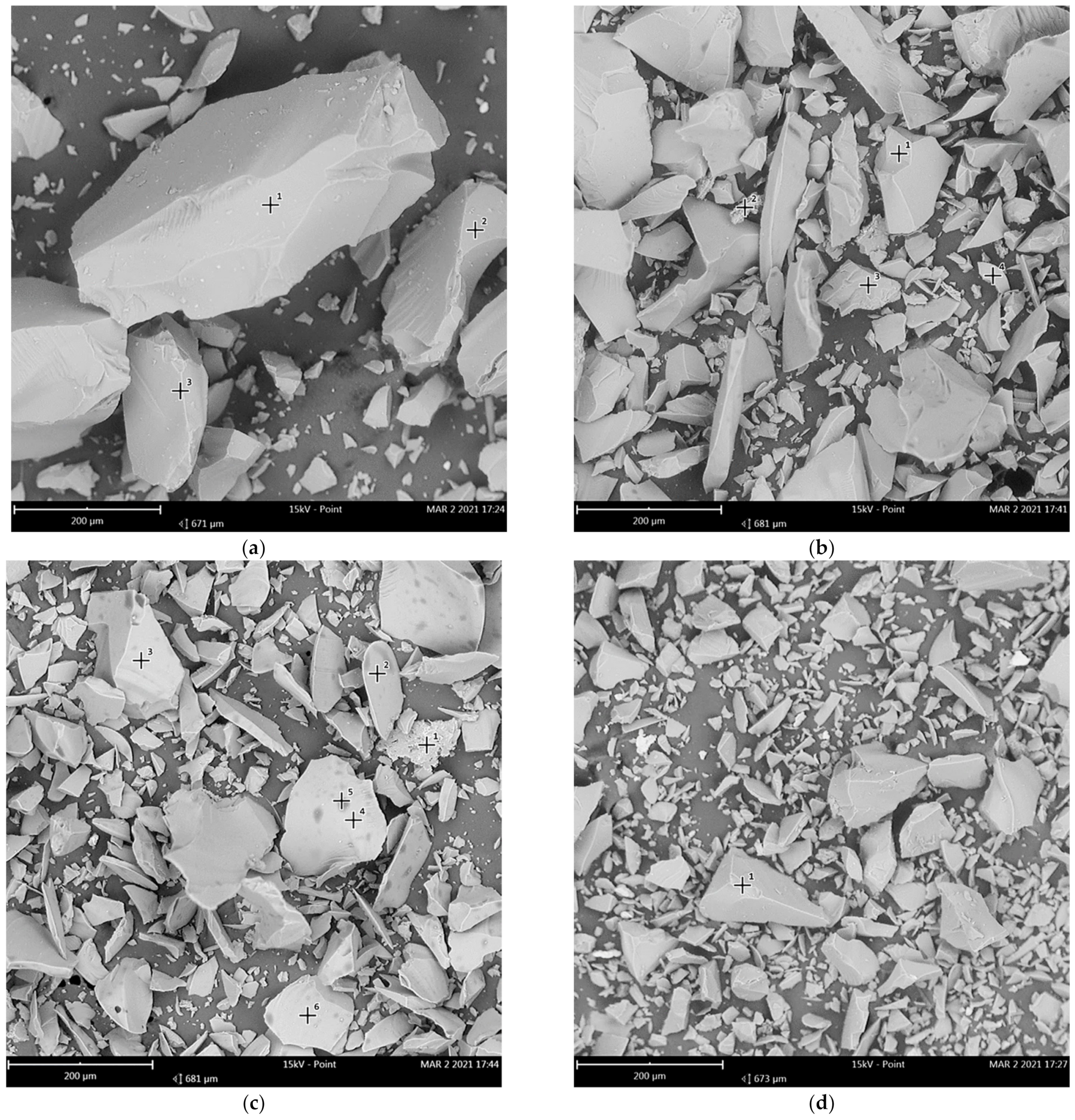

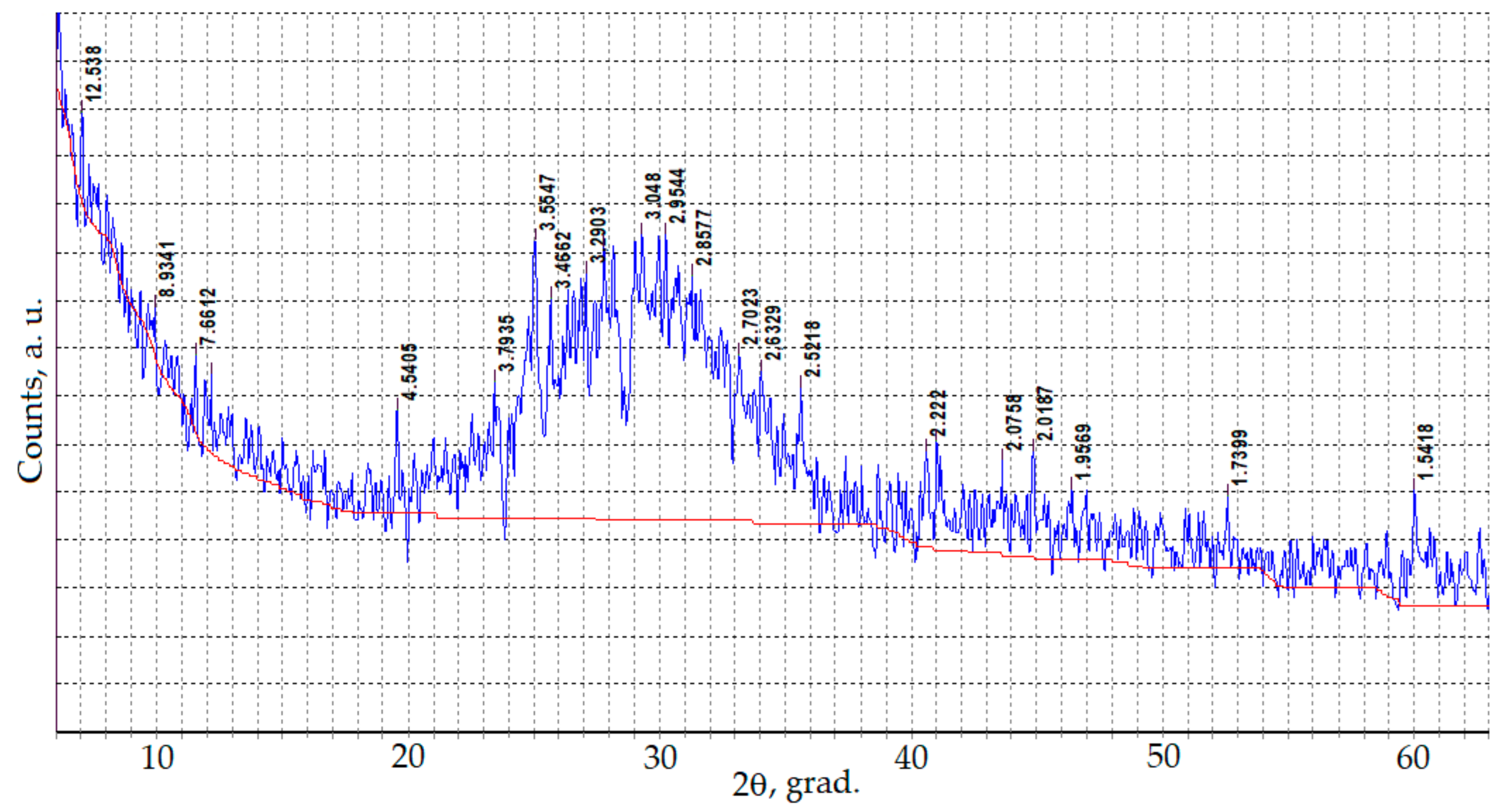



| Oxide | SiO2 | Al2O3 | Fe2O3 | CaO + MgO | Na2O + K2O | SO3 |
|---|---|---|---|---|---|---|
| Content, wt.% | 71.5–72.6 | 2–2.6 | 0.1–0.25 | 10–10.5 | 15.0–16.0 | 0.3–0.4 |
| Particle Size | Mass Fraction, wt.% | |||
|---|---|---|---|---|
| Coarse Cullet | Milled Cullet, Ssp = 450 m2/kg | Milled Cullet, Ssp = 500 m2/kg | Milled Cullet, Ssp = 550 m2/kg | |
| d > 1 mm | 10.5 | 0 | 0 | 0 |
| 0.2 < d < 1.0 mm | 21.5 | 5.2 | 1.7 | 1.9 |
| 0.063 < d < 0.2 mm | 49.3 | 76.1 | 89.7 | 91.3 |
| d < 0.063 mm | 13.2 | 18.7 | 8.6 | 6.8 |
| Sample | Glass Particle Size, μm | |||||
|---|---|---|---|---|---|---|
| Volume Mean D43 | Surface Mean D32 | Arithmetic Mean D10 | ||||
| μm3 | Ratio to the Reference Sample | μm2 | Ratio to the Reference Sample | μm | Ratio to the Reference Sample | |
| Milled cullet Ssp = 450 m2/kg | 46.68 | - | 19.21 | - | 15.45 | - |
| Milled cullet Ssp = 500 m2/kg | 22.77 | 2.05 | 11.43 | 1.68 | 12.16 | 1.27 |
| Milled cullet Ssp = 550 m2/kg | 22.34 | 2.09 | 11.08 | 1.73 | 11.61 | 1.33 |
| Parameter | Milled Glass to Crushed Glass (M/C) Ratio | ||||||
|---|---|---|---|---|---|---|---|
| 1.5 | 1.97 | 2.14 | 2.24 | 2.5 | 3.23 | 3.5 | |
| Thermal conductivity coefficient W/(m × K) | 0.05 | 0.06 | 0.06 | 0.09 | 0.095 | 0.05 | 0.015 |
| No | Liquid Glass Density, kg/m3 | Total Porosity, % | Open Porosity, % | Closed Porosity, % |
|---|---|---|---|---|
| 1 | 1350 | 68.7 | 14.63 | 54.07 |
| 2 | 1310 | 73.5 | 10.05 | 63.45 |
| 3 | 1230 | 78.9 | 10.77 | 68.13 |
| 4 | 1200 | 82.4 | 10.72 | 71.68 |
| 5 | 1130 | 85.6 | 8.90 | 76.7 |
| Liquid Glass Density, kg/m3 | Compressive Strength of Dry Cellular Concrete Rdry, MPa | Compressive Strength of Water-Saturated Cellular Concrete Rsat, MPa | Softening Coefficient, Cr |
|---|---|---|---|
| 1350 | 1.35 | 1.32 | 0.98 |
| 1310 | 1.21 | 1.17 | 0.97 |
| 1230 | 0.83 | 0.80 | 0.96 |
| 1200 | 0.57 | 0.55 | 0.96 |
| 1130 | 0.07 | 0.06 | 0.95 |
| Material | Characteristics | ||||
|---|---|---|---|---|---|
| Dry Density, kg/m3 | Compressive Strength, MPa | Thermal Conductivity, W/(K·m) | Total Porosity, % | Possible Applications | |
| Non-autoclaved ultra-lightweight glass-filled cellular concrete (present work) | 150–320 | 0.6–2 | 0.05–0.09 | 68–85 | Thermal and acoustic insulation, repairing composition, infills, non-bearing internal walls |
| Non-autoclaved ultra-lightweight foam concrete [44] | 100–300 | 0.1–1 | 0.043–0.078 | 70–80 | Thermal and acoustic insulation |
| Commercial autoclaved aerated concrete masonry [45] | 250–350 | 2–2.8 | 0.07–0.09 | ≤85 | Infills and claddings |
| Commercial non-autoclaved foamed concrete (from open sources) * | <500 | 0.5–1 | 0.15 | - | Roads and subbases, voids, mine shafts, basements and vaults, thermal insulators, complex formwork, tank filling |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Samchenko, S.V.; Korshunov, A.V. Formation of Cellular Concrete Structures Based on Waste Glass and Liquid Glass. Buildings 2024, 14, 17. https://doi.org/10.3390/buildings14010017
Samchenko SV, Korshunov AV. Formation of Cellular Concrete Structures Based on Waste Glass and Liquid Glass. Buildings. 2024; 14(1):17. https://doi.org/10.3390/buildings14010017
Chicago/Turabian StyleSamchenko, Svetlana V., and Andrey V. Korshunov. 2024. "Formation of Cellular Concrete Structures Based on Waste Glass and Liquid Glass" Buildings 14, no. 1: 17. https://doi.org/10.3390/buildings14010017
APA StyleSamchenko, S. V., & Korshunov, A. V. (2024). Formation of Cellular Concrete Structures Based on Waste Glass and Liquid Glass. Buildings, 14(1), 17. https://doi.org/10.3390/buildings14010017






