Experimental Study on MgO-Na2CO3 Combined Excitation Recycled Fine-Powder-Slag Cementitious System and Modification
Abstract
:1. Introduction
2. Experimental Materials and Methods
2.1. Raw Materials
2.2. Testing Method
2.2.1. Compressive Strength
2.2.2. pH
2.2.3. Setting Time and Fluidity
2.2.4. Dry Shrinkage
2.2.5. XRD
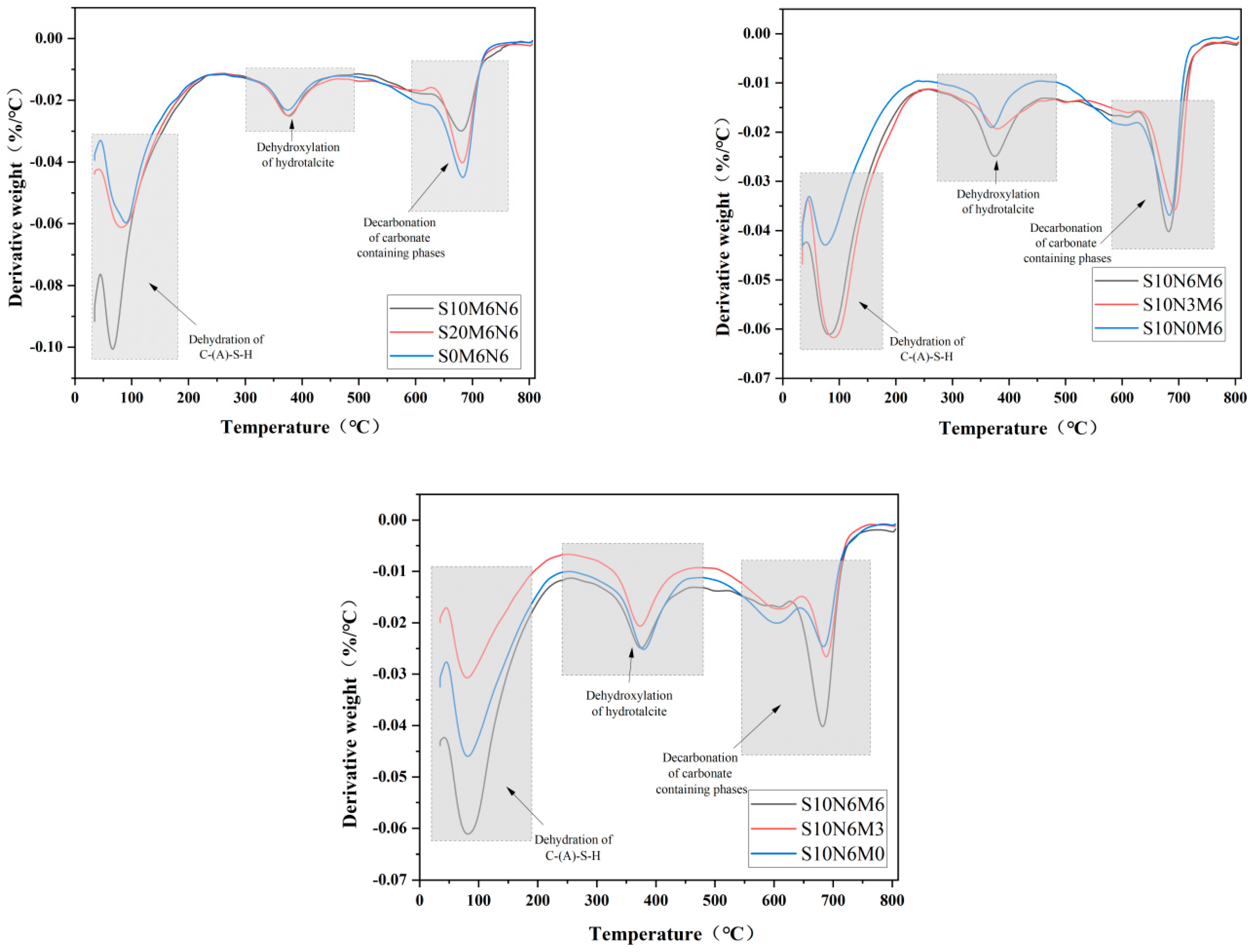
2.2.6. TG-DTG
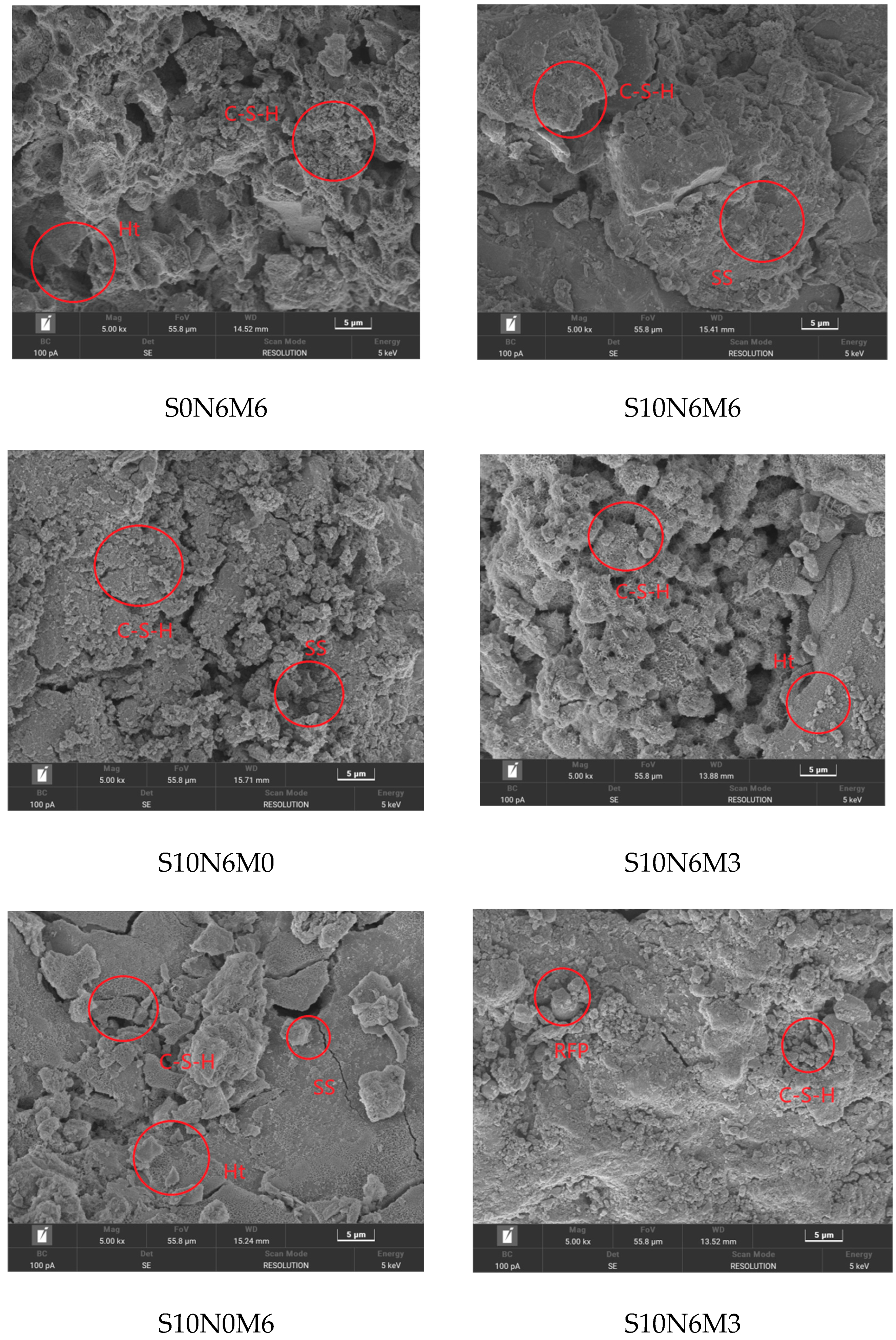
2.2.7. SEM-EDS
3. Results and Discussion
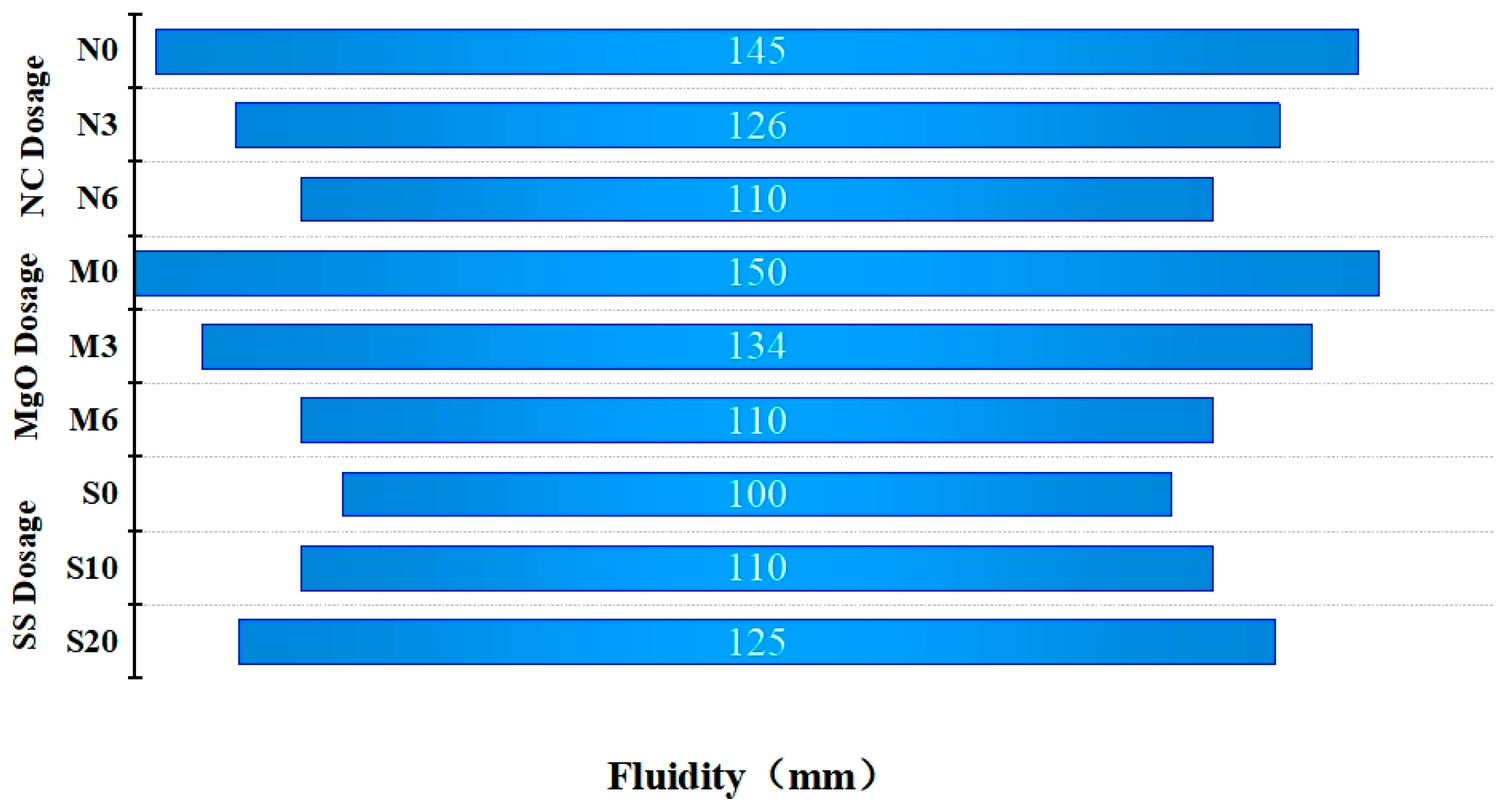
3.1. Setting Time and Fluidity
3.2. Compressive Strength
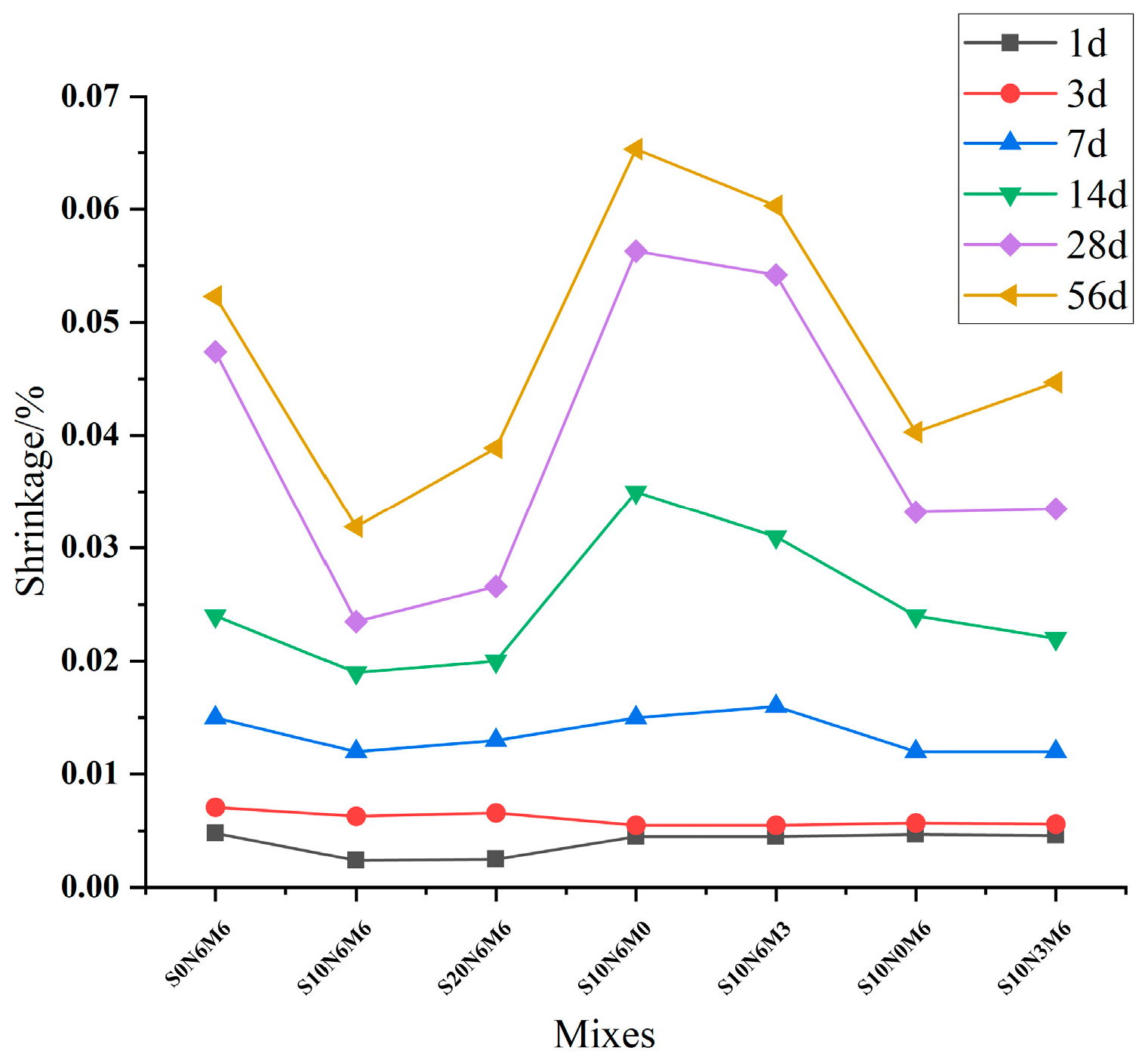
3.3. Dry Shrinkage
3.4. pH
3.5. Microscopic Testing and Analysis
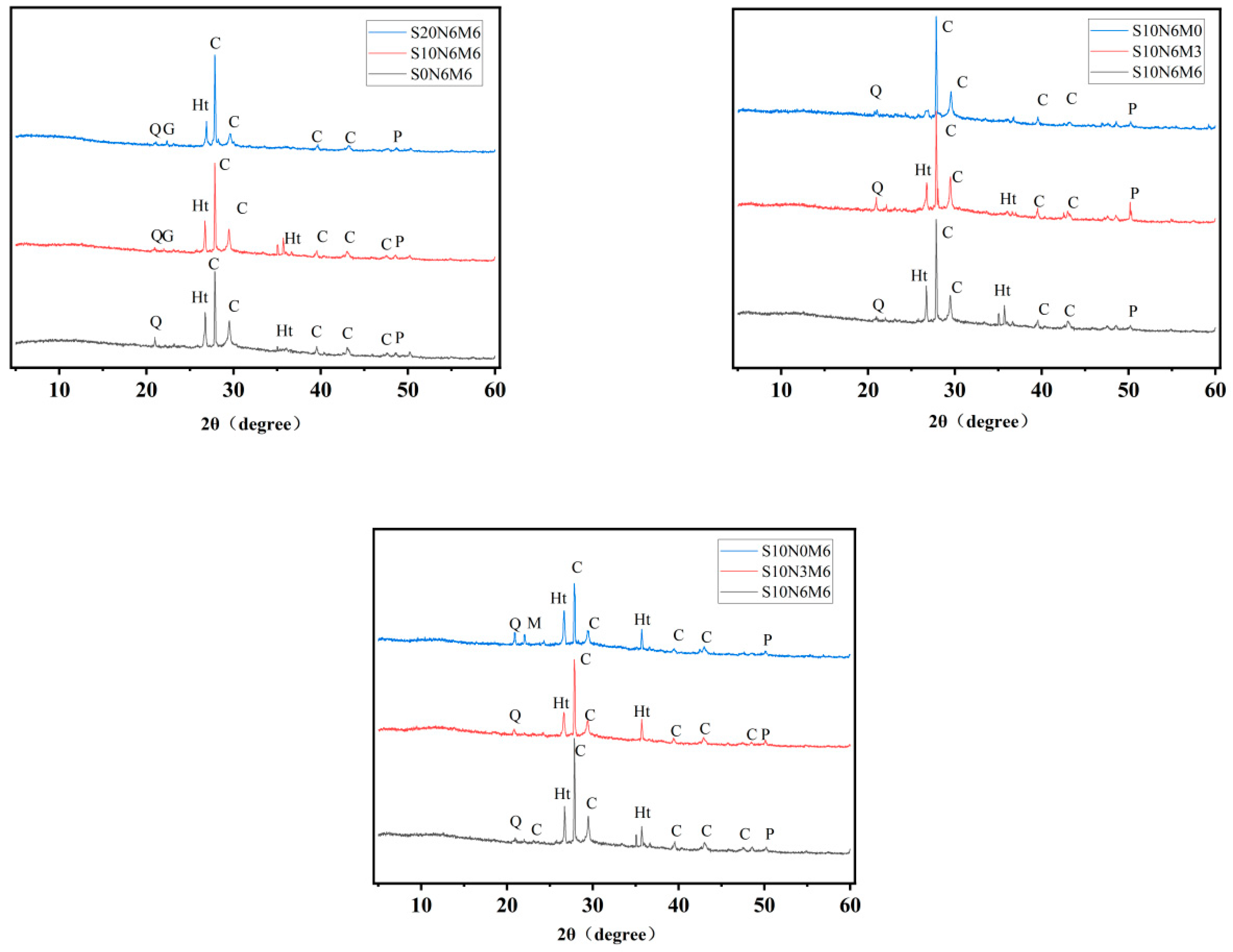
3.5.1. XRD
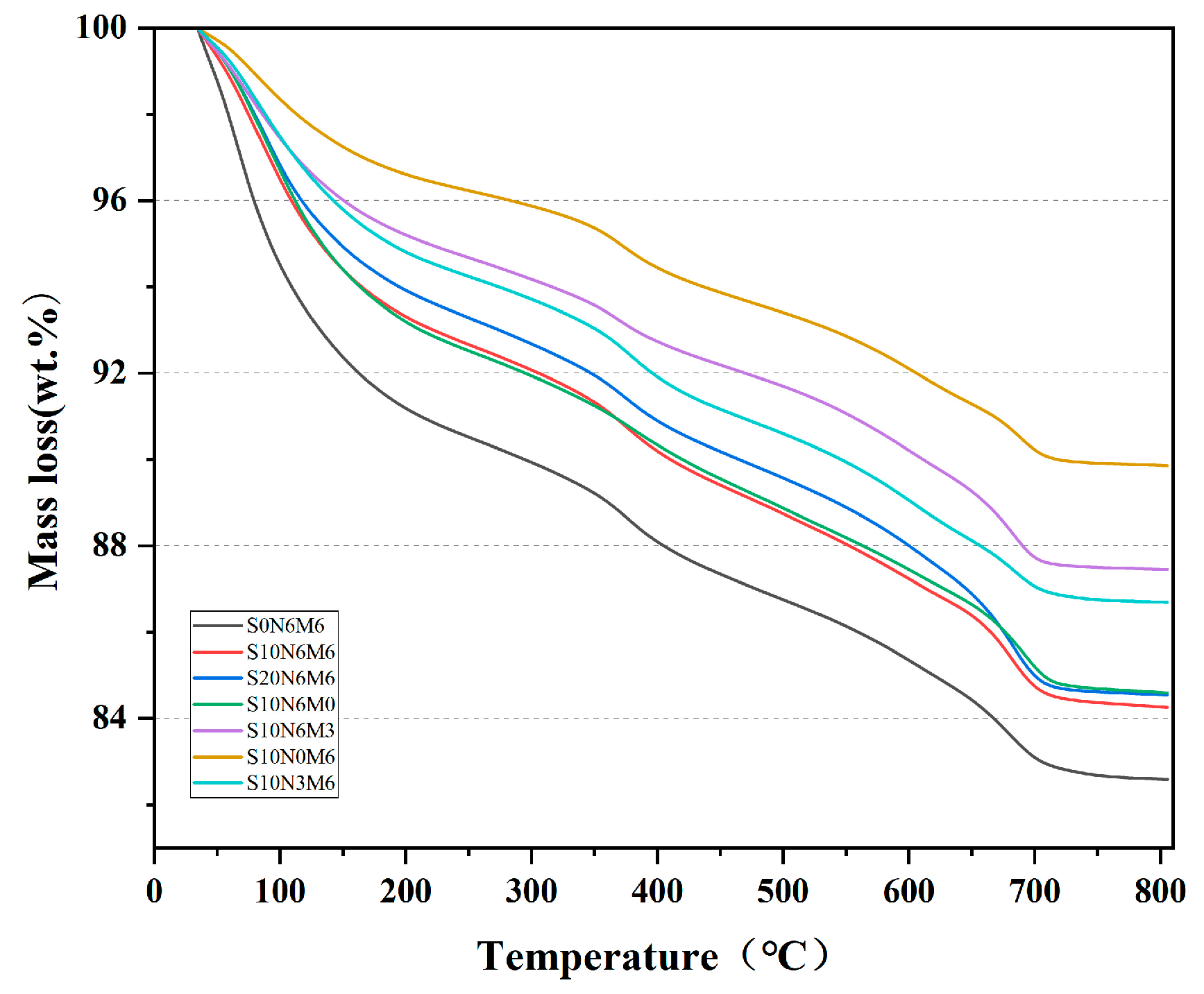
3.5.2. DG-DTG
3.5.3. SEM-EDS
3.6. Hydration Mechanism
4. Conclusions
- (1)
- Substituting 10% of slag with SS resulted in a slight reduction in early compressive strength development but exhibited higher strength compared to the Na2CO3-activated mixture at 28 days. This increased strength can be attributed to the generation of hydrotalcite, C-(A)-S-H, and C-(N, A)-S-H facilitated by Mg2+, Al3+, and Si4+ provided by MgO and SS.
- (2)
- The incorporation of MgO not only marginally decreases the solidification time of ternary composite cementitious materials but also significantly enhances the strength of the cementitious system. The addition of steel slag notably improves the fluidity of the cementitious system.
- (3)
- Rapid MgO hydration yields brucite, effectively filling pores and creating connection sites for gel products. The swift heat release during MgO dissolution accelerates the aluminosilicate precursor’s dissolution rate, resulting in a higher reaction rate between components.
- (4)
- Hydration products in BFS-RP activated using Na2CO3 primarily consist of C-(A)-S-H gel, hydrotalcite, and periclase. MgO plays a crucial role in promoting the formation of these hydration products. The introduction of CO32− by Na2CO3 and Mg2+ from reactive MgO hydrolysis significantly enhances hydrotalcite formation.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abdalqader, A.; Jin, F.; Al-Tabbaa, A. Performance of magnesia-modified sodium carbonate-activated slag/fly ash concrete. Cem. Concr. Compos. 2019, 103, 160–174. [Google Scholar] [CrossRef]
- Davidovits, J. Geopolymers. J. Therm. Anal. 1991, 37, 1633–1656. [Google Scholar] [CrossRef]
- Provis, J.L. Geopolymers and other alkali activated materials: Why, how, and what? Mater. Struct. 2014, 47, 11–25. [Google Scholar] [CrossRef]
- Amaral, L.F.; Oliveira, I.R.; Salomão, R.; Frollini, E.; Pandolfelli, V.C. Temperature and common-ion effect on magnesium oxide (MgO) hydration. Ceram. Int. 2010, 36, 1047–1054. [Google Scholar] [CrossRef]
- Barbosa, V.F.F.; MacKenzie, K.J.D. Thermal behaviour of inorganic geopolymers and composites derived from sodium polysialate. Mater. Res. Bull. 2003, 38, 319–331. [Google Scholar] [CrossRef]
- Duxson, P.; Fernández-Jiménez, A.; Provis, J.L.; Lukey, G.C.; Palomo, A.; van Deventer, J.S.J. Geopolymer technology: The current state of the art. J. Mater. Sci. 2007, 42, 2917–2933. [Google Scholar] [CrossRef]
- Scrivener, K.L.; Kirkpatrick, R.J. Innovation in use and research on cementitious material. Cem. Concr. Res. 2008, 38, 128–136. [Google Scholar] [CrossRef]
- Chen, Z.; Ye, H. Sequestration and release of nitrite and nitrate in alkali-activated slag: A route toward smart corrosion control. Cem. Concr. Res. 2021, 143, 106398. [Google Scholar] [CrossRef]
- Chen, Z.; Ye, H. Influence of metakaolin and limestone on chloride binding of slag activated by mixed magnesium oxide and sodium hydroxide. Cem. Concr. Compos. 2022, 127, 104397. [Google Scholar] [CrossRef]
- Lee, N.K.; Lee, H.K. Setting and mechanical properties of alkali-activated fly ash/slag concrete manufactured at room temperature. Constr. Build. Mater. 2013, 47, 1201–1209. [Google Scholar] [CrossRef]
- Gu, X.; Ge, X.; Liu, J.; Song, G.; Wang, S.; Hu, Z.; Wang, H. Study on the synergistic effect of calcium carbide residue -fly ash enhanced desulphurisation gypsum under high temperature maintenance condition. Constr. Build. Mater. 2024, 412, 134706. [Google Scholar] [CrossRef]
- Shi, C.; Qu, B.; Provis, J.L. Recent progress in low-carbon binders. Cem. Concr. Res. 2019, 122, 227–250. [Google Scholar] [CrossRef]
- Lin, Y.; He, T.; Da, Y.; Yang, R.; Zheng, D. Effects of recycled micro-powders mixing methods on the properties of recycled concrete. J. Build. Eng. 2023, 80, 107994. [Google Scholar] [CrossRef]
- Liu, M.; Yang, D.; Chen, L.; Chen, G.; Ma, Z. Effect of silicate modulus and alkali content on the microstructure and macroscopic properties of alkali-activated recycled powder mortar. Constr. Build. Mater. 2023, 397, 132365. [Google Scholar] [CrossRef]
- Zhang, J.; Guo, Z.; Zhu, Y.; Zhang, H.; Yan, M.; Liu, D.; Hao, J. Preparation and characterization of novel low-cost sensible heat storage materials with steel slag. J. Energy Storage 2024, 76, 109643. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, C.; Zhang, Y.; Wu, X.; Zhang, F.; Zhang, J.; Li, X. The mechanical strength, microstructure, and transport properties of steel slag reinforced loess soil system. Case Stud. Constr. Mater. 2024, 20, e02702. [Google Scholar] [CrossRef]
- Liu, J.; Song, G.; Ge, X.; Liu, B.; Liu, K.; Tian, Y.; Wang, X.; Hu, Z. Experimental Study on the Properties and Hydration Mechanism of Gypsum-Based Composite Cementitious Materials. Buildings 2024, 14, 314. [Google Scholar] [CrossRef]
- Zhuang, S.; Wang, Q.; Luo, T. A quantitative method to assess and predict the exothermic behavior of steel slag blended cement. Cem. Concr. Res. 2024, 175, 107373. [Google Scholar] [CrossRef]
- Zhu, H.; Liang, G.; Li, H.; Wu, Q.; Zhang, C.; Yin, Z.; Hua, S. Insights to the sulfate resistance and microstructures of alkali-activated metakaolin/slag pastes. Appl. Clay Sci. 2021, 202, 105968. [Google Scholar] [CrossRef]
- Ye, H.; Huang, L.; Chen, Z. Influence of activator composition on the chloride binding capacity of alkali-activated slag. Cem. Concr. Compos. 2019, 104, 103368. [Google Scholar] [CrossRef]
- Bian, Z.; Jin, G.; Ji, T. Effect of combined activator of Ca(OH)2 and Na2CO3 on workability and compressive strength of alkali-activated ferronickel slag system. Cem. Concr. Compos. 2021, 123, 104179. [Google Scholar] [CrossRef]
- Burciaga-Díaz, O.; Betancourt-Castillo, I.E.; Escalante-García, J.I. Limestone and class C fly ash blends activated with binary alkalis of Na2CO3–NaOH and MgO–NaOH: Reaction products and environmental impact. Cem. Concr. Compos. 2023, 137, 104949. [Google Scholar] [CrossRef]
- Akturk, B.; Abolfathi, M.; Ulukaya, S.; Kizilkanat, A.B.; Hooper, T.J.N.; Gu, L.; Yang, E.-H.; Unluer, C. Hydration kinetics and performance of sodium carbonate-activated slag-based systems containing reactive MgO and metakaolin under carbonation. Cem. Concr. Compos. 2022, 132, 104617. [Google Scholar] [CrossRef]
- Chen, Z.; Ye, H. The role of CaO and MgO incorporation in chloride resistance of sodium carbonate-activated slag. Cem. Concr. Compos. 2022, 132, 104625. [Google Scholar] [CrossRef]
- Dung, N.T.; Hooper, T.J.N.; Unluer, C. Enhancing the performance of MgO-activated slag-fly ash mixes by accelerated carbonation. J. CO2 Util. 2020, 42, 101356. [Google Scholar] [CrossRef]
- Wang, Y.-S.; Tae, S.-H.; Lin, R.-S.; Wang, X.-Y. Effects of Na2CO3 on engineering properties of cement–limestone powder–slag ternary blends. J. Build. Eng. 2022, 57, 104937. [Google Scholar] [CrossRef]
- GB/T17671-1999; Method of Testing Cements-Determination of Strength. Standardization Administration of China: Beijing, China, 1999.
- GB/T1346-2011; Test Methods for Water Requirement of Normal Consistency, Setting Time and Soundness of Portland Cement. Standardization Administration of China: Beijing, China, 2011.
- GB/T2419-2005; Test Method for Fluidity of Cement Mortar. National Standard: Beijing, China, 2005.
- JC/T603-2004; Standard Test Method for Drying Shinkage of Mortar. Building Materials Industry Standard: Beijing, China, 2004.
- Dung, N.T.; Hooper, T.J.N.; Unluer, C. Improving the carbonation resistance of Na2CO3-activated slag mixes via the use of reactive MgO and nucleation seeding. Cem. Concr. Compos. 2021, 115, 103832. [Google Scholar] [CrossRef]
- Yuan, X.-h.; Chen, W.; Lu, Z.-a.; Chen, H. Shrinkage compensation of alkali-activated slag concrete and microstructural analysis. Constr. Build. Mater. 2014, 66, 422–428. [Google Scholar] [CrossRef]
- Nied, D.; Enemark-Rasmussen, K.; L’Hopital, E.; Skibsted, J.; Lothenbach, B. Properties of magnesium silicate hydrates (M-S-H). Cem. Concr. Res. 2016, 79, 323–332. [Google Scholar] [CrossRef]
- Liu, T.; Li, S.; Chen, Y.; Brouwers, H.J.H.; Yu, Q. In-situ formation of layered double hydroxides in MgO–NaAlO2-activated GGBS/MSWI BA: Impact of Mg2+ on reaction mechanism and leaching behavior. Cem. Concr. Compos. 2023, 140, 105114. [Google Scholar] [CrossRef]
- Jin, F.; Gu, K.; Al-Tabbaa, A. Strength and hydration properties of reactive MgO-activated ground granulated blastfurnace slag paste. Cem. Concr. Compos. 2015, 57, 8–16. [Google Scholar] [CrossRef]




| Chemical Composition | MgO | Recycled Fine Powder | Blast Furnace Slag | Steel Slag |
|---|---|---|---|---|
| (%) by wt. | ||||
| SiO2 | 7.1 | 31.6 | 34.5 | 25.4 |
| Al2O3 | 0.4 | 4.8 | 17.7 | 5.3 |
| Fe2O3 | 0.3 | 3.5 | 1.0 | 32.2 |
| CaO | 2.4 | 31.3 | 34.0 | 9.2 |
| Na2O | - | 0.5 | 0.8 | 5.4 |
| MgO | 85.1 | 5.1 | 6.0 | 1.9 |
| SO3 | - | 0.9 | 1.6 | 3.5 |
| K2O | - | 0.7 | 1.3 | 2.5 |
| TiO2 | - | 0.2 | 0.8 | 0.3 |
| Mixes | RP | Slag | SS | NC | MgO | Water/Binder |
|---|---|---|---|---|---|---|
| S0N6M6 | 50 | 50 | 0 | 6 | 6 | 0.4 |
| S10N6M6 | 50 | 40 | 10 | 6 | 6 | |
| S20N6M6 | 50 | 30 | 20 | 6 | 6 | |
| S10N6M2 | 50 | 40 | 10 | 6 | 2 | |
| S10N6M4 | 50 | 40 | 10 | 6 | 4 | |
| S10N2M6 | 50 | 40 | 10 | 2 | 6 | |
| S10N4M6 | 50 | 40 | 10 | 4 | 6 |
| Mixes | Initial Setting Time/min | Final Setting Time/min |
|---|---|---|
| S0N6M6 | 600 | 900 |
| S10N6M6 | 620 | 930 |
| S20N6M6 | 700 | 990 |
| S10N6M0 | 1030 | 1410 |
| S10N6M3 | 790 | 1100 |
| S10N0M6 | >1d | >1d |
| S10N3M6 | 690 | 890 |
| Mixes | 1d/% | 3d/% | 7d/% | 14d/% | 28d/% | 56d/% |
|---|---|---|---|---|---|---|
| S0N6M6 | 0.0048 | 0.0071 | 0.015 | 0.024 | 0.0474 | 0.0523 |
| S10N6M6 | 0.0024 | 0.0063 | 0.012 | 0.019 | 0.0235 | 0.0319 |
| S20N6M6 | 0.0025 | 0.0066 | 0.013 | 0.020 | 0.0266 | 0.0389 |
| S10N6M0 | 0.0045 | 0.0055 | 0.015 | 0.035 | 0.0563 | 0.0653 |
| S10N6M3 | 0.0045 | 0.0055 | 0.016 | 0.031 | 0.0542 | 0.0603 |
| S10N0M6 | 0.0047 | 0.0057 | 0.012 | 0.024 | 0.0332 | 0.0403 |
| S10N3M6 | 0.0046 | 0.0056 | 0.012 | 0.022 | 0.0335 | 0.0447 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Tian, Y.; Ge, X.; Liu, B.; Liu, K.; Song, G. Experimental Study on MgO-Na2CO3 Combined Excitation Recycled Fine-Powder-Slag Cementitious System and Modification. Buildings 2024, 14, 592. https://doi.org/10.3390/buildings14030592
Liu J, Tian Y, Ge X, Liu B, Liu K, Song G. Experimental Study on MgO-Na2CO3 Combined Excitation Recycled Fine-Powder-Slag Cementitious System and Modification. Buildings. 2024; 14(3):592. https://doi.org/10.3390/buildings14030592
Chicago/Turabian StyleLiu, Jianping, Yulin Tian, Xiaowei Ge, Bing Liu, Kaixin Liu, and Ge Song. 2024. "Experimental Study on MgO-Na2CO3 Combined Excitation Recycled Fine-Powder-Slag Cementitious System and Modification" Buildings 14, no. 3: 592. https://doi.org/10.3390/buildings14030592







