Determination of ASR in Concrete Using Characterization Methods
Abstract
:1. Introduction
2. Materials and Methods
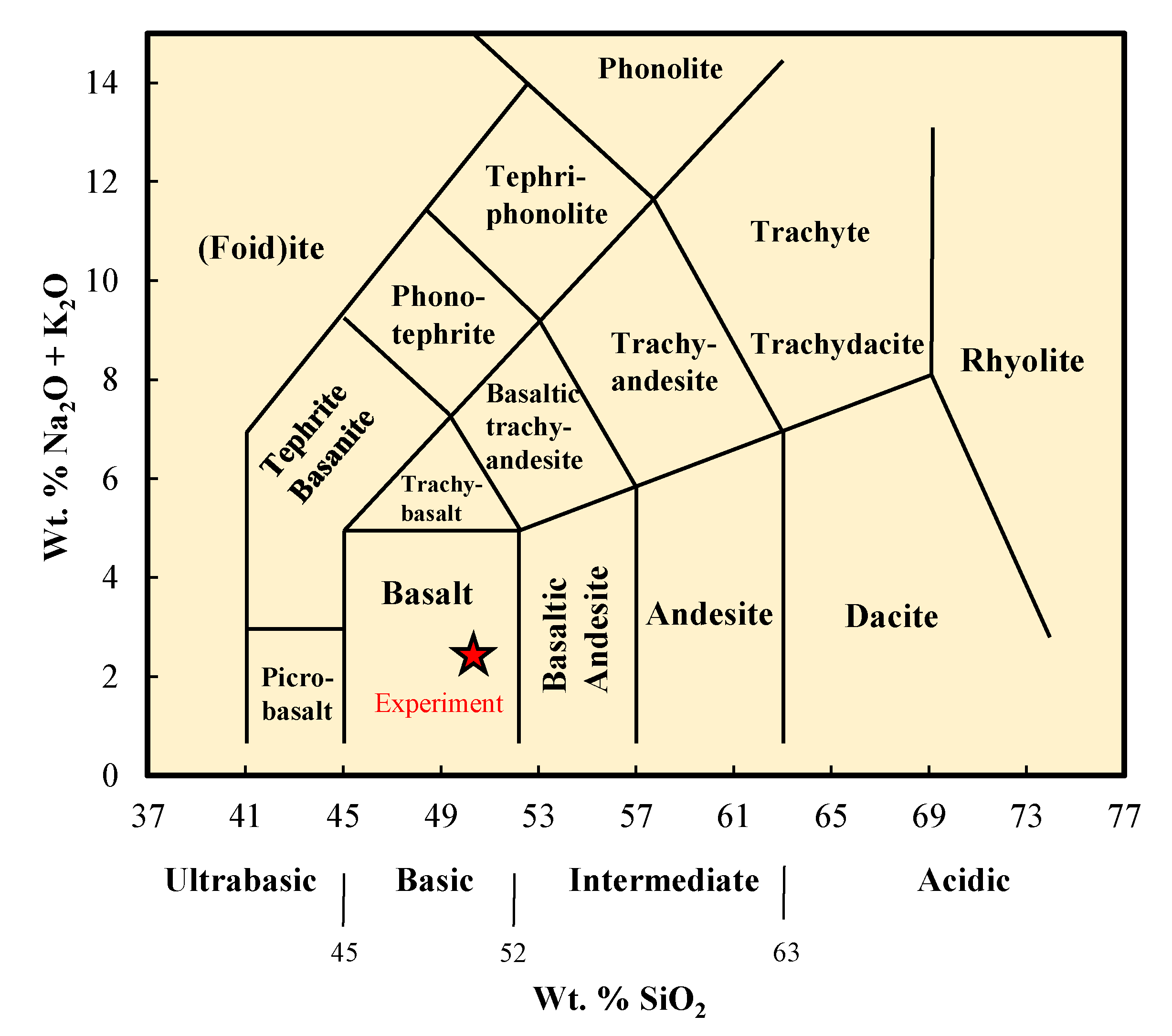
2.1. Materials
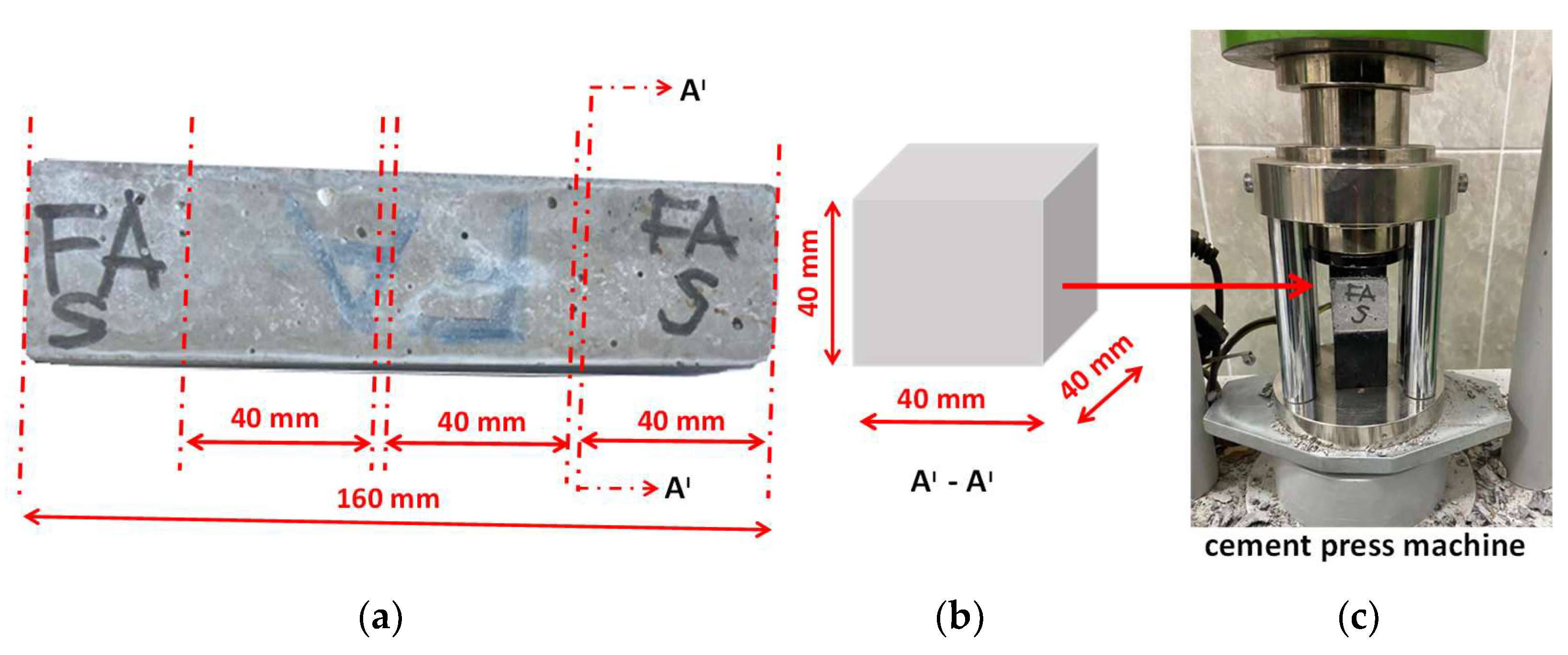
2.2. Preparation of Specimens
2.3. Mechanical Properties
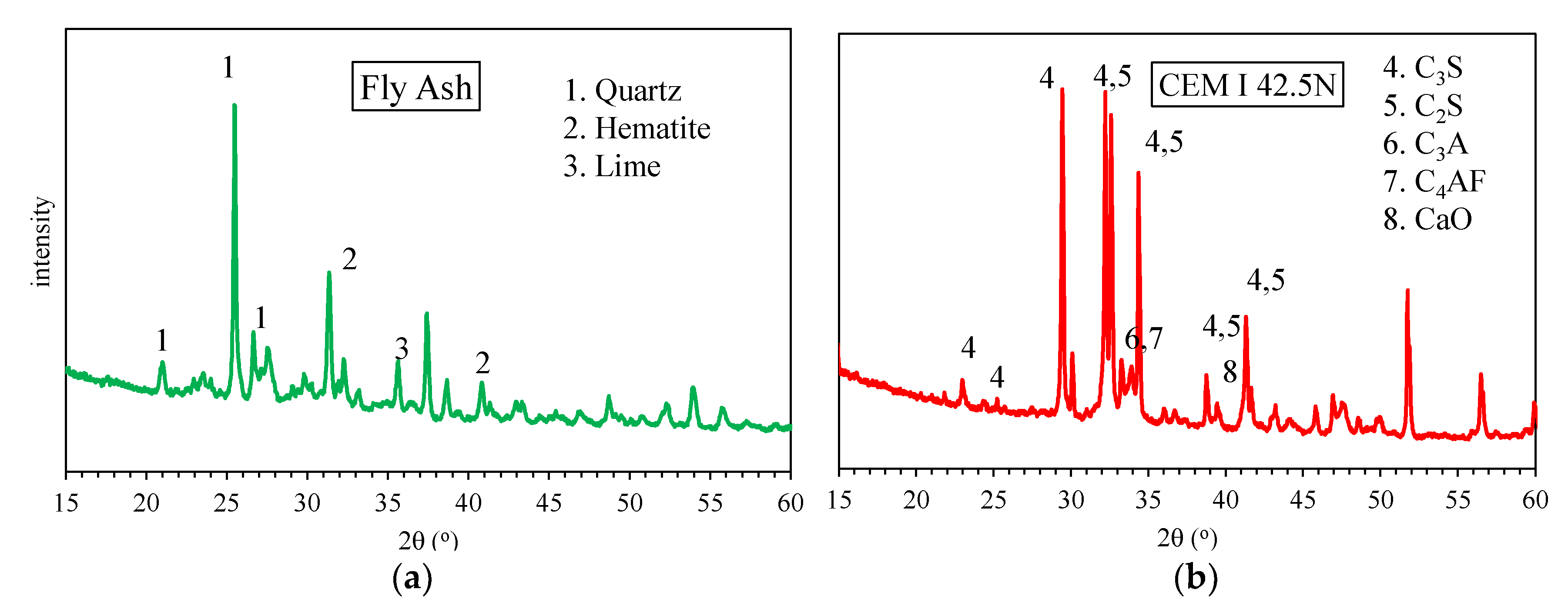
2.4. Mineralogical and Chemical Characterization
3. Results and Discussion
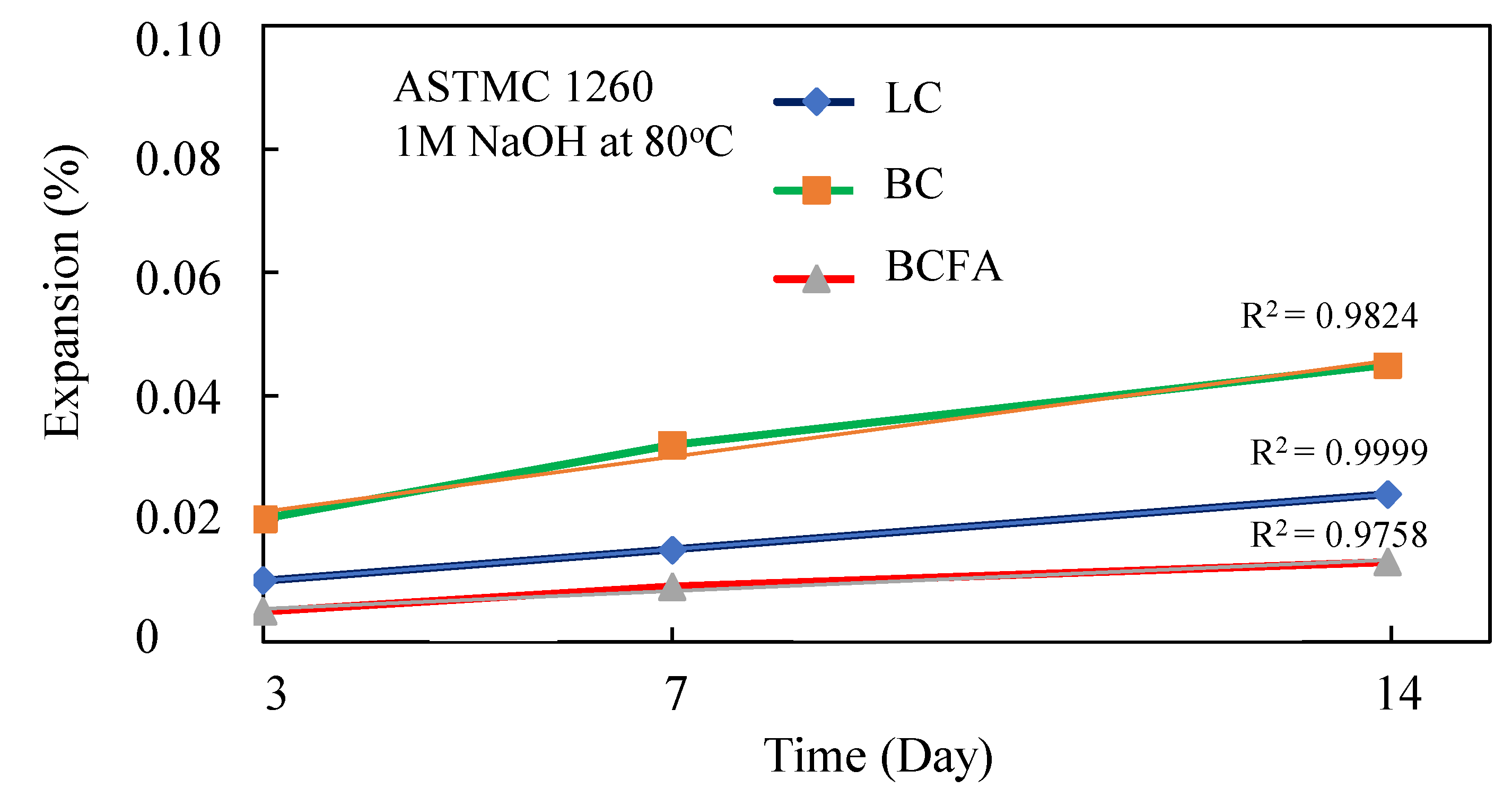
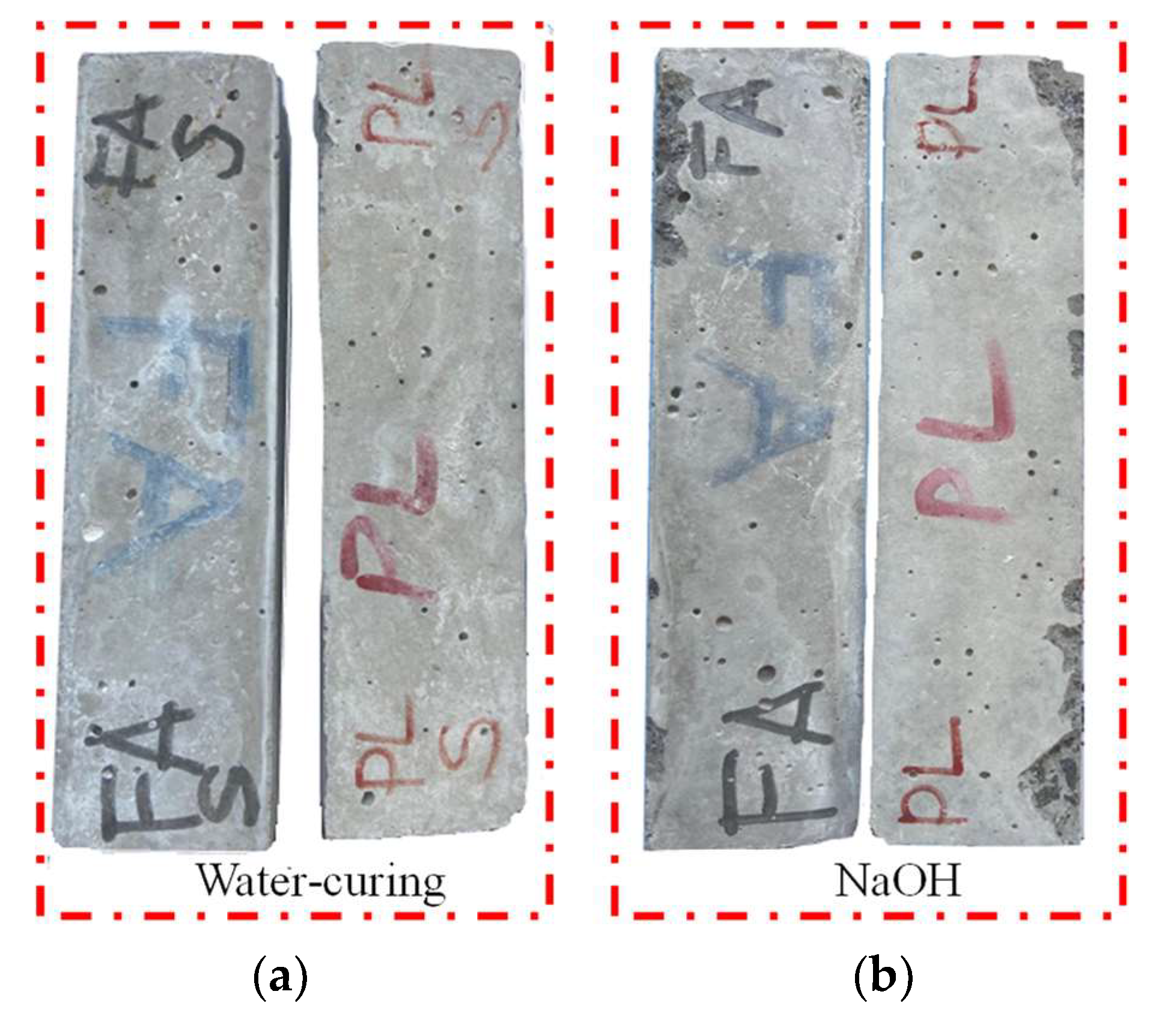
3.1. Accelerated Mortar Bar Testing (AMBT) ASTM C1260
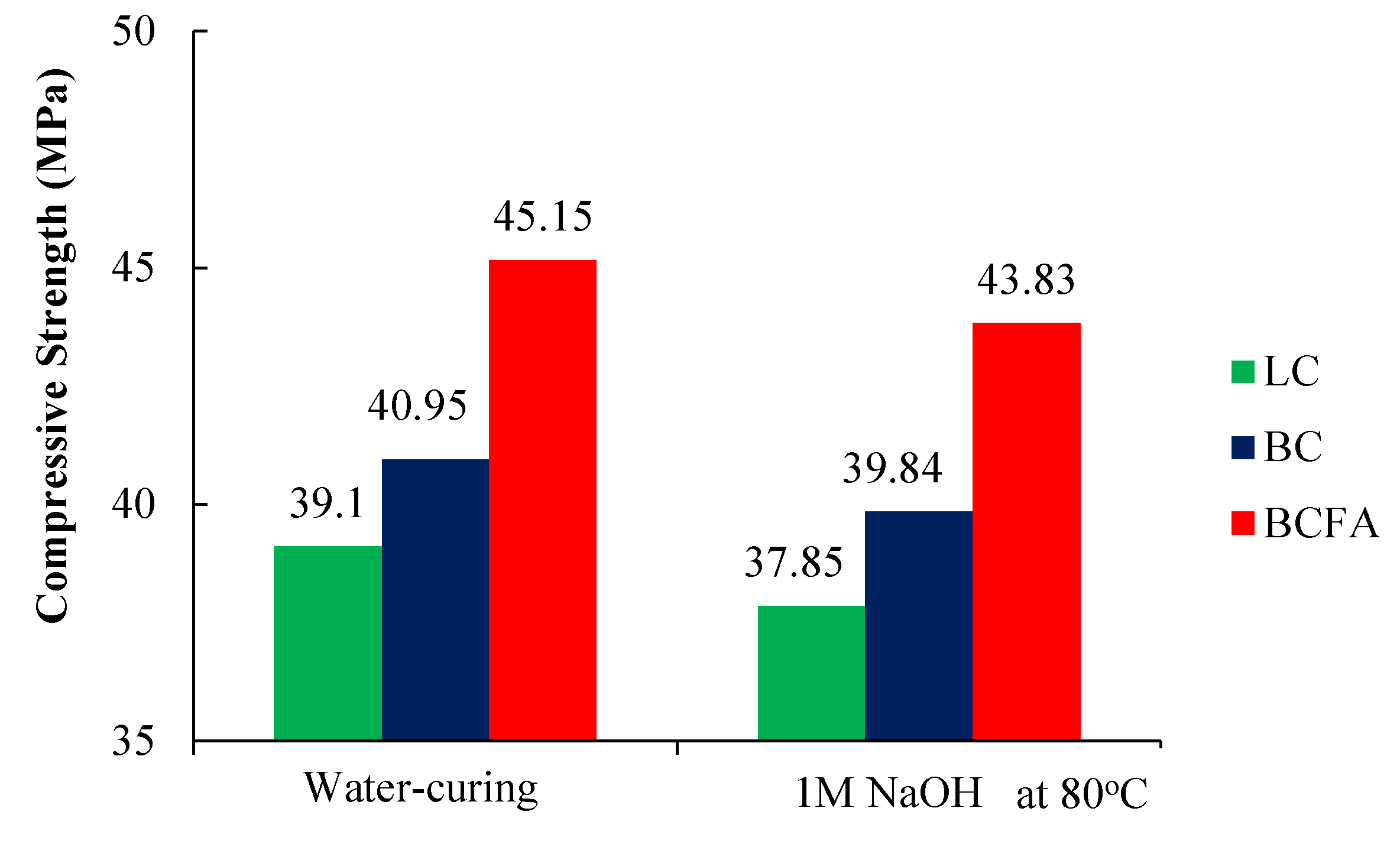
3.2. Compressive Strength
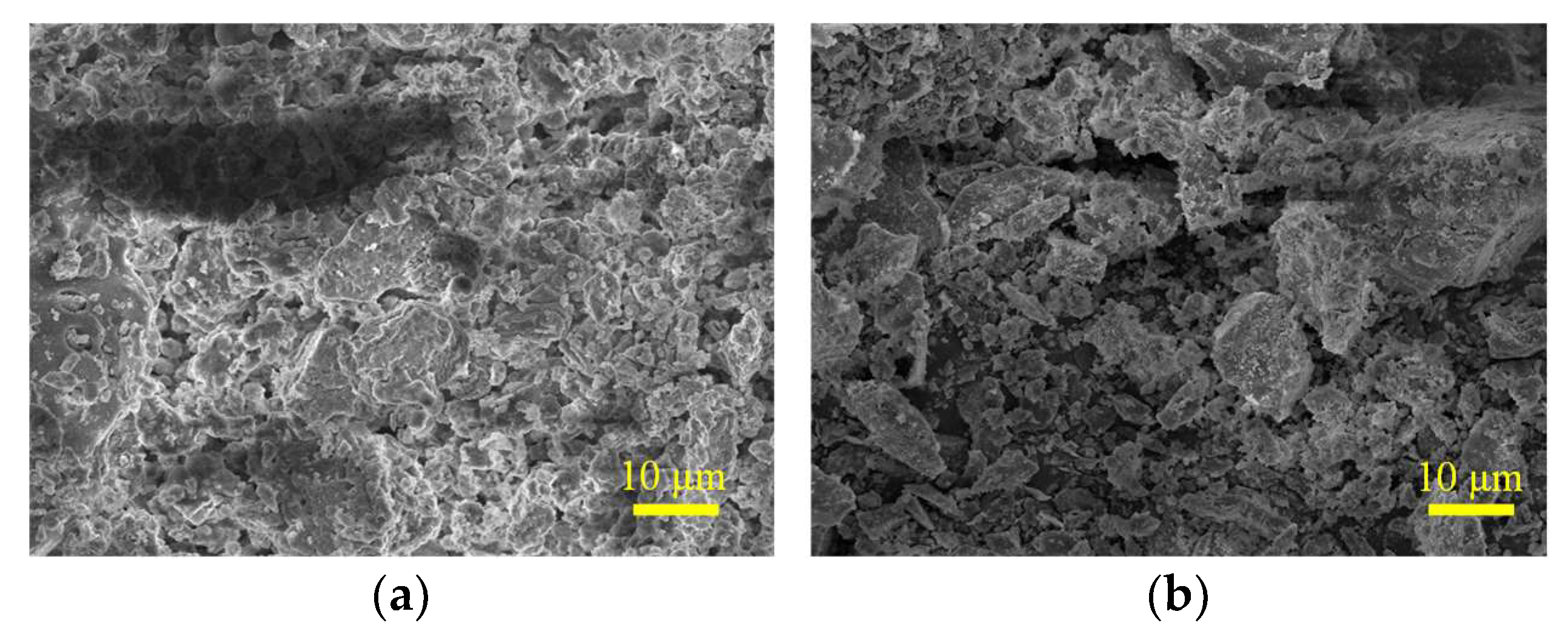
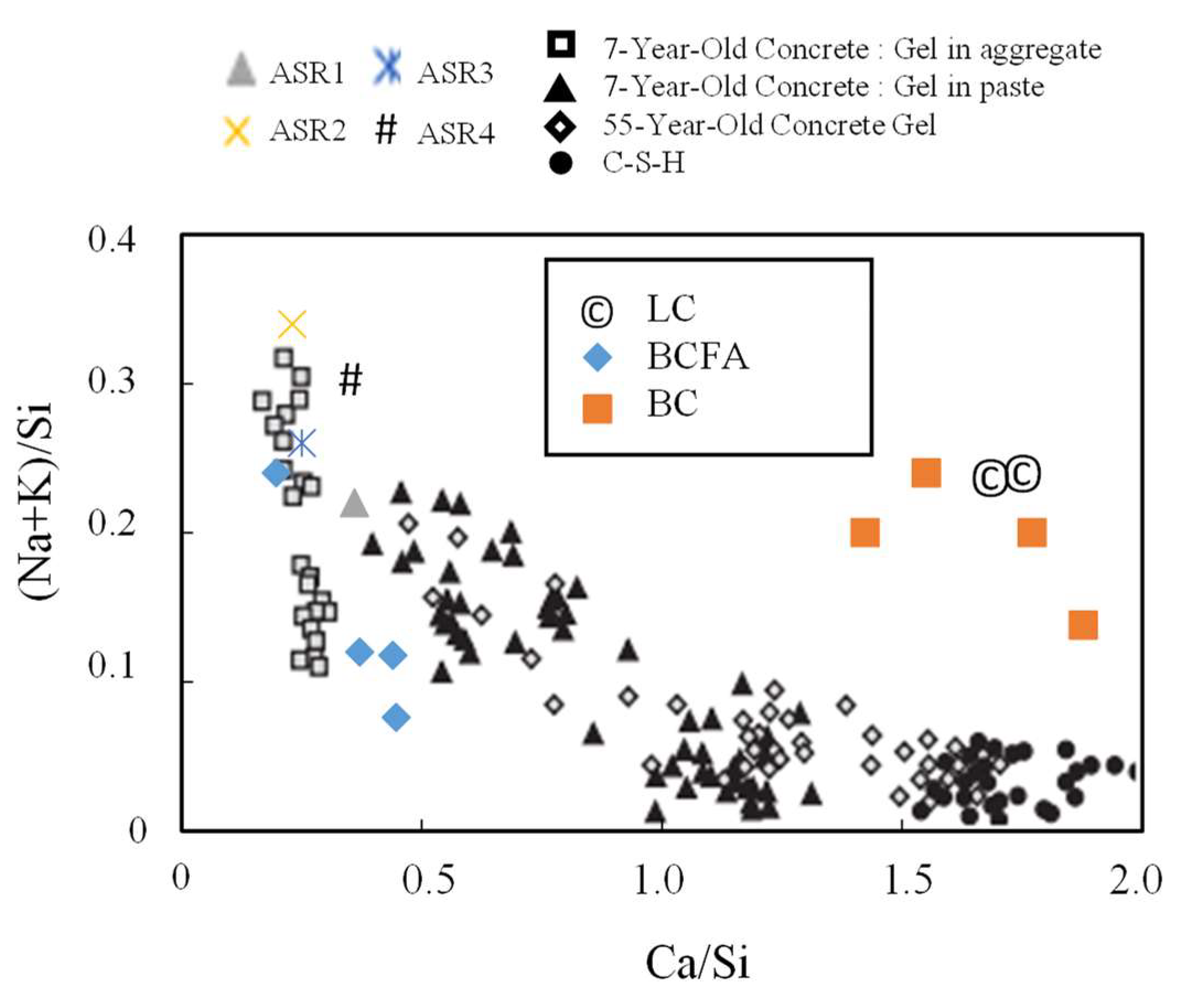
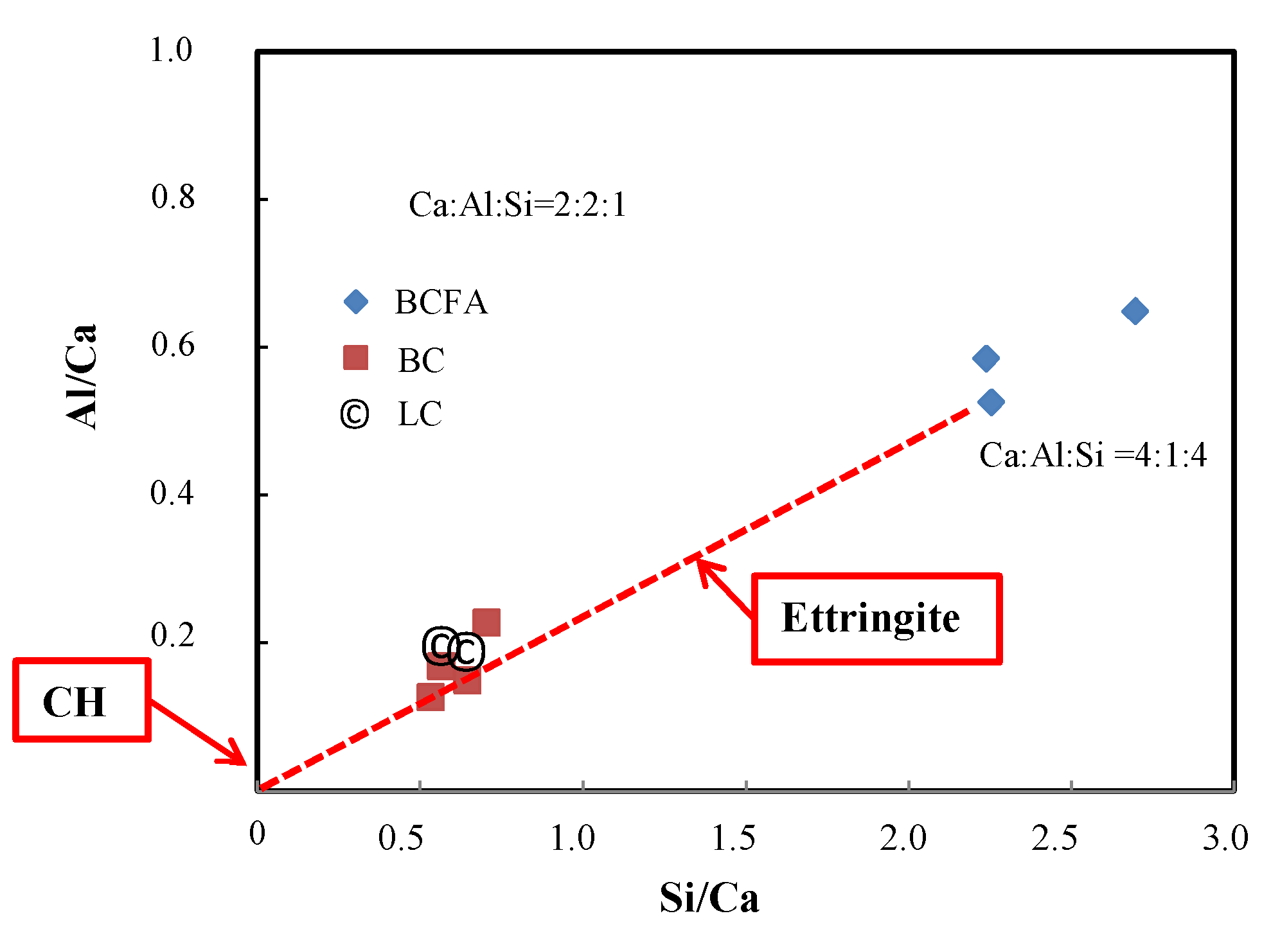
3.3. SEM-EDX Analysis
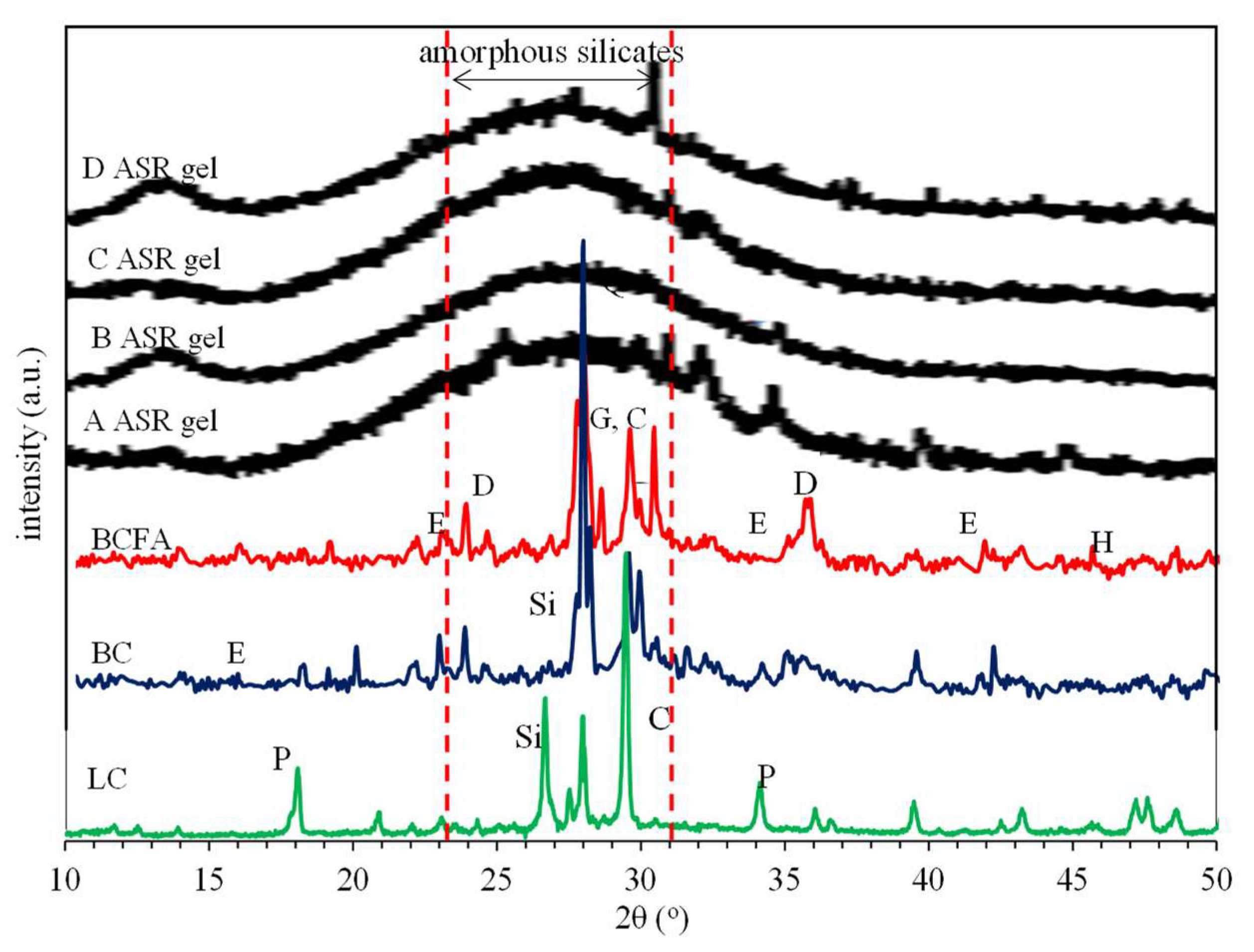
3.3.1. X-ray Diffraction (XRD)
3.3.2. FT-IR
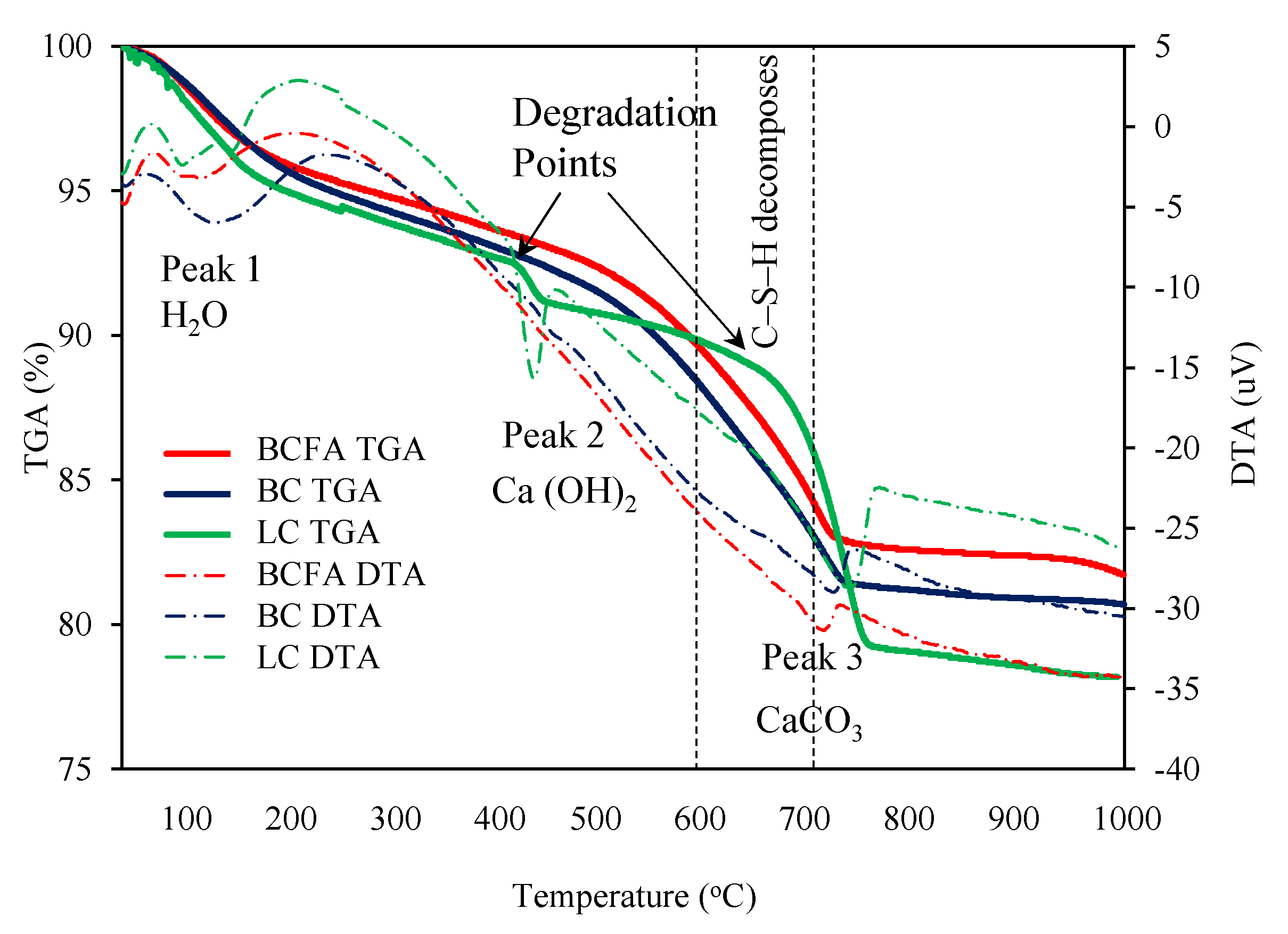
3.3.3. Thermogravimetric and Differential Thermal Analysis (TGA/DTA)
4. Conclusions
- As discussed above, the three indispensable factors for ASR are sufficient reactive silica, sufficient alkali metals, and sufficient moisture. However, ASR is difficult to understand due to its multifaceted complexity. Therefore, AMBT analysis alone may not be sufficient. Characterization studies such as SEM/EDX, XRD, FT-IR, and TGA can provide an understanding of ASR formation.
- The alkali–silica density in the medium as shown by the [(Na + K)/Si] − [Ca/Si] ratio in SEM/EDX analysis may provide some insight into the formation of ASR; however, it should be noted that this process is slow to proceed as the concrete ages and that the reaction product’s composition may eventually resemble C-S-H.
- Because of the well-known moisture retention characteristic resulting from the ASR maturation mechanism, the ASR footprint can be identified by the formation of noticeable water-induced peaks in TGA and FT-IR analyses.
- In the test conducted in accordance with ASTM C 1260 standards, it was determined that the volumetric expansion of basalt aggregate with basic petrographic structure in mortar was within the limit values, and no ASR gels were found in micro analysis. According to XRD analysis, it was determined that there was a decrease in ettringite and CH phases and an increase in C-S-H formation with the substitution of fly ash instead of cement. Therefore, it is possible to say that the substitution of basalt aggregate and fly ash increases the strength and durability of mortar.
- According to the results of the analyses, the addition of fly ash (FA) to mortars increased the strength and durability by increasing the amount of C-S-H gel. According to mechanical tests, 20% fly ash substitution instead of cement increased the compressive strength value up to 10%. However, AMBT analysis (1 M NaOH solution, 80 °C) showed a strength loss of about 3% in specimens kept for 14 days. In AMBT analysis, the volumetric expansion of basalt-based mortars decreased by 71% when 20% of fly ash was substituted for cement.
- It was concluded that igneous rocks showing basaltic properties in petrographic analysis can be used in the concrete industry since they are not reactive against ASR. In case reactive aggregates are used in concrete, it is possible to say that 20% fly ash replacement can be used as a measure against ASR.
Funding
Data Availability Statement
Conflicts of Interest
References
- Stanton, T.E. Influence of cement and aggregate on concrete expansion. Eng. News-Rec. 1940, 124, 171–173. [Google Scholar]
- Leemann, A.; Lura, P. E-modulus of the alkali-silica-reaction product determined by micro-indentation. Constr. Build. Mater. 2013, 44, 221–227. [Google Scholar] [CrossRef]
- Leemann, A. Raman microscopy of alkali-silica reaction (ASR) products formed in concrete. Cem. Concr. Res. 2017, 102, 41–47. [Google Scholar] [CrossRef]
- Shin, J.H.; Struble, L.J.; Kirkpatrick, R.J. Microstructural changes due to alkali-silica reaction during standard mortar test. Materials 2015, 8, 8292–8303. [Google Scholar] [CrossRef]
- Katayama, T. ASR gels and theır crystallıne phases ın concrete-universal products ın alkali-silica, alkali-silicate and alkali-carbonate reactions. In Proceedings of the 14th International Conference on Alkali-Aggregate Reaction (ICAAR), Austin, Texas, USA, 20–25 May 2012; pp. 20–25. [Google Scholar]
- Thaulow, N.; Jakobsen, U.H.; Clark, B. Composition of alkali silica gel and ettrıngıte ın concrete railroad ties: SEM-EDX and X-ray diffraction analyses. Cem. Concr. Res. 1996, 26, 309–318. [Google Scholar] [CrossRef]
- Peterson, K.; Gress, D.; Van Dam, T.; Sutter, L. Crystallized alkali-silica gel in concrete from the late 1890s. Cem. Concr. Res. 2006, 36, 1523–1532. [Google Scholar] [CrossRef]
- Leemann, A.; Shi, Z.; Lindgård, J. Characterization of amorphous and crystalline ASR products formed in concrete aggregates. Cem. Concr. Res. 2020, 137, 106190. [Google Scholar] [CrossRef]
- Mohammadi, A.; Ghiasvand, E.; Nili, M. Relation between mechanical properties of concrete and alkali-silica reaction (ASR); a review. Constr. Build. Mater. 2020, 258, 119567. [Google Scholar] [CrossRef]
- Fournier, B.; Bérubé, M.A.; Folliard, K.J.; Thomas, M. Report on the Diagnosis, Prognosis, and Mitigation of Alkali-Silica Reaction (ASR) in Transportation Structures (No. FHWA-HIF-09-004); Federal Highway Administration, Office of Pavement Technology: Cleveland, OH, USA, 2010.
- Bérubé, M.A.; Tremblay, C.; Fournier, B.; Thomas, M.D.; Stokes, D.B. Influence of lithium-based products proposed for counteracting ASR on the chemistry of pore solution and cement hydrates. Cem. Concr. Res. 2004, 34, 1645–1660. [Google Scholar] [CrossRef]
- Poyet, S.; Sellier, A.; Capra, B.; Foray, G.; Torrenti, J.M.; Cognon, H.; Bourdarot, E. Chemical modelling of Alkali Silica reaction: Influence of the reactive aggregate size distribution. Mater. Struct. Mater. Et Constr. 2007, 40, 229–239. [Google Scholar] [CrossRef]
- Mohammad Shahidul Islam, S.A. A Critical Assessment to the Performance of Alkali-Silica Reaction (ASR) in Concrete. Can. Chem. Trans. 2013, 1, 253–266. [Google Scholar] [CrossRef]
- Kawamura, M.; Fuwa, H. Effects of lithium salts on ASR gel composition and expansion of mortars. Cem. Concr. Res. 2003, 33, 913–919. [Google Scholar] [CrossRef]
- Hobbs, D.W. Alkali–Silica Reaction in Concrete. In Structure and Performance of Cement; Thomas Telford Publishing: London, UK, 2002; pp. 265–281. [Google Scholar]
- Ichikawa, T.; Miura, M. Modified model of alkali-silica reaction. Cem. Concr. Res. 2007, 37, 1291–1297. [Google Scholar] [CrossRef]
- Fanijo, E.O.; Kolawole, J.T.; Almakrab, A. Alkali-silica reaction (ASR) in concrete structures: Mechanisms, effects and evaluation test methods adopted in the United States. Case Stud. Constr. Mater. 2021, 15, e00563. [Google Scholar] [CrossRef]
- Katayama, T. Alkali Aggregate Reaction in The Vicinity of İzmir, Western Turkey, Alkali Aggregate Reaction In Concrete. In Proceedings of the 11th International Conference, Montréal, QC, Canada, 19–22 July 2000. [Google Scholar]
- Ma, P.; Liao, W.; Zhuo, Y.; Ma, H.; Zhu, Y.; Chen, G. Characterization of alkali-silica reaction (ASR) products and C-S-H using SWIR spectroscopy for nondestructive detection of ASR. Constr. Build. Mater. 2024, 416, 135207. [Google Scholar] [CrossRef]
- Karasin, A.; Hadzima-Nyarko, M.; Işık, E.; Doğruyol, M.; Karasin, I.B.; Czarnecki, S. The Effect of Basalt Aggregates and Mineral Admixtures on the Mechanical Properties of Concrete Exposed to Sulphate Attacks. Materials 2022, 15, 1581. [Google Scholar] [CrossRef]
- Shehata, M.H.; Thomas, M.D.A.; Bleszynski, R.F. The effects of fly ash composition on the chemistry of pore solution in hydrated cement pastes. Cem. Concr. Res. 1999, 29, 1915–1920. [Google Scholar] [CrossRef]
- Mehta, P.K.; Monteiro, P.J. Concrete: Microstructure, Properties, and Materials, 4th ed.; McGraw-Hill Companies: New York, NY, USA, 2014. [Google Scholar]
- Wen, J.; Dong, J.; Chang, C.; Xiao, X.; Zheng, W. Alkali−Silica Activity and Inhibition Measures of Concrete Aggregate in Northwest China. Crystals 2022, 12, 1013. [Google Scholar] [CrossRef]
- Šachlová, Š.; Kuchařová, A.; Pertold, Z.; Přikryl, R. Mıcroscopıc and Chemıcal Characterısatıon of ASR Induced by Quartz-Rıch Aggregates. In Proceedings of the 15th Euroseminar on Microscopy Applied to Building Materials, Delft, The Netherland, 16–19 June 2014. [Google Scholar]
- Andiç-Çakır, Ö. Investigation of Test Methods on Alkali Aggregate Reaction; Ege University Institute of Science: Bornova, Türkiye, 2007. [Google Scholar]
- Kambiz, R. Alkali-silica reaction in concrete a review. In Proceedings of the 2013 Ready Concrete Congress, İstanbul, Türkiye, 21–23 February 2013; pp. 289–311. [Google Scholar]
- Kazemi, P.; Nikudel, M.R.; Khamehchiyan, M.; Giri, P.; Taheri, S.; Clark, S.M. Assessment of Alkali–Silica Reaction Potential in Aggregates from Iran and Australia Using Thin-Section Petrography and Expansion Testing. Materials 2022, 15, 4289. [Google Scholar] [CrossRef]
- BS EN 196-6: 2018; Methods of testing cement is classified in these ICS categories: 91.100.10 Cement. Gypsum. Lime. Mortar. The British Standards Institution: London, UK, 2018.
- ASTM 204; Standard Test Methods for Fineness of Hydraulic Cement by Air-Permeability Apparatus. American Association State Highway and Transportation Officials Standard: Washington, DC, USA, 2013.
- Doğruyol, M.; Değer, M.K. Investigation of Basalt Rocks of Cizre Region in terms of Alkaline Silica (ASR). In Proceedings of the 1st International Conference on Scientific and Academic Research, Konya, Turkey, 10–13 December 2022. [Google Scholar]
- Değer, M.K.; Şırnak, T.C. The Effect of Basalt Aggregate of Cizre and Surroundings on Concrete Performance Compared to Dolomite Aggregate. Master’s Thesis, University Graduate School of Graduate Studies, Şırnak, Türkiye, 2023. [Google Scholar]
- BS 8007; Design of concrete structures for retaining aqueous liquids. BSI: London, UK, 1987.
- ASTM (American Society for Testing and Materials) Standards. Standard Test Method for the Resistance to Degradation of Small-Size Coarse Aggregates by Abrasion and Impact in the Los Angeles Machine, C131–03, v. 04.02; American Society for Testing and Materials: West Conshohocken, PA, USA, 2006. [Google Scholar]
- ASTM Standard. Test Method for Tensile Strength of Monolithic Advanced Ceramics at Ambient Temperatures, C 1273-05. Annual Book of ASTM Standards, Vol. 15.01; American Society of Testing and Materials: West Conshohocken, PA, USA, 2005. [Google Scholar]
- Kutchko, B.G.; Kim, A.G. Fly ash characterization by SEM-EDS. Fuel 2006, 85, 2537–2544. [Google Scholar] [CrossRef]
- Chancey, R.T.; Stutzman, P.; Juenger, M.C.G.; Fowler, D.W. Comprehensive phase characterization of crystalline and amorphous phases of a Class F fly ash. Cem. Concr. Res. 2010, 40, 146–156. [Google Scholar] [CrossRef]
- Li, X.; Ma, X.; Zhang, S.; Zheng, E. Mechanical properties and microstructure of class C fly ash-based geopolymer paste and mortar. Materials 2013, 6, 1485–1495. [Google Scholar] [CrossRef] [PubMed]
- Duxson, P.; Fernández-Jiménez, A.; Provis, J.L.; Lukey, G.C.; Palomo, A.; Van Deventer, J.S.J. Geopolymer technology: The current state of the art. J. Mater. Sci. 2007, 42, 2917–2933. [Google Scholar] [CrossRef]
- Granizo, M.L.; Alonso, S.; Blanco-Varela, M.T.; Palomo, A. Alkaline activation of metakaolin: Effect of calcium hydroxide in the products of reaction. J. Am. Ceram. Soc. Am. Ceram. Soc. 2002, 85, 225–231. [Google Scholar] [CrossRef]
- Yip, C.K.; Van Deventer, J.S.J. Microanalysis of calcium silicate hydrate gel formed within a geopolymeric binder. J. Mater. Sci. 2003, 38, 3851–3860. [Google Scholar] [CrossRef]
- Wang, S.D.; Scrivener, K.L.; Pratt, P.L. 0008-8846(94) 00046-8 Factors Affecting the Strength of Alkali-Activated Slag. Cem. Concr. Res. 1994, 24, 1033–1043. [Google Scholar] [CrossRef]
- Erdoğan, Y.E. Materials of Construction; M.E.T.U. Press: Ankara, Türkiye, 2002. [Google Scholar]
- Katayama, T. A Crıtıcal Revıew of Carbonate Rock Reactıons-Is Theır Reactıvıty Useful or Harmful. In Proceeding of the 9th International AAR Conference, London, UK, 27–31 July 1992; pp. 508–518. [Google Scholar]
- Rajabipour, F.; Giannini, E.; Dunant, C.; Ideker, J.H.; Thomas, M.D.A. Alkali-silica reaction: Current understanding of the reaction mechanisms and the knowledge gaps. Cem. Concr. Res. 2015, 75, 130–146. [Google Scholar] [CrossRef]
- ASTM C150-07; Standard Specification for Portland Cement. ASTM Standards: West Conshohocken, PA, USA, 2007.
- Tremblay, C.; Bérubé, M.A.; Fournier, B.; Thomas, M.D.; Folliard, K.J. Experimental investigation of the mechanisms by which LiNO3 is effective against ASR. Cem. Concr. Res. 2010, 40, 583–597. [Google Scholar] [CrossRef]
- le Maitre, R.W. A Classification of Igneous Rocks and Glossary of Terms; Blackwell Scientific Publ.: Oxford, UK, 1989. [Google Scholar]
- Katayama, T. Petrographic Study of the Alkali-aggregate Reactions in Concrete; University of Tokyo: Tokyo, Japan, 2012. [Google Scholar]
- Tapan, M. Alkali-silica reactivity of alkali volcanic rocks. Eur. J. Environ. Civ. Eng. 2015, 19, 94–108. [Google Scholar] [CrossRef]
- Korkanç, M.; Tuǧrul, A. Evaluation of selected basalts from the point of alkali-silica reactivity. Cem. Concr. Res. 2005, 35, 505–512. [Google Scholar] [CrossRef]
- ASTM C 1260; Standart Test Method for Potential Alkali Reactivity of Aggregates (Mortar-Bar Method). Annual Book of ASTM Standards: Philadelphia, PA, USA, 2007.
- Middendorf, B.; Hughes, J.J.; Callebaut, K.; Baronio, G.; Papayianni, I. Investigative methods for the characterisation of historic mortars - Part 1: Mineralogical characterization. Mater. Struct. Mater. Et Constr. 2005, 38, 761–769. [Google Scholar] [CrossRef]
- Shi, Z.; Leemann, A.; Rentsch, D.; Lothenbach, B. Synthesis of alkali-silica reaction product structurally identical to that formed in field concrete. Mater. Des. 2020, 190, 108562. [Google Scholar] [CrossRef]
- Leemann, A.; le Saout, G.; Winnefeld, F.; Rentsch, D.; Lothenbach, B. Alkali-Silica reaction: The Influence of calcium on silica dissolution and the formation of reaction products. J. Am. Ceram. Soc. 2011, 94, 1243–1249. [Google Scholar] [CrossRef]
- Wang, W.; Noguchi, T. Alkali-silica reaction (ASR) in the alkali-activated cement (AAC) system: A state-of-the-art review. Constr. Build. Mater. 2020, 252, 119105. [Google Scholar] [CrossRef]
- Thomas, M. The role of calcium hydroxide in alkali recycling in concrete. In Materials Science of Concrete Special Volume on Calcium Hydroxide in Concrete; Skalny, J., Gebauer, J., Odler, I., Eds.; American Ceramic Society: Westerville, OH, USA, 2001; pp. 269–280. [Google Scholar]
- Urhan, S. Alkali silica and pozzolanic reactions in concrete. Part 1: Interpretation of published results and an hypothesis concerning the mechanism. Cem. Concr. Res. 1987, 17, 141–152. [Google Scholar] [CrossRef]
- Hünger, K.J. The contribution of quartz and the role of aluminum for understanding the AAR with greywacke. Cem. Concr. Res. 2007, 37, 1193–1205. [Google Scholar] [CrossRef]
- García Lodeiro, I.; Fernández-Jimenez, A.; Palomo, A.; Macphee, D.E. Effect on fresh C-S-H gels of the simultaneous addition of alkali and aluminium. Cem. Concr. Res. 2010, 40, 27–32. [Google Scholar] [CrossRef]
- MTA. General Directorate of Mineral Research and Exploration; MTA: Ankara, Turkey, 1998. [Google Scholar]
- Smedskjaer, M.M.; Jensen, M.; Yue, Y.-Z. Theoretical calculation and measurement of the hardness of diopside. J. Am. Ceram. Soc. 2008, 91, 514–518. [Google Scholar] [CrossRef]
- Warr, L.N. IMA–CNMNC approved mineral symbols. Mineral. Mag. 2021, 85, 291–320. [Google Scholar] [CrossRef]
- Puchta, R. A brighter beryllium. Nat. Chem. 2011, 3, 416. [Google Scholar] [CrossRef] [PubMed]
- Deer, W.; Howie, R.; Zussman, J. An Introduction to the Rock Forming Minerals; Mineralogical Society of Great Britain and Ireland: Twickenham, UK, 1966; ISBN 0-582-44210-9. [Google Scholar]
- Tambelli, C.E.; Schneider, J.F.; Hasparyk, N.P.; Monteiro, P.J.M. Study of the structure of alkali-silica reaction gel by high-resolution NMR spectroscopy. J. Non-Cryst. Solids 2006, 352, 3429–3436. [Google Scholar] [CrossRef]
- Hamoudi, A.; Khouchaf, L.; Depecker, C.; Revel, B.; Montagne, L.; Cordier, P. Microstructural evolution of amorphous silica following alkali-silica reaction. J. Non-Cryst. Solids 2008, 354, 5074–5078. [Google Scholar] [CrossRef]
- Imaoka, M.; Hasegawa, H.; Yasui, I. X-ray diffraction study of the structure of silicate glasses. II: Alkali Disilicate Glasses. Pascal Francis Bibliogr. Databases 1983, 24, 72–78. [Google Scholar]
- Serra, J.; González, P.; Liste, S.; Serra, C.; Chiussi, S.; León, B.; Pérez-Amor, M.; Ylänen, H.O.; Hupa, M. FTIR and XPS studies of bioactive silica based glasses. J. Non-Cryst. Solids 2003, 332, 20–27. [Google Scholar] [CrossRef]
- Takadama, H.; Kim, H.M.; Kokubo, T.; Nakamura, T. Mechanism of biomineralization of apatite on a sodium silicate glass: TEM-EDX study in vitro. Chem. Mater. 2001, 13, 1108–1113. [Google Scholar] [CrossRef]
- Uchino, T.; Sakka, T.; Hotta, K.; Lwasaki, M. Attenuated Total Reflectance Fourier-Transform Infrared Spectra of a Hydrated Sodium Silicate Glass. J. Am. Ceram. Soc. 1989, 72, 2173–2175. [Google Scholar] [CrossRef]
- Webster, F.X.; Kiemle, D.J. Spectrometric Identification of Organic Compounds; John Wiley & Sons: Hoboken, NJ, USA, 2005. [Google Scholar]
- Ricol, S.; Vernaz, E.; Barboux, P. Synthesis of Gels in the System Na20-ZrO2-SiO2; Kluwer Academic Publishers: Alphen aan den Rijn, The Netherlands, 1997. [Google Scholar]
- Ping, Y.; Kirkpatrick, R.J.; Brent, P.; McMillan, P.F.; Cong, X. Structure of calcium silicate hydrate (C-S-H): Near-, mid-, and far-infrared spectroscopy. J. Am. Ceram. Soc. 1999, 82, 742–748. [Google Scholar] [CrossRef]
- Singh, M.; Waghmare, S.; Kumar, S.V. Characterization of lime plasters used in 16th century Mughal monument. J. Archaeol. Sci. 2014, 42, 430–434. [Google Scholar] [CrossRef]
- Choudhary, R.; Gupta, R.; Nagar, R. Impact on fresh, mechanical, and microstructural properties of high strength self-compacting concrete by marble cutting slurry waste, fly ash, and silica fume. Constr. Build. Mater. 2020, 239, 117888. [Google Scholar] [CrossRef]
- Chollet, M.; Horgnies, M. Analyses of the surfaces of concrete by Raman and FT-IR spectroscopies: Comparative study of hardened samples after demoulding and after organic post-treatment. Surf. Interface Anal. 2011, 43, 714–725. [Google Scholar] [CrossRef]
- Horgnies, M.; Willieme, P.; Gabet, O. Influence of the surface properties of concrete on the adhesion of coating: Characterization of the interface by peel test and FT-IR spectroscopy. Prog. Org. Coat. 2011, 72, 360–379. [Google Scholar] [CrossRef]
- Bhat, P.A.; Debnath, N.C. Theoretical and experimental study of structures and properties of cement paste: The nanostructural aspects of CSH. J. Phys. Chem. Solids 2011, 72, 920–933. [Google Scholar] [CrossRef]
- Horgnies, M.; Chen, J.J.; Bouillon, C. Overview about the Use of Fourier Transform Infrared Spectroscopy to Study Cementitious Materials; WIT Transactions on Engineering Sciences: London, UK, 2013. [Google Scholar]
- Hou, X.; Struble, L.J.; Kirkpatrick, R.J. Kanemite as a Model for ASR gel. In Proceeding of the 12th International Conference on Alkali Aggregate in Concrete (ICAAR), Beijing, China, 15–19 October 2004. [Google Scholar]
- Bažant, Z.P.; Kaplan, M.F. Concrete at High Temperatures: Material Properties and Mathematical Models; Longman Group Limited google schola: London, UK, 1996. [Google Scholar]
- Noumowe, A. Effet de Hautes Températures (20–600 C) Sur le Béton: Cas Particulier du Béton a Hautes Performances. Doctoral Dissertation, INSA, New Delhi, India, 1995. [Google Scholar]
- Platret, G. Suivi de l’hydratation du ciment et de l’évolution des phases solides dans les bétons par analyse thermique, caractéristiques microstructurales et propriétés relativesa la durabilité des bétons. Méthode D’essai 2002, 58. [Google Scholar]
- Zhang, B. Effects of moisture evaporation (weight loss) on fracture properties of high performance concrete subjected to high temperatures. Fire Saf. J. 2011, 46, 543–549. [Google Scholar] [CrossRef]
- Savva, A.; Manita, P.; Sideris, K.K. Influence of elevated temperatures on the mechanical properties of blended cement concretes prepared with limestone and siliceous aggregates. Cem. Concr. Compos. 2005, 27, 239–248. [Google Scholar] [CrossRef]
- Moon, J.; Bae, S.; Celik, K.; Yoon, S.; Kim, K.H.; Kim, K.S.; Monteiro, P.J.M. Characterization of natural pozzolan-based geopolymeric binders. Cem. Concr. Compos. 2014, 53, 97–104. [Google Scholar] [CrossRef]
- Kupwade-Patil, K.; Palkovic, S.D.; Bumajdad, A.; Soriano, C.O. Büyüköztürk, Use of silica fume and natural volcanic ash as a replacement to Portland cement: Micro and pore structural investigation using NMR, XRD, FTIR and X-ray microtomography. Constr. Build. Mater. 2018, 158, 574–590. [Google Scholar] [CrossRef]
- Annaba, K.; El Mendili, Y.; Stout, H.; Ech-chebab, A.; Ouaki, B.; Cherkaoui, M. Florence, Mechanical, electrochemical (EIS), and microstructural characterization of reinforced concrete incorporating natural volcanic pozzolan. Case Stud. Constr. Mater. 2023, 19, e02620. [Google Scholar] [CrossRef]
- Karaşin, A.; Doğruyol, M. An experimental study on strength and durability for utilization of fly ash in concrete mix. Adv. Mater. Sci. Eng. 2014, 2014, 417514. [Google Scholar] [CrossRef]





| Component | SiO2 | Al2O3 | Fe2O3 | CaO | CaCO3 | MgO | SO3 | LOI 1 | Na2Oeq * |
|---|---|---|---|---|---|---|---|---|---|
| Type 1 Portland cement | 18.12 | 5.21 | 3.03 | 62.06 | - | 2.70 | 3.21 | 3.98 | 0.72 |
| FA | 21.65 | 33.55 | 8.76 | 27.50 | 4.50 | 0.05 | 1.29 | 2.00 | |
| Basalt | 50.63 | 10.51 | 6.49 | - | 21.55 | 3.32 | - | 2.08 | 2.08 |
| 3CaO·SiO2 (C3S) | 2CaO·SiO2 (C2S) | 3CaO·Al2O3 (C3A) | 4CaO·Al2O3·Fe2O3 (C4AF) |
|---|---|---|---|
| 66.40 | 1.87 | 8.68 | 9.22 |
| Acceptance Limits | ||
|---|---|---|
| Compressive strength (MPa) | 92 | |
| Schmidt hammer (MPa) [31] | 50 | |
| Water absorption (%) | 2.70 | <3.0 [32] |
| LA abrasion loss (%) | 27 | <50 (500 cycles) [33] |
| Density (g/cm3) | 2.78 | <2.70 [34] |
| Sample Code | Sieves | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| No: 4 | No: 8 | No: 16 | No: 30 | No: 50 | |||||||
| 10% | 25% | 25% | 25% | 15% | |||||||
| Aggregate | C 1 | FA 2 | W 3 | W/B 4 | C/A 5 | ||||||
| Gram | |||||||||||
| Limestone-based | LC | 99 | 247.5 | 247.5 | 247.5 | 148.5 | 440 | - | 206.8 | 0.47 | 2.25 |
| Basalt-based | BC | 99 | 247.5 | 247.5 | 247.5 | 148.5 | 440 | - | 206.8 | 0.47 | 2.25 |
| BCFA | 99 | 247.5 | 247.5 | 247.5 | 148.5 | 352 | 88 | 206.8 | 0.47 | 2.25 | |
| Water Curing | 1M NaOH 80 °C | Strength Change (CSL), % | |
|---|---|---|---|
| LC | 39.10 Mpa | 37.85 Mpa | −3.19 |
| BC | 40.95 Mpa | 39.84 Mpa | −2.79 |
| BCFA | 45.15 Mpa | 43.83 Mpa | −3.01 |
| FAE * | +10.25% | +10.01% |
| Specimens | ||||
|---|---|---|---|---|
| LC | 0.24 | 1.72 | 16.50 | 0.20 |
| BC | 0.21 | 1.76 | 4.48 | 0.17 |
| BCFA | 0.12 | 0.45 | 4.75 | 1.17 |
| ASR 1 | 0.22 | 0.36 | 0.43 | 0.07 |
| ASR 2 | 0.34 | 0.23 | 0.76 | - |
| ASR 3 | 0.26 | 0.25 | 0.22 | - |
| ASR 4 | 0.30 | 0.39 | 0.97 | - |
| Wave Number Range (cm−1) | Assignment | Compound Formation | References |
|---|---|---|---|
| 530–558 | Si-O out-of-plane bending | Ettringite | [73,74,75] |
| 882–890 | CO32- | Carbonates | [75] |
| 950–1004 | Si-O stretching and vibration | C-S-H | [73,75,76,77] |
| 1520–1524 | CO32- | Calcium carbonate | [76,77] |
| 1580–1646 | H-O-H | - | [75,78] |
| 1640–1650 | C-H bending | Chemically bonded water | [75,78] |
| 3200–3400 | O-H | H2O | [76,79] |
| 3618–3627 | O-H | Portlandite | [74,76] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Doğruyol, M. Determination of ASR in Concrete Using Characterization Methods. Buildings 2024, 14, 657. https://doi.org/10.3390/buildings14030657
Doğruyol M. Determination of ASR in Concrete Using Characterization Methods. Buildings. 2024; 14(3):657. https://doi.org/10.3390/buildings14030657
Chicago/Turabian StyleDoğruyol, Murat. 2024. "Determination of ASR in Concrete Using Characterization Methods" Buildings 14, no. 3: 657. https://doi.org/10.3390/buildings14030657
APA StyleDoğruyol, M. (2024). Determination of ASR in Concrete Using Characterization Methods. Buildings, 14(3), 657. https://doi.org/10.3390/buildings14030657






