Experimental Study on Improving the Performance of Cement Mortar with Self-Synthesized Viscosity-Reducing Polycarboxylic Acid Superplasticizer
Abstract
1. Introduction
2. Experiment
2.1. Materials and Sample Preparation
2.1.1. Cement
2.1.2. Self-Synthesized VRPCE
2.1.3. Cement Paste
2.1.4. Cement Mortar
2.2. Methods
2.2.1. GPC
2.2.2. TOC
2.2.3. Zeta Potential
2.2.4. Cement Particle Size
2.2.5. XRD
2.2.6. MIP
2.2.7. TG
2.2.8. SEM
2.2.9. Fluidity
2.2.10. Compressive Strength
2.2.11. Shrinkage
2.2.12. Creep
3. Results
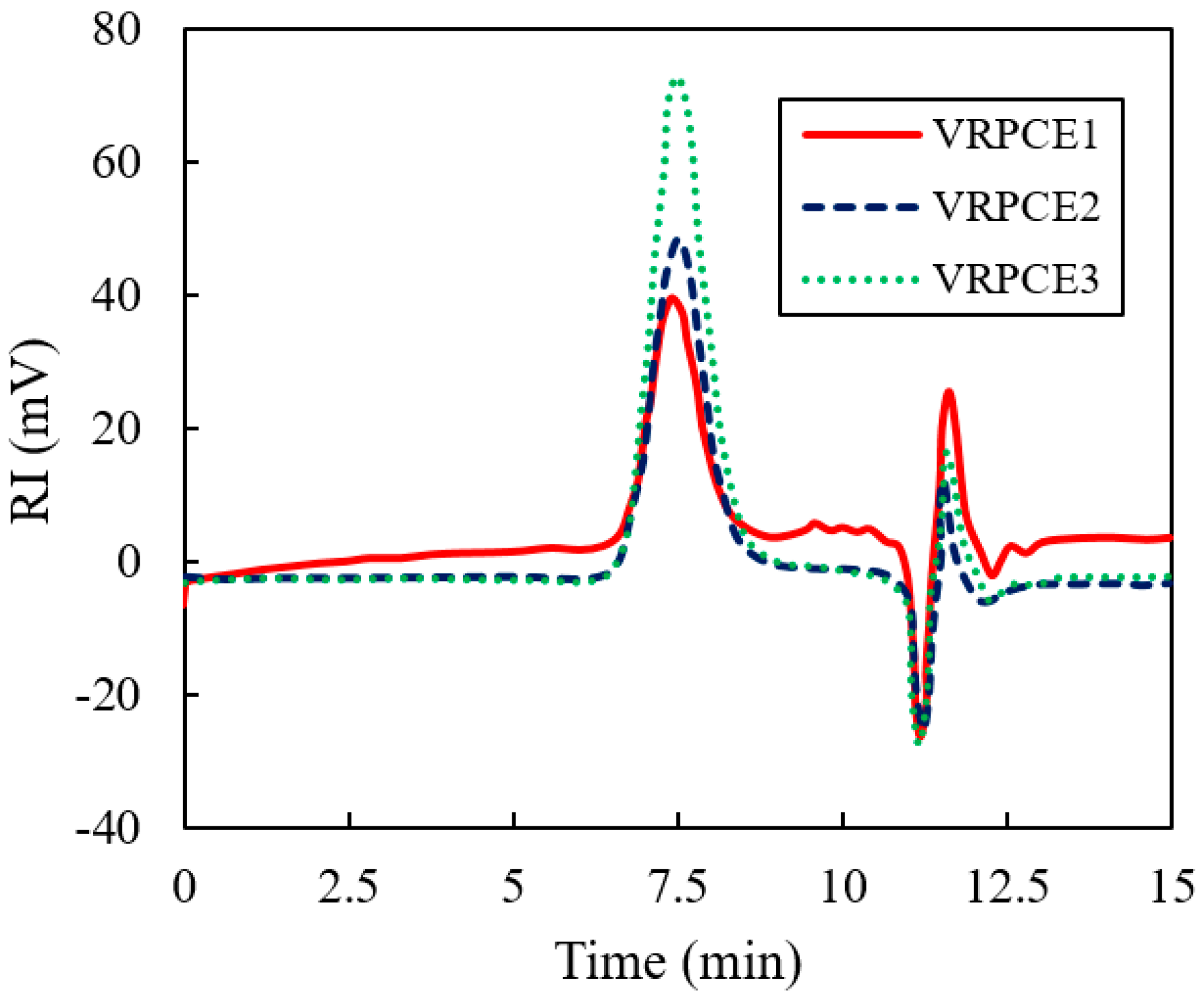
3.1. Molecular Weight and Molecular Weight Distribution
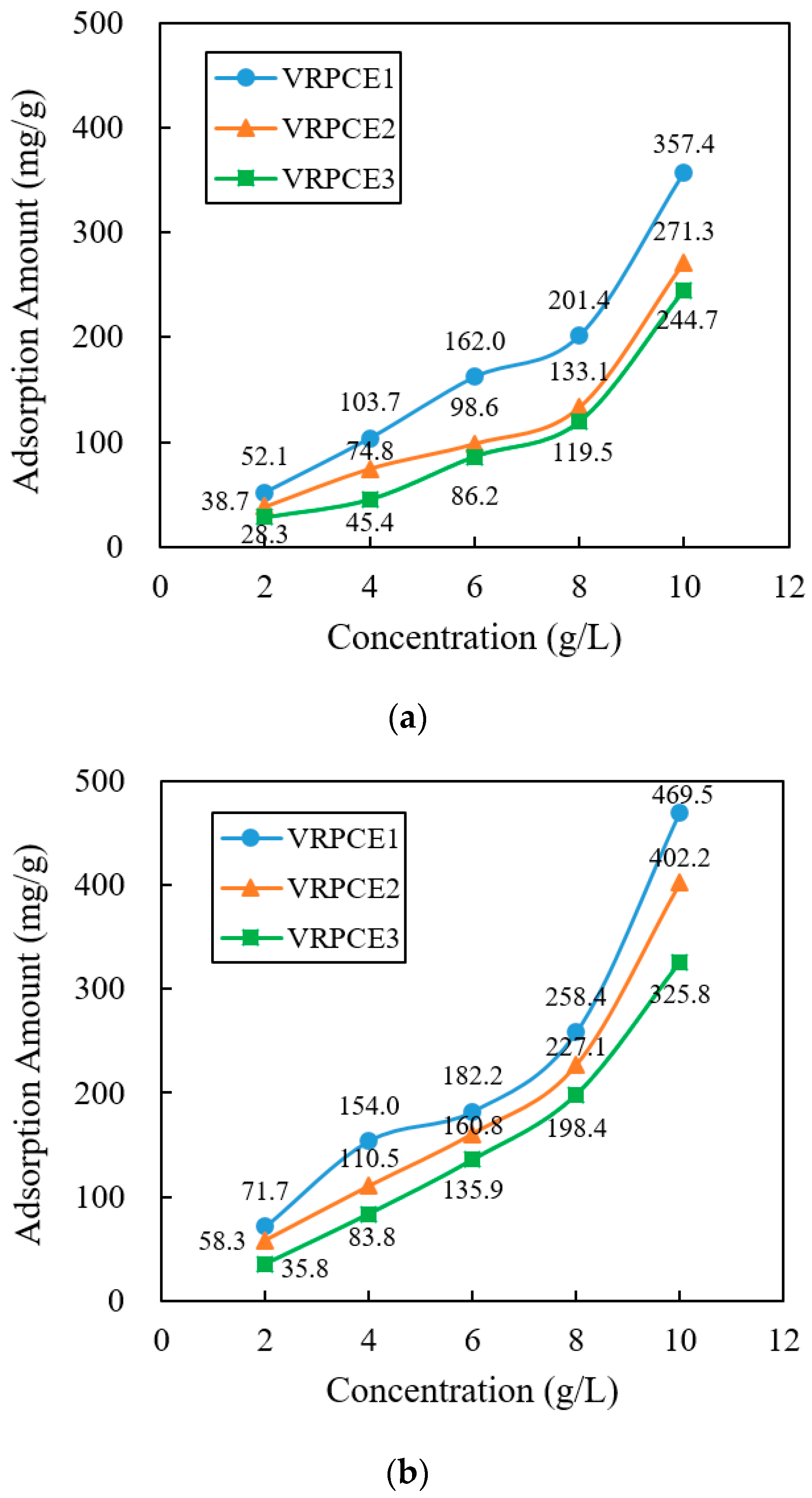
3.2. Adsorption
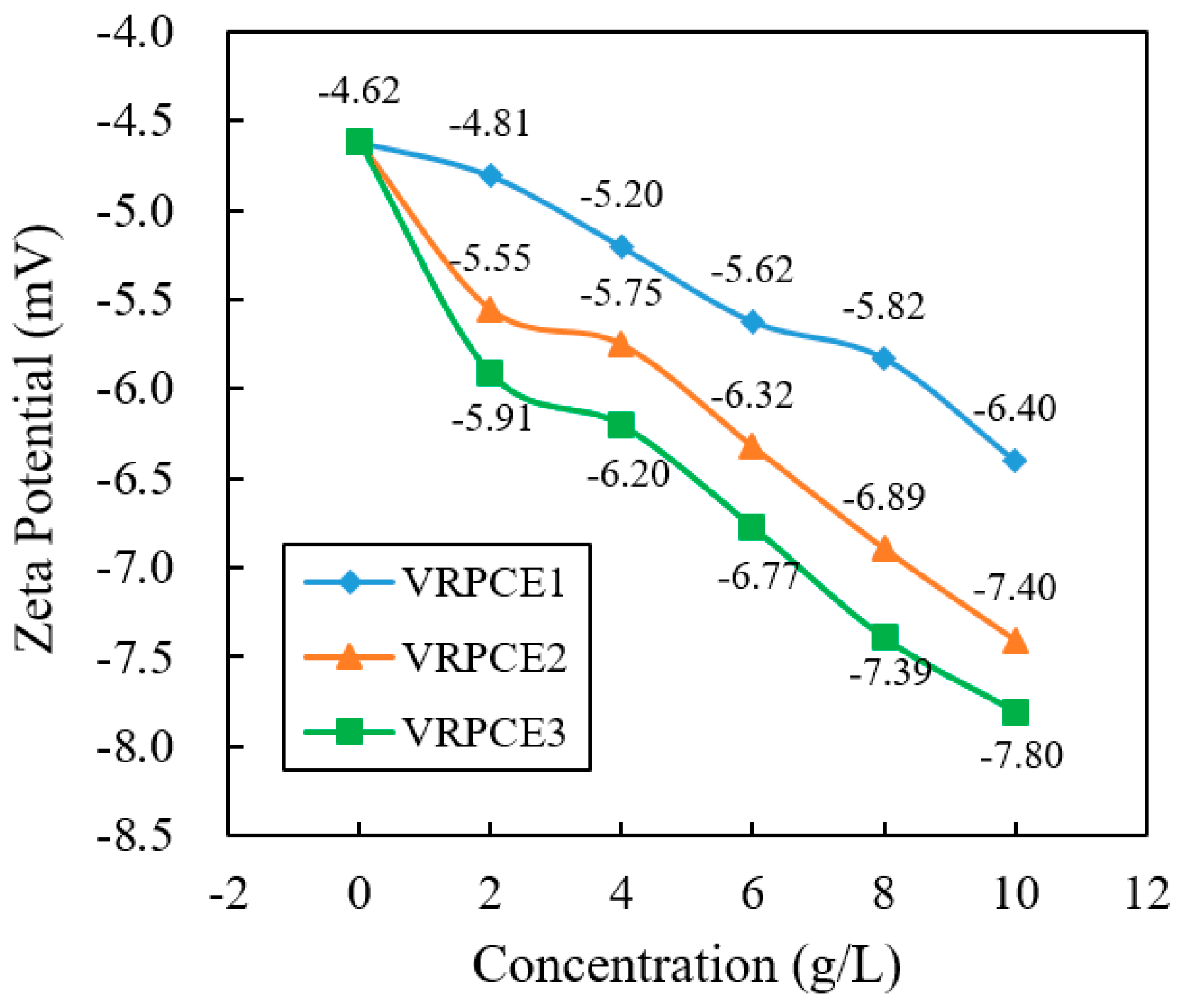
3.3. Dispersion
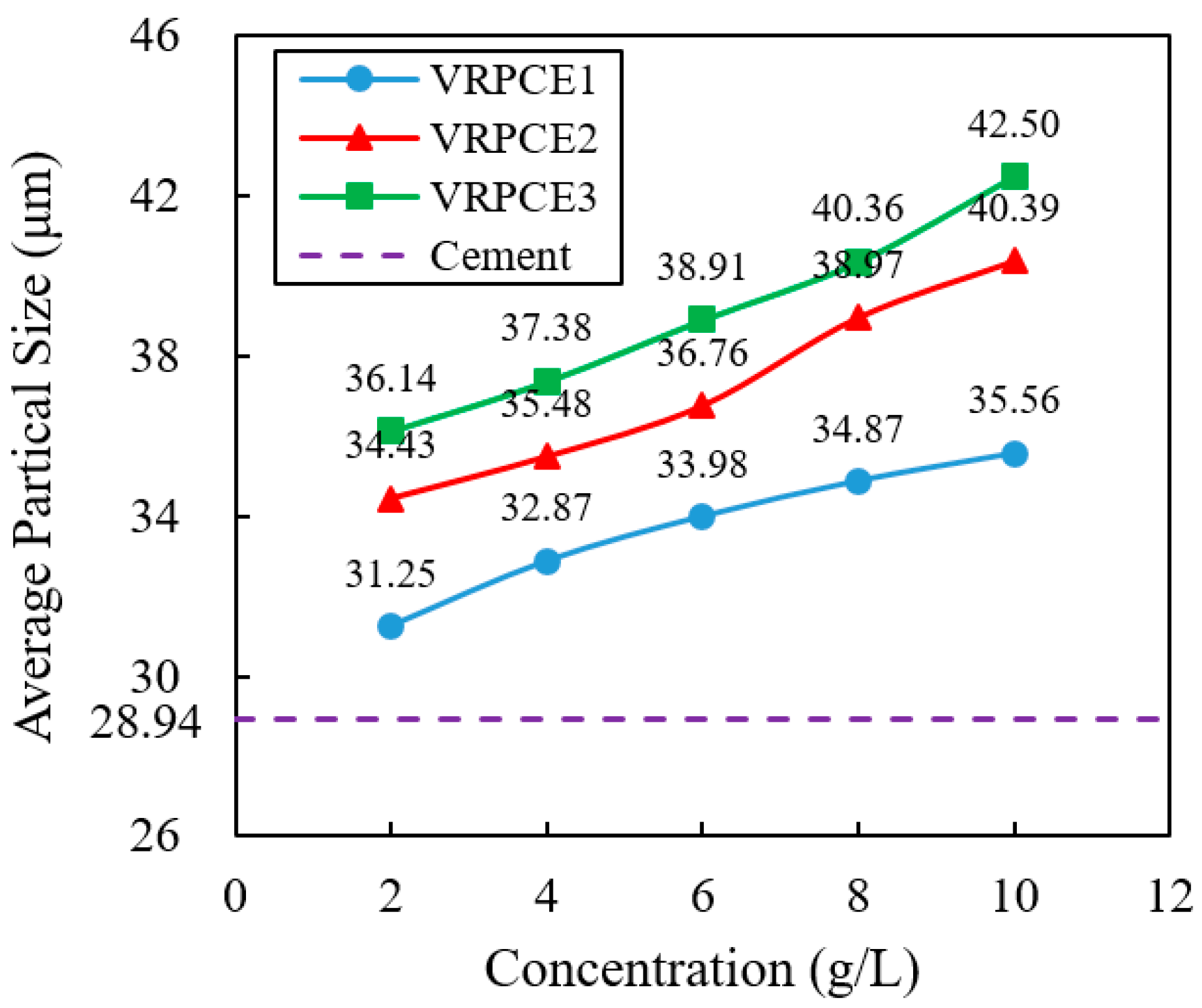
3.4. Particle Size
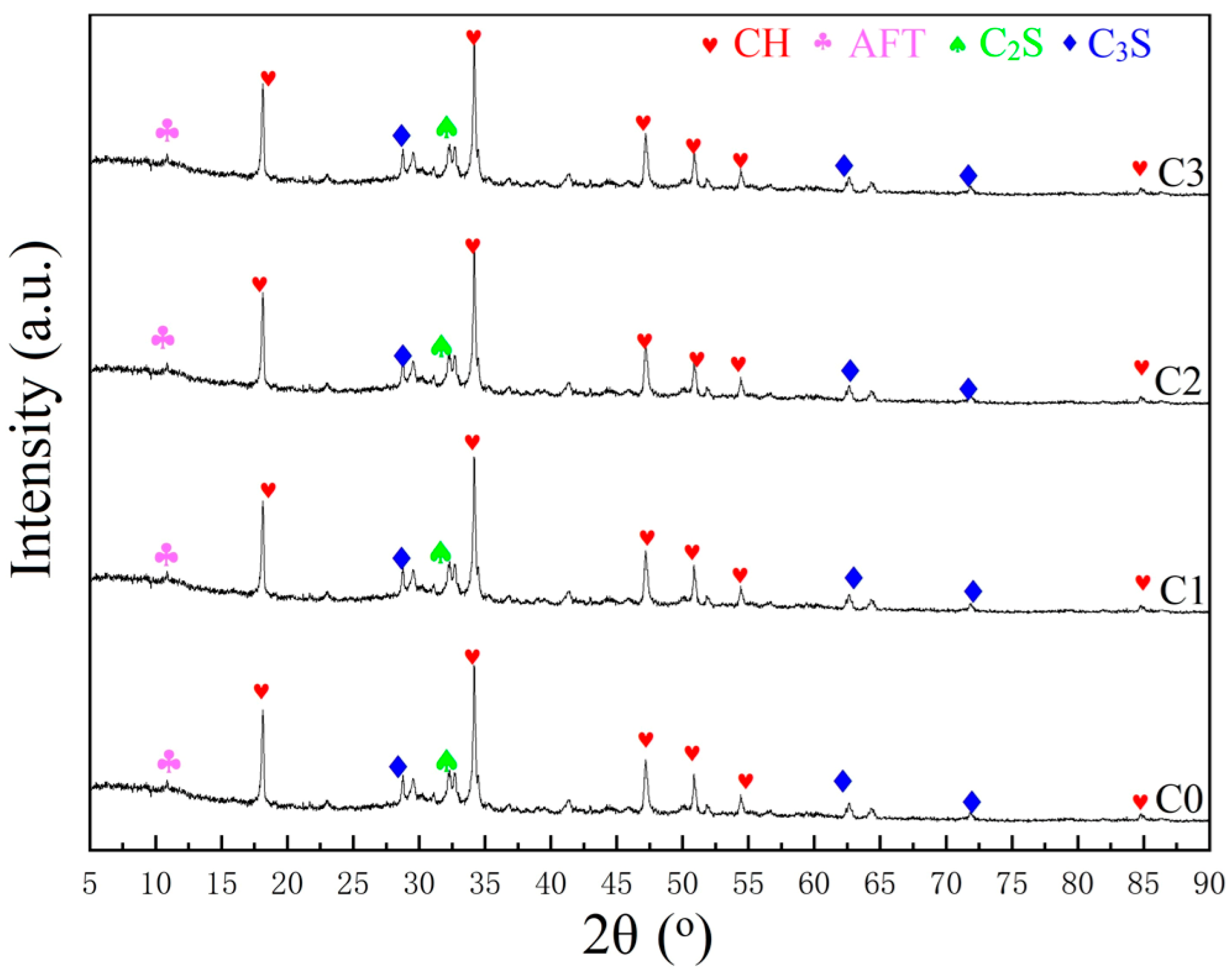
3.5. Compositions
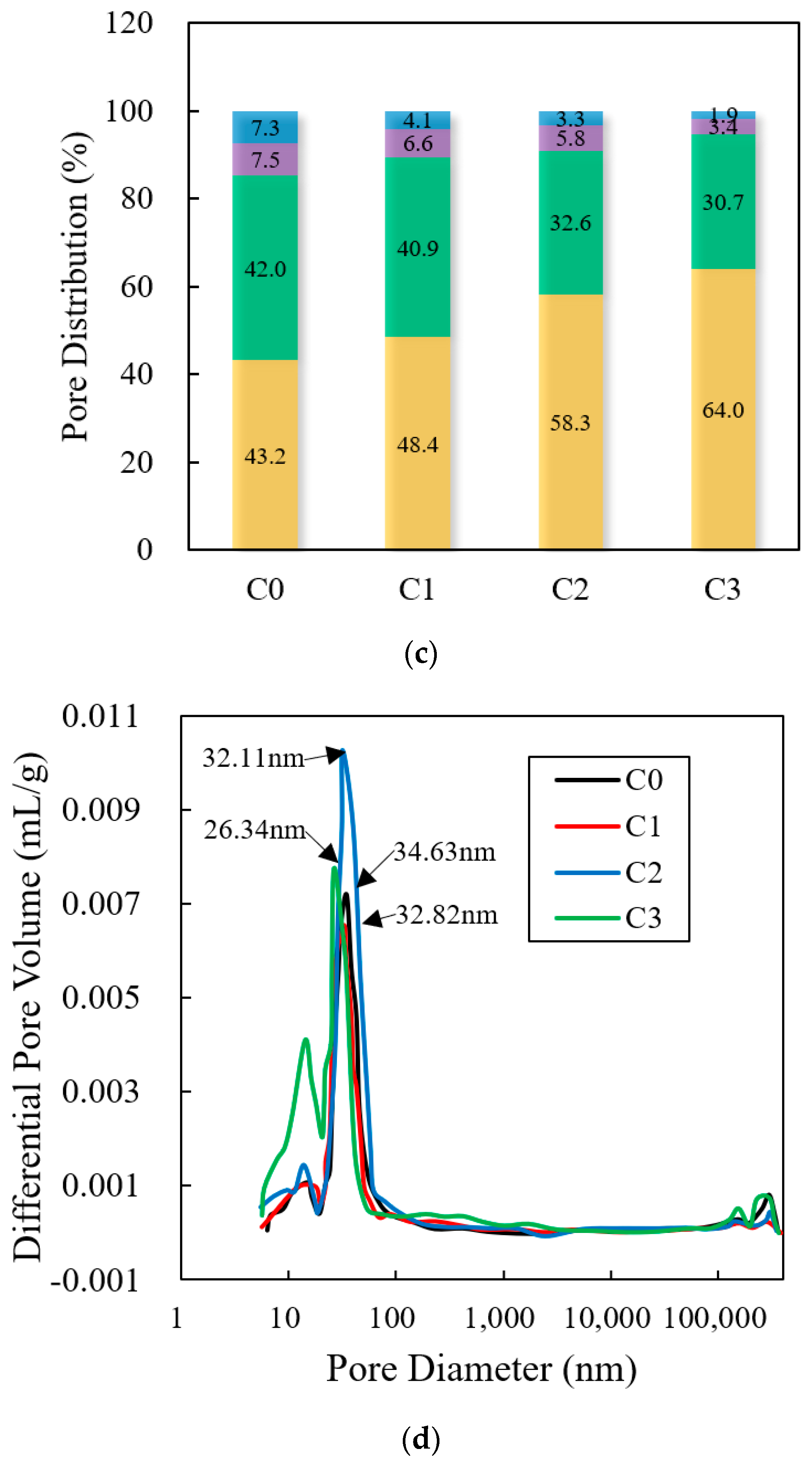
3.6. Pores
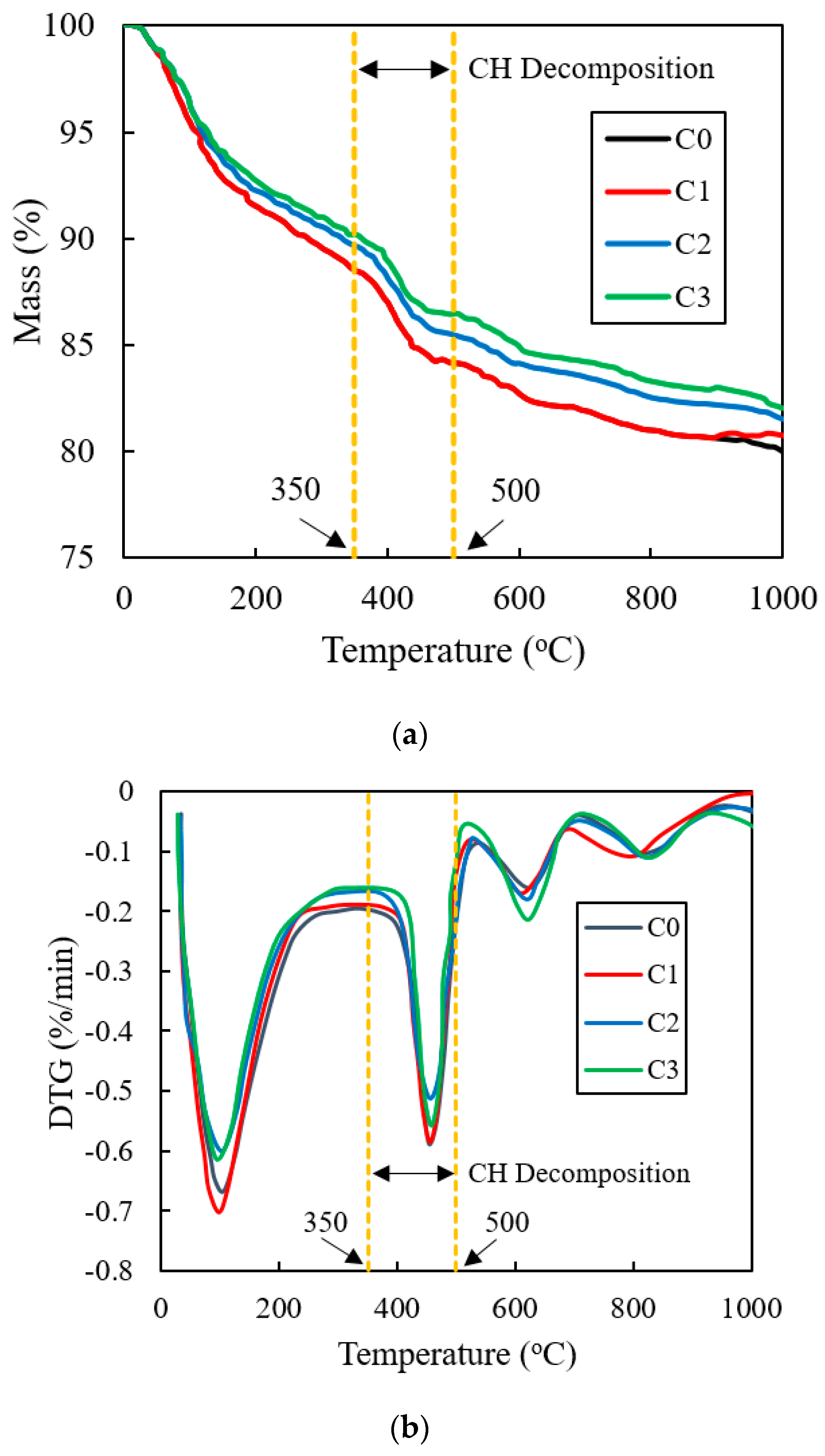
3.7. TG
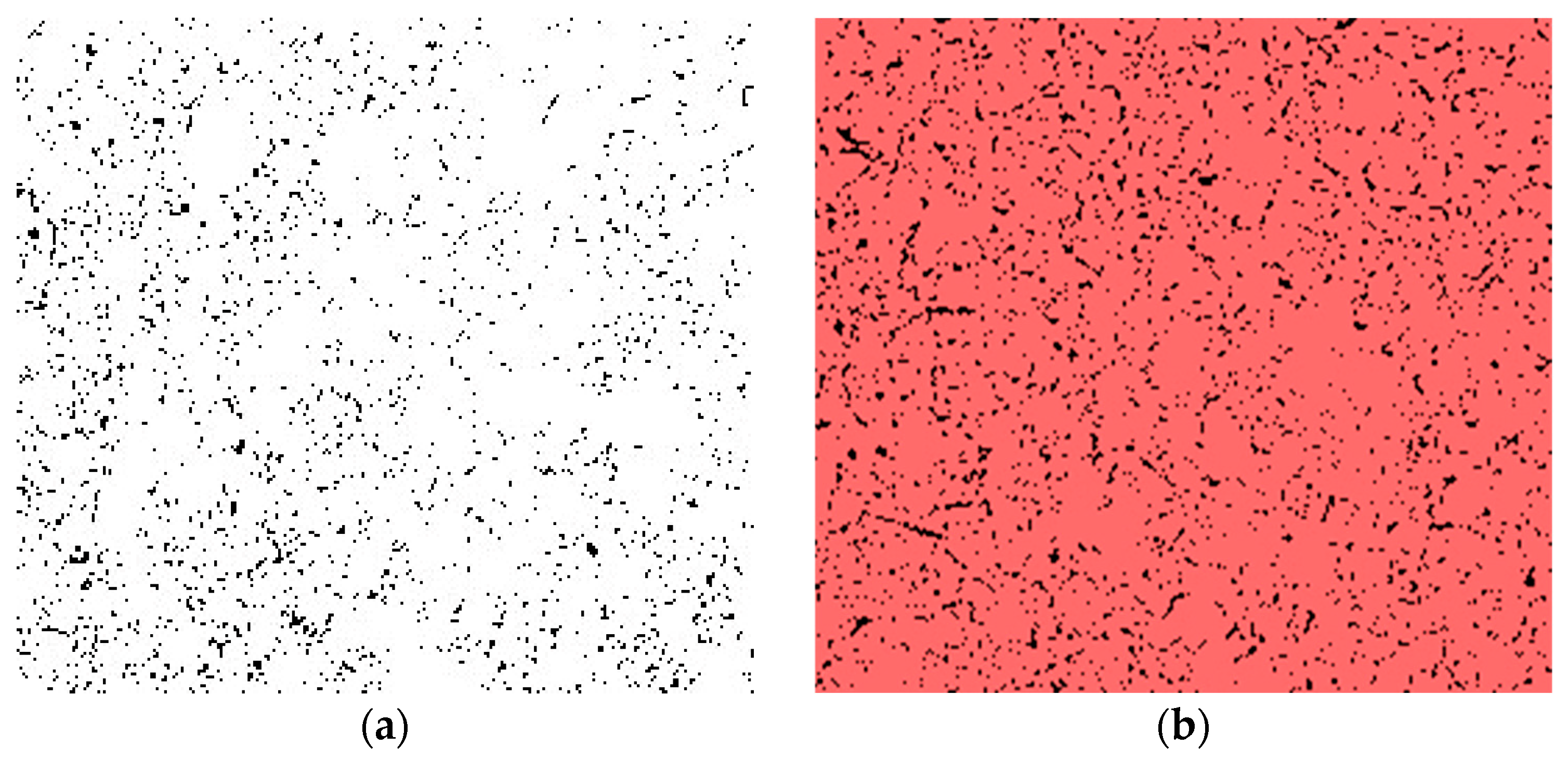
3.8. SEM
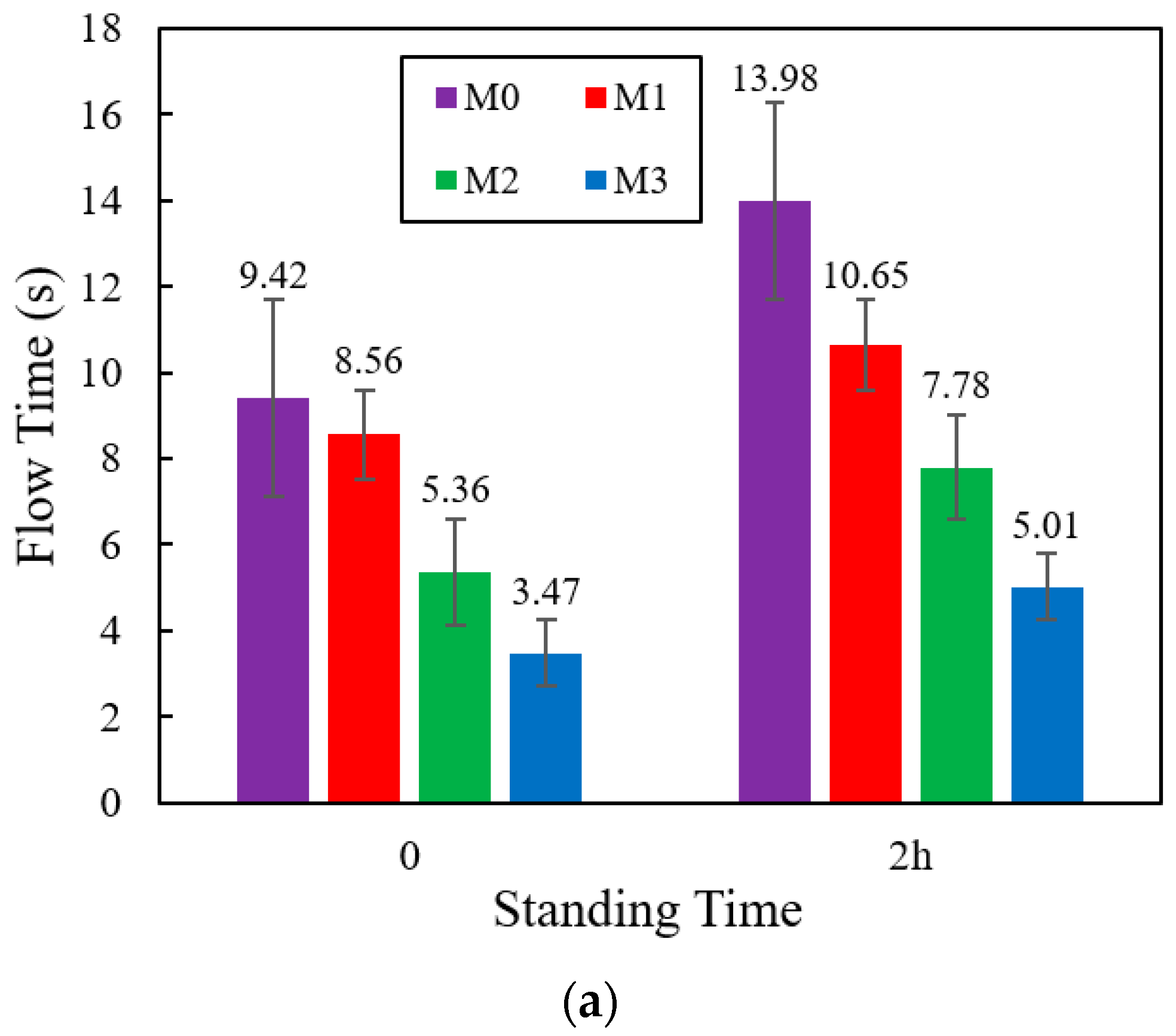
3.9. Fluidity
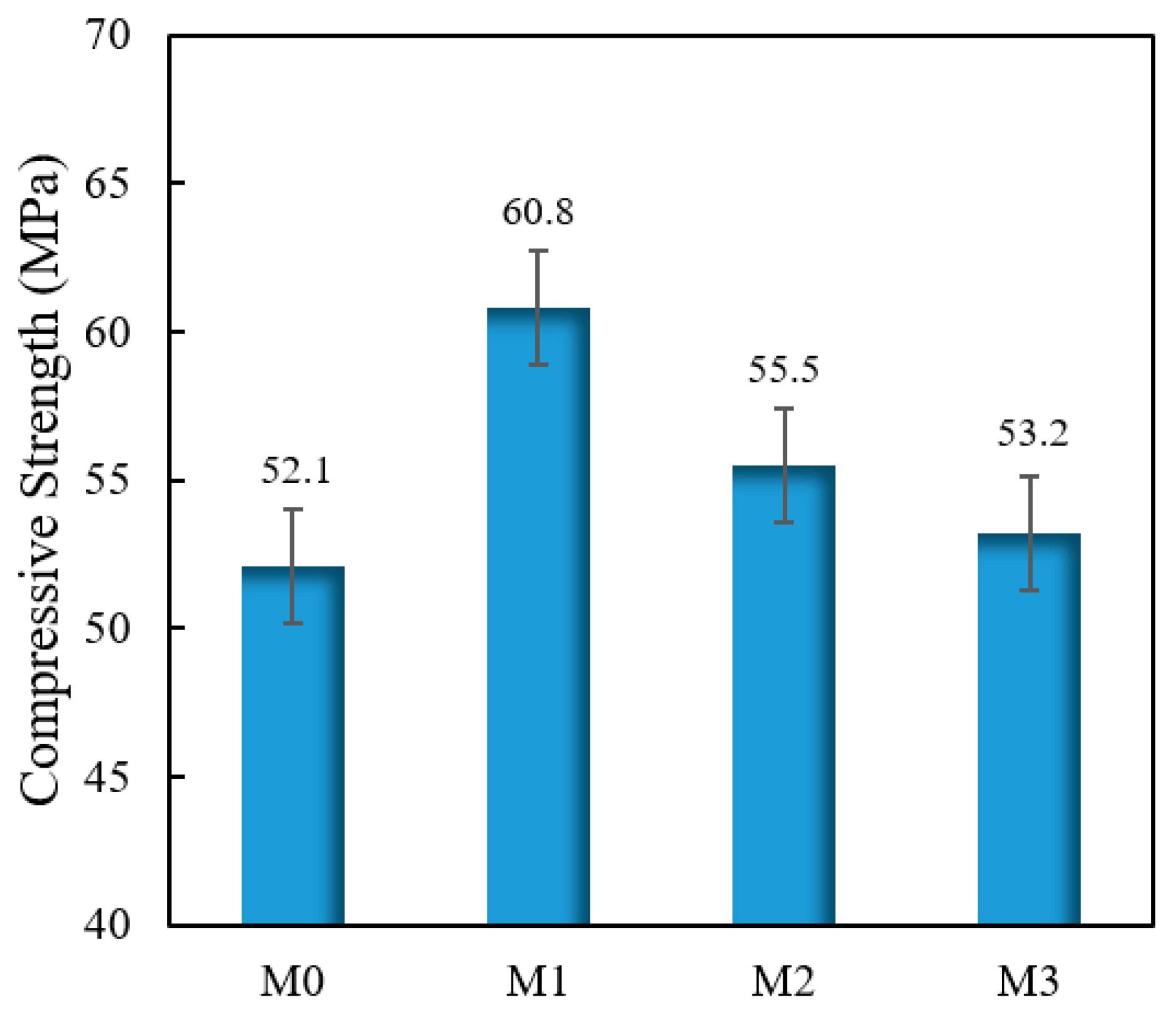
3.10. Compressive Strength
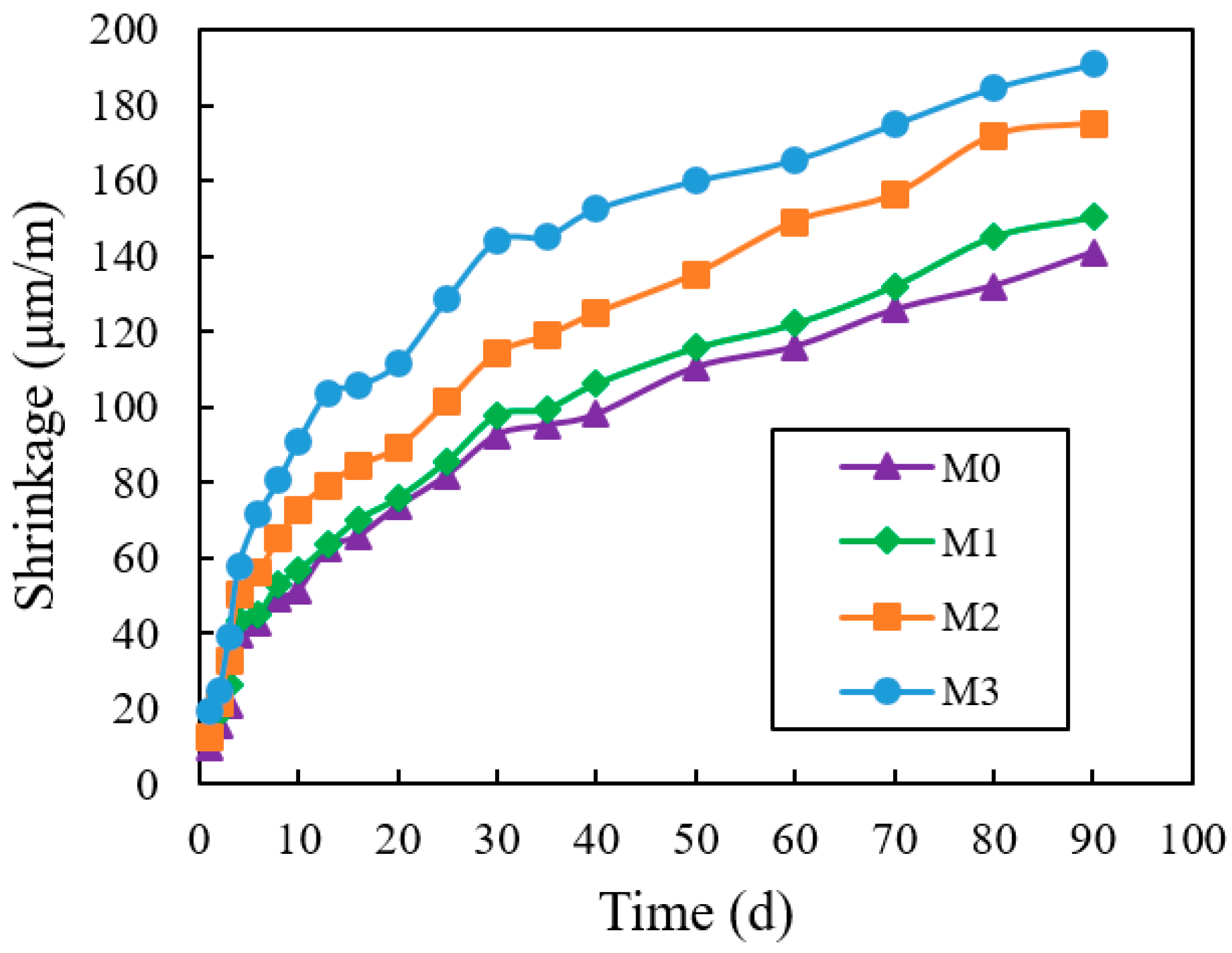
3.11. Shrinkage
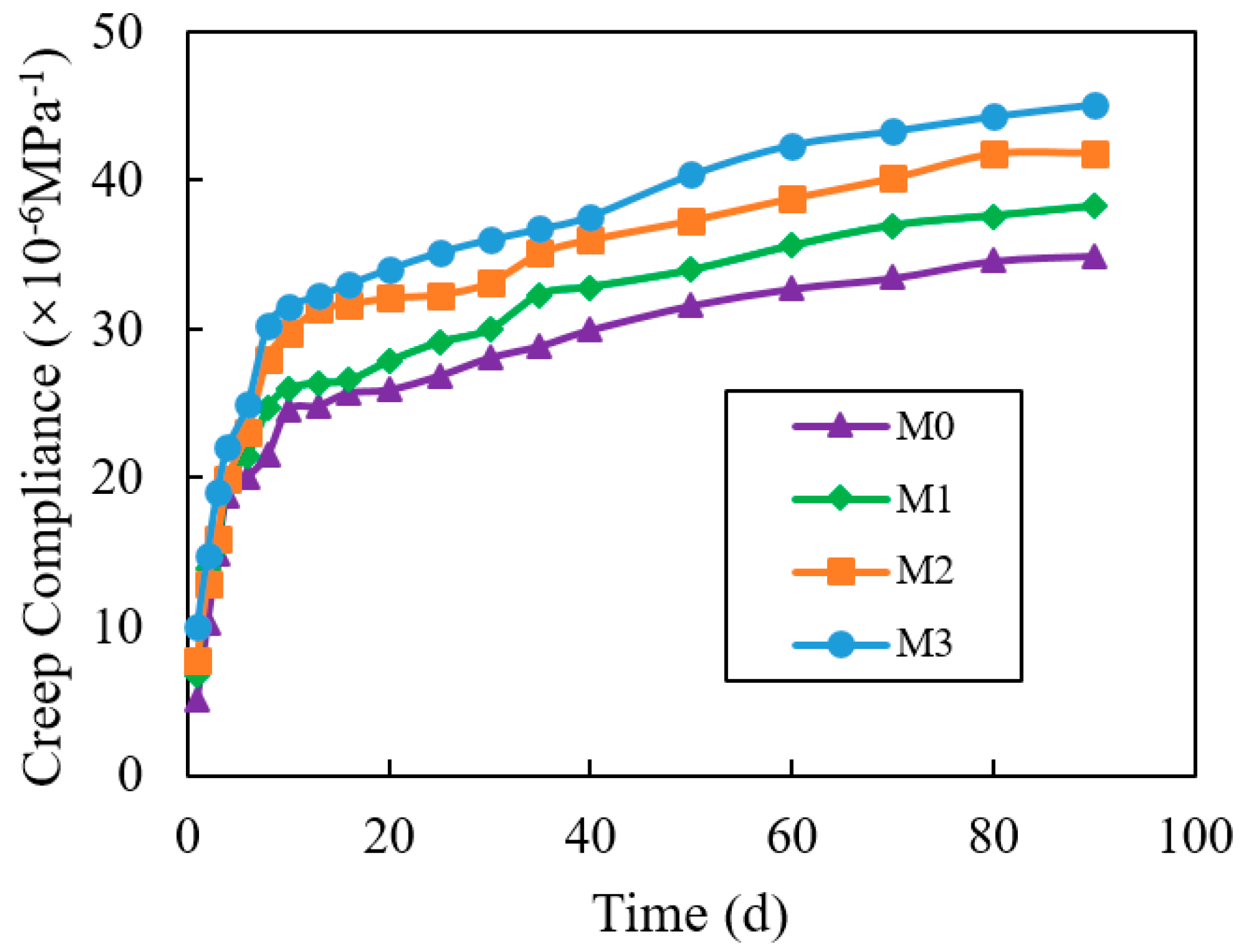
3.12. Creep
4. Discussion
4.1. Molecular Weight and Molecular Weight Distribution
4.2. Adsorption
4.3. Dispersion
4.4. Particle Size
4.5. Compositions
4.6. Pores
4.7. TG
4.8. SEM
4.9. Fluidity
4.10. Compressive Strength
4.11. Shrinkage
4.12. Creep
5. Conclusions
- Reducing the content of HPEG and increasing the content of AA can reduce the number average molecular weight and weight average molecular weight of the VRPCE from 46,162 to 34,053, and 82,186 to 71,985. Moreover, the content variation of HPEG and AA had a small impact on the polymer dispersibility index which was in the range of 2.0–2.2;
- When the concentration of the VRPCEs increased by five times, the adsorption capacity of the VRPCE1, VRPCE2, and VRPCE3 onto cement particles increased by 5.86, 6.01, and 7.64 times, respectively. Therefore, the effect on increasing the adsorption amount of cement particles was VRPCE1 > VRPCE2 > VRPCE3. When the concentration of VRPCEs was 10 g/L, the absolute zeta potential values of the cement particles within the VRPCEs increased by 33.1%, 33.3%, and 32.0% compared to that when the concentration of the VRPCEs was 2 g/L. When the concentration of the VRPCEs was 10 g/L, the particle size of the cement increased by 22.87%, 39.56%, and 46.86%. Hence, the effect of increasing the absolute value of zeta potential on the surface of cement particles and the particle size of cement particles were VRPCE1 < VRPCE2 < VRPCE3;
- The porosity of C0, C1, C2, and C3 gradually increased, increasing by 0.03%, 30.0%, and 56.9%, compared to C0. The mass loss values of C1, C2, and C3 increased by 23.0%, 37.0%, and 55.5%, compared with C0. Therefore, the effect of improving the hydration degree of cement, increasing the porosity, and optimizing the pore structure was VRPCE1 < VRPCE2 < VRPCE3;
- When the standing time was 2 h, the flow time of M1, M2, and M3 decreased by 23.8%, 44.3%, and 64.2%, compared to M0. The compressive strength of M1, M2, and M3 increased by 16.7%, 6.5%, and 2.1%, compared with M0. The shrinkage of M1, M2, and M3 increased by 6.6%, 24.2%, 35.4%. compared to M0. The creep degrees of M1, M2, and M3 decreased by 7.1%, 15.2%, and 22.5%, compared to M0. Hence, the impact on increasing the fluidity and shrinkage of cement mortar, reducing compressive strength and creep, was VRPCE1 < VRPCE2 < VRPCE3.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yi, N.H.; Kim, J.H.J.; Han, T.S.; Cho, Y.G.; Lee, J.H. Blast-resistant characteristics of ultra-high strength concrete and reactive powder concrete. Constr. Build. Mater. 2012, 28, 694–707. [Google Scholar] [CrossRef]
- Riedel, W.; Noldgen, M.; Strassburger, E.; Thoma, K.; Fehling, E. Local damage to Ultra High Performance Concrete structures caused by an impact of aircraft engine missiles. Nucl. Eng. Des. 2010, 240, 2633–2642. [Google Scholar] [CrossRef]
- Wang, C.; Yang, C.H.; Liu, F.; Wan, C.J.; Pu, X.C. Preparation of Ultra-High Performance Concrete with common technology and materials. Cem. Concr. Compos. 2012, 34, 538–544. [Google Scholar] [CrossRef]
- Emdadi, A.; Mehdipour, I.; Libre, N.A.; Shekarchi, M. Optimized workability and mechanical properties of FRCM by using fiber factor approach: Theoretical and experimental study. Mater. Struct. 2015, 48, 1149–1161. [Google Scholar] [CrossRef]
- Mardani-Aghabaglou, A.; Tuyan, M.; Yilmaz, G.; Arioz, O.; Ramyar, K. Effect of different types of superplasticizer on fresh, rheological and strength properties of self-consolidating concrete. Constr. Build. Mater. 2013, 47, 1020–1025. [Google Scholar] [CrossRef]
- Shi, C.J.; Wu, Z.M.; Xiao, J.F.; Wang, D.H.; Huang, Z.Y.; Fang, Z. A review on ultra high performance concrete: Part I. Raw materials and mixture design. Constr. Build. Mater. 2015, 101, 741–751. [Google Scholar] [CrossRef]
- Li, J.Y.; Yao, Y. A study on creep and drying shrinkage of high performance concrete. Cem. Concr. Res. 2001, 31, 1203–1206. [Google Scholar] [CrossRef]
- Sobuz, H.R.; Visintin, P.; Ali, M.S.M.; Singh, M.; Griffith, M.C.; Sheikh, A.H. Manufacturing ultra-high performance concrete utilising conventional materials and production methods. Constr. Build. Mater. 2016, 111, 251–261. [Google Scholar] [CrossRef]
- Wen, X.D.; Feng, L.; Hu, D.Y.; Wang, K.; Zhenya, Z.Y. Effect of side-chain length in polycarboxylic superplasticizer on the early-age performance of cement-based materials. Constr. Build. Mater. 2019, 211, 26–32. [Google Scholar] [CrossRef]
- Yamada, K.; Takahashi, T.; Hanehara, S.; Matsuhisa, M. Effects of the chemical structure on the properties of polycarboxylate-type superplasticizer. Cem. Concr. Res. 2000, 30, 197–207. [Google Scholar] [CrossRef]
- Zingg, A.; Winnefeld, F.; Holzer, L.; Pakusch, J.; Becker, S.; Figi, R.; Gauckler, L. Interaction of polycarboxylate-based superplasticizers with cements containing different C(3)A amounts. Cem. Concr. Compos. 2009, 31, 153–162. [Google Scholar] [CrossRef]
- Tan, H.B.; Gu, B.Q.; Guo, Y.L.; Ma, B.G.; Huang, J.; Ren, J.; Zou, F.B.; Guo, Y.F. Improvement in compatibility of polycarboxylate superplasticizer with poor-quality aggregate containing montmorillonite by incorporating polymeric ferric sulfate. Constr. Build. Mater. 2018, 162, 566–575. [Google Scholar] [CrossRef]
- Atarashi, D.; Yamada, K.; Itoh, A.; Miyauchi, M.; Sakai, E. Interaction between Montmorillonite and Chemical Admixture. J. Adv. Concr. Technol. 2015, 13, 325–331. [Google Scholar] [CrossRef][Green Version]
- Liu, J.Z.; Wang, K.J.; Zhang, Q.Q.; Han, F.Y.; Sha, J.F.; Liu, J.P. Influence of superplasticizer dosage on the viscosity of cement paste with low water-binder ratio. Constr. Build. Mater. 2017, 149, 359–366. [Google Scholar] [CrossRef]
- Plank, J.; Sachsenhauser, B. Experimental determination of the effective anionic charge density of polycarboxylate superplasticizers in cement pore solution. Cem. Concr. Res. 2009, 39, 1–5. [Google Scholar] [CrossRef]
- Alonso, M.M.; Puertas, F. Adsorption of PCE and PNS superplasticisers on cubic and orthorhombic C(3)A. Effect of sulfate. Constr. Build. Mater. 2015, 78, 324–332. [Google Scholar] [CrossRef]
- Karihaloo, B.L.; Ghanbari, A. Mix proportioning of self-compacting high- and ultra-high-performance concretes with and without steel fibres. Mag. Concr. Res. 2012, 64, 1089–1100. [Google Scholar] [CrossRef]
- Poe, G.D.; Jarrett, W.L.; Scales, C.W.; McCormick, C.L. Enhanced coil expansion and intrapolymer complex formation of linear poly(methacrylic acid) containing poly(ethylene glycol) grafts. Macromolecules 2004, 37, 2603–2612. [Google Scholar] [CrossRef]
- Alonso, M.M.; Palacios, M.; Puertas, F. Compatibility between polycarboxylate-based admixtures and blended-cement pastes. Cem. Concr. Compos. 2013, 35, 151–162. [Google Scholar] [CrossRef]
- Perez-Nicolas, M.; Duran, A.; Navarro-Blasco, I.; Fernandez, J.M.; Sirera, R.; Alvarez, J.I. Study on the effectiveness of PNS and LS superplasticizers in air lime-based mortars. Cem. Concr. Res. 2016, 82, 11–22. [Google Scholar] [CrossRef]
- Lei, L.; Plank, J. Synthesis and Properties of a Vinyl Ether-Based Polycarboxylate Superplasticizer for Concrete Possessing Clay Tolerance. Ind. Eng. Chem. Res. 2014, 53, 1048–1055. [Google Scholar] [CrossRef]
- Zhang, Q.Q.; Liu, J.Z.; Liu, J.P.; Han, F.Y.; Lin, W. Effect of Superplasticizers on Apparent Viscosity of Cement-Based Material with a Low Water-Binder Ratio. J. Mater. Civ. Eng. 2016, 28, 1–7. [Google Scholar] [CrossRef]
- Zhang, Y.R.; Luo, X.; Kong, X.M.; Wang, F.Z.; Gao, L. Rheological properties and microstructure of fresh cement pastes with varied dispersion media and superplasticizers. Powder Technol. 2018, 330, 219–227. [Google Scholar] [CrossRef]
- Peng, X.Y.; Yi, C.H.; Deng, Y.H.; Qiu, X.Q. Synthesis and Evaluation of Polycarboxylate-Type Superplasticizers with Different Carboxylic Contents Used in a Cement System. Int. J. Polym. Mater. 2011, 60, 923–938. [Google Scholar] [CrossRef]
- He, Y.; Zhang, X.; Kong, Y.N.; Wang, X.F.; Shui, L.L.; Wang, H.R. Influence of Polycarboxylate Superplasticizer on Rheological Behavior in Cement Paste. J. Wuhan Univ. Technol.-Mater. Sci. Ed. 2018, 33, 932–937. [Google Scholar] [CrossRef]
- Plank, J.; Pollmann, K.; Zouaoui, N.; Andres, P.R.; Schaefer, C. Synthesis and performance of methacrylic ester based polycarboxylate superplasticizers possessing hydroxy terminated poly(ethylene glycol) side chains. Cem. Concr. Res. 2008, 38, 1210–1216. [Google Scholar] [CrossRef]
- Huang, Z.; Yang, Y.; Ran, Q.P.; Liu, J.P. Preparing hyperbranched polycarboxylate superplasticizers possessing excellent viscosity-reducing performance through in situ redox initialized polymerization method. Cem. Concr. Compos. 2018, 93, 323–330. [Google Scholar] [CrossRef]
- Dalas, F.; Pourchet, S.; Nonat, A.; Rinaldi, D.; Sabio, S.; Mosquet, M. Fluidizing efficiency of comb-like superplasticizers: The effect of the anionic function, the side chain length and the grafting degree. Cem. Concr. Res. 2015, 71, 115–123. [Google Scholar] [CrossRef]
- Qian, S.S.; Yao, Y.; Wang, Z.M.; Cui, S.P.; Liu, X.; Jiang, H.D.; Guo, Z.L.; Lai, G.H.; Xu, Q.; Guan, J.N. Synthesis, characterization and working mechanism of a novel polycarboxylate superplasticizer for concrete possessing reduced viscosity. Constr. Build. Mater. 2018, 169, 452–461. [Google Scholar] [CrossRef]
- Janowska-Renkas, E. The effect of superplasticizers’ chemical structure on their efficiency in cement pastes. Constr. Build. Mater. 2013, 38, 1204–1210. [Google Scholar] [CrossRef]
- Du, K.L.; Hou, H.H.; Liu, Z.Y.; Li, X.H.; Wen, Q.R. Preparation and Properties of Anti-mud and Viscosity-Reducing polycarboxylate superplasticizer. In Proceedings of the 5th International Conference on Advances in Energy, Environment and Chemical Engineering (AEECE), Shanghai, China, 16–18 August 2019; pp. 1–5. [Google Scholar]
- Chen, Y.; Chen, Y.; Liu, Y.; Tao, J.; Liu, R.; Li, Z.; Liu, F.; Li, M. Effect of the Alkyl Density of Acrylic Acid Ester on the Viscosity-Reducing Effect of Polycarboxylate Superplasticizer. Molecules 2023, 28, 7293. [Google Scholar] [CrossRef]
- Chen, J.; Yang, C.; He, Y.; Wang, F.; Zeng, C. Effects and Mechanism of Hyperbranched Phosphate Polycarboxylate Superplasticizers on Reducing Viscosity of Cement Paste. Materials 2024, 17, 1896. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Qian, C.; Zheng, C.; Jiang, H.; Yu, Z.; Wu, Z.; Zhou, Z. Synthesis and Performance of Polycarboxylate Superplasticizer with Viscosity-Reducing and Low-Shrinkage Properties for Fair-Faced Concrete. Materials 2024, 17, 2685. [Google Scholar] [CrossRef]
- ISO 19596: 2017; Admixtures for Concrete. International Standardization Organization: Geneva, Switzerland, 2017.
- ISO 13320: 2020; Particle Size Analysis—Laser Diffraction Methods. International Standardization Organization: Geneva, Switzerland, 2020.
- ISO 679: 2009; Cement—Test Methods—Determination of Strength. International Standardization Organization: Geneva, Switzerland, 2009.
- Plank, J.; Hirsch, C. Impact of zeta potential of early cement hydration phases on superplasticizer adsorption. Cem. Concr. Res. 2007, 37, 537–542. [Google Scholar] [CrossRef]
- Hou, D.S.; Jia, Y.T.; Yu, J.; Wang, P.G.; Liu, Q.F. Transport Properties of Sulfate and Chloride Ions Confined between Calcium Silicate Hydrate Surfaces: A Molecular Dynamics Study. J. Phys. Chem. C 2018, 122, 28021–28032. [Google Scholar] [CrossRef]
- Yamada, K.; Ogawa, S.; Hanehara, S. Controlling of the adsorption and dispersing force of polycarboxylate-type superplasticizer by sulfate ion concentration in aqueous phase. Cem. Concr. Res. 2001, 31, 375–383. [Google Scholar] [CrossRef]
- Soin, A.V.; Catalan, L.J.J.; Kinrade, S.D. A combined QXRD/TG method to quantify the phase composition of hydrated portland cements (vol 48, pg 17, 2013). Cem. Concr. Res. 2013, 50, 88. [Google Scholar] [CrossRef]
- Jin, S.S.; Zhang, J.X.; Han, S. Fractal analysis of relation between strength and pore structure of hardened mortar. Constr. Build. Mater. 2017, 135, 1–7. [Google Scholar] [CrossRef]
- Silva, F.G.S.; Fiuza, R.A.; da Silva, J.S.; de Brito, C.; Andrade, H.M.C.; Goncalves, J.P. Consumption of calcium hydroxide and formation of C-S-H in cement pastes. J. Therm. Anal. Calorim. 2014, 116, 287–293. [Google Scholar] [CrossRef]
- Lyu, K.; She, W.; Miao, C.; Chang, H.; Gu, Y. Quantitative characterization of pore morphology in hardened cement paste via SEM-BSE image analysis. Constr. Build. Mater. 2019, 202, 589–602. [Google Scholar] [CrossRef]
- Ma, Y.H.; Jiao, D.W.; Sha, S.N.; Zhou, B.B.; Liu, Y.; Shi, C.J. Effect of anchoring groups of polycarboxylate ether superplasticizer on the adsorption and dispersion of cement paste containing montmorillonite. Cem. Concr. Compos. 2022, 134, 104737. [Google Scholar] [CrossRef]
- Walkley, B.; Geddes, D.A.; Matsuda, T.; Provis, J.L. Reversible Adsorption of Polycarboxylates on Silica Fume in High pH, High Ionic Strength Environments for Control of Concrete Fluidity. Langmuir 2022, 38, 1662–1671. [Google Scholar] [CrossRef] [PubMed]
- Benboudjema, F.; Meftah, F.; Torrenti, J.M. Interaction between drying, shrinkage, creep and cracking phenomena in concrete. Eng. Struct. 2005, 27, 239–250. [Google Scholar] [CrossRef]




| Composition | SiO2 | Al2O3 | Fe2O3 | CaO | MgO | SO3 | K2O | Na2O | LOI |
|---|---|---|---|---|---|---|---|---|---|
| wt.% | 21.92 | 4.46 | 3.43 | 64.55 | 1.98 | 0.34 | 0.65 | 0.46 | 2.21 |
| Type | HPEG3000 | AA | PFM | MDMA |
|---|---|---|---|---|
| VRPCE1 | 87 | 9 | 1 | 3 |
| VRPCE2 | 81 | 15 | 1 | 3 |
| VRPCE3 | 79 | 17 | 1 | 3 |
| Code | VRPCE Type | Water/Cement | VRPCE Content (%) |
|---|---|---|---|
| C0 | - | 0.25 | 0 |
| C1 | VRPCE1 | 0.25 | 0.3 |
| C2 | VRPCE2 | 0.25 | 0.3 |
| C3 | VRPCE3 | 0.25 | 0.3 |
| Code | VRPCE Type | Water/Cement | Cement/Sand | VRPCE Content (%) |
|---|---|---|---|---|
| M0 | - | 0.25 | 0.5 | 0 |
| M1 | VRPCE1 | 0.25 | 0.5 | 0.3 |
| M2 | VRPCE2 | 0.25 | 0.5 | 0.3 |
| M3 | VRPCE3 | 0.25 | 0.5 | 0.3 |
| Code | Mn | Mw | PDI |
|---|---|---|---|
| VRPCE1 | 40,162 | 82,186 | 2.05 |
| VRPCE2 | 37,510 | 80,598 | 2.15 |
| VRPCE3 | 34,053 | 71,985 | 2.11 |
| Code | C0 | C1 | C2 | C3 |
|---|---|---|---|---|
| Porosity (%) | 7.02 | 7.04 | 9.12 | 11.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Shen, Y.; Li, Y.; Tian, Y. Experimental Study on Improving the Performance of Cement Mortar with Self-Synthesized Viscosity-Reducing Polycarboxylic Acid Superplasticizer. Buildings 2024, 14, 2418. https://doi.org/10.3390/buildings14082418
Wang Z, Shen Y, Li Y, Tian Y. Experimental Study on Improving the Performance of Cement Mortar with Self-Synthesized Viscosity-Reducing Polycarboxylic Acid Superplasticizer. Buildings. 2024; 14(8):2418. https://doi.org/10.3390/buildings14082418
Chicago/Turabian StyleWang, Zigeng, Yonghao Shen, Yue Li, and Yuan Tian. 2024. "Experimental Study on Improving the Performance of Cement Mortar with Self-Synthesized Viscosity-Reducing Polycarboxylic Acid Superplasticizer" Buildings 14, no. 8: 2418. https://doi.org/10.3390/buildings14082418
APA StyleWang, Z., Shen, Y., Li, Y., & Tian, Y. (2024). Experimental Study on Improving the Performance of Cement Mortar with Self-Synthesized Viscosity-Reducing Polycarboxylic Acid Superplasticizer. Buildings, 14(8), 2418. https://doi.org/10.3390/buildings14082418







