Effect of Active MgO on Compensated Drying Shrinkage and Mechanical Properties of Alkali-Activated Fly Ash–Slag Materials
Abstract
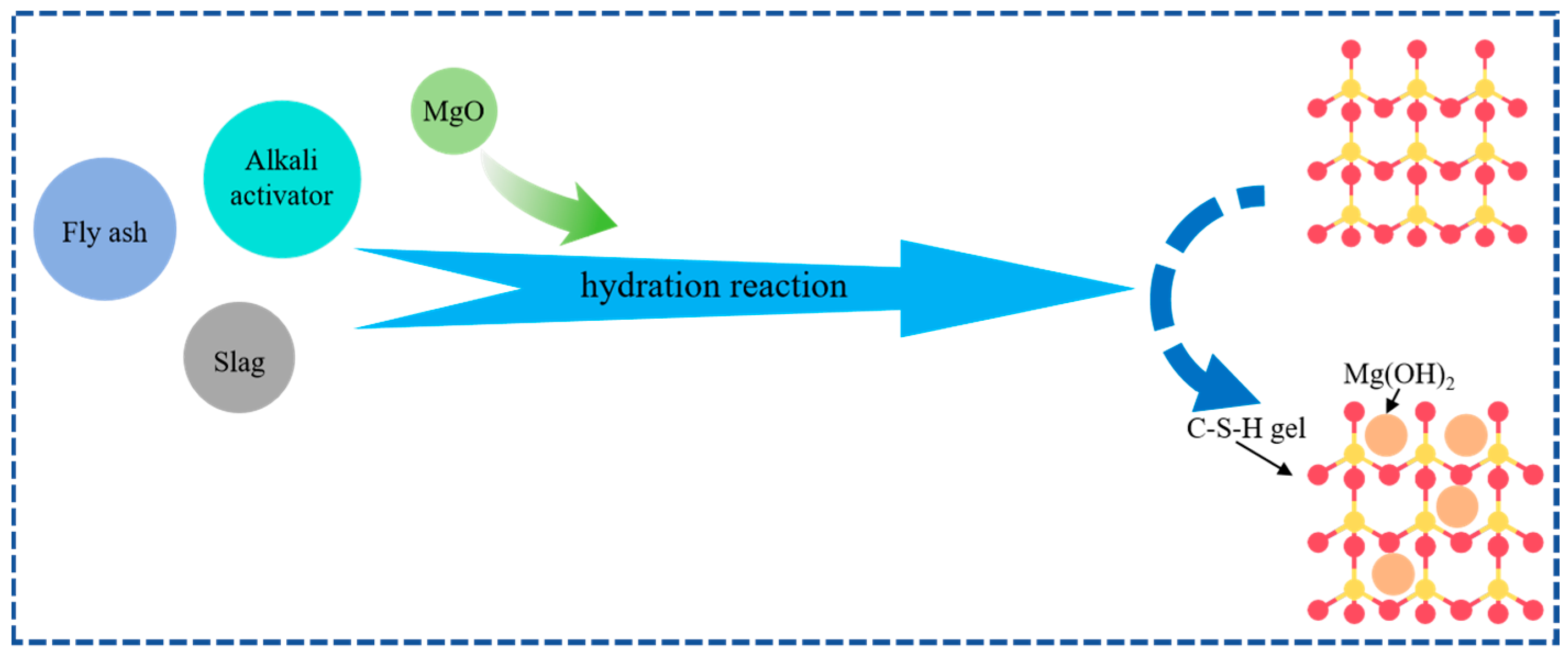
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation Process
2.3. Testing Methods
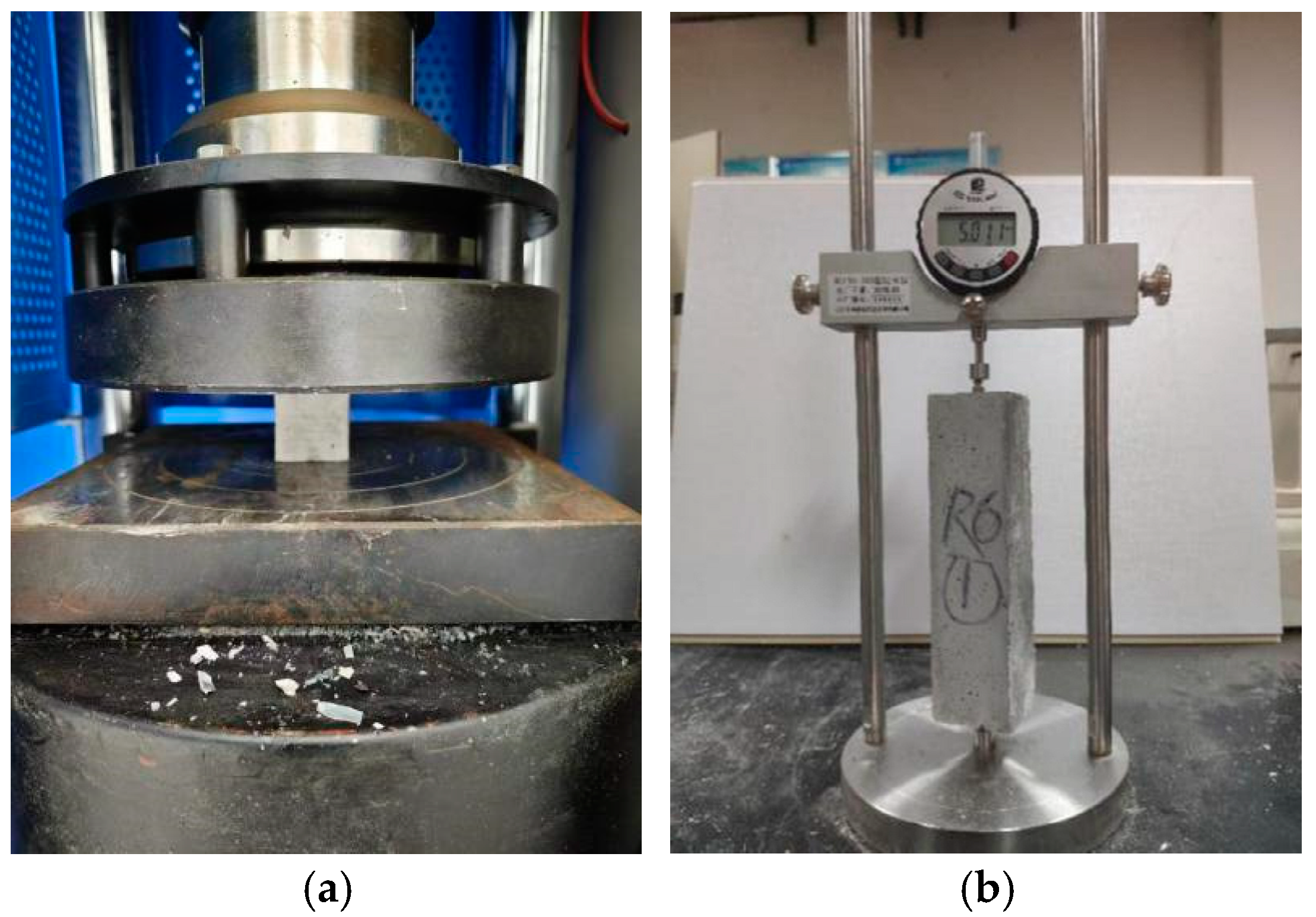
2.3.1. Compressive Strength Tests
2.3.2. Drying Shrinkage Tests
2.3.3. Microscopic Tests
3. Results and Analysis
3.1. Mechanical Properties’ Analysis
3.2. Analysis of the Change in Active-Mg-Compensated Drying Shrinkage
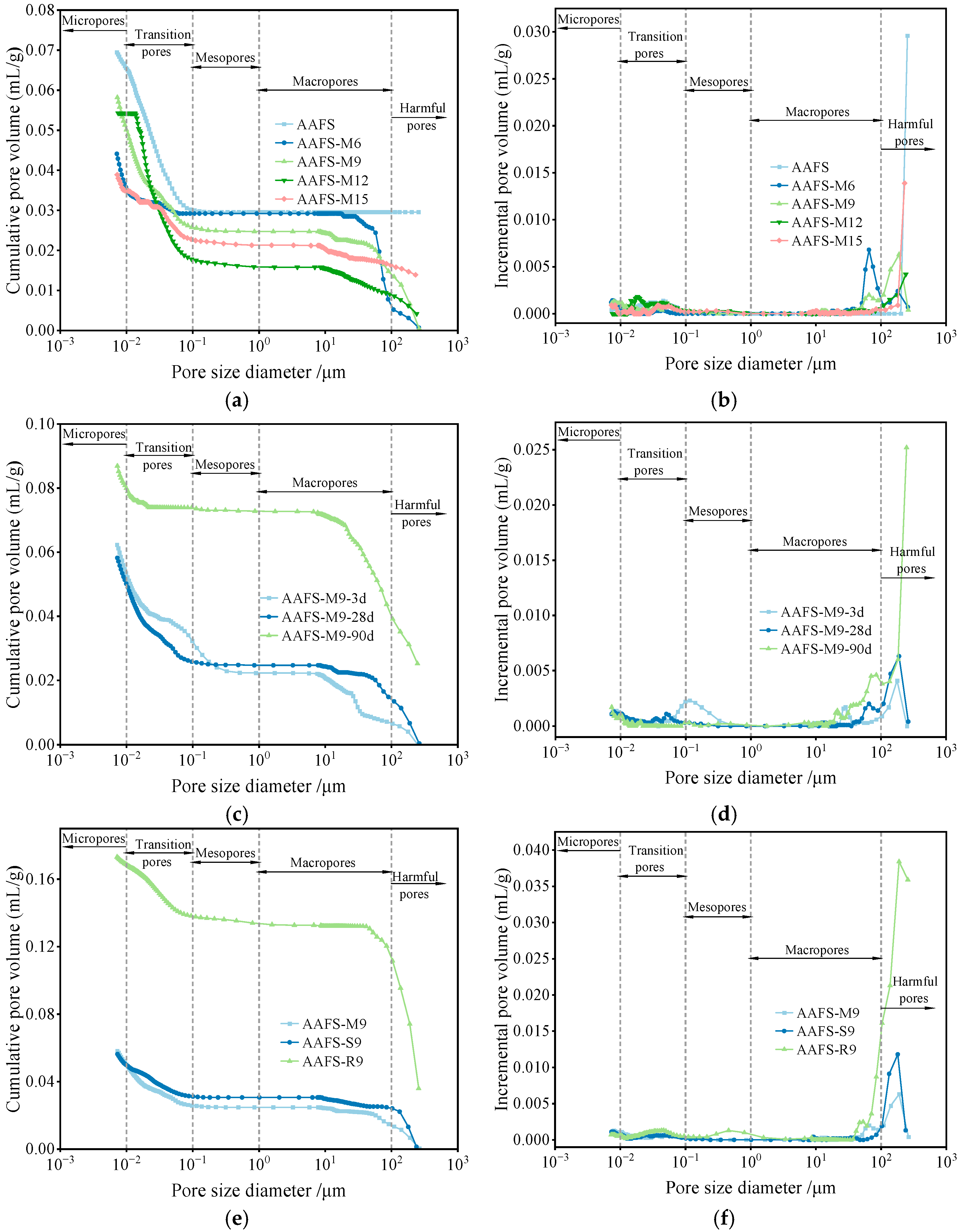
3.3. MIP Analysis
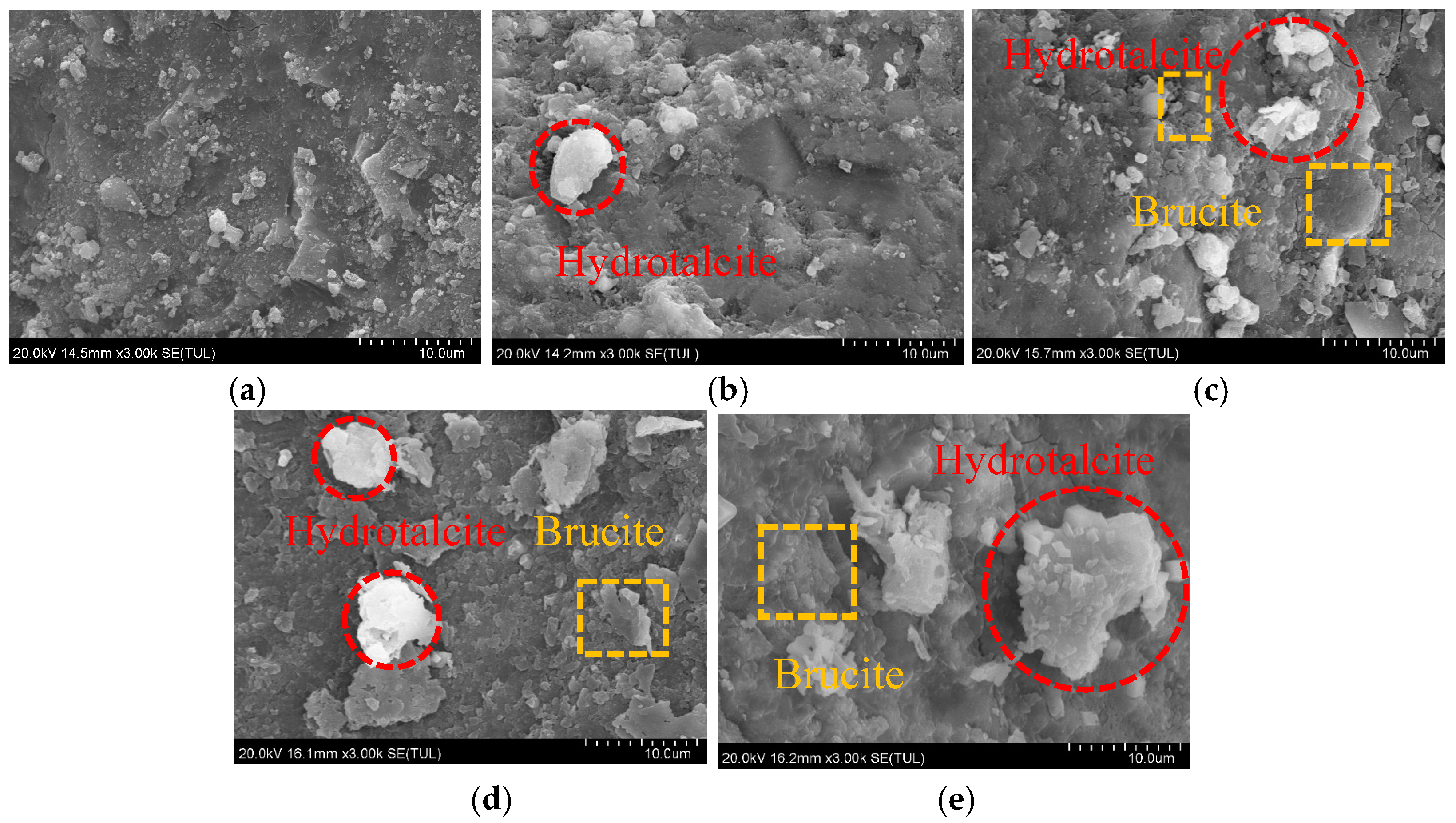
3.4. SEM Analysis
4. Conclusions
- (1)
- The addition of active MgO can enhance the compressive strength of AAMs to some extent, but it decreases when the content is too high. The flexural strength exhibited a decreasing trend, except for a noticeable increase at a content of 9%. This study found that AAMs with 9% M-MgO exhibited superior mechanical properties.
- (2)
- The incorporation of active MgO induces significant expansion behavior in AAMs, which increases with content. The shrinkage rates of R-M-S MgO samples decreased to 15.8%, 19.1%, and 32.2%, indicating a positive correlation between the expansion behavior of AAMs and the activity of MgO.
- (3)
- High-temperature drying, high-altitude terrain, and wind can accelerate moisture evaporation within AAMs, promoting drying shrinkage and crack formation. Compared to methods such as using steel reinforcement to inhibit drying shrinkage, expansive MgO agents offer advantages such as lower cost, easier application, and significant effectiveness. As green materials, AAMs contribute to lowering carbon emissions, reducing energy consumption, saving energy, and reducing emissions.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Farhan, K.; Johari, M.; Demirboğa, R. Assessment of important parameters involved in the synthesis of geopolymer composites: A review. Constr. Build. Mater. 2020, 264, 120276. [Google Scholar] [CrossRef]
- Shi, C.; Qu, B.; Provis, J. Recent progress in low-carbon binders. Cem. Concr. Res. 2019, 122, 227–250. [Google Scholar] [CrossRef]
- Tositti, L.; Masi, G.; Morozzi, P.; Zappi, A.; Bignozzi, M. Cleaner, sustainable, and safer: Green potential of alkali-activated materials in current building industry, radiological good practice, and a few tips. Constr. Build. Mater. 2023, 409, 133879. [Google Scholar] [CrossRef]
- Yang, J.; Sun, Z.; Belie, N.; Snoeck, D. Internal curing and its application to alkali-activated materials: A literature review. Cem. Concr. Compos. 2024, 145, 105360. [Google Scholar] [CrossRef]
- Masson-Delmotte, V.; Zhai, P.; Pirani, S.; Connors, C.; Péan, S.; Berger, N.; Caud, Y.; Chen, L.; Goldfarb, M.; Scheel Monteiro, P.; et al. Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change; IPCC: Geneva, Switzerland, 2021. [Google Scholar]
- Valente, M.; Sambucci, M.; Sibai, A. Geopolymers vs. cement matrix materials: How nanofiller can help a sustainability approach for smart construction applications—A review. Nanomaterials 2021, 11, 2007. [Google Scholar] [CrossRef]
- Khalid, H.; Lee, N.; Park, S.; Abbas, N.; Lee, H. Synthesis of geopolymer-supported zeolites via robust one-step method and their adsorption potential. J. Hazard. Mater. 2018, 353, 522–533. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.; Shi, H.; Dick, W. Compressive strength and microstructural characteristics of class C fly ash geopolymer. Cem. Concr. Compos. 2010, 32, 142–147. [Google Scholar] [CrossRef]
- Provis, J.; Deventer, J. Alkali Activated Materials: State-of-the-Art Report, RILEM TC 224-AAM; Springer: Dordrecht, The Netherlands, 2013. [Google Scholar]
- Elzeadani, M.; Bompa, D.; Elghazouli, A. One part alkali activated materials: A state-of-the-art review. J. Build. Eng. 2022, 57, 104871. [Google Scholar] [CrossRef]
- Shi, C. Corrosion resistance of alkali-activated slag cement. Adv. Cem. Res. 2003, 15, 77–81. [Google Scholar] [CrossRef]
- El-Didamony, H.; Amer, A.; Ela-ziz, H. Properties and durability of alkali-activated slag pastes immersed in sea water. Ceram. Int. 2012, 38, 3773–3780. [Google Scholar] [CrossRef]
- Provis, J. Alkali-activated materials. Cem. Concr. Res. 2018, 114, 40–48. [Google Scholar] [CrossRef]
- Arbi, K.; Nedeljkovic, M.; Zuo, Y.; Ye, G. A review on the durability of alkali-activated fly ash/slag systems: Advances, issues, and perspectives. Ind. Eng. Chem. Res. 2016, 55, 5439–5453. [Google Scholar] [CrossRef]
- Zhang, P.; Gao, Z.; Wang, J.; Guo, J.; Hu, S.; Ling, Y. Properties of fresh and hardened fly ash/slag based geopolymer concrete: A review. J. Clean. Prod. 2020, 270, 122389. [Google Scholar] [CrossRef]
- Ding, Y.; Dai, J.; Shi, C. Mechanical properties of alkali-activated concrete: A state-of-the-art review. Constr. Build. Mater. 2016, 127, 68–79. [Google Scholar] [CrossRef]
- Ma, H.; Wu, C. Mechanical and microstructural properties of alkali-activated fly ash-slag material under sustained moderate temperature effect. Cem. Concr. Compos. 2022, 134, 104744. [Google Scholar] [CrossRef]
- Mastali, M.; Kinnunen, P.; Dalvand, A.; Firouz, R.M.; Illikainen, M. Drying shrinkage in alkali-activated binders–a critical review. Constr. Build. Mater. 2018, 190, 533–550. [Google Scholar] [CrossRef]
- Sant, G. The influence of temperature on autogenous volume changes in cementitious materials containing shrinkage reducing admixtures. Cem. Concr. Compos. 2012, 34, 855–865. [Google Scholar] [CrossRef]
- Liu, H.; Bu, Y.; Sanjayan, J.; Nazari, A.; Shen, Z. Suitability of polyacrylamide superabsorbent polymers as the internal curing agent of well cement. Constr. Build. Mater. 2016, 112, 253–260. [Google Scholar] [CrossRef]
- Cao, F.; Miao, M.; Yan, P. Hydration characteristics and expansive mechanism of MgO expansive agents. Constr. Build. Mater. 2018, 183, 234–242. [Google Scholar] [CrossRef]
- Ma, H.; Chen, H.; Zhu, H.; Shi, Y.; Ni, Y.; Huo, Q.; Hang, Z. Study on the drying shrinkage of alkali-activated coal gangue-slag mortar and its mechanisms. Constr. Build. Mater. 2019, 225, 204–213. [Google Scholar]
- Wang, Z.; Park, S.; Khalid, H.; Lee, H. Hydration properties of alkali-activated fly ash/slag binders modified by MgO with different reactivity. J. Build. Eng. 2021, 44, 103252. [Google Scholar] [CrossRef]
- Gu, L.; Qin, X.; Feng, J. Experimental studies on the volume stability of MgO expansion agent in concrete. J. Build. Eng. 2023, 79, 107866. [Google Scholar] [CrossRef]
- Cui, Y.; Li, Y.; Wang, Q. Engineering performance and expansion mechanism of MgO expansion agent in ultra-high performance concrete (UHPC). J. Build. Eng. 2023, 68, 106079. [Google Scholar] [CrossRef]
- Mo, L.; Fang, J.; Hou, W.; Ji, X.; Yang, J.; Fan, T.; Wang, H. Synergetic effects of curing temperature and hydration reactivity of MgO expansive agents on their hydration and expansion behaviours in cement pastes. Constr. Build. Mater. 2019, 207, 206–217. [Google Scholar] [CrossRef]
- Sherir, M.; Hossain, K.; Lachemi, M. Self-healing and expansion characteristics of cementitious composites with high volume fly ash and MgO-type expansive agent. Constr. Build. Mater. 2016, 127, 80–92. [Google Scholar] [CrossRef]
- Hwang, C.; Vo, D.; Tran, V.; Yehualaw, M. Effect of high MgO content on the performance of alkali-activated fine slag under water and air curing conditions. Constr. Build. Mater. 2018, 186, 503–513. [Google Scholar] [CrossRef]
- Ma, H.; Li, X.; Zheng, X.; Niu, X.; Fang, Y. Drying Effect of active MgO on the hydration kinetics characteristics and microstructures of alkali-activated fly ash-slag materials. Constr. Build. Mater. 2022, 361, 129677. [Google Scholar] [CrossRef]
- Jin, F.; Gu, K.; Al-Tabbaa, A. Strength and drying shrinkage of active MgO modified alkali-activated slag paste. Constr. Build. Mater. 2014, 51, 395–404. [Google Scholar] [CrossRef]
- Huang, K.; Shi, X.; Zollinger, D.; Mirsayar, M.; Wang, A.; Mo, L. Use of MgO expansion agent to compensate concrete shrinkage in jointed reinforced concrete pavement under high-altitude environmental conditions. Constr. Build. Mater. 2019, 202, 528–536. [Google Scholar] [CrossRef]
- Lee, N.; Jang, J.; Lee, H. Shrinkage characteristics of alkali-activated fly ash/slag paste and mortar at early ages. Cem. Concr. Compos. 2014, 53, 239–248. [Google Scholar] [CrossRef]
- He, J.; Zheng, W.; Bai, W.; Hu, T.; He, J.; Song, X. Effect of active MgO on hydration and properties of alkali-activated slag pastes with different activators. Constr. Build. Mater. 2021, 271, 121608. [Google Scholar] [CrossRef]
- Zhao, H.; Xiang, Y.; Xie, D.; Xu, W.; Wang, Y.; Li, H.; Tian, Q.; Liu, J. Effects of CaO-based and MgO-based expansion agent, curing temperature and restraint degree on pore structure of early-age mortar. Constr. Build. Mater. 2020, 257, 119572. [Google Scholar] [CrossRef]
- Huang, K.; Deng, M.; Mo, L.; Wang, Y. Early age stability of concrete pavement by using hybrid fiber together with MgO expansion agent in high altitude locality. Constr. Build. Mater. 2013, 48, 685–690. [Google Scholar] [CrossRef]
- Mo, L.; Deng, M.; Tang, M. Effects of calcination condition on expansion property of MgO-type expansive agent used in cement-based materials. Cem. Concr. Res. 2010, 40, 437–446. [Google Scholar] [CrossRef]
- Ye, Y.; Li, L.; Song, Y.; Yu, H.; Shi, T.; Ye, Q. Effects of nano MgO and light-burnt MgO on mechanical properties and long-term expansion of paste mixed with Portland cement and slag. Constr. Build. Mater. 2024, 443, 137756. [Google Scholar] [CrossRef]
- Atis, C.; Bilim, C.; Celik, O.; Karahan, O. Influence of activator on the strength and drying shrinkage of alkali-activated slag mortar. Constr. Build. Mater. 2009, 23, 548–555. [Google Scholar] [CrossRef]
- Matalkah, F.; Salem, T.; Shaafaey, M.; Soroushian, P. Drying shrinkage of alkali activated binders cured at room temperature. Constr. Build. Mater. 2019, 201, 563–570. [Google Scholar] [CrossRef]
- Abdel-Gawwad, H.; Mohammed, M.; Alomayri, T. Single and dual effects of magnesia and alumina nano-particles on strength and drying shrinkage of alkali activated slag. Constr. Build. Mater. 2019, 228, 116827. [Google Scholar] [CrossRef]
- Palacios, M.; Puertas, F. Effect of shrinkage-reducing admixtures on the properties of alkali-activated slag mortars and pastes. Cem. Concr. Res. 2007, 37, 691–702. [Google Scholar] [CrossRef]
- Fan, J.; Zhang, B. Repair of ordinary Portland cement concrete using alkali activated slag/fly ash: Freeze-thaw resistance and pore size evolution of adhesive interface. Constr. Build. Mater. 2021, 300, 124334. [Google Scholar] [CrossRef]
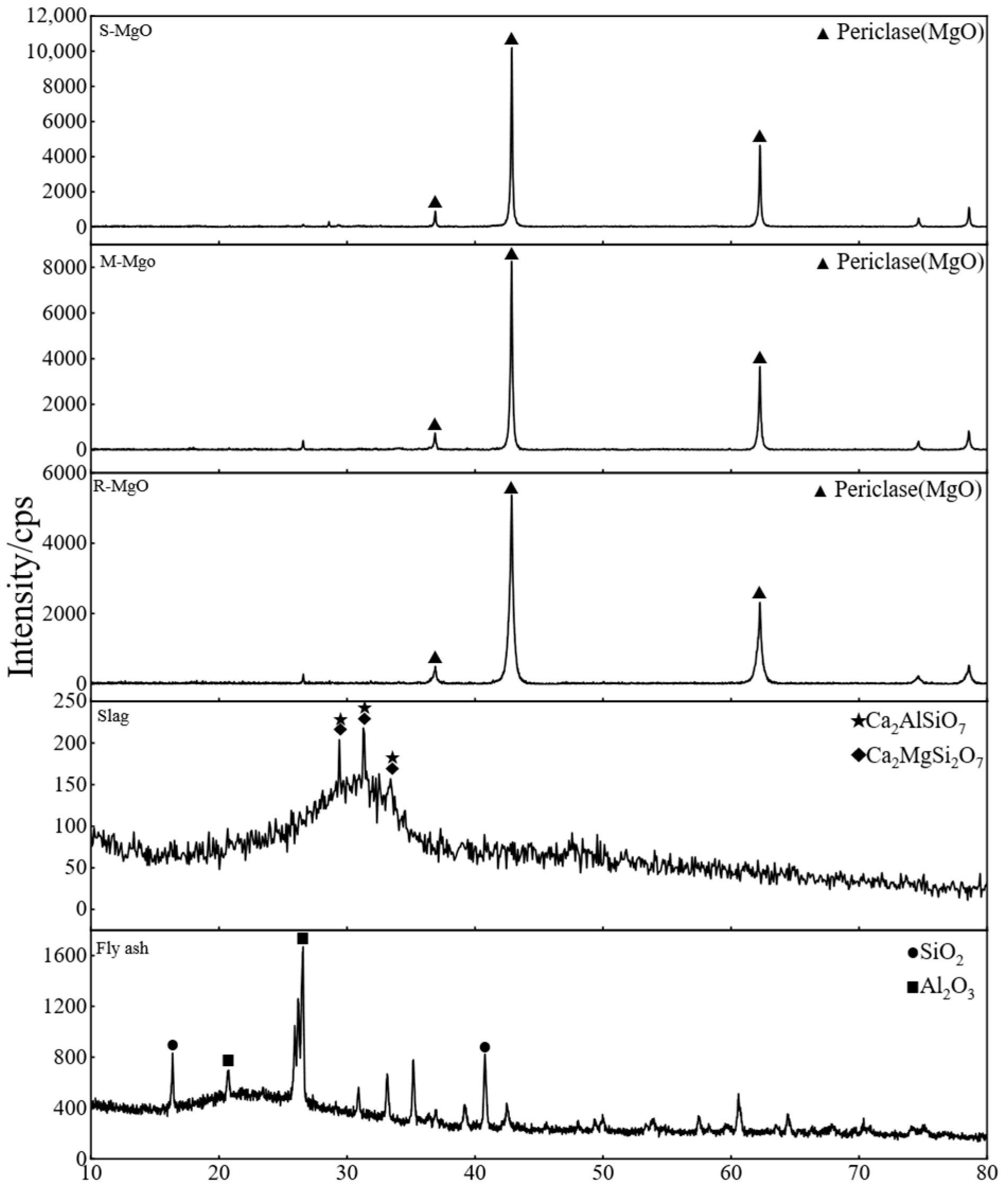






| Material | CaO | MgO | Al2O3 | SiO2 | Fe2O3 | SO3 | LOI |
|---|---|---|---|---|---|---|---|
| Slag | 35.58 | 7.16 | 16.32 | 36.10 | 0.23 | 1.71 | 2.9 |
| Fly ash | 2.66 | 0.24 | 32.79 | 55.71 | 4.43 | — | 0.97 |
| R-MgO | 1.31 | 92.65 | 0.7 | 1 | 1 | 0.2 | 1.6 |
| M-MgO | 1.3 | 90.92 | 0.5 | 2.2 | 0.8 | 0.1 | 2.2 |
| S-MgO | 2.6 | 90.04 | 0.5 | 2.7 | 1 | 0.1 | 1.9 |
| Sample No. | Fly Ash | Slag | MgO Type | MgO Content |
|---|---|---|---|---|
| AAFS | 50% | 50% | — | 0 |
| AAFS-S6 | 47% | 47% | S | 6% |
| AAFS-S9 | 45.5% | 45.5% | S | 9% |
| AAFS-S12 | 44% | 44% | S | 12% |
| AAFS-S15 | 42.5% | 42.5% | S | 15% |
| AAFS-M6 | 47% | 47% | M | 6% |
| AAFS-M9 | 45.5% | 45.5% | M | 9% |
| AAFS-M12 | 44% | 44% | M | 12% |
| AAFS-M15 | 42.5% | 42.5% | M | 15% |
| AAFS-R6 | 47% | 47% | R | 6% |
| AAFS-R9 | 45.5% | 45.5% | R | 9% |
| AAFS-R12 | 44% | 44% | R | 12% |
| AAFS-R15 | 42.5% | 42.5% | R | 15% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, H.; Li, S.; Lei, Z.; Wu, J.; Yuan, X.; Niu, X. Effect of Active MgO on Compensated Drying Shrinkage and Mechanical Properties of Alkali-Activated Fly Ash–Slag Materials. Buildings 2025, 15, 256. https://doi.org/10.3390/buildings15020256
Ma H, Li S, Lei Z, Wu J, Yuan X, Niu X. Effect of Active MgO on Compensated Drying Shrinkage and Mechanical Properties of Alkali-Activated Fly Ash–Slag Materials. Buildings. 2025; 15(2):256. https://doi.org/10.3390/buildings15020256
Chicago/Turabian StyleMa, Hongqiang, Shiru Li, Zelong Lei, Jialong Wu, Xinhua Yuan, and Xiaoyan Niu. 2025. "Effect of Active MgO on Compensated Drying Shrinkage and Mechanical Properties of Alkali-Activated Fly Ash–Slag Materials" Buildings 15, no. 2: 256. https://doi.org/10.3390/buildings15020256
APA StyleMa, H., Li, S., Lei, Z., Wu, J., Yuan, X., & Niu, X. (2025). Effect of Active MgO on Compensated Drying Shrinkage and Mechanical Properties of Alkali-Activated Fly Ash–Slag Materials. Buildings, 15(2), 256. https://doi.org/10.3390/buildings15020256




