Designing for Neonates’ Wellness: Differences in the Reverberation Time Between an Incubator Located in an Open Unit and in a Private Room of a NICU
Abstract
:1. Introduction
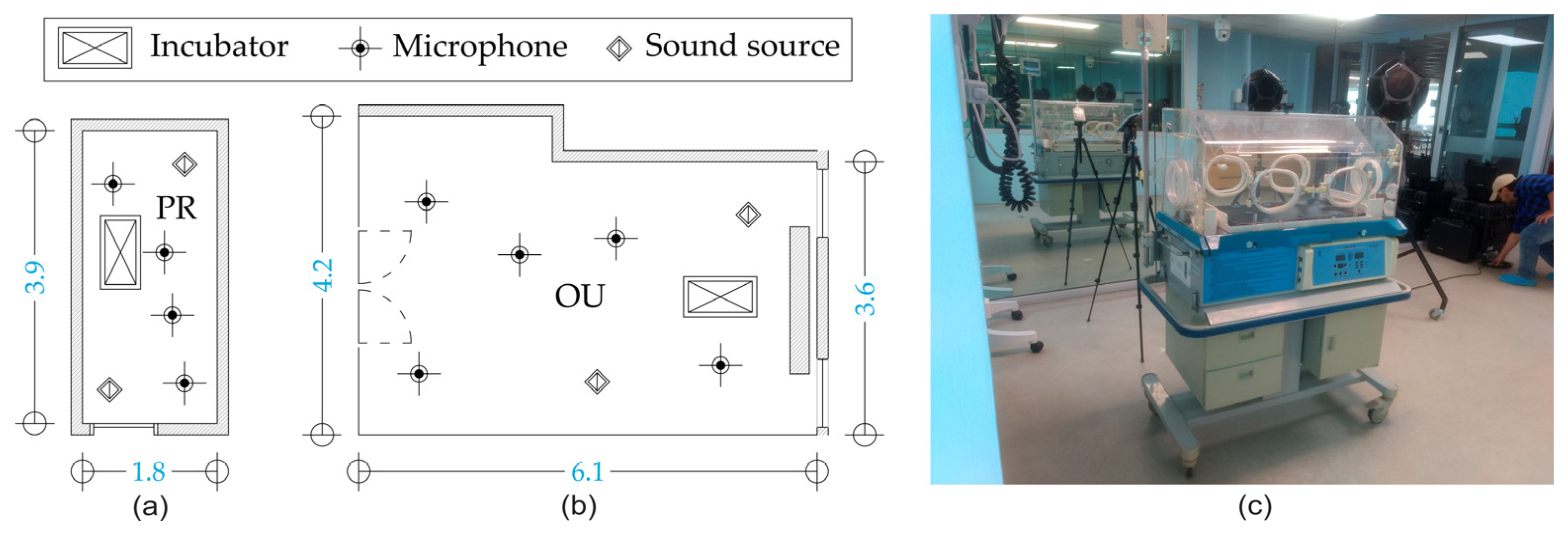
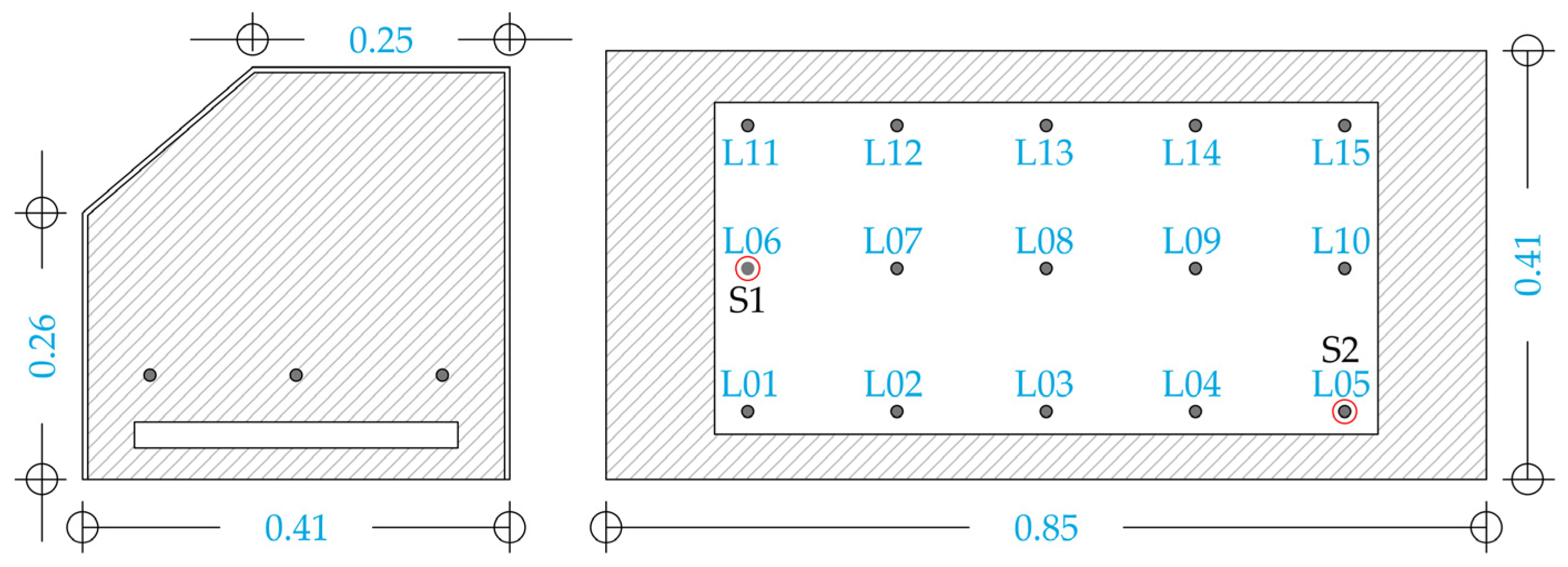
2. Materials and Methods
2.1. Reverberation Time Measurements
2.2. Acoustic Insulation Measurements
2.3. Rooms and Incubator Description
2.4. Statistical Analysis
3. Results
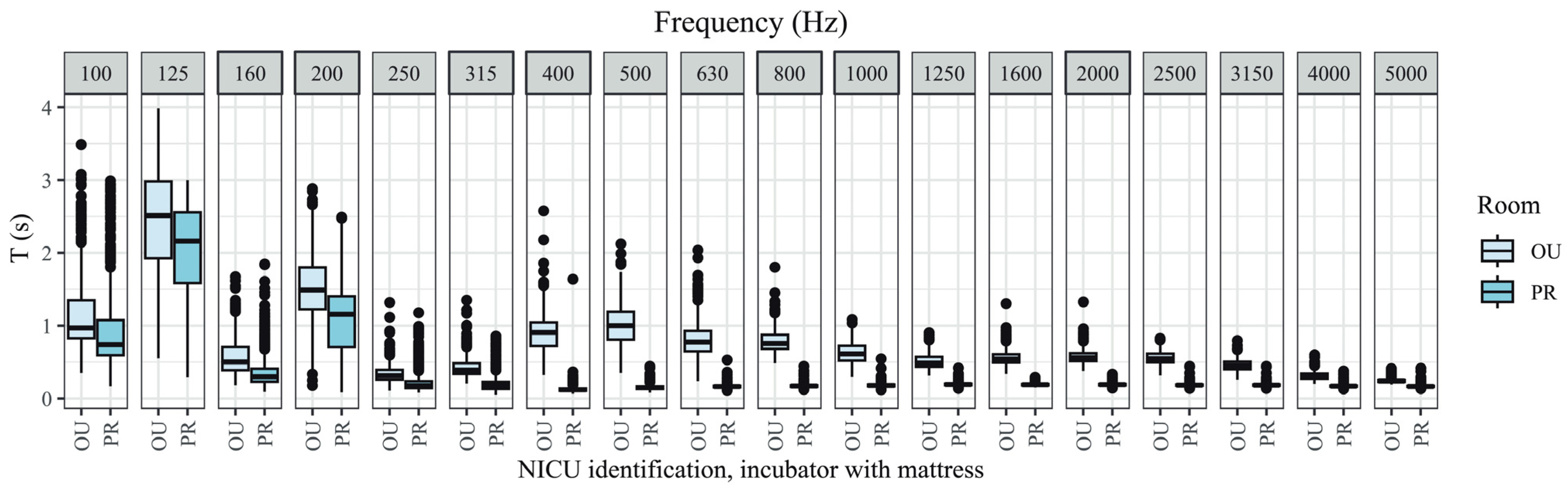
3.1. T of Incubator Without Mattress in Open Unit and Private Room
3.2. T of Incubator with Mattress in Open Unit and Private Room
3.3. T of Open Unit and Private Room
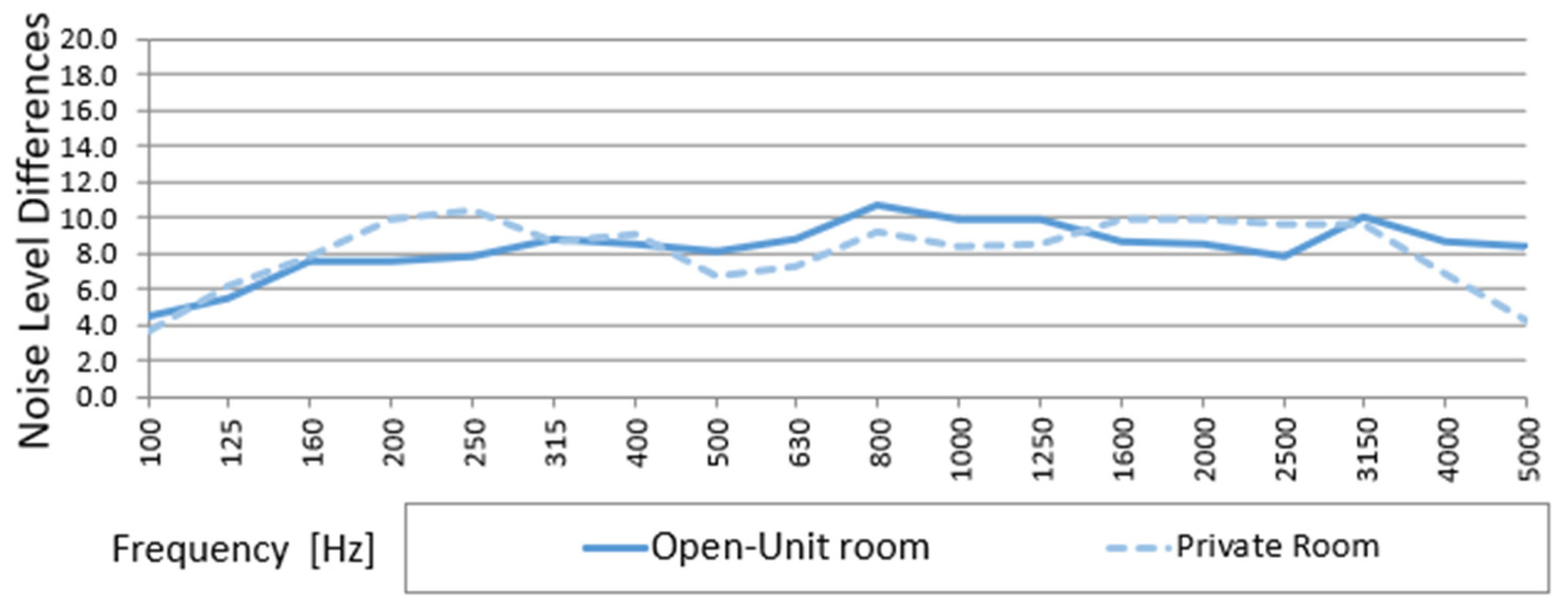
3.4. Noise Level Differences (D) Between Outside and Inside the Incubator in Open Unit and Private Room
4. Discussion
4.1. Acoustic Coupling and Poor Acoustic Insulation
4.2. Flutter Echo
4.3. Resonant Frequencies
4.4. Combined Effects and Possible Consequences on Neonatal Health
4.5. Neuroscience and NICU Design
4.6. Sustainable Development Goals and NICU Design
4.7. Limitations of the Study
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McCallig, M.; Pakrashi, V.; Durkin, C. Evaluation of noise levels and noise sources in an Irish neonatal intensive care unit. Ann. Work. Expo. Health 2024, 68, 550–555. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.W.; Ortmann, A.J.; Clark, W.W. Noise in the Neonatal Intensive Care Unit: A New Approach to Examining Acoustic Events. Noise Health 2018, 20, 121–130. [Google Scholar] [PubMed]
- Garinis, A.C.; Liao, S.; Cross, C.P.; Galati, J.; Middaugh, J.L.; Mace, J.C.; Wood, A.-M.; McEvoy, L.; Moneta, L.; Lubianski, T.; et al. Effect of gentamicin and levels of ambient sound on hearing screening outcomes in the neonatal intensive care unit: A pilot study. Int. J. Pediatr. Otorhinolaryngol. 2017, 97, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, A.; Matsuo, H. Sound Environments Surrounding Preterm Infants Within an Occupied Closed Incubator. J. Pediatr. Nurs. 2016, 31, e149–e154. [Google Scholar] [CrossRef]
- Restin, T.; Gaspar, M.; Bassler, D.; Kurtcuoglu, V.; Scholkmann, F.; Haslbeck, F.B. Newborn Incubators Do Not Protect from High Noise Levels in the Neonatal Intensive Care Unit and Are Relevant Noise Sources by Themselves. Children 2021, 8, 704. [Google Scholar] [CrossRef]
- Santos, J.; Carvalhais, C.; Xavier, A.; Silva, M.V. Assessment and characterization of sound pressure levels in Portuguese neonatal intensive care units. Arch. Environ. Occup. Health 2018, 73, 121–127. [Google Scholar] [CrossRef]
- Fernández-Zacarías, F.; Puyana-Romero, V.; Hernández-Molina, R. The Importance of Noise Attenuation Levels in Neonatal Incubators. Acoustics 2022, 4, 821–833. [Google Scholar] [CrossRef]
- Mayhew, K.J.; Lawrence, S.L.; Squires, J.E.; Harrison, D. Elevated Sound Levels in the Neonatal Intensive Care Unit: What Is Causing the Problem? Adv. Neonatal Care 2022, 22, E207–E216. [Google Scholar] [CrossRef]
- Vohr, B.R. Screening the Newborn for Hearing Loss; UpToDate: Waltham, MA, USA, 2020. [Google Scholar]
- Sibrecht, G.; Wróblewska-Seniuk, K.; Bruschettini, M. Noise or sound management in the neonatal intensive care unit for preterm or very low birth weight infants. Cochrane Database Syst. Rev. 2024, 2024, CD010333. [Google Scholar]
- Cardoso, S.M.S.; Kozlowski, L.d.C.; de Lacerda, A.B.M.; Marques, J.M.; Ribas, A. Newborn physiological responses to noise in the neonatal unit. Braz. J. Otorhinolaryngol. 2015, 81, 583–588. [Google Scholar] [CrossRef]
- Kuhn, P.; Zores, C.; Pebayle, T.; Hoeft, A.; Langlet, C.; Escande, B.; Astruc, D.; Dufour, A. Infants born very preterm react to variations of the acoustic environment in their incubator from a minimum signal-to-noise ratio threshold of 5 to 10 dBA. Pediatr. Res. 2012, 71, 386–392. [Google Scholar] [CrossRef] [PubMed]
- Wharrad, H.J.; Davis, A.C. Behavioural and Autonomic Responses to Sound in Pre-Term and Full-Term Babies. Br. J. Audiol. 1997, 31, 315–329. [Google Scholar] [CrossRef] [PubMed]
- Vranekovic, G.; Hock, E.; Isaac, P.; Cordero, L. Heart rate variability and cardiac response to an auditory stimulus. Biol. Neonate 1974, 24, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Stanley, N.; Graven, M. Sound and the Developing Infant in the NICU: Conclusions and Recommendations for Care. J. Perinatol. 2000, 20, 88–93. [Google Scholar]
- Lahav, A.; Skoe, E. An acoustic gap between the NICU and womb: A potential risk for compromised neuroplasticity of the auditory system in preterm infants. Front. Neurosci. 2014, 8, 381. [Google Scholar] [CrossRef]
- Lejeune, F.; Parra, J.; Berne-audéoud, F.; Marcus, L.; Barisnikov, K.; Gentaz, E.; Debillon, T. Sound Interferes with the Early Tactile Manual Abilities of Preterm Infants. Sci. Rep. 2016, 6, 23329. [Google Scholar] [CrossRef]
- Lai, T.T.; Bearer, C.F. Iatrogenic Environmental Hazards in the Neonatal Intensive Care Unit. Clin. Perinatol. 2008, 35, 163–181. [Google Scholar] [CrossRef]
- Long, J.G.; Lucey, J.F.; Philip, A.G.S. Noise and Hypoxemia in the Intensive Care Nursery. Pediatrics 1980, 65, 143–145. [Google Scholar] [CrossRef]
- Wachman, E.M.; Lahav, A. The effects of noise on preterm infants in the NICU. Arch. Dis. Child.-Fetal Neonatal Ed. 2011, 96, F305–F309. [Google Scholar] [CrossRef]
- Williams, A.L.; Sanderson, M.; Lai, D.; Selwyn, B.J.; Lasky, R. Intensive Care Noise and Mean Arterial Blood Pressure in Extremely Low-Birth-Weight Neonates. Am. J. Perinatol. 2008, 26, 323–329. [Google Scholar] [CrossRef]
- Peng, N.-H.; Bachman, J.; Jenkins, R.; Chen, C.H.; Chang, Y.C.; Chang, Y.S.; Wang, T.M. Relationships Between Environmental Stressors Stress Biobehavioral Responses of Preterm Infants in NICU. Adv. Neonatal Care 2013, 13, 2–10. [Google Scholar] [CrossRef] [PubMed]
- Beira Jiménez, J.L. Afección Acústica de Pacientes en Incubadoras. Caso de Estudio: Neonatos Prematuros en el Hospital Universitario Puerta del Mar de Cádiz; Universidad de Cádiz: Cádiz, Spain, 2021. [Google Scholar]
- Aaron, J.N.; Carlisle, C.C.; Carskadon, M.A.; Meyer, J.; Hill, N.S.; Millman, R.P. Environmental noise as a cause of sleep disruption in an intermediate respiratory care unit. Sleep 1996, 19, 707–710. [Google Scholar] [CrossRef] [PubMed]
- Balk, S.J.; Bochner, R.E.; Ramdhanie, M.A.; Reilly, B.K. Preventing Excessive Noise Exposure in Infants, Children, and Adolescents. Pediatrics 2023, 152, e2023063752. [Google Scholar] [CrossRef] [PubMed]
- Muñoz Illescas, M.L.; Sevilla Salgado, S.; Pérez Lafuente, E. Tecnología y mínima manipulación en prematuros. Enfermería Integr. Col. Enfermería Val. 2017, 116, 41–45. [Google Scholar]
- Guerra Rodríguez, A.E. Alteraciones del Sueño en los Pacientes Pediátricos Hospitalizado; Universidad Autónoma de Nuevo León: San Nicolás de los Garza, Mexico, 2023. [Google Scholar]
- Singh, K.; Parashar, K.; Srishti, K. The Long-Term Impact of Neonatal Intensive Care Unit (Nicu) Environments on Infant Development: A Comprehensive Review with Gap Analysis. Int. J. Curr. Sci. 2024, 14, 613–619. [Google Scholar]
- Valdés-de la Torre, G.E.; Martina Luna, M.; Braverman Bronstein, A.; Iglesias Leboreiro, J.; Bernárdez Zapata, I. Medición comparativa de la intensidad de ruido dentro y fuera de incubadoras cerradas. Perinatol. Reprod. Humana 2018, 32, 65–69. [Google Scholar] [CrossRef]
- American Academy of Pediatrics. Committee on Environmental Health. Noise: A Hazard for the Fetus and Newborn. Pediatrics 1997, 100, 724–727. [Google Scholar] [CrossRef]
- Busch-Vishniac, I.J.; West, J.E.; Barnhill, C.; Hunter, T.; Orellana, D.; Chivukula, R. Noise levels in Johns Hopkins Hospital. J. Acoust. Soc. Am. 2005, 118, 3629–3645. [Google Scholar] [CrossRef]
- Fortes-Garrido, J.C.; Velez-Pereira, A.M.; Gázquez, M.; Hidalgo-Hidalgo, M.; Bolívar, J.P. The characterization of noise levels in a neonatal intensive care unit and the implications for noise management. J. Environ. Health Sci. Eng. 2014, 12, 104. [Google Scholar] [CrossRef]
- Hassanein, S.M.A.; El Raggal, N.M.; Shalaby, A.A. Neonatal nursery noise: Practice-based learning and improvement. J. Matern. Neonatal Med. 2013, 26, 392–395. [Google Scholar] [CrossRef]
- Benini, F.; Magnavita, V.; Lago, P.; Arslan, E.; Pisan, P. Evaluation of noise in the neonatal intensive care unit. Am. J. Perinatol. 1996, 13, 37–41. [Google Scholar] [CrossRef] [PubMed]
- Givrad, S.; Hartzel, G.; Scala, M. Promoting infant mental health in the neonatal intensive care unit (NICU): A review of nurturing factors and interventions for NICU infant-parent relationships. Early Hum Dev. 2021, 154, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Webb, A.R.; Heller, H.T.; Benson, C.B.; Lahav, A. Mother’s voice and heartbeat sounds elicit auditory plasticity in the human brain before full gestation. Proc. Natl. Acad. Sci. USA 2015, 112, 3152–3157. [Google Scholar] [CrossRef] [PubMed]
- Yetkin, A.K.; Gunes, B.; Erbas, O. Prenatal Auditory Stimulation and Its Significance for Newborns. J. Exp. Basic. Med. Sci. 2023, 4, 96–103. [Google Scholar]
- Plante-Hébert, J.; Boucher, V.J.; Jemel, B. The processing of intimately familiar and unfamiliar voices: Specific neural responses of speaker recognition and identification. PLoS ONE 2021, 16, e0250214. [Google Scholar] [CrossRef]
- Balsan, M.J.; Burns, J.; Kimock, F.; Hirsch, E.; Unger, A.; Telesco, R.; Bloch-Salisbury, E. A pilot study to assess the safety, efficacy and ease of use of a novel hearing protection device for hospitalized neonates. Early Hum. Dev. 2021, 156, 105365. [Google Scholar] [CrossRef]
- Pineda, R.G.; Stransky, K.E.; Rogers, C.; Duncan, M.H.; Smith, G.C.; Neil, J.; Inder, T. The Single Patient Room in the NICU: Maternal and Family Effects. J. Perinatol. 2012, 32, 545–551. [Google Scholar] [CrossRef]
- Szymczak, S.E.; Shellhaas, R.A. Impact of NICU design on environmental noise. J. Neonatal Nurs. 2014, 20, 77–81. [Google Scholar] [CrossRef]
- Carvalhais, C.; Santos, J.; Vieira, M.; Xavier, A. Is There Sufficient Training of Health Care Staff on Noise Reduction in Neonatal Intensive Care Units? a Pilot Study From Neonoise. J. Toxicol. Environ. Health Part A Curr. Issues 2015, 78, 897–903. [Google Scholar] [CrossRef]
- Liu, W.F. The impact of a noise reduction quality improvement project upon sound levels in the open-unit-design neonatal intensive care unit. J. Perinatol. 2010, 30, 489–496. [Google Scholar] [CrossRef]
- Wang, D.; Aubertin, C.; Barrowman, N.; Moreau, K.; Dunn, S.; Harrold, J. Reduction of noise in the neonatal intensive care unit using sound-activated noise meters. Arch. Dis. Child. Fetal Neonatal Ed. 2014, 99, 515–516. [Google Scholar] [CrossRef] [PubMed]
- Fusch, G.; Mohamed, S.; Bakry, A.; Li, E.W.; Dutta, S.; El Helou, S.; Fusch, C. Analysis of noise levels in the neonatal intensive care unit: The impact of clinical microsystems. Eur. J. Pediatr. 2024, 183, 1245–1254. [Google Scholar] [CrossRef] [PubMed]
- Fucile, S.; Patterson, C.; Dow, K. Noise reduction in the neonatal intensive care unit: An exploratory study. J. Neonatal Nurs. 2023, 29, 330–333. [Google Scholar] [CrossRef]
- Coston, A.D.; Aune, C. Reducing Noise in the Neonatal Intensive Care Unit. Pediatrics 2019, 144, 154. [Google Scholar] [CrossRef]
- Bergman, I.; Hirsch, R.P.; Fria, T.J.; Shapiro, S.M.; Holzman, I.; Painter, M.J. Cause of hearing loss in the high-risk premature infant. J. Pediatr. 1985, 106, 95–101. [Google Scholar] [CrossRef]
- American Academy of Pediatrics. Guidelines for Perinatal Care, 8th ed.; American Academy of Pediatrics: Itasca, IL, USA, 2020. [Google Scholar]
- Moreira Pinheiro, E.; Guinsburg, R.; de Araujo Nabuco, M.A.; Yoshiko Kakehashi, T. Noise at the Neonatal Intensive Care Unit and inside the interior of the incubator. Rev. Lat. Am. Enfermagem 2011, 19, 1214–1221. (In Spanish) [Google Scholar] [CrossRef]
- Carvalhais, C.; Silva, M.V.; Silva, J.; Xavier, A.; Santos, J. Noise in neonatal intensive care units: A short review. In Proceedings of the Euronoise 2018, Crete, Greece, 27–31 May 2018; pp. 545–550. Available online: http://www.euronoise2018.eu/docs/papers/95_Euronoise2018.pdf (accessed on 16 April 2025).
- Philbin, M.K. Planning the acoustic environment of a neonatal intensive care unit. Clin. Perinatol. 2004, 31, 331–352. [Google Scholar] [CrossRef]
- Lichtig, I.; Maki, K. Estudos de níveis de ruídos ambientais e de ruídos gerados pelas incubadoras em uma unidade de terapia intensiva. Pediatria 1992, 14, 30–34. [Google Scholar]
- DOliveira Rodarte, M.; Silvan Scochi, C.G.; Moraes Leite, A.; Ide Fujinaga, C.; Zamberlan, N.E.; Correa Castral, T. O ruído gerado durante a manipulação das incubadoras: Implicações para o cuidado de enfermagem. Rev. Lat. Am. Enferm. 2005, 13, 79–85. [Google Scholar] [CrossRef]
- Barceló, C.; Molina, E.; Mendoza, J.G.; Dueñas, E.; Plá, E. Estructura física de los sonidos continuos y de impulso en incubadoras infantiles de uso nacional. Rev. Cub. Ped. 1986, 58, 575–590. [Google Scholar]
- Puyana-Romero, V.; Núñez-Solano, D.; Hernández, R.; Fernández-Zacarías, F.; Beira-Jiménez, J.L.; Garzón, C.; Muñoz, E.J. Reverberation time measurements of a neonatal incubator. Appl. Acoust. 2020, 167, 107374. [Google Scholar] [CrossRef]
- Fernández Zacarías, F.; Beira Jiménez, J.L.; Bustillo Velázquez-Gaztelu, P.J.; Hernández Molina, R.; Lubián López, S. Noise level in neonatal incubators: A comparative study of three models. Int. J. Pediatr. Otorhinolaryngol. 2018, 107, 150–154. [Google Scholar] [CrossRef] [PubMed]
- Puyana-Romero, V.; Núñez-Solano, D.; Hernández-Molina, R.; Jara-Muñoz, E. Influence of the NICU on the Acoustic Isolation of a Neonatal Incubator. Front. Pediatr. 2020, 8, 588. [Google Scholar] [CrossRef]
- Rodríguez-Montaño, V.M.; Puyana-Romero, V.; Hernández-Molina, R.; Beira-Jiménez, J.L. The Noise: A Silent Threat to the Recovery of Patients in Neonatal Intensive Care Units. Buildings 2024, 14, 2778. [Google Scholar] [CrossRef]
- Núñez-Solano, D.; Puyana-Romero, V.; Ordoñez-Andrade, C.; Bravo-Monayo, L.; Garzón-Pico, C. Impulse response simulation of a small room and in situ measurements validation. In Proceedings of the 147th Audio Engineering Society Convention, New York, NY, USA, 16–19 October 2019; pp. 1–7. [Google Scholar]
- Kleiner, M.; Tichy, J. Acoustics of Small Rooms; CRC Press: Boca Raton, FL, USA, 2014; pp. 1–453. [Google Scholar]
- Everest, F.A. The Master Handbook of Acoustics, 4th ed.; McGraw-Hill Education: New York, NY, USA, 2000. [Google Scholar]
- Pätynen, J.; Katz, B.F.G.; Lokki, T. Investigations on the balloon as an impulse source. J. Acoust. Soc. Am. 2011, 129, EL27–EL33. [Google Scholar] [CrossRef]
- Griesinger, D. Beyond MLS-Occupied Hall Measurement with FFT Techniques. In Audio Engineering Society Convention 101; Audio Engineering Society: New York, NY, USA, 1996. [Google Scholar]
- Cheenne, D.J.; Ardila, M.; Lee, C.G.; Bridgewater, B. A qualitative and quantitative analysis of impulse responses from balloon bursts. J. Acoust. Soc. Am. 2008, 123, 3909. [Google Scholar] [CrossRef]
- ISO 3382-2; Measurement of Room Acoustic Parameters. Part 2: Reverberation Time in Ordinary Rooms. International Organization for Standardization: Geneva, Switzerland, 2008.
- Nash, A. On the acoustical characteristics of a balloon. In Proceedings of the International Symposium on Room Acoustics, Seville, Spain, 10–12 September 2007; pp. 2761–2762. [Google Scholar]
- Llinares, J.; Llopis, A.; Sancho, J. Acústica Arquitectónica y Urbanística; Universitat Politècnica de València: València, Spain, 2008. [Google Scholar]
- Puyana-Romero, V.; Tamayo-Guamán, L.M.; Núñez-Solano, D.; Hernández-Molina, R.; Ciaburro, G. Artificial Neural Network-Based Model to Characterize the Reverberation Time of a Neonatal Incubator. In Innovations in Machine and Deep Learning: Case Studies and Applications; Rivera, G., Rosete, A., Dorronsoro, B., Rangel-Valdez, N., Eds.; Springer Nature: Cham, Switzerland, 2023; pp. 305–322. [Google Scholar] [CrossRef]
- Berzborn, M.; Bomhardt, R.; Klein, J.; Richter, J.G.; Vorländer, M. The ITA-Toolbox: An Open Source MATLAB Toolbox for Acoustic Measurements and Signal Processing. In Proceedings of the 43th Annual German Congress on Acoustics, Kiel, Germany, 6–9 March 2017; pp. 222–225. Available online: http://www.ita-toolbox.org/publications/ITA-Toolbox_paper2017.pdf (accessed on 16 April 2025).
- ISO 16283-1:2014; Acoustics—Field Measurement of Sound Insulation in Buildings and of Building Elements—Part 1: Airborne Sound Insulation. International Organization for Standardization: Geneva, Switzerland, 2014.
- Berger, V.; Zhou, Y. Kolmogorov–Smirnov Test: Overview. In Wiley StatsRef: Statistics Reference Online; Wiley: Hoboken, NJ, USA, 2014. [Google Scholar]
- Ostertagová, E.; Ostertag, O.; Kováč, J. Methodology and Application of the Kruskal-Wallis Test. Appl. Mech. Mater. 2014, 611, 115–120. [Google Scholar] [CrossRef]
- Lydersen, S. Adjustment of p values for multiple hypotheses: Why, when and how. Ann. Rheum. Dis. 2024, 83, 1254–1255. [Google Scholar] [CrossRef]
- Chen, S.Y.; Feng, Z.; Yi, X. A general introduction to adjustment for multiple comparisons. J. Thorac. Dis. 2017, 9, 1725–1729. [Google Scholar] [CrossRef]
- Cohen, J. Using Effect Size—Or Why the P Value Is Not Enough. J. Grad. Med. Educ. 2012, 4, 279–282. [Google Scholar]
- Coe, R. It’s the Effect Size, Stupid: What effect size is and why it is important. In Proceedings of the Conference of the British Educational Research Association, Exeter, UK, 11–14 September 2002. [Google Scholar]
- Heričko, T.; Šumak, B. Exploring Maintainability Index Variants for Software Maintainability Measurement in Object-Oriented Systems. Appl. Sci. 2023, 13, 2972. [Google Scholar] [CrossRef]
- Kerby, D.S. The Simple Difference Formula: An Approach to Teaching Nonparametric Correlation. Compr. Psychol. 2014, 3, 1. [Google Scholar] [CrossRef]
- Parker, R.I.; Hagan-Burke, S. Useful Effect Size Interpretations for Single Case Research. Behav. Ther. 2007, 38, 95–105. [Google Scholar] [CrossRef]
- Ben-Shachar, M.S.; Makowski, D.; Daniel Lüdecke Patil, I.; Wiernik, B.M.; Thériault, R.; Kelley, K.; Stanley, D.; Caldwell, A.; Burnett, J.; Karreth, J. Package ‘Effectsize ’. BugReports. 2024, pp. 87–91. Available online: https://easystats.github.io/effectsize/ (accessed on 16 April 2025).
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; Routledge: New York, NY, USA, 1988; 567p. [Google Scholar] [CrossRef]
- Wasserstein, R.L.; Lazar, N.A. The ASA’s Statement on p-Values: Context, Process, and Purpose. Am. Stat. 2016, 70, 129–133. [Google Scholar] [CrossRef]
- Gibbons, J.D.; Pratt, J.W. P-values: Interpretation and Methodology. Am. Stat. 2012, 29, 37–41. [Google Scholar]
- Berger, J.O.; Sellke, T. Testing a point null hypothesis: The irreconcilability of P values and evidence. J. Am. Stat. Assoc. 1987, 82, 112–122. [Google Scholar] [CrossRef]
- Sellke, T.; Bayarri, M.J.; Berger, J.O. Calibration of p Values for Testing Precise Null Hypotheses. Am. Stat. 2001, 55, 62–71. [Google Scholar] [CrossRef]
- Delampady, M. Lower Bounds on Bayes Factors for Interval Null Hypotheses. J. Am. Stat. Assoc. 1989, 84, 120–124. [Google Scholar] [CrossRef]
- Delapady, M.; Berger, J.O. Lower bounds of Bayes factors for multinomial distributions, with applications to Chi-Squared tests of fit. Ann. Stat. 1980, 8, 1295–1316. [Google Scholar]
- Rouder, J.N.; Speckman, P.L.; Sun, D.; Morey, R.D.; Iverson, G. Bayesian t tests for accepting and rejecting the null hypothesis. Psychon. Bull. Rev. 2009, 16, 225–237. [Google Scholar] [CrossRef]
- Bayesian Independent—Sample Inference—IBM Documentation. Available online: https://www.ibm.com/docs/en/spss-statistics/25.0.0?topic=statistics-bayesian-independent-sample-inference#fntarg_2 (accessed on 29 April 2024).
- Edwards, W.; Lindman, H.; Savage, L.J. Bayesian statistical inference for psychological research. Psychol. Rev. 1963, 70, 193–242. [Google Scholar] [CrossRef]
- Sabine, W.C. Collected Papers on Acoustics; Harvard University Press: Cambridge, MA, USA, 1922; p. 279. [Google Scholar]
- Inacio, O. Fundamentals of Room Acoustics; Engineering Acoustics: Barcelona, Spain, 2005. [Google Scholar]
- Zhang, Y.; Du, J.; Liu, Y.; Yang, T.; Liu, Z. Sound transmission between rooms coupled through partition with elastically restrained edges. In Internoise 2014; Australian Acoustical Society: Melbourne, Australia, 2014; pp. 1–12. [Google Scholar]
- Knudsen, V.O.; Harris, C.M. Reverberation in coupled spaces. In Acoustical Designing in Architecture; Acoustical Society of America (ASA): Honolulu, HI, USA, 1978. [Google Scholar]
- Kuttruff, H. Room Acoustics; Spon Press: London, UK, 2009; p. 389. [Google Scholar]
- Long, M. Architectural Acoustics; Stern, R., Levy, M., Eds.; Elsevier Academic Press: Amsterdam, The Netherlands, 2006. [Google Scholar]
- Longoni, H.C.; Turra, B. Identificación, Análisis y Control del Eco Flotante. 2012. Universidad Tecnológica Nacional Facultad Regional Córdoba, Mayo 2012, Argentina. Available online: https://www.profesores.frc.utn.edu.ar/electronica/fundamentosdeacusticayelectroacustica/pub/file/FAyE0812E1-Longoni-Turra(1).pdf (accessed on 16 April 2025).
- Fastl, H.; Zwicker, E. Psychoacoustics: Facts and Models; Springer: Cham, Switzerland, 2007. [Google Scholar]
- Puyana-Romero, V.; Núñez-Solano, D.; Fernández-Zacarías, F.; Jara-Muñoz, E.; Hernández-Molina, R. The Importance of Reverberation for the Design of Neonatal Incubators. Front. Pediatr. 2021, 9, 4–11. [Google Scholar] [CrossRef] [PubMed]
- Buchenau, U.; D’Angelo, G.; Carini, G.; Liu, X.; Ramos, M.A. Sound absorption in glasses. Rev. Phys. 2022, 9, 100078. [Google Scholar] [CrossRef]
- de Paiva, A. Neuroscience for Architecture: How Building Design Can Influence Behaviors and Performance. J. Civ. Eng. Archit. 2018, 12, 132–138. [Google Scholar]
- Zhang, X.; Spear, E.; Hsu, H.H.L.; Gennings, C.; Stroustrup, A. NICU-based stress response and preterm infant neurobehavior: Exploring the critical windows for exposure. Pediatr. Res. 2022, 92, 1470–1478. [Google Scholar] [CrossRef]
- Kahneman, D. Thinking, Fast and Slow; Farrar, Straus and Giroux: New York, NY, USA, 2011; p. 499. [Google Scholar]
- Haan Mde Dumontheil, I.; Johnson, M.H. Developmental Cognitive Neuroscience: An Introduction, 5th ed.; The Wiley Handbook of Developmental Psychopathology; Wiley: Hoboken, NJ, USA, 2023; pp. 179–196. [Google Scholar]
- Ulrich, R.S.; Zimring, C.; Zhu, X.; DuBose, J.; Seo, H.B.; Choi, Y.S.; Quan, X.; Joseph, A. A review of the research literature on evidence-based healthcare design. HERD 2008, 1, 61–125. [Google Scholar] [CrossRef]
- Attaianese, E. Environmental Design and Human Performance. A Literature Review. In Proceedings of the 20th Congress of the International Ergonomics Association (IEA 2018), Florence, Italy, 26–30 August 2018; Bagnara, S., Tartaglia, R., Albolino, S., Alexander, T., Fujita, Y., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 486–495. [Google Scholar]
- United Nations Department of Economic and Social Affairs. The 17 Goals. 2024. Sustainable Development Goals. Available online: https://sdgs.un.org/goals (accessed on 5 July 2024).
- Krueger, C.; Schue, S.; Parker, L. Neonatal intensive care unit sound levels before and after structural reconstruction. MCN Am. J. Matern. Child. Nurs. 2007, 32, 358–362. [Google Scholar] [CrossRef]
- Deng, Z.; Xie, H.; Kang, J. The acoustic environment in typical hospital wards in China. Appl. Acoust. 2023, 203, 109202. [Google Scholar] [CrossRef]
- Meng, Q.; Wu, Y. Sound Environment and Acoustic Perception in Hospitals. In Indoor Sound Environment and Acoustic Perception [Internet]; Meng, Q., Wu, Y., Eds.; Springer Nature: Singapore, 2024; pp. 125–163. [Google Scholar] [CrossRef]
- White, R.; Smith, J.; Shepley, M. Recommended Standards for Newborn ICU Design. J. Perinatol. 2013, 33, S2–S16. [Google Scholar] [CrossRef]
- Annerstedt, M.; Jönsson, P.; Wallergård, M.; Johansson, G.; Karlson, B.; Grahn, P.; Hansen, Å.M.; Währborg, P. Inducing physiological stress recovery with sounds of nature in a virtual reality forest—Results from a pilot study. Physiol. Behav. 2013, 118, 240–250. [Google Scholar] [CrossRef]
- Pallasmaa, J. The Eyes of the Skin: Architecture and the Senses; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2012. [Google Scholar]
- Zumthor, P. (Ed.) Thinking Architecture; Birkhäuser: Basel, Switzerland, 2006. [Google Scholar]



| Without Mattress | |||||||
|---|---|---|---|---|---|---|---|
| Frequency | Mann–Whitney U | Z | p-Value | Corrected p-Value | N | rc (Rank-Biserial) | Interpretation [82] |
| F100 | 156,788.000 | −6.748 | 0.000 | 0.000 | 1308 | 0.222 | small |
| F125 | 199,059.500 | −4.150 | 0.000 | 0.000 | 1380 | 0.132 | small |
| F160 | 44,704.000 | −25.189 | 0.000 | 0.000 | 1367 | 0.801 | large |
| F200 | 123,737.000 | −12.633 | 0.000 | 0.000 | 1308 | 0.408 | moderate |
| F250 | 18,428.000 | −28.885 | 0.000 | 0.000 | 1367 | 0.918 | large |
| F315 | 38,887.000 | −26.223 | 0.000 | 0.000 | 1385 | 0.831 | large |
| F400 | 3615.500 | −31.190 | 0.000 | 0.000 | 1395 | 0.985 | large |
| F500 | 43.500 | −31.677 | 0.000 | 0.000 | 1394 | 1.000 | large |
| F630 | 10.000 | −31.689 | 0.000 | 0.000 | 1395 | 1.000 | large |
| F800 | 173.500 | −31.682 | 0.000 | 0.000 | 1397 | 0.999 | large |
| F1000 | 109.000 | −31.691 | 0.000 | 0.000 | 1397 | 1.000 | large |
| F1250 | 9976.000 | −30.355 | 0.000 | 0.000 | 1397 | 0.957 | large |
| F1600 | 4387.000 | −31.112 | 0.000 | 0.000 | 1397 | 0.981 | large |
| F2000 | 4678.000 | −31.072 | 0.000 | 0.000 | 1397 | 0.980 | large |
| F2500 | 22.000 | −31.703 | 0.000 | 0.000 | 1397 | 1.000 | large |
| F3150 | 1218.000 | −31.541 | 0.000 | 0.000 | 1397 | 0.995 | large |
| F4000 | 17,018.000 | −29.402 | 0.000 | 0.000 | 1397 | 0.927 | large |
| F5000 | 20,5451.500 | −3.894 | 0.000 | 0.000 | 1397 | 0.123 | large |
| Bayes Factor Two Independent Samples Test (Method = Rouder) | Posterior Distribution Characterization for Independent Sample Mean | |||||
|---|---|---|---|---|---|---|
| Mean Difference (PR-OU) | Pooled Std. Error Difference | Bayes Factor | Interpretation | 95% Credible Interval | ||
| Lower Bound | Upper Bound | |||||
| F100 | −0.504 | 0.056 | 0.000 | Extreme evidence for H1 | −0.629 | −0.380 |
| F125 | −0.284 | 0.079 | 0.038 | Very strong evidence for H1 | −0.430 | −0.139 |
| F160 | −0.887 | 0.031 | 0.000 | Extreme evidence for H1 | −0.957 | −0.818 |
| F200 | −0.659 | 0.054 | 0.000 | Extreme evidence for H1 | −0.762 | −0.556 |
| F250 | −0.782 | 0.020 | 0.000 | Extreme evidence for H1 | −0.827 | −0.738 |
| F315 | −0.363 | 0.014 | 0.000 | Extreme evidence for H1 | −0.396 | −0.330 |
| F400 | −0.818 | 0.014 | 0.000 | Extreme evidence for H1 | −0.851 | −0.785 |
| F500 | −0.832 | 0.012 | 0.000 | Extreme evidence for H1 | −0.859 | −0.804 |
| F630 | −0.629 | 0.008 | 0.000 | Extreme evidence for H1 | −0.647 | −0.611 |
| F800 | −0.513 | 0.006 | 0.000 | Extreme evidence for H1 | −0.529 | −0.498 |
| F1000 | −0.409 | 0.005 | 0.000 | Extreme evidence for H1 | −0.421 | −0.397 |
| F1250 | −0.189 | 0.004 | 0.000 | Extreme evidence for H1 | −0.199 | −0.180 |
| F1600 | −0.280 | 0.004 | 0.000 | Extreme evidence for H1 | −0.289 | −0.272 |
| F2000 | −0.248 | 0.004 | 0.000 | Extreme evidence for H1 | −0.257 | −0.238 |
| F2500 | −0.319 | 0.005 | 0.000 | Extreme evidence for H1 | −0.330 | −0.308 |
| F3150 | −0.235 | 0.004 | 0.000 | Extreme evidence for H1 | −0.245 | −0.224 |
| F4000 | −0.105 | 0.004 | 0.000 | Extreme evidence for H1 | −0.116 | −0.095 |
| F5000 | −0.023 | 0.004 | 0.000 | Extreme evidence for H1 | −0.033 | −0.013 |
| With Mattress | |||||||
|---|---|---|---|---|---|---|---|
| Frequency | Mann–Whitney U | Z | p-Value | Corrected p-Value | N | rc (Rank-Biserial) | Interpretation [82] |
| F100 | 162,864.500 | −6.994 | 0.000 | 0.000 | 1323 | 0.226 | small |
| F125 | 175,593.000 | −3.543 | 0.000 | 0.000 | 1270 | 0.116 | small |
| F160 | 123,386.000 | −13.640 | 0.000 | 0.000 | 1343 | 0.436 | moderate |
| F200 | 125,964.500 | −12.852 | 0.000 | 0.000 | 1326 | 0.413 | moderate |
| F250 | 121,114.500 | −14.383 | 0.000 | 0.000 | 1359 | 0.458 | moderate |
| F315 | 30,467.000 | −26.748 | 0.000 | 0.000 | 1329 | 0.858 | large |
| F400 | 563.000 | −31.305 | 0.000 | 0.000 | 1355 | 0.997 | large |
| F500 | 53.000 | −31.498 | 0.000 | 0.000 | 1370 | 1.000 | large |
| F630 | 290.000 | −31.536 | 0.000 | 0.000 | 1379 | 0.999 | large |
| F800 | 1.000 | −31.592 | 0.000 | 0.000 | 1381 | 1.000 | large |
| F1000 | 348.500 | −31.544 | 0.000 | 0.000 | 1381 | 0.998 | large |
| F1250 | 305.500 | −31.550 | 0.000 | 0.000 | 1381 | 0.999 | large |
| F1600 | 1.000 | −31.584 | 0.000 | 0.000 | 1380 | 1.000 | large |
| F2000 | 2.000 | −31.584 | 0.000 | 0.000 | 1380 | 1.000 | large |
| F2500 | 274.000 | −31.546 | 0.000 | 0.000 | 1380 | 0.999 | large |
| F3150 | 360.000 | −31.526 | 0.000 | 0.000 | 1379 | 0.998 | large |
| F4000 | 9233.000 | −30.323 | 0.000 | 0.000 | 1381 | 0.960 | large |
| F5000 | 61,175.500 | −23.169 | 0.000 | 0.000 | 1380 | 0.734 | large |
| Bayes Factor Two Independent Samples Test (Method = Rouder) | Posterior Distribution Characterization for Independent Sample Mean | |||||
|---|---|---|---|---|---|---|
| Mean Difference (PR-OU) | Pooled Std, Error Difference | Bayes Factor | Interpretation | 95% Credible Interval | ||
| Lower Bound | Upper Bound | |||||
| F100 | −0.164 | 0.035 | 0.000 | Extreme evidence for H1 | −0.236 | −0.092 |
| F125 | −0.253 | 0.056 | 0.001 | Extreme evidence for H1 | −0.366 | −0.140 |
| F160 | −0.147 | 0.013 | 0.000 | Extreme evidence for H1 | −0.173 | −0.121 |
| F200 | −0.373 | 0.027 | 0.000 | Extreme evidence for H1 | −0.426 | −0.320 |
| F250 | −0.067 | 0.008 | 0.000 | Extreme evidence for H1 | −0.082 | −0.052 |
| F315 | −0.183 | 0.007 | 0.000 | Extreme evidence for H1 | −0.197 | −0.169 |
| F400 | −0.736 | 0.009 | 0.000 | Extreme evidence for H1 | −0.757 | −0.715 |
| F500 | −0.800 | 0.010 | 0.000 | Extreme evidence for H1 | −0.823 | −0.777 |
| F630 | −0.589 | 0.009 | 0.000 | Extreme evidence for H1 | −0.611 | −0.568 |
| F800 | −0.565 | 0.006 | 0.000 | Extreme evidence for H1 | −0.578 | −0.552 |
| F1000 | −0.394 | 0.005 | 0.000 | Extreme evidence for H1 | −0.406 | −0.382 |
| F1250 | −0.266 | 0.004 | 0.000 | Extreme evidence for H1 | −0.275 | −0.258 |
| F1600 | −0.320 | 0.004 | 0.000 | Extreme evidence for H1 | −0.328 | −0.312 |
| F2000 | −0.332 | 0.003 | 0.000 | Extreme evidence for H1 | −0.340 | −0.324 |
| F2500 | −0.323 | 0.003 | 0.000 | Extreme evidence for H1 | −0.330 | −0.315 |
| F3150 | −0.225 | 0.003 | 0.000 | Extreme evidence for H1 | −0.232 | −0.219 |
| F4000 | −0.094 | 0.002 | 0.000 | Extreme evidence for H1 | −0.099 | −0.089 |
| F5000 | −0.027 | 0.001 | 0.000 | Extreme evidence for H1 | −0.029 | −0.024 |
| In OU and PR (Incubator Without Mattress) | |||||||
|---|---|---|---|---|---|---|---|
| Frequency | Mann–Whitney U | Z | p-Value | Corrected p-Value | N | rc (Rank-Biserial) | Interpretation [82] |
| F100 | 24.000 | −5.589 | 0.000 | 0.000 | 157 | 0.986 | large |
| F125 | 22.500 | −5.599 | 0.000 | 0.000 | 157 | 0.987 | large |
| F160 | 50.000 | −5.418 | 0.000 | 0.000 | 157 | 0.971 | large |
| F200 | 20.000 | −5.616 | 0.000 | 0.000 | 157 | 0.988 | large |
| F250 | 24.000 | −5.589 | 0.000 | 0.000 | 157 | 0.986 | large |
| F315 | 24.000 | −5.589 | 0.000 | 0.000 | 157 | 0.986 | large |
| F400 | 13.000 | −5.662 | 0.000 | 0.000 | 157 | 0.992 | large |
| F500 | 20.000 | −5.616 | 0.000 | 0.000 | 157 | 0.988 | large |
| F630 | 12.000 | −5.669 | 0.000 | 0.000 | 157 | 0.993 | large |
| F800 | 20.000 | −5.616 | 0.000 | 0.000 | 157 | 0.988 | large |
| F1000 | 22.500 | −5.599 | 0.000 | 0.000 | 157 | 0.987 | large |
| F1250 | 13.000 | −5.662 | 0.000 | 0.000 | 157 | 0.992 | large |
| F1600 | 171.000 | −4.618 | 0.000 | 0.000 | 157 | 0.896 | large |
| F2000 | 24.000 | −5.590 | 0.000 | 0.000 | 157 | 0.986 | large |
| F2500 | 12.000 | −5.669 | 0.000 | 0.000 | 157 | 0.993 | large |
| F3150 | 350.000 | −3.436 | 0.001 | 0.001 | 157 | 0.774 | medium |
| F4000 | 300.000 | −3.766 | 0.000 | 0.000 | 157 | 0.809 | medium |
| F5000 | 460.000 | −2.709 | 0.007 | 0.007 | 157 | 0.692 | small |
| Bayes Factor Two Independent Samples Test (Method = Rouder) | Posterior Distribution Characterization for Independent Sample Mean | |||||
|---|---|---|---|---|---|---|
| Mean Difference (PR-OU) | Pooled Std. Error Difference | Bayes Factor | Interpretation | 95% Credible Interval | ||
| Lower Bound | Upper Bound | |||||
| F100 | −0.433 | 0.071 | 0.000 | Extreme evidence for H1 | −0.484 | −0.381 |
| F125 | −1.356 | 0.115 | 0.000 | Extreme evidence for H1 | −1.427 | −1.285 |
| F160 | −0.420 | 0.079 | 0.000 | Strong evidence for H1 | −0.466 | −0.373 |
| F200 | −1.420 | 0.093 | 0.000 | Extreme evidence for H1 | −1.473 | −1.366 |
| F250 | −0.888 | 0.083 | 0.000 | Extreme evidence for H1 | −0.937 | −0.840 |
| F315 | −0.498 | 0.084 | 0.000 | Extreme evidence for H1 | −0.546 | −0.450 |
| F400 | −0.763 | 0.089 | 0.000 | Extreme evidence for H1 | −0.815 | −0.712 |
| F500 | −0.812 | 0.085 | 0.000 | Extreme evidence for H1 | −0.862 | −0.762 |
| F630 | −0.679 | 0.073 | 0.000 | Extreme evidence for H1 | −0.721 | −0.637 |
| F800 | −0.621 | 0.077 | 0.000 | Extreme evidence for H1 | −0.665 | −0.577 |
| F1000 | −0.460 | 0.068 | 0.000 | Extreme evidence for H1 | −0.499 | −0.421 |
| F1250 | −0.409 | 0.071 | 0.000 | Extreme evidence for H1 | −0.449 | −0.368 |
| F1600 | −0.322 | 0.069 | 0.000 | Extreme evidence for H1 | −0.361 | −0.283 |
| F2000 | −0.455 | 0.079 | 0.000 | Extreme evidence for H1 | −0.500 | −0.411 |
| F2500 | −0.447 | 0.063 | 0.000 | Extreme evidence for H1 | −0.483 | −0.411 |
| F3150 | −0.274 | 0.079 | 0.023 | Very strong evidence for H1 | −0.319 | −0.230 |
| F4000 | −0.251 | 0.066 | 0.008 | Extreme evidence for H1 | −0.288 | −0.214 |
| F5000 | −0.134 | 0.050 | 0.195 | Moderate evidence of H0 | −0.163 | −0.106 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Puyana-Romero, V.; Nuñez-Solano, D.; Hernández-Molina, R.; Fernández-Zacarías, F.; Jimenez, J.; Ciaburro, G. Designing for Neonates’ Wellness: Differences in the Reverberation Time Between an Incubator Located in an Open Unit and in a Private Room of a NICU. Buildings 2025, 15, 1411. https://doi.org/10.3390/buildings15091411
Puyana-Romero V, Nuñez-Solano D, Hernández-Molina R, Fernández-Zacarías F, Jimenez J, Ciaburro G. Designing for Neonates’ Wellness: Differences in the Reverberation Time Between an Incubator Located in an Open Unit and in a Private Room of a NICU. Buildings. 2025; 15(9):1411. https://doi.org/10.3390/buildings15091411
Chicago/Turabian StylePuyana-Romero, Virginia, Daniel Nuñez-Solano, Ricardo Hernández-Molina, Francisco Fernández-Zacarías, Juan Jimenez, and Giuseppe Ciaburro. 2025. "Designing for Neonates’ Wellness: Differences in the Reverberation Time Between an Incubator Located in an Open Unit and in a Private Room of a NICU" Buildings 15, no. 9: 1411. https://doi.org/10.3390/buildings15091411
APA StylePuyana-Romero, V., Nuñez-Solano, D., Hernández-Molina, R., Fernández-Zacarías, F., Jimenez, J., & Ciaburro, G. (2025). Designing for Neonates’ Wellness: Differences in the Reverberation Time Between an Incubator Located in an Open Unit and in a Private Room of a NICU. Buildings, 15(9), 1411. https://doi.org/10.3390/buildings15091411






