Haemophilin-Producing Strains of Haemophilus haemolyticus Protect Respiratory Epithelia from NTHi Colonisation and Internalisation
Abstract
:1. Introduction
2. Results
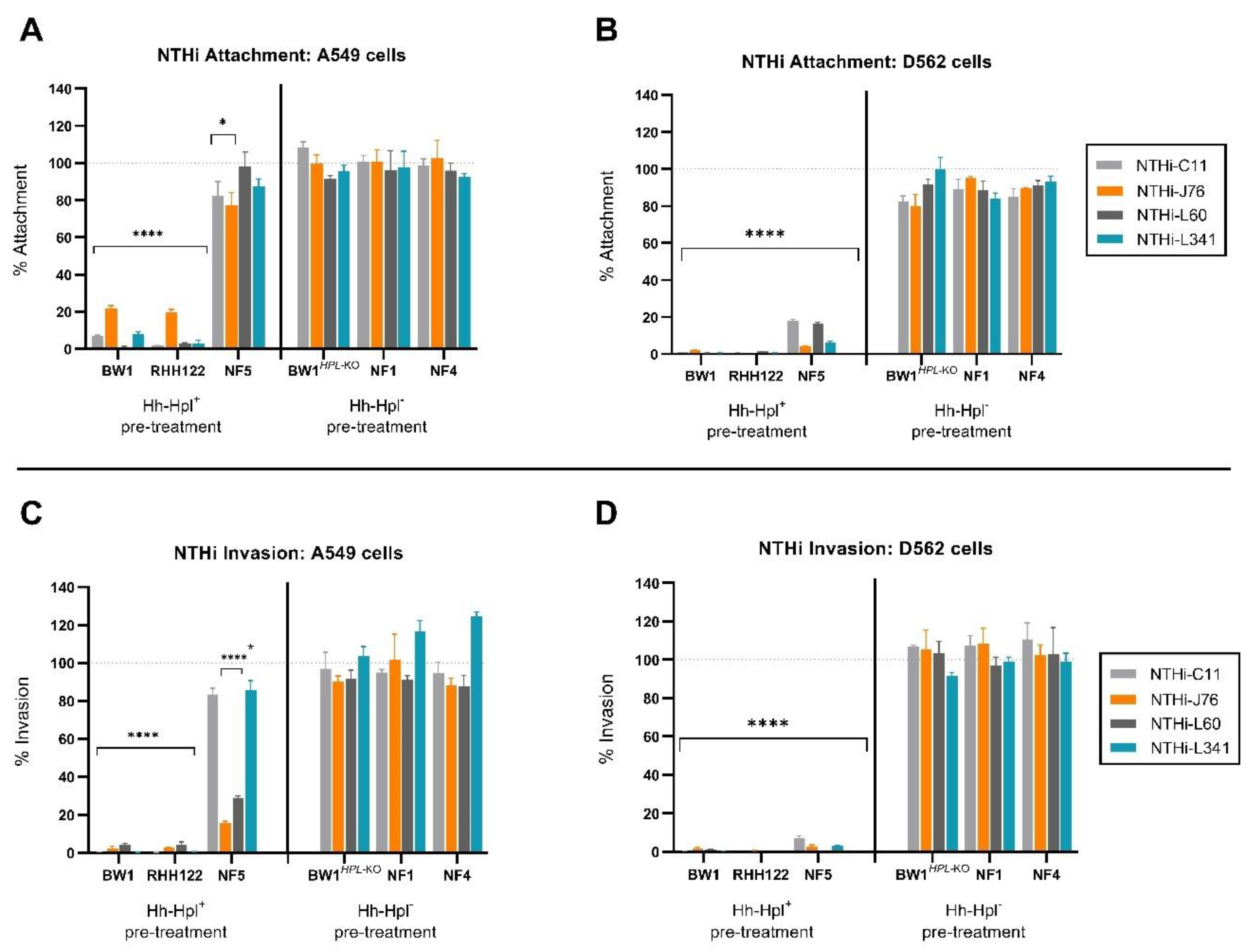
2.1. Cell Monolayers Pre-Treated with Hpl-Producing Strains of Hh Were Protected from NTHi Attachment and Invasion
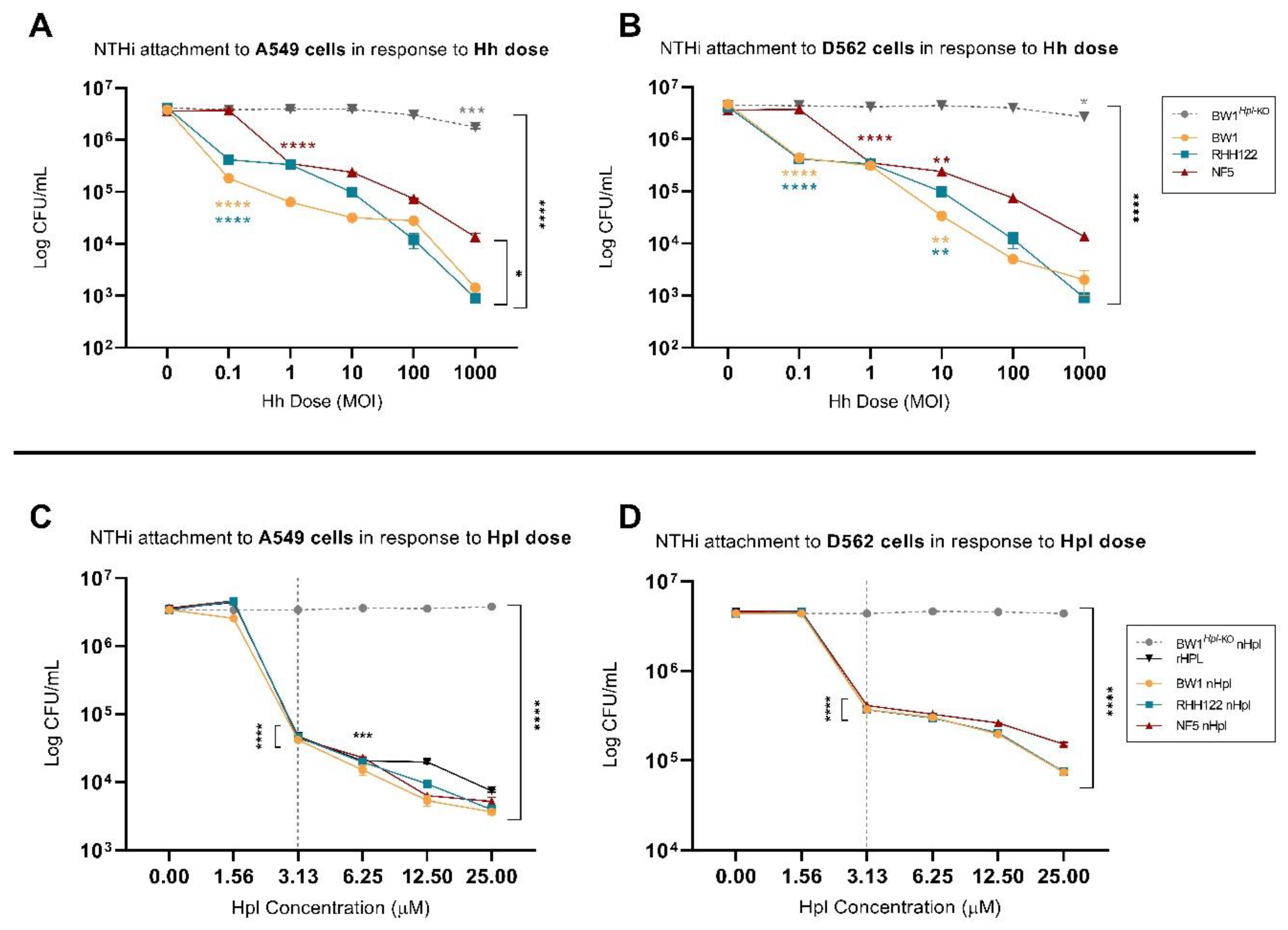
2.2. Small Treatment Doses of Hh-BW1 or Hh-RHH122 Were Sufficient to Inhibit NTHi Interactions with Model Epithelium Cell Lines
2.3. Purified Hpl Inhibits NTHi Interactions with Model Epithelium Cell Lines
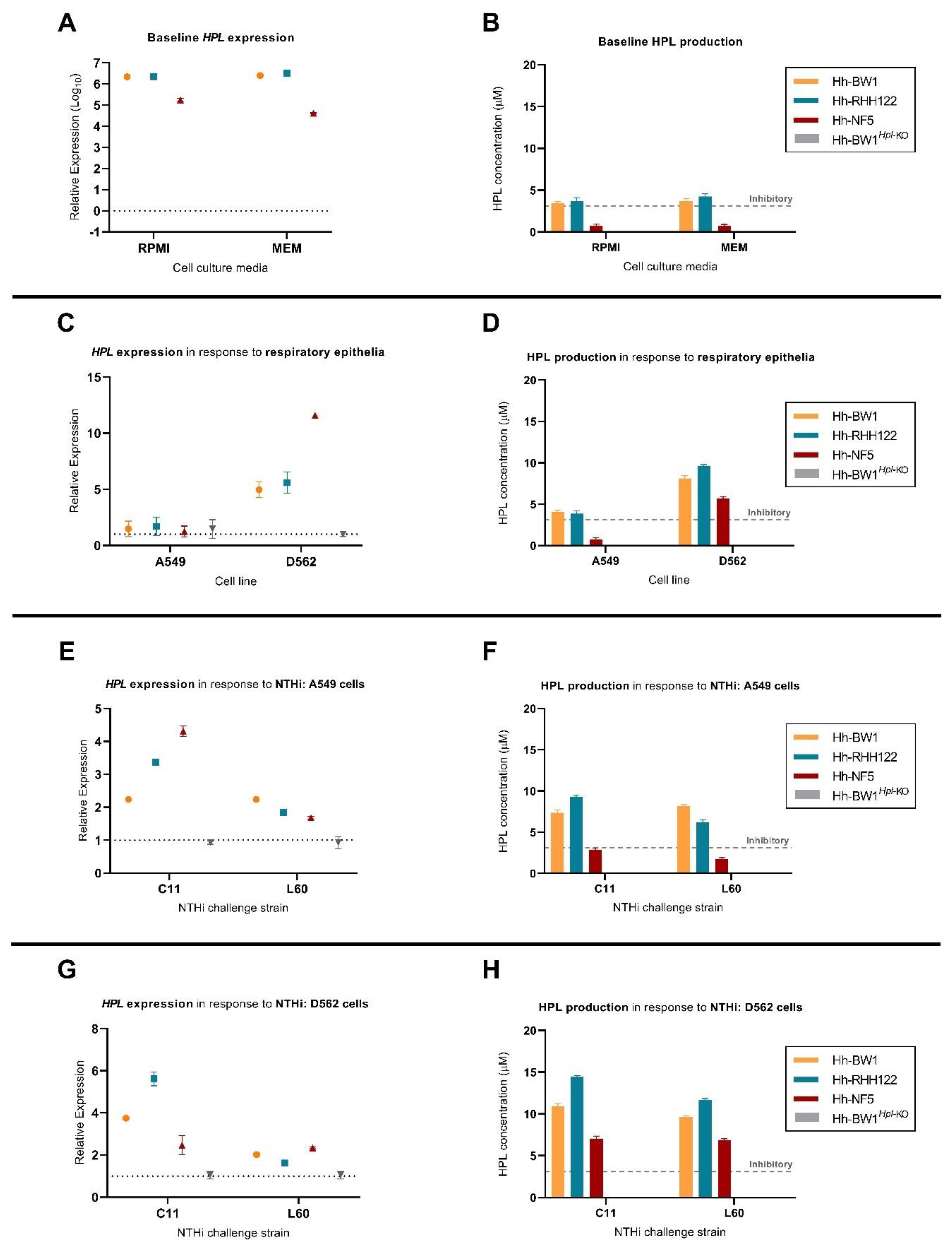
2.4. Expression of Hpl Is Stimulated in Response to D562 Cell Culture and NTHi Challenge
3. Discussion
4. Materials and Methods
4.1. Microbial Strains
4.2. Microbial Growth Conditions and Propagation of Haem-Starved Populations
4.3. Generation of Nalidixic Acid-Resistant Mutants
4.4. Epithelial Cell Culture and Maintenance
4.5. Baseline Attachment and Invasion of Respiratory Epithelial Cell Lines
4.6. Bacterial and Cell Viability
4.7. Preparation and Quantification of Recombinant and Native Hpl
4.8. Competitive Cell Association and Invasion
4.9. Expression Analysis
4.10. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Mukundan, D.; Ecevit, Z.; Patel, M.; Marrs, C.F.; Gilsdorf, J.R. Pharyngeal Colonization Dynamics of Haemophilus influenzae and Haemophilus haemolyticus in Healthy Adult Carriers. J. Clin. Microbiol. 2007, 45, 3207–3217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Eldere, J.; Slack, M.P.E.; Ladhani, S.N.; Cripps, A.W. Non-typeable Haemophilus influenzae, an under-recognised pathogen. Lancet Infect. Dis. 2014, 14, 1281–1292. [Google Scholar] [CrossRef] [Green Version]
- Witherden, E.A.; Tristram, S.G. Prevalence and mechanisms of β-lactam resistance in Haemophilus haemolyticus. J. Antimicrob. Chemother. 2013, 68, 1049–1053. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ito, M.; Hotomi, M.; Maruyama, Y.; Hatano, M.; Sugimoto, H.; Yoshizaki, T.; Yamanaka, N. Clonal spread of β-lactamase-producing amoxicillin–clavulanate-resistant (BLPACR) strains of non-typeable Haemophilus influenzae among young children attending a day care in Japan. Int. J. Pediatr. Otorhinolaryngol. 2010, 74, 901–906. [Google Scholar] [CrossRef] [Green Version]
- Dabernat, H.; Delmas, C. Epidemiology and evolution of antibiotic resistance of Haemophilus influenzae in children 5 years of age or less in France, 2001–2008: A retrospective database analysis. Eur. J. Clin. Microbiol. Infect. Dis. 2012, 31, 2745–2753. [Google Scholar] [CrossRef]
- Hare, K.; Leach, A.; Morris, P.; Smith-Vaughan, H.; Torzillo, P.; Bauert, P.; Cheng, A.; McDonald, M.; Brown, N.; Chang, A.; et al. Impact of recent antibiotics on nasopharyngeal carriage and lower airway infection in Indigenous Australian children with non-cystic fibrosis bronchiectasis. Int. J. Antimicrob. Agents 2012, 40, 365–369. [Google Scholar] [CrossRef] [Green Version]
- Atkinson, C.; Tristram, S. Antimicrobial resistance in cystic fibrosis isolates of Haemophilus influenzae. Br. J. Biomed. Sci. 2016, 73, 87–89. [Google Scholar] [CrossRef]
- Ikeda, M.; Enomoto, N.; Hashimoto, D.; Fujisawa, T.; Inui, N.; Nakamura, Y.; Suda, T.; Nagata, T. Nontypeable Haemophilus influenzae exploits the interaction between protein-E and vitronectin for the adherence and invasion to bronchial epithelial cells. BMC Microbiol. 2015, 15, 1–11. [Google Scholar] [CrossRef]
- Smith-Vaughan, H.; Byun, R.; Nadkarni, M.A.; Jacques, N.A.; Hunter, N.; Halpin, S.; Morris, P.S.; Leach, A.J. Measuring nasal bacterial load and its association with otitis media. BMC Ear Nose Throat Disord. 2006, 6, 10. [Google Scholar] [CrossRef] [Green Version]
- Smith-Vaughan, H.C.; Binks, M.J.; Marsh, R.L.; Kaestli, M.; Ward, L.; Hare, K.M.; Pizzutto, S.J.; Thornton, R.B.; Morris, P.S.; Leach, A.J. Dominance of Haemophilus influenzae in ear discharge from Indigenous Australian children with acute otitis media with tympanic membrane perforation. BMC Ear Nose Throat Disord. 2013, 13, 12. [Google Scholar] [CrossRef] [Green Version]
- Kirkham, L.-A.S.; Wiertsema, S.P.; Mowe, E.N.; Bowman, J.M.; Riley, T.V.; Richmond, P.C. Nasopharyngeal Carriage of Haemophilus haemolyticus in Otitis-Prone and Healthy Children. J. Clin. Microbiol. 2010, 48, 2557–2559. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Desai, H.; Eschberger, K.; Wrona, C.; Grove, L.; Agrawal, A.; Grant, B.; Yin, J.; Parameswaran, G.I.; Murphy, T.; Sethi, S. Bacterial Colonization Increases Daily Symptoms in Patients with Chronic Obstructive Pulmonary Disease. Ann. Am. Thorac. Soc. 2014, 11, 303–309. [Google Scholar] [CrossRef] [PubMed]
- Hotomi, M.; Arai, J.; Billal, D.S.; Takei, S.; Ikeda, Y.; Ogami, M.; Kono, M.; Beder, L.B.; Toya, K.; Kimura, M.; et al. Nontypeable Haemophilus influenzae isolated from intractable acute otitis media internalized into cultured human epithelial cells. Auris Nasus Larynx 2010, 37, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Pickering, J.L.; Amy, P.; Karli, J.C.; Camilla, G.; Peter, C.R.; Guicheng, Z.; Ruth, B.T.; Lee-Ann, S.K. Haemophilus haemolyticus interaction with host cells is different to nontypeable Haemophilus in-fluenzae and prevents NTHi association with epithelial cells. Front. Cell. Infect. Microbiol. 2016, 6, 50. [Google Scholar]
- Singh, N.K.; Kunde, D.A.; Tristram, S. Effect of epithelial cell type on in vitro invasion of non-typeable Haemophilus influenzae. J. Microbiol. Methods 2016, 129, 66–69. [Google Scholar] [CrossRef]
- Swords, W.E.; Buscher, B.A.; Ii, K.V.S.; Preston, A.; Nichols, W.A.; Weiser, J.N.; Gibson, B.W.; Apicella, M.A. Non-typeable Haemophilus influenzae adhere to and invade human bronchial epithelial cells via an interaction of lipooligosaccharide with the PAF receptor. Mol. Microbiol. 2000, 37, 13–27. [Google Scholar] [CrossRef]
- Morey, P.; Victoria, C.; Pau, M.-L.; Antonio, L.-G.; Veronica, R.; Carles, S.; Jose, A.B.; Junkal, G. Evidence for a non-replicative intracellular stage of nontypable Haemophilus influenzae in epithelial cells. Microbiology 2011, 157, 234–250. [Google Scholar] [CrossRef] [Green Version]
- Van Schilfgaarde, M.; Eijik, P.; Regelink, A.; UlsenVan, P.; Everts, V.; Dankert, J.; Van Alphen, L. Haemophilus influenzaelocalized in epithelial cell layers is shielded from antibiotics and an-tibody-mediated bactericidal activity. Microb. Pathog. 1999, 26, 249–262. [Google Scholar] [CrossRef]
- Groeneveld, K.; Loek, A.; Paul, P.E.; Gert, V.; Henk, M.J.; Zanen, H.C. Endogenous and exogenous reinfections by Haemophilus influenzae in patients with chronic ob-structive pulmonary disease: The effect of antibiotic treatment on persistence. J. Infect. Dis. 1990, 161, 512–517. [Google Scholar] [CrossRef]
- Murphy, T.F.; Aimee, L.B.; Andrew, T.S.; Sanjay, S. Persistent colonization by Haemophilus influenzae in chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2004, 170, 266–272. [Google Scholar] [CrossRef]
- Ahearn, C.P.; Gallo, M.C.; Murphy, T.F. Insights on persistent airway infection by non-typeable Haemophilus influ-enzae in chronic obstructive pulmonary disease. Pathog. Dis. 2017, 75, ftx042. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moller, L.V.; Tiemens, W.; Van der Bij, W.; Kooi, K.; Wever, B.; Dankert, J.; Van Alphen, L. Haemophilus influenzae in lung explants of patients with end-stage pulmonary disease. Am. J. Respir. Crit. Care Med. 1998, 157, 950–956. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Su, Y.-C.; Jalalvand, F.; Thegerström, J.; Riesbeck, K. The Interplay Between Immune Response and Bacterial Infection in COPD: Focus Upon Non-typeable Haemophilus influenzae. Front. Immunol. 2018, 9, 2530. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choby, J.E.; Skaar, E.P. Haem synthesis and acquisition in bacterial pathogens. J. Mol. Biol. 2016, 428, 3408–3428. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Contreras, H.; Chim, N.; Credali, A.; Goulding, C.W. Haem uptake in bacterial pathogens. Curr. Opin. Chem. Biol. 2014, 19, 34–41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Richard, K.L.; Kelley, B.R.; Johnson, J.G. Haem Uptake and Utilization by Gram-Negative Bacterial Pathogens. Front. Cell. Infect. Microbiol. 2019, 9, 81. [Google Scholar] [CrossRef] [PubMed]
- Hariadi, N.I.; Lixin, Z.; Mayuri, P.; Sara, A.S.; Gregg, S.D.; Carl, F.M.; Janet, G. Comparative profile of haem acquisition genes in disease-causing and colonizing nontypeable Hae-mophilus influenzae and Haemophilus haemolyticus. J. Clin. Microbiol. 2015, 53, 2132–2137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loeb, M.R. Ferrochelatase activity and protoporphyrin IX utilization in Haemophilus influenzae. J. Bacteriol. 1995, 177, 3613–3615. [Google Scholar] [CrossRef] [Green Version]
- Cloonan, S.M. The “iron”-y of iron overload and iron deficiency in chronic obstructive pulmonary disease. American J. Respir. Crit. Care Med. 2017, 196, 1103–1112. [Google Scholar] [CrossRef] [Green Version]
- Whitby, P.W.; Sim, E.K.; Morton, D.J.; Patel, A.J.; Stull, T.L. Transcription of genes encoding iron and haem acquisition proteins of Haemophilus influenzae during acute otitis media. Infect. Immun. 1997, 65, 4696–4700. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez-Arce, I.; Tamim, A.-J.; Begona, E.; Ariadna, F.-C.; Celia, G.-C.; Sara, M.; Susanna, T.-H.; Kristian, R.; Garmendia, J. Moonlighting of Haemophilus influenzae haem acquisition systems contributes to the host air-way-pathogen interplay in a coordinated manner. Virulence 2019, 10, 315–333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morton, D.J.; Seale, T.W.; Bakaletz, L.O.; Jurcisek, J.A.; Smith, A.; VanWagoner, T.M.; Whitby, P.W.; Stull, T.L. The haem-binding protein (HbpA) of Haemophilus influenzae as a virulence determinant. Int. J. Med Microbiol. 2009, 299, 479–488. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vogel, A.R.; Blake, R.S.; Forrest, K.R.; Samantha, W.S.; Rachel, L.G.; Sheryl, S.J.; Kevin, M.M. SapF-mediated haem-iron utilization enhances persistence and coordinates biofilm architecture of Haemophilus. Front. Cell. Infect. Microbiol. 2012, 2, 42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Whitby, P.W.; VanWagoner, T.M.; Seale, T.W.; Morton, D.J.; Stull, T.L. Comparison of transcription of the Haemophilus influenzae iron/haem modulon genes in vitro and in vivo in the chinchilla middle ear. BMC Genom. 2013, 14, 925. [Google Scholar] [CrossRef] [Green Version]
- Seale, T.W.; Morton, D.J.; Whitby, P.W.; Wolf, R.; Kosanke, S.D.; VanWagoner, T.M.; Stull, T.L. Complex Role of Hemoglobin and Hemoglobin-Haptoglobin Binding Proteins in Haemophilus influenzae Virulence in the Infant Rat Model of Invasive Infection. Infect. Immun. 2006, 74, 6213–6225. [Google Scholar] [CrossRef] [Green Version]
- Szelestey, B.R.; Heimlich, D.R.; Raffel, F.K.; Justice, S.S.; Mason, K.M. Haemophilus Responses to Nutritional Immunity: Epigenetic and Morphological Contribution to Biofilm Architecture, Invasion, Persistence and Disease Severity. PLoS Pathog. 2013, 9, e1003709. [Google Scholar] [CrossRef]
- Stites, S.W.; Plautz, M.W.; Bailey, K.; O’Brien-Ladner, A.R.; Wesselius, L.J. Increased Concentrations of Iron and Isoferritins in the Lower Respiratory Tract of Patients with Stable Cystic Fibrosis. Am. J. Respir. Crit. Care Med. 1999, 160, 796–801. [Google Scholar] [CrossRef]
- Latham, R.D.; Gell, D.A.; Fairbairn, R.L.; Lyons, A.B.; Shukla, S.D.; Cho, K.Y.; Jones, D.A.; Harkness, N.M.; Tristram, S.G. An isolate of Haemophilus haemolyticus produces a bacteriocin-like substance that inhibits the growth of nontypeable Haemophilus influenzae. Int. J. Antimicrob. Agents 2017, 49, 503–506. [Google Scholar] [CrossRef]
- Latham, R.D.; Torrado, M.; Atto, B.; Walshe, J.L.; Wilson, R.; Guss, J.M.; Mackay, J.P.; Tristram, S.; Gell, D.A. A haem-binding protein produced by Haemophilus haemolyticus inhibits non-typeable Haemophilus influenzae. Mol. Microbiol. 2019, 113, 381–398. [Google Scholar] [CrossRef]
- Atto, B.; Latham, R.D.; Kunde, D.; Gell, D.A.; Tristram, S. In Vitro Anti-NTHi Activity of Haemophilin-Producing Strains of Haemophilus haemolyticus. Pathogens 2020, 9, 243. [Google Scholar] [CrossRef] [Green Version]
- Okabe, T.; Yamazaki, Y.; Shiotani, M.; Suzuki, T.; Shiohara, M.; Kasuga, E.; Notake, S.; Yanagisawa, H. An amino acid substitution in PBP-3 in Haemophilus influenzae associate with the invasion to bronchial epithelial cells. Microbiol. Res. 2010, 165, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Bandi, V.; Apicella, M.A.; Mason, E.; Murphy, T.F.; Siddiqi, A.; Atmar, R.L.; Greenberg, S.B. Nontypeable Haemophilus influenzae in the lower respiratory tract of patients with chronic bronchitis. Am. J. Respir. Crit. Care Med. 2001, 164, 2114–2119. [Google Scholar] [CrossRef] [PubMed]
- King, P. Haemophilus influenzae and the lung (Haemophilus and the lung). Clin. Transl. Med. 2012, 1, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mokrzan, E.M.; Johnson, T.J.; Bakaletz, L.O. Expression of the Nontypeable Haemophilus influenzae Type IV Pilus Is Stimulated by Coculture with Host Respiratory Tract Epithelial Cells. Infect. Immun. 2019, 87. [Google Scholar] [CrossRef]
- Hibbing, M.E.; Fuqua, C.; Parsek, M.R.; Peterson, S.B. Bacterial competition: Surviving and thriving in the microbial jungle. Nat. Rev. Genet. 2009, 8, 15–25. [Google Scholar] [CrossRef] [Green Version]
- Leinweber, A.; Weigert, M.; Kümmerli, R. The bacterium Pseudomonas aeruginosa senses and gradually responds to interspecific competition for iron. Evolution 2018, 72, 1515–1528. [Google Scholar] [CrossRef]
- Trejo-Hernández, A.; Andrade-Domínguez, A.; Hernández, M.; Encarnación, S. Interspecies competition triggers virulence and mutability in Candida albicans–Pseudomonas aeruginosa mixed biofilms. ISME J. 2014, 8, 1974–1988. [Google Scholar] [CrossRef] [Green Version]
- Anderson, R.; Wang, X.; Briere, E.C.; Katz, L.S.; Cohn, A.C.; Clark, T.A.; Messonnier, N.E.; Mayer, L.W. Haemophilus haemolyticus Isolates Causing Clinical Disease. J. Clin. Microbiol. 2012, 50, 2462–2465. [Google Scholar] [CrossRef] [Green Version]
- Fenger, M.G.; Winnie, R.; Hanne, V.O.; Niels, N.-L. Low occurrence of ‘non-haemolytic Haemophilus haemolyticus’ misidentified as Haemophilus influ-enzae in cystic fibrosis respiratory specimens, and frequent recurrence of persistent H. influenzae clones despite antimi-crobial treatment. Int. J. Med Microbiol. 2012, 302, 315–319. [Google Scholar] [CrossRef]
- Hotomi, M.; Kono, M.; Togawa, A.; Arai, J.; Takei, S.; Ikeda, Y.; Ogami, M.; Murphy, T.F.; Yamanaka, N. Haemophilus influenzae and Haemophilus haemolyticus in tonsillar cultures of adults with acute pharyngotonsillitis. Auris Nasus Larynx 2010, 37, 594–600. [Google Scholar] [CrossRef]
- Zhang, B.; Kunde, D.; Tristram, S. Haemophilus haemolyticus is infrequently misidentified as Haemophilus influenzae in diagnostic specimens in Australia. Diagn. Microbiol. Infect. Dis. 2014, 80, 272–273. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.-P.; Loosmore, S.M.; Underdown, B.J.; Klein, M.H. Nasopharyngeal Colonization with Nontypeable Haemophilus influenzae in Chinchillas. Infect. Immun. 1998, 66, 1973–1980. [Google Scholar] [CrossRef] [Green Version]
- Hood, D.; Richard, M.; Tom, P.; Caroline, R.; Debbie, W.; Ali, A.; Michael, C.; Sara, W.; Martin, F.; Brown, S.D.M.; et al. A new model for non-typeable Haemophilus influenzae middle ear infection in the Junbo mutant mouse. Dis. Model Mech. 2016, 9, 69–79. [Google Scholar] [CrossRef] [Green Version]
- De Gier, C.; Granland, C.; Pickering, J.; Walls, T.; Bhuiyan, M.U.; Mills, N.; Richmond, P.C.; Best, E.; Thornton, R.B.; Kirkham, L.-A.S. PCV7- and PCV10-Vaccinated Otitis-Prone Children in New Zealand Have Similar Pneumococcal and Haemophilus influenzae Densities in Their Nasopharynx and Middle Ear. Vaccines 2019, 7, 14. [Google Scholar] [CrossRef] [Green Version]
- Hill, A.T.; Campbell, E.J.; Hill, S.L.; Bayley, D.L.; A Stockley, R. Association between airway bacterial load and markers of airway inflammation in patients with stable chronic bronchitis. Am. J. Med. 2000, 109, 288–295. [Google Scholar] [CrossRef]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef]
- Pfaffl, M.W.; Horgan, G.W.; Dempfle, L. Relative expression software tool (REST©) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic Acids Res. 2002, 30, e36. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Atto, B.; Kunde, D.; Gell, D.A.; Tristram, S. Haemophilin-Producing Strains of Haemophilus haemolyticus Protect Respiratory Epithelia from NTHi Colonisation and Internalisation. Pathogens 2021, 10, 29. https://doi.org/10.3390/pathogens10010029
Atto B, Kunde D, Gell DA, Tristram S. Haemophilin-Producing Strains of Haemophilus haemolyticus Protect Respiratory Epithelia from NTHi Colonisation and Internalisation. Pathogens. 2021; 10(1):29. https://doi.org/10.3390/pathogens10010029
Chicago/Turabian StyleAtto, Brianna, Dale Kunde, David A Gell, and Stephen Tristram. 2021. "Haemophilin-Producing Strains of Haemophilus haemolyticus Protect Respiratory Epithelia from NTHi Colonisation and Internalisation" Pathogens 10, no. 1: 29. https://doi.org/10.3390/pathogens10010029




