In Silico Analysis of Possible Interaction between Host Genomic Transcription Factors (TFs) and Zika Virus (ZikaSPH2015) Strain with Combinatorial Gene Regulation; Virus Versus Host—The Game Reloaded
Abstract
:1. Introduction
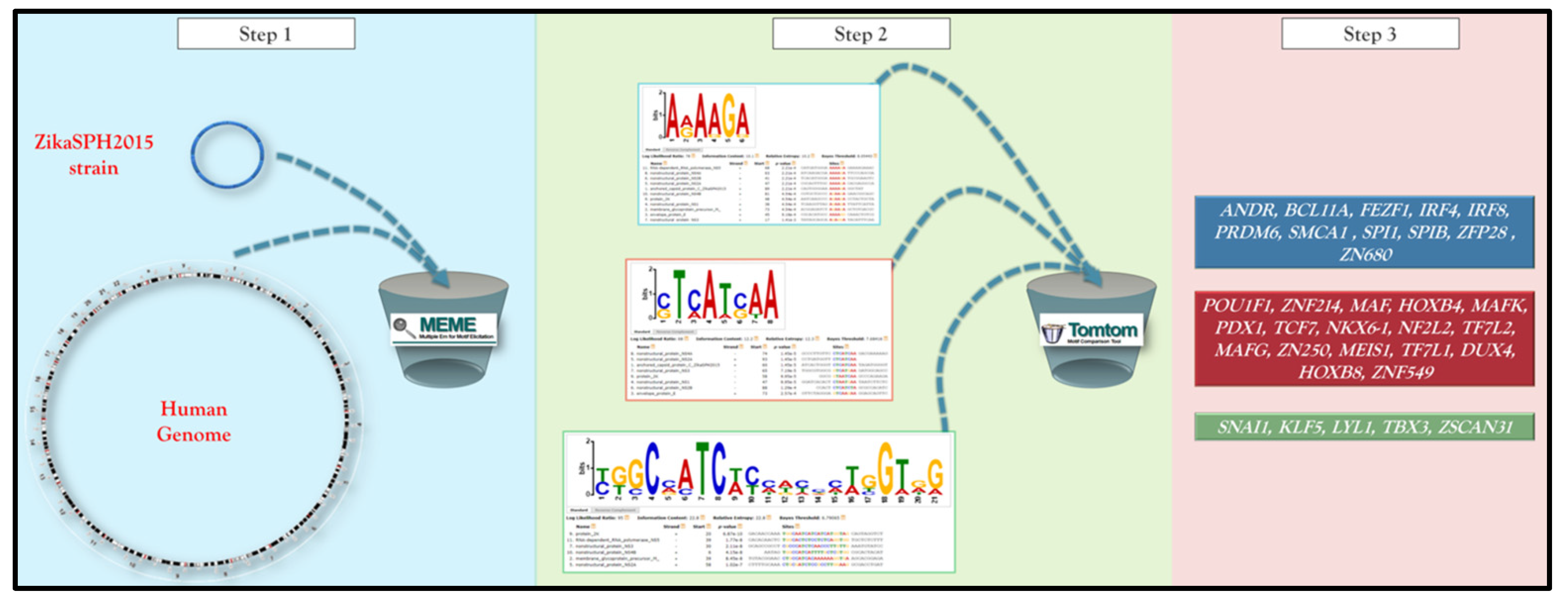
2. Materials and Methods
3. Results
4. Discussion and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Laureti, M.; Narayanan, D.; Rodriguez-Andres, J.; Fazakerley, J.K.; Kedzierski, L. Flavivirus Receptors: Diversity, Identity, and Cell Entry. Front. Immunol. 2018, 9, 2180. [Google Scholar] [CrossRef] [Green Version]
- Baud, D.; Gubler, D.J.; Schaub, B.; Lanteri, M.C.; Musso, D. An update on Zika virus infection. Lancet 2017, 390, 2099–2109. [Google Scholar] [CrossRef] [Green Version]
- Panchaud, A.; Stojanov, M.; Ammerdorffer, A.; Vouga, M.; Baud, D. Emerging Role of Zika Virus in Adverse Fetal and Neonatal Outcomes. Clin. Microbiol. Rev. 2016, 29, 659–694. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mittal, R.; Nguyen, D.; Debs, L.H.; Patel, A.P.; Liu, G.; Jhaveri, V.M.; S Kay, S.I.; Mittal, J.; Bandstra, E.S.; Younis, R.T.; et al. Zika Virus: An Emerging Global Health Threat. Front. Cell. Infect. Microbiol. 2017, 7, 486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- EDAC. Zika Virus Epidemic in the Americas: Potential Association with Microcephaly and Guillain-Barré Syndrome. 2015. Available online: https://www.ecdc.europa.eu/en/publications-data/rapid-risk-assessment-zika-virus-epidemic-americas-potential-association (accessed on 13 January 2021).
- Pyzocha, N.J.; Chinchen, S.E.; Maurer, D.M. Zany Over Zika Virus: An Overview of Diagnosis and Treatment Modalities. Curr. Sports. Med. Rep. 2017, 16, 109–113. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, C.; Napoli, C.; Venturi, G.; Pupella, S.; Lombardini, L.; Calistri, P.; Monaco, F.; Cagarelli, R.; Angelini, P.; Bellini, R.; et al. West Nile virus transmission: Results from the integrated surveillance system in Italy, 2008 to 2015. Euro Surveill. 2016, 21, 30340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prisant, N.; Joguet, G.; Herrmann-Stock, C.; Moriniere, C.; Pavili, L.; Lurel, S.; Bujan, L. Upper and lower genital tract Zika virus screening in a large cohort of reproductive-age women during the Americas epidemic. Reprod. Biomed. Online 2019, 39, 624–632. [Google Scholar] [CrossRef] [PubMed]
- Hermanns, K.; Göhner, C.; Kopp, A.; Schmidt, A.; Merz, W.M.; Markert, U.R.; Junglen, S.; Drosten, C. Zika virus infection in human placental tissue explants is enhanced in the presence of dengue virus antibodies in-vitro. Emerg. Microbes Infect. 2018, 7, 198. [Google Scholar] [CrossRef] [Green Version]
- Sager, G.; Gabaglio, S.; Sztul, E.; Belov, G.A. Role of Host Cell Secretory Machinery in Zika Virus Life Cycle. Viruses 2018, 10, 559. [Google Scholar] [CrossRef] [Green Version]
- Harwing, A.; Landick, R.; Berkhout, B. The battle of RNA synthesis: Virus versus host. Viruses 2017, 9, 309. [Google Scholar] [CrossRef] [Green Version]
- Wei, H.; Zhou, M.M. Viral-encoded enzymes that target host chromatin functions. Biochim. Biophys. Acta 2010, 1799, 296–301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cunha, M.S.; Esposito, D.L.A.; Rocco, I.M.; Maeda, A.Y.; Vasami, F.G.S.; Nogueira, J.S.; de Souza, R.P.; Suzuki, A.; Addas-Carvalho, M.; Barjas-Castro Mde, L.; et al. First complete genome sequence of Zika virus (Flaviviridae, Flavivirus) from an autochthonous transmission in Brazil. Genome Announc. 2016, 4, e00032-16. [Google Scholar] [CrossRef] [Green Version]
- Chetta, M.; Rosati, A.; Marzullo, L.; Tarsitano, M.; Bukvic, N. A SARS-CoV-2 host infection model network based on genomic Human Transcription Factors (TFs) depletion. Heliyon 2020, 6, e05010. [Google Scholar] [CrossRef] [PubMed]
- Chetta, M.; Di Pietro, L.; Bukvic, N.; Lattanzi, W. Rising Roles of Small Noncoding RNAs in Cotranscriptional Regulation: In Silico Study of miRNA and piRNA Regulatory Network in Humans. Genes 2020, 11, 482. [Google Scholar] [CrossRef]
- Machanick, P.; Bailey, T.L. MEME-ChIP: Motif analysis of large DNA datasets. Bioinformatics 2011, 27, 1696–1697. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, S.; Stamatoyannopoulos, J.A.; Bailey, T.L.; Noble, W.S. Quantifying similarity between motifs. Genome Biol. 2007, 8, R24. [Google Scholar] [CrossRef] [Green Version]
- Kuhn, M.; von Mering, C.; Campillos, M.; Jensen, L.J.; Bork, P. STITCH: Interaction networks of chemicals and proteins. Nucleic Acids Res. 2008, 36, D684–D688. [Google Scholar] [CrossRef]
- Soblet, J.; Dimov, I.; Graf von Kalckreuth, C.; Cano-Chervel, J.; Baijot, S.; Pelc, K.; Sottiaux, M.; Vilain, C.; Smits, G.; Deconinck, N. BCL11A frameshift mutation associated with dyspraxia and hypotonia affecting the fine, gross, oral, and speech motor systems. Am. J. Med. Genet. A 2018, 176, 201–208. [Google Scholar] [CrossRef] [Green Version]
- Fang, Q.; George, A.S.; Brinkmeier, M.L.; Mortensen, A.H.; Gergics, P.; Cheung, L.Y.; Daly, A.Z.; Ajmal, A.; Pérez Millán, M.I.; Ozel, A.B.; et al. Genetics of Combined Pituitary Hormone Deficiency: Roadmap into the Genome Era. Endocr. Rev. 2016, 37, 636–675. [Google Scholar] [CrossRef] [Green Version]
- Sofos, E.; Pescosolido, M.F.; Quintos, J.B.; Abuelo, D.; Gunn, S.; Hovanes, K.; Morrow, E.M.; Shur, N.A. A novel familial 11p15.4 microduplication associated with intellectual disability, dysmorphic features, and obesity with involvement of the ZNF214 gene. Am. J. Med. Genet. A. 2012, 158A, 50–58. [Google Scholar] [CrossRef]
- Li, N.; Subrahmanyan, L.; Smith, E.; Yu, X.; Zaidi, S.; Choi, M.; Mane, S.; Nelson-Williams, C.; Behjati, M.; Kazemi, M.; et al. Mutations in the Histone Modifier PRDM6 Are Associated with Isolated Nonsyndromic Patent Ductus Arteriosus. Am. J. Hum. Genet. 2016, 98, 1082–1091. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, L.; Zhu, C.; Luo, K.; Li, Y.; Pi, H.; Yuan, W.; Wang, Y.; Huang, C.; Liu, M.; Wu, X. Identification and characterization of two novel zinc finger genes, ZNF359 and ZFP28, in human development. Biochem. Biophys. Res. Commun. 2002, 295, 862–868. [Google Scholar] [CrossRef]
- de Oliveira Dias, J.R.; Ventura, C.V.; de Paula Freitas, B.; Prazeres, J.; Ventura, L.O.; Bravo-Filho, V.; Aleman, T.; Ko, A.I.; Zin, A.; Belfort, R., Jr.; et al. Zika and the Eye: Pieces of a Puzzle. Prog. Retin. Eye. Res. 2018, 66, 85–106. [Google Scholar] [CrossRef] [PubMed]
- Aymé, S.; Philip, N. Fine-Lubinsky syndrome: A fourth patient with brachycephaly, deafness, cataract, microstomia and mental retardation. Clin. Dysmorphol. 1996, 5, 55–60. [Google Scholar] [PubMed]
- Niceta, M.; Stellacci, E.; Gripp, K.W.; Zampino, G.; Kousi, M.; Anselmi, M.; Traversa, A.; Ciolfi, A.; Stabley, D.; Bruselles, A.; et al. Mutations impairing GSK3-mediated MAF phosphorylation cause cataract, deafness, intellectual disability, seizures, and a Down syndrome-like facies. Am. J. Hum. Genet. 2015, 96, 816–825. [Google Scholar] [CrossRef] [Green Version]
- Lim, K.R.Q.; Nguyen, Q.; Yokota, T. DUX4 Signalling in the Pathogenesis of Facioscapulohumeral Muscular Dystrophy. Int. J. Mol. Sci. 2020, 21, 729. [Google Scholar] [CrossRef] [Green Version]
- Alaiti, M.A.; Orasanu, G.; Tugal, D.; Lu, Y.; Jain, M.K. Kruppel-like factors and vascular inflammation: Implications for atherosclerosis. Curr. Atheroscler. Rep. 2012, 14, 438–449. [Google Scholar] [CrossRef] [Green Version]
- Gilmour, J.; Assi, S.A.; Jaegle, U.; Kulu, D.; van de Werken, H.; Clarke, D.; Westhead, D.R.; Philipsen, S.; Bonifer, C. A crucial role for the ubiquitously expressed transcription factor Sp1 at early stages of hematopoietic specification. Development 2014, 141, 2391–2401. [Google Scholar] [CrossRef] [Green Version]
- Shukla, V.; Lu, R. IRF4 and IRF8: Governing the virtues of B Lymphocytes. Front. Biol. 2014, 9, 269–282. [Google Scholar] [CrossRef] [Green Version]
- Sichien, D.; Scott, C.L.; Martens, L.; Vanderkerken, M.; Van Gassen, S.; Plantinga, M.; Joeris, T.; De Prijck, S.; Vanhoutte, L.; Vanheerswynghels, M.; et al. IRF8 Transcription Factor Controls Survival and Function of Terminally Differentiated Conventional and Plasmacytoid Dendritic Cells, Respectively. Immunity 2016, 45, 626–640. [Google Scholar] [CrossRef] [Green Version]
- Cisse, B.; Caton, M.L.; Lehner, M.; Maeda, T.; Scheu, S.; Locksley, R.; Holmberg, D.; Zweier, C.; den Hollander, N.S.; Kant, S.G.; et al. Transcription factor E2-2 is an essential and specific regulator of plasmacytoid dendritic cell development. Cell 2008, 135, 37–48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harly, C.; Kenney, D.; Wang, Y.; Ding, Y.; Zhao, Y.; Awasthi, P.; Bhandoola, A. A Shared Regulatory Element Controls the Initiation of Tcf7 Expression During Early T Cell and Innate Lymphoid Cell Developments. Front. Immunol. 2020, 11, 470. [Google Scholar] [CrossRef] [PubMed]
- Lai, J.J.; Lai, K.P.; Zeng, W.; Chuang, K.H.; Altuwaijri, S.; Chang, C. Androgen receptor influences on body defense system via modulation of innate and adaptive immune systems: Lessons from conditional AR knockout mice. Am. J. Pathol. 2012, 181, 1504–1512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nunes Dos Santos, K.; Florentino, R.M.; França, A.; Lima Filho, A.C.M.; Santos, M.L.D.; Missiaggia, D.; Fonseca, M.C.; Brasil Costa, I.; Vidigal, P.V.T.; Nathanson, M.H.; et al. Polymorphism in the Promoter Region of NFE2L2 Gene Is a Genetic Marker of Susceptibility to Cirrhosis Associated with Alcohol Abuse. Int. J. Mol. Sci. 2019, 3589. [Google Scholar] [CrossRef] [Green Version]
- Johung, K.; Goodwin, E.C.; Di Maio, D. Human papillomavirus E7 repression in cervical carcinoma cells initiates a transcriptional cascade driven by the retinoblastoma family, resulting in senescence. J. Virol. 2007, 81, 2102–2116. [Google Scholar] [CrossRef] [Green Version]
- Capron, C.; Lécluse, Y.; Kaushik, A.L.; Foudi, A.; Lacout, C.; Sekkai, D.; Godin, I.; Albagli, O.; Poullion, I.; Svinartchouk, F.; et al. The SCL relative LYL-1 is required for fetal and adult hematopoietic stem cell function and B-cell differentiation. Blood 2006, 107, 4678–4686. [Google Scholar] [CrossRef] [Green Version]
- Goldman, O.; Valdes, V.J.; Ezhkova, E.; Gouon-Evans, V. The mesenchymal transcription factor SNAI-1 instructs human liver specification. Stem. Cell. Res. 2016, 17, 62–68. [Google Scholar] [CrossRef] [Green Version]
- Eckler, M.J.; Chen, B. Fez family transcription factors: Controlling neurogenesis and cell fate in the developing mammalian nervous system. Bioessays 2014, 36, 788–797. [Google Scholar] [CrossRef] [Green Version]
- Oshima, M.; Endoh, M.; Endo, T.A.; Toyoda, T.; Nakajima-Takagi, Y.; Sugiyama, F.; Koseki, H.; Kyba, M.; Iwama, A.; Osawa, M. Genome-wide analysis of target genes regulated by HoxB4 in hematopoietic stem and progenitor cells developing from embryonic stem cells. Blood 2011, 117, e142–e150. [Google Scholar] [CrossRef] [Green Version]
- Gaston-Massuet, C.; McCabe, M.J.; Scagliotti, V.; Young, R.M.; Carreno, G.; Gregory, L.C.; Jayakody, S.A.; Pozzi, S.; Gualtieri, A.; Basu, B.; et al. Transcription factor 7-like 1 is involved in hypothalamo-pituitary axis development in mice and humans. Proc. Natl. Acad. Sci. USA 2016, 113, E548–E557. [Google Scholar] [CrossRef] [Green Version]
- Karaca, E.; Harel, T.; Pehlivan, D.; Jhangiani, S.N.; Gambin, T.; Coban Akdemir, Z.; Gonzaga-Jauregui, C.; Erdin, S.; Bayram, Y.; Campbell, I.M.; et al. Genes that Affect Brain Structure and Function Identified by Rare Variant Analyses of Mendelian Neurologic Disease. Neuron 2015, 88, 499–513. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Motahari, Z.; Martinez-De Luna, R.I.; Viczian, A.S.; Zuber, M.E. Tbx3 represses bmp4 expression and, with Pax6, is required and sufficient for retina formation. Development 2016, 143, 3560–3572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luo, X.J.; Mattheisen, M.; Li, M.; Huang, L.; Rietschel, M.; Børglum, A.D.; Als, T.D.; van den Oord, E.J.; Aberg, K.A.; Mors, O.; et al. Systematic Integration of Brain eQTL and GWAS Identifies ZNF323 as a Novel Schizophrenia Risk Gene and Suggests Recent Positive Selection Based on Compensatory Advantage on Pulmonary Function. Schizophr. Bull. 2015, 41, 1294–1308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nicolino, M.; Claiborn, K.C.; Senée, V.; Boland, A.; Stoffers, D.A.; Julier, C. A novel hypomorphic PDX1 mutation responsible for permanent neonatal diabetes with subclinical exocrine deficiency. Diabetes 2010, 59, 733–740. [Google Scholar] [CrossRef] [Green Version]
- Bansal, V.S.; Raja, C.P.; Venkataraman, K.; Vijayalakshmi, M.A. Genes involved in pancreatic islet cell rejuvenation. Indian J Med. Res. 2013, 137, 695–703. [Google Scholar]
- Zhuang, Y.; Niu, F.; Liu, D.; Sun, J.; Zhang, X.; Zhang, J.; Guo, S. Associations of TCF7L2 gene polymorphisms with the risk of diabetic nephropathy: A case-control study. Medicine (Baltimore) 2018, 97, e8388. [Google Scholar] [CrossRef]
- Lengner, C.J.; Welstead, G.G.; Jaenisch, R. The pluripotency regulator Oct4: A role in somatic stem cells? Cell Cycle 2008, 7, 725–728. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vouga, M.; Baud, D. Imaging of congenital Zika virus infection: The route to identification of prognostic factors. Prenat. Diagn. 2016, 36, 799–811. [Google Scholar] [CrossRef]
- França, G.V.A.; Schuler-Faccini, L.; Oliveira, W.K.; Henriques, C.M.; Carmo, E.H.; Pedi, V.D.; Nunes, M.L.; Castro, M.C.; Serruya, S.; Silveira, M.F.; et al. Congenital Zika virus syndrome in Brazil: A case series of the first 1501 livebirths with complete investigation. Lancet 2016, 388, 891–897. [Google Scholar] [CrossRef] [Green Version]
- Eppes, C.; Rac, M.; Dunn, J.; Versalovic, J.; Murray, K.O.; Suter, M.A.; Sanz Cortes, M.; Espinoza, J.; Seferovic, M.D.; Lee, W.; et al. Testing for Zika virus infection in pregnancy: Key concepts to deal with an emerging epidemic. Am. J. Obstet. Gynecol. 2017, 216, 209–225. [Google Scholar] [CrossRef] [Green Version]
- van der Linden, V.; Pessoa, A.; Dobyns, W.; Barkovich, A.J.; Júnior, H.V.; Filho, E.L.; Ribeiro, E.M.; Leal, M.C.; Coimbra, P.P.; Aragão, M.F.; et al. Description of 13 infants born during October 2015–January 2016 with congenital Zika virus infection without microcephaly at birth Brazil. Morb. Mortal. Wkly. Rep. 2016, 65, 1343–1348. [Google Scholar] [CrossRef] [PubMed]
- Teng, Y.; Liu, S.; Guo, X.; Liu, S.; Jin, Y.; He, T.; Bi, D.; Zhang, P.; Lin, B.; An, X.; et al. An Integrative Analysis Reveals a Central Role of P53 Activation via MDM2 in Zika Virus Infection Induced Cell Death. Front. Cell. Infect. Microbiol. 2017, 7, 327. [Google Scholar] [CrossRef] [PubMed]
- Chesnut, M.; Muñoz, L.S.; Harris, G.; Freeman, D.; Gama, L.; Pardo, C.A.; Pamies, D. In vitro and in silico Models to Study Mosquito-Borne Flavivirus Neuropathogenesis. Prevention, and Treatment. Front. Cell. Infect. Microbiol. 2019, 9, 223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, A.; Singh, H.N.; Pareek, V.; Raza, K.; Dantham, S.; Kumar, P.; Mochan, S.; Faiq, M.A. A possible mechanism of zika virus associated microcephaly: Imperative role of retinoic acid response element (RARE) consensus sequence repeats in the viral genome. Front. Hum. Neurosci. 2016, 10, 403. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pylro, V.S.; Oliveira, F.S.; Morais, D.K.; Cuadros-Orellana, S.; Pais, F.S.; Medeiros, J.D.; Geraldo, J.A.; Gilbert, J.; Volpini, A.C.; Fernandes, G.R. ZIKV–CDB: A collaborative database to guide research linking SncRNAs and ZIKA virus disease symptoms. PLoS Negl. Trop. Dis. 2016, 10, e0004817. [Google Scholar] [CrossRef]
- Tsai, K.; Cullen, B.R. Epigenetic and epitranscriptomic regulation of viral replication. Nat. Rev. Microbiol. 2020, 18, 559–570. [Google Scholar] [CrossRef]
- Kalayci, S.; Selvan, M.E.; Ramos, I.; Cotsapas, C.; Harris, E.; Kim, E.Y.; Montgomery, R.R.; Poland, G.; Pulendran, B.; Tsang, J.; et al. ImmuneRegulation: A web-based tool for identifying human immune regulatory elements. Nucleic Acids Res. 2019, 47, 142–150. [Google Scholar] [CrossRef] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chetta, M.; Tarsitano, M.; Vicari, L.; Saracino, A.; Bukvic, N. In Silico Analysis of Possible Interaction between Host Genomic Transcription Factors (TFs) and Zika Virus (ZikaSPH2015) Strain with Combinatorial Gene Regulation; Virus Versus Host—The Game Reloaded. Pathogens 2021, 10, 69. https://doi.org/10.3390/pathogens10010069
Chetta M, Tarsitano M, Vicari L, Saracino A, Bukvic N. In Silico Analysis of Possible Interaction between Host Genomic Transcription Factors (TFs) and Zika Virus (ZikaSPH2015) Strain with Combinatorial Gene Regulation; Virus Versus Host—The Game Reloaded. Pathogens. 2021; 10(1):69. https://doi.org/10.3390/pathogens10010069
Chicago/Turabian StyleChetta, Massimiliano, Marina Tarsitano, Laura Vicari, Annalisa Saracino, and Nenad Bukvic. 2021. "In Silico Analysis of Possible Interaction between Host Genomic Transcription Factors (TFs) and Zika Virus (ZikaSPH2015) Strain with Combinatorial Gene Regulation; Virus Versus Host—The Game Reloaded" Pathogens 10, no. 1: 69. https://doi.org/10.3390/pathogens10010069
APA StyleChetta, M., Tarsitano, M., Vicari, L., Saracino, A., & Bukvic, N. (2021). In Silico Analysis of Possible Interaction between Host Genomic Transcription Factors (TFs) and Zika Virus (ZikaSPH2015) Strain with Combinatorial Gene Regulation; Virus Versus Host—The Game Reloaded. Pathogens, 10(1), 69. https://doi.org/10.3390/pathogens10010069






