Immune Function in Critically Ill Septic Children
Abstract
:1. Introduction
2. Innate and Adaptive Immune Systems
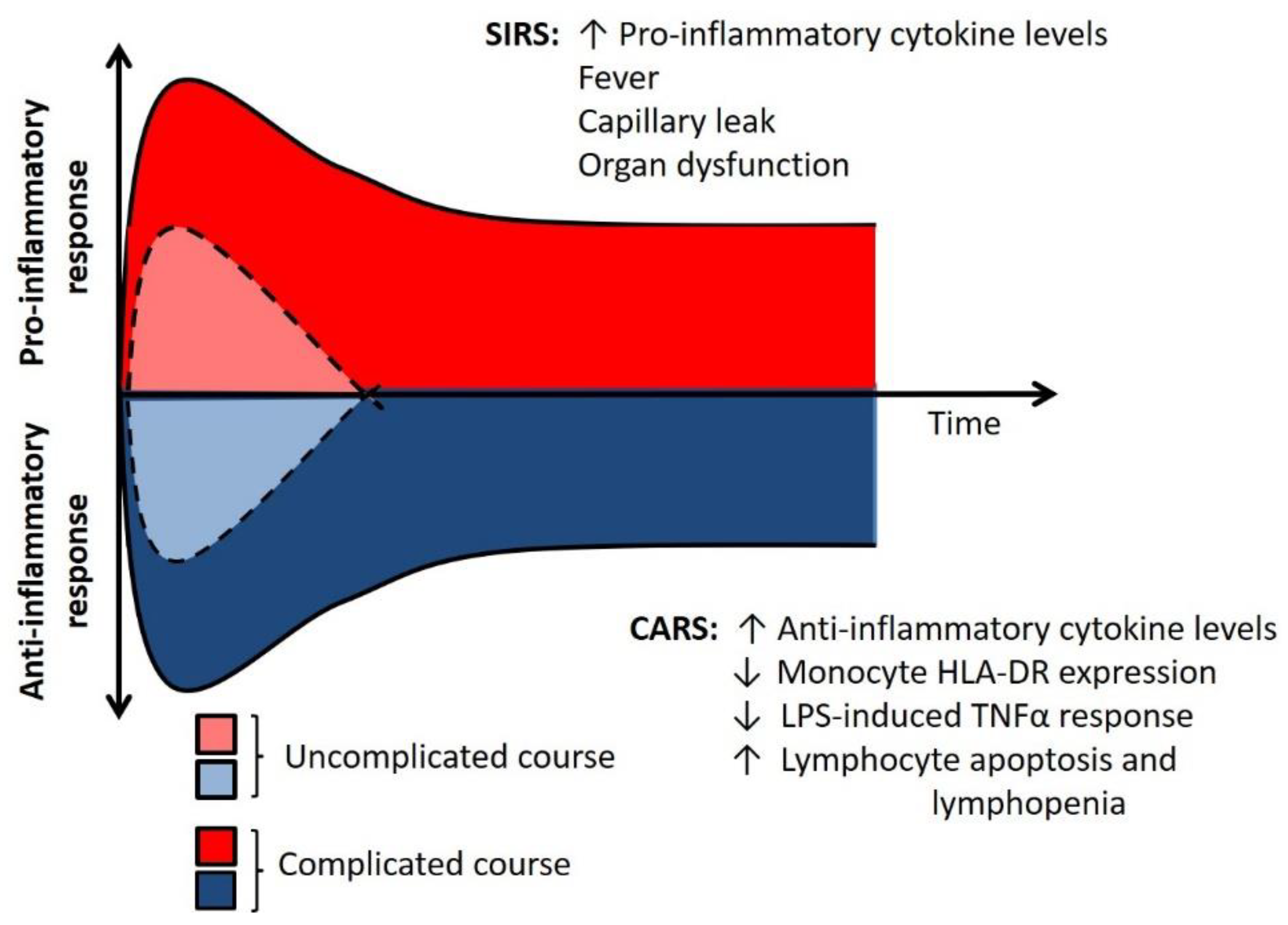
3. Two Sides of the Immune Response
4. Immunoparalysis
5. Gene Expression
6. Immunothrombosis
7. Developmental Effects on Immune Response
8. Risk Factors for Immunoparalysis
8.1. Host Specific
8.2. Infection Specific
8.3. Treatment Specific
9. Current Research and Future Directions
Author Contributions
Funding
Conflicts of Interest
References
- Weiss, S.L.; Fitzgerald, J.C.; Pappachan, J.; Wheeler, D.; Jaramillo-Bustamante, J.C.; Salloo, A.; Singhi, S.C.; Erickson, S.; Roy, J.A.; Bush, J.L.; et al. Global Epidemiology of Pediatric Severe Sepsis: The Sepsis Prevalence, Outcomes, and Therapies Study. Am. J. Respir. Crit. Care Med. 2015, 191, 1147–1157. [Google Scholar] [CrossRef]
- Singer, M.; Deutschman, C.S.; Seymour, C.W.; Shankar-Hari, M.; Annane, D.; Bauer, M.; Bellomo, R.; Bernard, G.R.; Chiche, J.-D.; Coopersmith, C.M.; et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA 2016, 315, 801–810. [Google Scholar] [CrossRef] [PubMed]
- Workman, J.K.; Ames, S.G.; Reeder, R.W.; Korgenski, E.K.; Masotti, S.M.; Bratton, S.L.; Larsen, G.Y. Treatment of Pediatric Septic Shock With the Surviving Sepsis Campaign Guidelines and PICU Patient Outcomes. Pediatr. Crit. Care Med. 2016, 17, e451–e458. [Google Scholar] [CrossRef] [Green Version]
- Weiss, S.L.; Fitzgerald, J.C.; Balamuth, F.; Alpern, E.R.; Lavelle, J.; Chilutti, M.; Grundmeier, R.; Nadkarni, V.M.; Thomas, N.J. Delayed Antimicrobial Therapy Increases Mortality and Organ Dysfunction Duration in Pediatric Sepsis. Crit. Care Med. 2014, 42, 2409–2417. [Google Scholar] [CrossRef]
- Weiss, S.L.; Balamuth, F.; Hensley, J.; Fitzgerald, J.C.; Bush, J.; Nadkarni, V.M.; Thomas, N.J.; Hall, M.; Muszynski, J. The Epidemiology of Hospital Death Following Pediatric Severe Sepsis: When, Why, and How Children with Sepsis Die. Pediatr. Crit. Care Med. J. Soc. Crit. Care Med. World Fed. Pediatr. Intensive Crit. Care Soc. 2017, 18, 823–830. [Google Scholar] [CrossRef]
- Dimeloe, S.; Burgener, A.-V.; Grählert, J.; Hess, C. T-Cell Metabolism Governing Activation, Proliferation and Differentiation; a Modular View. Immunology 2017, 150, 35–44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, J. T Helper Cell Differentiation, Heterogeneity, and Plasticity. Cold Spring Harb. Perspect. Biol. 2018, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schulte, W.; Bernhagen, J.; Bucala, R. Cytokines in Sepsis: Potent Immunoregulators and Potential Therapeutic Targets—An Updated View. Mediators Inflamm. 2013, 2013. [Google Scholar] [CrossRef]
- Shapiro, N.; Howell, M.D.; Bates, D.W.; Angus, D.C.; Ngo, L.; Talmor, D. The Association of Sepsis Syndrome and Organ Dysfunction with Mortality in Emergency Department Patients with Suspected Infection. Ann. Emerg. Med. 2006, 48, 583–590. e1. [Google Scholar] [CrossRef]
- Hall, M.W.; Knatz, N.L.; Vetterly, C.; Tomarello, S.; Wewers, M.D.; Volk, H.D.; Carcillo, J.A. Immunoparalysis and Nosocomial Infection in Children with Multiple Organ Dysfunction Syndrome. Intensive Care Med. 2011, 37, 525–532. [Google Scholar] [CrossRef] [Green Version]
- Jensen, I.J.; Sjaastad, F.V.; Griffith, T.S.; Badovinac, V.P. Sepsis-Induced T Cell Immunoparalysis: The Ins and Outs of Impaired T Cell Immunity. J. Immunol. Baltim. Md 1950 2018, 200, 1543–1553. [Google Scholar] [CrossRef]
- Hall, M.W.; Geyer, S.M.; Guo, C.-Y.; Panoskaltsis-Mortari, A.; Jouvet, P.; Ferdinands, J.; Shay, D.K.; Nateri, J.; Greathouse, K.; Sullivan, R.; et al. Innate Immune Function and Mortality in Critically Ill Children with Influenza: A Multicenter Study. Crit. Care Med. 2013, 41, 224–236. [Google Scholar] [CrossRef]
- Muszynski, J.A.; Nofziger, R.; Moore-Clingenpeel, M.; Greathouse, K.; Anglim, L.; Steele, L.; Hensley, J.; Hanson-Huber, L.; Nateri, J.; Ramilo, O.; et al. Early Immune Function and Duration of Organ Dysfunction in Critically III Children with Sepsis. Am. J. Respir. Crit. Care Med. 2018, 198, 361–369. [Google Scholar] [CrossRef]
- Carcillo, J.A.; Berg, R.A.; Wessel, D.; Pollack, M.; Meert, K.; Hall, M.; Newth, C.; Lin, J.C.; Doctor, A.; Shanley, T.; et al. A Multicenter Network Assessment of Three Inflammation Phenotypes in Pediatric Sepsis-Induced Multiple Organ Failure. Pediatr. Crit. Care Med. J. Soc. Crit. Care Med. World Fed. Pediatr. Intensive Crit. Care Soc. 2019, 20, 1137–1146. [Google Scholar] [CrossRef]
- Novak, T.; Hall, M.W.; McDonald, D.R.; Newhams, M.M.; Mistry, A.J.; Panoskaltsis-Mortari, A.; Mourani, P.M.; Loftis, L.L.; Weiss, S.L.; Tarquinio, K.M.; et al. RIG-I and TLR4 Responses and Adverse Outcomes in Pediatric Influenza-Related Critical Illness. J. Allergy Clin. Immunol. 2020, 145, 1673–1680.e11. [Google Scholar] [CrossRef] [Green Version]
- Volk, H.D.; Reinke, P.; Krausch, D.; Zuckermann, H.; Asadullah, K.; Müller, J.M.; Döcke, W.D.; Kox, W.J. Monocyte Deactivation--Rationale for a New Therapeutic Strategy in Sepsis. Intensive Care Med. 1996, 22 (Suppl 4), S474–S481. [Google Scholar] [CrossRef] [PubMed]
- Monneret, G.; Lepape, A.; Voirin, N.; Bohé, J.; Venet, F.; Debard, A.-L.; Thizy, H.; Bienvenu, J.; Gueyffier, F.; Vanhems, P. Persisting Low Monocyte Human Leukocyte Antigen-DR Expression Predicts Mortality in Septic Shock. Intensive Care Med. 2006, 32, 1175–1183. [Google Scholar] [CrossRef]
- Landelle, C.; Lepape, A.; Voirin, N.; Tognet, E.; Venet, F.; Bohé, J.; Vanhems, P.; Monneret, G. Low Monocyte Human Leukocyte Antigen-DR Is Independently Associated with Nosocomial Infections after Septic Shock. Intensive Care Med. 2010, 36, 1859–1866. [Google Scholar] [CrossRef] [PubMed]
- Boeddha, N.P.; Kerklaan, D.; Dunbar, A.; van Puffelen, E.; Nagtzaam, N.M.A.; Vanhorebeek, I.; Van den Berghe, G.; Hazelzet, J.A.; Joosten, K.F.; Verbruggen, S.C.; et al. HLA-DR Expression on Monocyte Subsets in Critically Ill Children. Pediatr. Infect. Dis. J. 2018, 37, 1034–1040. [Google Scholar] [CrossRef] [PubMed]
- Döcke, W.-D.; Höflich, C.; Davis, K.A.; Röttgers, K.; Meisel, C.; Kiefer, P.; Weber, S.U.; Hedwig-Geissing, M.; Kreuzfelder, E.; Tschentscher, P.; et al. Monitoring Temporary Immunodepression by Flow Cytometric Measurement of Monocytic HLA-DR Expression: A Multicenter Standardized Study. Clin. Chem. 2005, 51, 2341–2347. [Google Scholar] [CrossRef] [Green Version]
- Manzoli, T.F.; Troster, E.J.; Ferranti, J.F.; Sales, M.M. Prolonged Suppression of Monocytic Human Leukocyte Antigen-DR Expression Correlates with Mortality in Pediatric Septic Patients in a Pediatric Tertiary Intensive Care Unit. J. Crit. Care 2016, 33, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Ye, Y.; Tang, G.; Li, J. ΔHLA-DR Less than 400 MAb/Cell Would Be Better than the Threshold Value of 1000 MAb/Cell in Predicting Mortality in Pediatric Septic Patients. J. Crit. Care 2017, 39, 289–290. [Google Scholar] [CrossRef]
- Felmet, K.A.; Hall, M.W.; Clark, R.S.B.; Jaffe, R.; Carcillo, J.A. Prolonged Lymphopenia, Lymphoid Depletion, and Hypoprolactinemia in Children with Nosocomial Sepsis and Multiple Organ Failure. J. Immunol. Baltim. Md 1950 2005, 174, 3765–3772. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muszynski, J.A.; Nofziger, R.; Greathouse, K.; Steele, L.; Hanson-Huber, L.; Nateri, J.; Hall, M.W. Early Adaptive Immune Suppression in Children with Septic Shock: A Prospective Observational Study. Crit. Care Lond. Engl. 2014, 18, R145. [Google Scholar] [CrossRef] [Green Version]
- Wong, H.R.; Cvijanovich, N.Z.; Anas, N.; Allen, G.L.; Thomas, N.J.; Bigham, M.T.; Weiss, S.L.; Fitzgerald, J.; Checchia, P.A.; Meyer, K.; et al. Developing a Clinically Feasible Personalized Medicine Approach to Pediatric Septic Shock. Am. J. Respir. Crit. Care Med. 2015, 191, 309–315. [Google Scholar] [CrossRef]
- Wong, H.R.; Cvijanovich, N.; Lin, R.; Allen, G.L.; Thomas, N.J.; Willson, D.F.; Freishtat, R.J.; Anas, N.; Meyer, K.; Checchia, P.A.; et al. Identification of Pediatric Septic Shock Subclasses Based on Genome-Wide Expression Profiling. BMC Med. 2009, 7, 34. [Google Scholar] [CrossRef] [Green Version]
- Bline, K.E.; Moore-Clingenpeel, M.; Hensley, J.; Steele, L.; Greathouse, K.; Anglim, L.; Hanson-Huber, L.; Nateri, J.; Muszynski, J.A.; Ramilo, O.; et al. Hydrocortisone Treatment Is Associated with a Longer Duration of MODS in Pediatric Patients with Severe Sepsis and Immunoparalysis. Crit. Care Lond. Engl. 2020, 24, 545. [Google Scholar] [CrossRef] [PubMed]
- Katayama, Y.; Takahashi, M.; Kuwayama, H. Helicobacter Pylori Causes Runx3 Gene Methylation and Its Loss of Expression in Gastric Epithelial Cells, Which Is Mediated by Nitric Oxide Produced by Macrophages. Biochem. Biophys. Res. Commun. 2009, 388, 496–500. [Google Scholar] [CrossRef]
- Shames, S.R.; Bhavsar, A.P.; Croxen, M.A.; Law, R.J.; Mak, S.H.C.; Deng, W.; Li, Y.; Bidshari, R.; de Hoog, C.L.; Foster, L.J.; et al. The Pathogenic Escherichia Coli Type III Secreted Protease NleC Degrades the Host Acetyltransferase P300. Cell. Microbiol. 2011, 13, 1542–1557. [Google Scholar] [CrossRef] [PubMed]
- Saeed, S.; Quintin, J.; Kerstens, H.H.D.; Rao, N.A.; Aghajanirefah, A.; Matarese, F.; Cheng, S.-C.; Ratter, J.; Berentsen, K.; van der Ent, M.A.; et al. Epigenetic Programming of Monocyte-to-Macrophage Differentiation and Trained Innate Immunity. Science 2014, 345, 1251086. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Menachery, V.D.; Schäfer, A.; Burnum-Johnson, K.E.; Mitchell, H.D.; Eisfeld, A.J.; Walters, K.B.; Nicora, C.D.; Purvine, S.O.; Casey, C.P.; Monroe, M.E.; et al. MERS-CoV and H5N1 Influenza Virus Antagonize Antigen Presentation by Altering the Epigenetic Landscape. Proc. Natl. Acad. Sci. USA 2018, 115, E1012–E1021. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sullivan, K.E.; Reddy, A.B.M.; Dietzmann, K.; Suriano, A.R.; Kocieda, V.P.; Stewart, M.; Bhatia, M. Epigenetic Regulation of Tumor Necrosis Factor Alpha. Mol. Cell. Biol. 2007, 27, 5147–5160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Topper, M.J.; Vaz, M.; Marrone, K.A.; Brahmer, J.R.; Baylin, S.B. The Emerging Role of Epigenetic Therapeutics in Immuno-Oncology. Nat. Rev. Clin. Oncol. 2020, 17, 75–90. [Google Scholar] [CrossRef] [PubMed]
- Iba, T.; Levi, M.; Levy, J.H. Sepsis-Induced Coagulopathy and Disseminated Intravascular Coagulation. Semin. Thromb. Hemost. 2020, 46, 089–095. [Google Scholar] [CrossRef]
- Delabranche, X.; Stiel, L.; Severac, F.; Galoisy, A.-C.; Mauvieux, L.; Zobairi, F.; Lavigne, T.; Toti, F.; Anglès-Cano, E.; Meziani, F.; et al. Evidence of Netosis in Septic Shock-Induced Disseminated Intravascular Coagulation. Shock. Inj. Inflamm. Sepsis Lab. Clin. Approaches 2017, 47, 313–317. [Google Scholar] [CrossRef]
- Lever, R.; Lo, W.T.; Faraidoun, M.; Amin, V.; Brown, R.A.; Gallagher, J.; Page, C.P. Size-Fractionated Heparins Have Differential Effects on Human Neutrophil Function in Vitro. Br. J. Pharmacol. 2007, 151, 837–843. [Google Scholar] [CrossRef] [Green Version]
- Simonsen, K.A.; Anderson-Berry, A.L.; Delair, S.F.; Davies, H.D. Early-Onset Neonatal Sepsis. Clin. Microbiol. Rev. 2014, 27, 21–47. [Google Scholar] [CrossRef] [Green Version]
- Wynn, J.L.; Cvijanovich, N.Z.; Allen, G.L.; Thomas, N.J.; Freishtat, R.J.; Anas, N.; Meyer, K.; Checchia, P.A.; Lin, R.; Shanley, T.P.; et al. The Influence of Developmental Age on the Early Transcriptomic Response of Children with Septic Shock. Mol. Med. Camb. Mass 2011, 17, 1146–1156. [Google Scholar] [CrossRef]
- Sood, B.G.; Shankaran, S.; Schelonka, R.L.; Saha, S.; Benjamin, D.K.; Sánchez, P.J.; Adams-Chapman, I.; Stoll, B.J.; Thorsen, P.; Skogstrand, K.; et al. Cytokine Profiles of Preterm Neonates with Fungal and Bacterial Sepsis. Pediatr. Res. 2012, 72, 212–220. [Google Scholar] [CrossRef]
- Kleiner, G.; Marcuzzi, A.; Zanin, V.; Monasta, L.; Zauli, G. Cytokine Levels in the Serum of Healthy Subjects. Available online: https://www.hindawi.com/journals/mi/2013/434010/ (accessed on 21 January 2021).
- Smith, C.L.; Dickinson, P.; Forster, T.; Craigon, M.; Ross, A.; Khondoker, M.R.; France, R.; Ivens, A.; Lynn, D.J.; Orme, J.; et al. Identification of a Human Neonatal Immune-Metabolic Network Associated with Bacterial Infection. Nat. Commun. 2014, 5, 4649. [Google Scholar] [CrossRef] [Green Version]
- Lawn, J.E.; Cousens, S.; Zupan, J. 4 Million Neonatal Deaths: When? Where? Why? Lancet 2005, 365, 891–900. [Google Scholar] [CrossRef]
- Klein, S.L.; Flanagan, K.L. Sex Differences in Immune Responses. Nat. Rev. Immunol. 2016, 16, 626–638. [Google Scholar] [CrossRef]
- Oertelt-Prigione, S. The Influence of Sex and Gender on the Immune Response. Autoimmun. Rev. 2012, 11, A479–A485. [Google Scholar] [CrossRef]
- Haghmorad, D.; Salehipour, Z.; Nosratabadi, R.; Rastin, M.; Kokhaei, P.; Mahmoudi, M.B.; Amini, A.A.; Mahmoudi, M. Medium-Dose Estrogen Ameliorates Experimental Autoimmune Encephalomyelitis in Ovariectomized Mice. J. Immunotoxicol. 2016, 13, 885–896. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hodeib, S.; Herberg, J.A.; Levin, M.; Sancho-Shimizu, V. Human Genetics of Meningococcal Infections. Hum. Genet. 2020, 139, 961–980. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kernan, K.F.; Ghaloul-Gonzalez, L.; Shakoory, B.; Kellum, J.A.; Angus, D.C.; Carcillo, J.A. Adults with Septic Shock and Extreme Hyperferritinemia Exhibit Pathogenic Immune Variation. Genes Immun. 2019, 20, 520–526. [Google Scholar] [CrossRef]
- Berends, E.T.M.; Zheng, X.; Zwack, E.E.; Ménager, M.M.; Cammer, M.; Shopsin, B.; Torres, V.J. Staphylococcus Aureus Impairs the Function of and Kills Human Dendritic Cells via the LukAB Toxin. mBio 2019, 10. [Google Scholar] [CrossRef] [Green Version]
- Arcanjo, A.F.; Nico, D.; de Castro, G.M.M.; da Silva Fontes, Y.; Saltarelli, P.; Decote-Ricardo, D.; Nunes, M.P.; Ferreira-Pereira, A.; Palatnik-de-Sousa, C.B.; Freire-de-Lima, C.G.; et al. Dependency of B-1 Cells in the Maintenance of Splenic Interleukin-10 Producing Cells and Impairment of Macrophage Resistance in Visceral Leishmaniasis. Front. Microbiol. 2017, 8, 978. [Google Scholar] [CrossRef] [Green Version]
- Prandota, J. Furosemide: Progress in Understanding Its Diuretic, Anti-Inflammatory, and Bronchodilating Mechanism of Action, and Use in the Treatment of Respiratory Tract Diseases. Am. J. Ther. 2002, 9, 317–328. [Google Scholar] [CrossRef]
- Yuengsrigul, A.; Chin, T.W.; Nussbaum, E. Immunosuppressive and Cytotoxic Effects of Furosemide on Human Peripheral Blood Mononuclear Cells. Ann. Allergy Asthma Immunol. Off. Publ. Am. Coll. Allergy Asthma Immunol. 1999, 83, 559–566. [Google Scholar] [CrossRef]
- Francois, B.; Jeannet, R.; Daix, T.; Walton, A.H.; Shotwell, M.S.; Unsinger, J.; Monneret, G.; Rimmelé, T.; Blood, T.; Morre, M.; et al. Interleukin-7 Restores Lymphocytes in Septic Shock: The IRIS-7 Randomized Clinical Trial. JCI Insight 2018, 3, e98960. [Google Scholar] [CrossRef] [PubMed]
- Hotchkiss, R.S.; Colston, E.; Yende, S.; Angus, D.C.; Moldawer, L.L.; Crouser, E.D.; Martin, G.S.; Coopersmith, C.M.; Brakenridge, S.; Mayr, F.B.; et al. Immune Checkpoint Inhibition in Sepsis: A Phase 1b Randomized, Placebo-Controlled, Single Ascending Dose Study of Anti-PD-L1 (BMS-936559). Crit. Care Med. 2019, 47, 632–642. [Google Scholar] [CrossRef] [PubMed]
- Hall, M.W. Immune Modulation in Pediatric Sepsis. J. Pediatr. Intensive Care 2019, 8, 42–50. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Genetic Polymorphism | Downstream Effect |
| TNFα promoter (G→A at nucleotide -308) | Increased risk of sepsis among trauma patients |
| TLR4 (A→G at nucleotide +896) | Reduced LPS responsiveness, increased risk to Gram-negative sepsis |
| MASP2 deficiency | Decreased mannose-binding lectin protein, higher risk of sepsis and fatal outcome |
| BPI Taq gene | Predictor of sepsis severity in children in PICU |
| SNP in TLR1 at position -7202 | Increased cytokine response and higher risk of mortality in sepsis |
| Epigenetic Variant | Downstream Effect |
| Dimethylation of histone 3 at lysine residue 9 | Decreased macrophage response to LPS challenge |
| Histone methylation at promoter region of IL-12 gene | Decreased IL-12 protein production by dendritic cells in response to TLR stimulus, promote Th2 response |
| Acetylation of histone 3 and 4 proximal to IFNγ promoter | Increased differentiation of naïve T cells to Th1 cells |
| Decreased histone acetylation of Foxp3 gene | Upregulated expression of T regulatory cells |
| Drug(s) | Mechanism |
|---|---|
| Overt Immunomodulation | |
| Suppression of the immune response | |
| Tacrolimus, cyclosporine | Calcineurin inhibition-induced lymphocyte suppression |
| Sirolimus, everolimus | mTOR inhibition-induced lymphocyte suppression |
| Glucocorticoids | Lymphocyte apoptosis, suppression of pro-inflammatory gene transcription |
| Myeloablative chemotherapy | Bone marrow suppression |
| Anti-leukocyte monoclonal antibodies (e.g., rituximab and anti-thymocyte globulin) | Depletion of specific immune cell populations |
| Anti-cytokine agents (e.g., tocilizumab and anakinra) | Pathway-specific inhibition of inflammatory pathways |
| Enhancement of the immune response | |
| Granulocyte colony-stimulating factor | Increased numbers and function of neutrophils |
| Granulocyte macrophage colony-stimulating factor | Increased numbers and function of antigen-presenting cells (e.g., monocytes and dendritic cells) |
| Interferon-γ | Activation of innate and adaptive immune cells |
| Occult Immunomodulation | |
| Suppression of the immune response | |
| Antimicrobials | Bone marrow suppression (e.g., β-lactams, sulfonamides, and ganciclovir) |
| Catecholamines | Stimulation of β adrenergic receptors |
| Furosemide | Inhibition of cytokine production |
| Insulin | Inhibition of cytokine production |
| Opioids | Leukocyte apoptosis, inhibition of cytokine production, induction of TGFβ |
| Barbiturates | Inhibition of neutrophil function |
| Enhancement of the immune response | |
| Antimicrobials | Release of PAMPs through pathogen lysis (Jarisch–Herxheimer reaction) |
| Catecholamines | Stimulation of α adrenergic receptors |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bline, K.E.; Hall, M.W. Immune Function in Critically Ill Septic Children. Pathogens 2021, 10, 1239. https://doi.org/10.3390/pathogens10101239
Bline KE, Hall MW. Immune Function in Critically Ill Septic Children. Pathogens. 2021; 10(10):1239. https://doi.org/10.3390/pathogens10101239
Chicago/Turabian StyleBline, Katherine Elizabeth, and Mark W. Hall. 2021. "Immune Function in Critically Ill Septic Children" Pathogens 10, no. 10: 1239. https://doi.org/10.3390/pathogens10101239
APA StyleBline, K. E., & Hall, M. W. (2021). Immune Function in Critically Ill Septic Children. Pathogens, 10(10), 1239. https://doi.org/10.3390/pathogens10101239





