Selection of Endophytic Beauveria bassiana as a Dual Biocontrol Agent of Tomato Pathogens and Pests
Abstract
:1. Introduction
2. Results
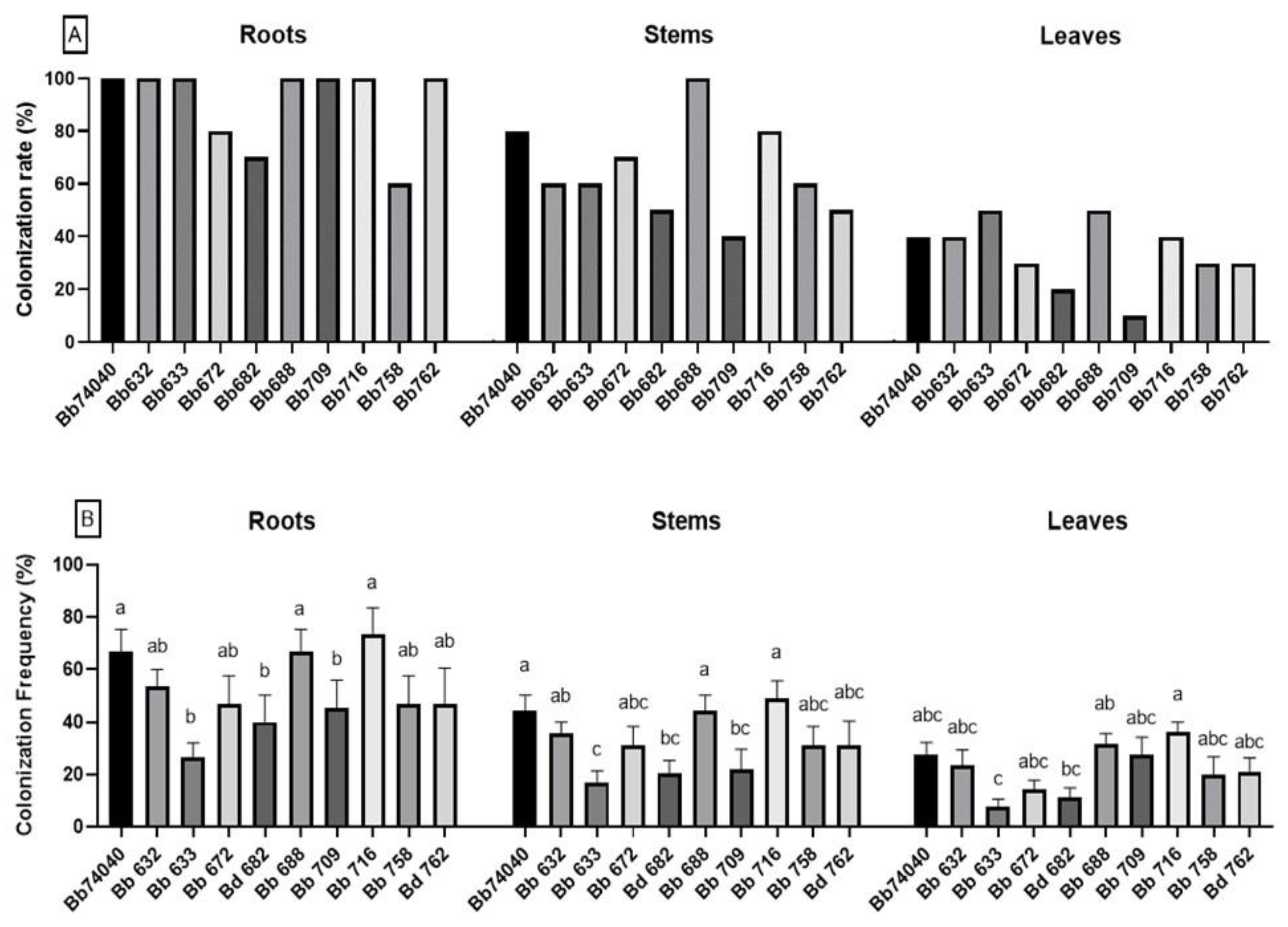
2.1. Induction and Assessment of Endophytic Colonization
2.2. Biocontrol of Tomato Foliar Pathogens—Test In Vitro
2.3. Selection of Potential EF and Biocontrol B. bassiana Strains
2.4. Plant Growth Promotion Assay
2.5. Biocontrol of Tomato Foliar Pathogens—Tests in Planta
2.6. Biocontrol of the Aphid M. euphorbiae—Test in Planta
3. Discussion
4. Conclusions
5. Materials and Methods
5.1. Fungal Isolates: Origins, Identification, and Culture Methods
5.2. B. bassiana Conidia Production on Rice
5.3. Induction and Assessment of Endophytic Colonization
5.4. Biocontrol of Tomato Foliar Pathogens—Tests In Vitro
5.5. B. bassiana Strain Choice for In Vivo Assay
5.6. Plant-Growth Promotion Assay
5.7. Biocontrol of Tomato Foliar Pathogens—Tests in Planta
5.8. Insect Rearing
5.9. Biocontrol of the Aphid M. euphorbiae—Tests in Planta
5.10. Data Analyses
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Struik, P.C.; Kuyper, T.W. Sustainable intensification in agriculture: The richer shade of green. A review. Agron. Sustain. Dev. 2017, 37, 1–15. [Google Scholar] [CrossRef]
- Foley, J.A.; Ramankutty, N.; Brauman, K.A.; Cassidy, E.S.; Gerber, J.S.; Johnston, M.; Mueller, N.D.; O’Connell, C.; Ray, D.K.; West, P.C.; et al. Solutions for a cultivated planet. Nature 2011, 478, 337–342. [Google Scholar] [CrossRef] [Green Version]
- Fraser, E.D.G. The challenge of feeding a diverse and growing population. Physiol. Behav. 2020, 221, 112908. [Google Scholar] [CrossRef]
- Fraser, E.; Legwegoh, A.; Krishna, K.C.; CoDyre, M.; Dias, G.; Hazen, S.; Johnson, R.; Martin, R.; Ohberg, L.; Sethuratnam, S.; et al. Biotechnology or organic? Extensive or intensive? Global or local? A critical review of potential pathways to resolve the global food crisis. Trends Food Sci. Technol. 2016, 48, 78–87. [Google Scholar] [CrossRef]
- Finkel, O.M.; Castrillo, G.; Paredes, S.H.; González, I.S.; Dangl, J.L. Understanding and exploiting plant beneficial microbes. Curr. Opin. Plant Biol. 2017, 38, 155–163. [Google Scholar] [CrossRef]
- Parnell, J.J.; Berka, R.; Young, H.A.; Sturino, J.M.; Kang, Y.; Barnhart, D.M.; DiLeo, M.V. From the Lab to the Farm: An Industrial Perspective of Plant Beneficial Microorganisms. Front. Plant Sci. 2016, 7, 1110. [Google Scholar] [CrossRef] [PubMed]
- Egamberdieva, D.; Wirth, S.J.; Alqarawi, A.A.; Abd-Allah, E.F.; Hashem, A. Phytohormones and beneficial microbes: Essential components for plants to balance stress and fitness. Front. Microbiol. 2017, 8, 1–14. [Google Scholar] [CrossRef]
- Govind, G.; Singh, P.S.; Narendra, K.A.; Sunil, K.S.; Vinod, S. Plant Growth Promoting Rhizobacteria (PGPR): Current and Future Prospects for Development of Sustainable Agriculture. J. Microb. Biochem. Technol. 2015, 7, 096–102. [Google Scholar] [CrossRef]
- Berg, G. Plant-microbe interactions promoting plant growth and health: Perspectives for controlled use of microorganisms in agriculture. Appl. Microbiol. Biotechnol. 2009, 84, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Medina, A.; Van Wees, S.C.M.; Pieterse, C.M.J. Airborne signals by Trichoderma fungi stimulate iron uptake responses in roots resulting in priming of jasmonic acid-dependent defences in shoots of Arabidopsis thaliana and Solanum lycopersicum. Plant. Cell. Environ. 2017, 40, 2691–2705. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pascale, A.; Vinale, F.; Manganiello, G.; Nigro, M.; Lanzuise, S.; Ruocco, M.; Marra, R.; Lombardi, N.; Woo, S.L.; Lorito, M. Trichoderma and its secondary metabolites improve yield and quality of grapes. Crop Prot. 2017, 92, 176–181. [Google Scholar] [CrossRef] [Green Version]
- Bamisile, B.S.; Dash, C.K.; Akutse, K.S.; Keppanan, R.; Wang, L. Fungal endophytes: Beyond herbivore management. Front. Microbiol. 2018, 9, 1–11. [Google Scholar] [CrossRef] [Green Version]
- McKinnon, A.C.; Saari, S.; Moran-Diez, M.E.; Meyling, N.V.; Raad, M.; Glare, T.R. Beauveria bassiana as an endophyte: A critical review on associated methodology and biocontrol potential. BioControl 2017, 62, 1–17. [Google Scholar] [CrossRef]
- Ownley, B.H.; Gwinn, K.D.; Vega, F.E. Endophytic fungal entomopathogens with activity against plant pathogens: Ecology and evolution. In The Ecology of Fungal Entomopathogens; Springer: Dordrecht, The Netherlands, 2010; ISBN 9789048139651. [Google Scholar]
- Saikkonen, K.; Saari, S.; Helander, M. Defensive mutualism between plants and endophytic fungi? Fungal Divers 2010, 41, 101–113. [Google Scholar] [CrossRef]
- Sinno, M.; Ranesi, M.; Gioia, L.; D’errico, G.; Woo, S.L. Endophytic fungi of tomato and their potential applications for crop improvement. Agriculture 2020, 10, 587. [Google Scholar] [CrossRef]
- Rodriguez, R.J.; White, J.F.; Arnold, A.E., Jr.; Redman, R.S. Fungal endophytes: Diversity and functional roles. New Phytol. 2009, 182, 314–330. [Google Scholar] [CrossRef]
- Jaber, L.R.; Ownley, B.H. Can we use entomopathogenic fungi as endophytes for dual biological control of insect pests and plant pathogens? Biol. Control 2018, 116, 36–45. [Google Scholar] [CrossRef]
- Gouda, S.; Das, G.; Sen, S.K.; Shin, H.S.; Patra, J.K. Endophytes: A treasure house of bioactive compounds of medicinal importance. Front. Microbiol. 2016, 7, 1538. [Google Scholar] [CrossRef] [Green Version]
- Segaran, G.; Sathiavelu, M. Fungal endophytes: A potent biocontrol agent and a bioactive metabolites reservoir. Biocatal. Agric. Biotechnol. 2019, 21, 101284. [Google Scholar] [CrossRef]
- Kumar, S.; Kaushik, N. Metabolites of endophytic fungi as novel source of biofungicide: A review. Phytochem. Rev. 2012, 11, 507–522. [Google Scholar] [CrossRef]
- Vega, F.E. Insect pathology and fungal endophytes. J. Invertebr. Pathol. 2008, 98, 277–279. [Google Scholar] [CrossRef] [PubMed]
- Barra-Bucarei, L.; France, A.; Millas, P. Crossing frontiers: Endophytic entomopathogenic fungi for biological control of plant diseases. In Endophytes for a Growing World; Cambridge University Press: Cambridge, UK, 2019; p. 67. [Google Scholar]
- Pieterse, C.M.J.; Poelman, E.H.; Van Wees, S.C.M.; Dicke, M. Induced plant responses to microbes and insects. Front. Plant Sci. 2013, 4, 475. [Google Scholar] [CrossRef] [Green Version]
- Pieterse, C.M.; Zamioudis, C.; Berendsen, R.L.; Weller, D.M.; Van Wees, S.C.; Bakker, P.A. Induced systemic resistance by beneficial microbes. Annu. Rev. Phytopathol. 2014, 52, 347–375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Loon, L.C.; Bakker, P.; Pieterse, C.M.J. Systemic resistance induced by rhizosphere bacteria. Annu. Rev. Phytopathol. 1998, 36, 453–483. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Durrant, W.E.; Dong, X. Systemic acquired resistance. Ann. Rev. Phytopathol. 2004, 42, 185–209. [Google Scholar] [CrossRef] [PubMed]
- Conrath, U.; Beckers, G.J.M.; Flors, V.; García-Agustín, P.; Jakab, G.; Mauch, F.; Newman, M.A.; Pieterse, C.M.J.; Poinssot, B.; Pozo, M.J.; et al. Priming: Getting ready for battle. Mol. Plant-Microbe Interact. 2006, 19, 1062–1071. [Google Scholar] [CrossRef] [Green Version]
- Conrath, U.; Beckers, G.J.; Langenbach, C.J.; Jaskiewicz, M.R. Priming for enhanced defense. Ann. Rev. Phytopathol. 2015, 53, 97–119. [Google Scholar] [CrossRef]
- Vos, C.M.; Yang, Y.; De Coninck, B.; Cammue, B.P.A. Fungal (-like) biocontrol organisms in tomato disease control. Biol. Control 2014, 74, 65–81. [Google Scholar] [CrossRef]
- Manganiello, G.; Sacco, A.; Ercolano, M.R.; Vinale, F.; Lanzuise, S.; Pascale, A.; Napolitano, M.; Lombardi, N.; Lorito, M.; Woo, S.L. Modulation of tomato response to Rhizoctonia solani by Trichoderma harzianum and its secondary metabolite harzianic acid. Front. Microbiol. 2018, 9, 1966. [Google Scholar] [CrossRef]
- Faria, M.R.d.; Wraight, S.P. Mycoinsecticides and Mycoacaricides: A comprehensive list with worldwide coverage and international classification of formulation types. Biol. Control 2007, 43, 237–256. [Google Scholar] [CrossRef]
- Saranraj, P.; Jayaparakash, A. Agrobeneficial entomopathogenic fungi—Beauveria bassiana: A review. Asian J. Multidiscip. Res. 2017, 3, 1051–1087. [Google Scholar] [CrossRef]
- Quesada-Moraga, E.; Landa, B.B.; Munoz-Ledesma, J.; Jimenez-Diaz, R.M.; Santiago-Alvarez, C. Endophytic colonization of opium poppy, Papaver somniferum, by an entomopathogenic Beauveria bassiana strain. Mycopathologia 2006, 161, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Quesada-Moraga, E.; Munoz-Ledesma, F.J.; Santiago-Alvarez, C. Systemic protection of Papaver somniferum L. against Iraella luteipes (Hymenoptera: Cynipidae) by an endophytic strain of Beauveria bassiana (Ascomycota: Hypocreales). Environ. Entomol. 2009, 38, 723–730. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quesada-Moraga, E.; López-Díaz, C.; Landa, B.B. The hidden habit of the entomopathogenic fungus Beauveria bassiana: First demonstration of vertical plant transmission. PLoS ONE 2014, 9, 8–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vega, F.E.; Goettel, M.S.; Chandler, D.; Jackson, M.A.; Keller, S.; Koike, M.; Maniania, N.K.; Monzon, A.; Ownley, B.H.; Pell, J.K.; et al. Fungal entomopathogens: New insights on their ecology. Fungal Ecol. 2009, 2, 149–159. [Google Scholar] [CrossRef] [Green Version]
- Vidal, S.; Jaber, L.R. Entomopathogenic fungi as endophytes: Plant-endophyte-herbivore interactions and prospects for use in biological control. Curr. Sci. 2015, 109, 46–54. [Google Scholar]
- Ownley, B.H.; Pereira, R.M.; Klingeman, W.E.; Quigley, N.B.; Leckie, B.M. Beauveria bassiana, a dual purpose biological control with activity against insect pests and plant pathogens. Emerg. Concepts Plant Health Manag. 2004, 2004, 255–269. [Google Scholar]
- Ownley, B.H.; Griffin, M.R.; Klingeman, W.E.; Gwinn, K.D.; Moulton, J.K.; Pereira, R.M. Beauveria bassiana: Endophytic colonization and plant disease control. J. Invertebr. Pathol. 2008, 98, 267–270. [Google Scholar] [CrossRef]
- Wei, Q.Y.; Li, Y.Y.; Xu, C.; Wu, Y.X.; Zhang, Y.R.; Liu, H. Endophytic colonization by Beauveria bassiana increases the resistance of tomatoes against Bemisia tabaci. Arthropod-Plant Interact. 2020, 14, 289–300. [Google Scholar] [CrossRef] [Green Version]
- Raman, A.; Suryanarayanan, T.S. Fungus–plant interaction influences plant-feeding insects. Fungal Ecol. 2017, 29, 123–132. [Google Scholar] [CrossRef]
- Allegrucci, N.; Velazquez, M.S.; Russo, M.L.; Perez, E.; Scorsetti, A.C. Endophytic colonization of tomato by the entomopathogenic fungus Beauveria bassiana: The use of different inoculation techniques and their effects on the tomato leafminer Tuta absoluta (Lepidoptera: Gelechiidae). J. Plant Prot. Res. 2017, 57, 206–211. [Google Scholar] [CrossRef] [Green Version]
- Tefera, T.; Vidal, S. Effect of inoculation method and plant growth medium on endophytic colonization of sorghum by the entomopathogenic fungus Beauveria bassiana. BioControl. 2009, 54, 663–669. [Google Scholar] [CrossRef] [Green Version]
- Posada, F.; Aime, M.C.; Peterson, S.W.; Rehner, S.A.; Vega, F.E. Inoculation of coffee plants with the fungal entomopathogen Beauveria bassiana (Ascomycota: Hypocreales). Mycol. Res. 2007, 111, 748–757. [Google Scholar] [CrossRef] [Green Version]
- Parsa, S.; Ortiz, V.; Vega, F.E. Establishing Fungal Entomopathogens as Endophytes: Towards Endophytic Biological Control. J. Vis. Exp. 2013, 74, 50360. [Google Scholar] [CrossRef] [Green Version]
- Qayyum, M.A.; Wakil, W.; Arif, M.J.; Sahi, S.T.; Dunlap, C.A. Infection of Helicoverpa armigera by endophytic Beauveria bassiana colonizing tomato plants. Biol. Control 2015, 90, 200–207. [Google Scholar] [CrossRef]
- Afandhi, A.; Widjayanti, T.; Emi, A.A.L.; Tarno, H.; Afiyanti, M.; Handoko, R.N.S. Endophytic fungi Beauveria bassiana Balsamo accelerates growth of common bean (Phaeseolus vulgaris L.). Chem. Biol. Technol. Agric. 2019, 6, 1–6. [Google Scholar] [CrossRef]
- Yun, H.G.; Kim, D.J.; Gwak, W.S.; Shin, T.Y.; Woo, S.D. Entomopathogenic fungi as dual control agents against both the pest Myzus persicae and phytopathogen. Botrytis Cinerea. Mycobiol. 2017, 45, 192–198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vega, F.E. The use of fungal entomopathogens as endophytes in biological control: A review. Mycologia 2018, 110, 4–30. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.K.; Singh, A.K.; Kumar, A. Disease management of tomato through PGPB: Current trends and future perspective. 3 Biotech 2017, 7, 1–10. [Google Scholar] [CrossRef] [PubMed]
- He, D.C.; Zhan, J.S.; Xie, L.H. Problems, challenges and future of plant disease management: From an ecological point of view. J. Integr. Agric. 2016, 15, 705–715. [Google Scholar] [CrossRef]
- Prabhukarthikeyan, S.R.; Keerthana, U.; Archana, S.; Raguchander, T. Induced resistance in tomato plants to Helicoverpa armigera by mixed formulation of bacillus subtilis and Beauveria bassiana. Res. J. Biotechnol. 2017, 12, 53–59. [Google Scholar]
- Powell, W.; Klingeman, W.; Ownley, B.; Gwinn, K. Evidence of endophytic Beauveria bassiana in seed-treated tomato plants acting as a systemic entomopathogen to larval Helicoverpa zea (Lepidop- tera: Noctuidae). J. Entomol. Sci. 2009, 44, 391–396. [Google Scholar] [CrossRef]
- Brownbridge, M.; Reay, S.D.; Nelson, T.L.; Glare, T.R. Persistence of Beauveria bassiana (Ascomycota: Hypocreales) as an endophyte following inoculation of radiata pine seed and seedlings. Biol. Control 2012, 61, 194–200. [Google Scholar] [CrossRef]
- Resquín-Romero, G.; Garrido-Jurado, I.; Delso, C.; Ríos-Moreno, A.; Quesada-Moraga, E. Transient endophytic colonizations of plants improve the outcome of foliar applications of mycoinsecticides against chewing insects. J. Invertebr. Pathol. 2016, 136, 23–31. [Google Scholar] [CrossRef]
- Garrido-Jurado, I.; Resquín-Romero, G.; Amarilla, S.P.; Ríos-Moreno, A.; Carrasco, L.; Quesada-Moraga, E. Transient endophytic colonization of melon plants by entomopathogenic fungi after foliar application for the control of Bemisia tabaci Gennadius (Hemiptera: Aleyrodidae). J. Pest Sci. 2017, 90, 319–330. [Google Scholar] [CrossRef]
- Omukoko, C.A. Biocontrol Mechanisms of Endophytic Beauveria bassiana in three Tomato (Lycopersum esculentum) varieties C. World Dev. 2018, 1, 43–52. [Google Scholar] [CrossRef]
- Sánchez-Rodríguez, A.R.; Del Campillo, M.C.; Quesada-Moraga, E. Beauveria bassiana: An entomopathogenic fungus alleviates Fe chlorosis symptoms in plants grown on calcareous substrates. Sci. Hortic. 2015, 197, 193–202. [Google Scholar] [CrossRef]
- Shrivastava, G.; Ownley, B.H.; Augé, R.M.; Toler, H.; Dee, M.; Vu, A.; Köllner, T.G.; Chen, F. Colonization by arbuscular mycorrhizal and endophytic fungi enhanced terpene production in tomato plants and their defense against a herbivorous insect. Symbiosis 2015, 65, 65–74. [Google Scholar] [CrossRef]
- Jonkers, W.; Rodriguez Estrada, A.E.; Lee, K.; Breakspear, A.; May, G.; Kistler, H.C. Metabolome and transcriptome of the interaction between Ustilago maydis and Fusarium verticillioides in vitro. Appl. Environ. Microbiol. 2012, 78, 3656–3667. [Google Scholar] [CrossRef] [Green Version]
- Yan, J.F.; Broughton, S.J.; Yang, S.L.; Gange, A.C. Do endophytic fungi grow through their hosts systemically? Fungal Ecol. 2015, 13, 53–59. [Google Scholar] [CrossRef]
- Barra-Bucarei, L.; Gerding, M. Antifungal Activity of Beauveria bassiana Endophyte against Botrytis cinerea in Two Solanaceae Crops. Microorganisms 2020, 8, 65. [Google Scholar] [CrossRef] [Green Version]
- Jaber, L.R. Seed inoculation with endophytic fungal entomopathogens promotes plant growth and reduces crown and root rot (CRR) caused by Fusarium culmorum in wheat. Planta 2018, 248, 1525–1535. [Google Scholar] [CrossRef] [PubMed]
- Culebro-Ricaldi, J.M.; Ruíz-Valdiviezo, V.M.; Rodríguez-Mendiola, M.A.; Ávila-Miranda, M.E.; Gutiérrez- Miceli, F.A.; Cruz-Rodríguez, R.I.; Dendooven, L.; Montes-Molina, J.A. Antifungal properties of Beauveria bassiana strains against Fusarium oxysporum f. sp. lycopersici race 3 in tomato crop. J. Environ. Biol. 2017, 38, 821–827. [Google Scholar] [CrossRef]
- Azadi, N.; Shirzad, A.; Mohammadi, H. A study of some biocontrol mechanisms of Beauveria bassiana against Rhizoctonia dis-ease on tomato. Acta Biol. Szeged. 2016, 60, 119–127. [Google Scholar]
- Orole, O.O.; Adejumo, T.O. Activity of fungal endophytes against four maize wilt pathogens. Afr. J. Microbiol. Res. 2009, 3, 969–973. [Google Scholar]
- Bark, Y.G.; Lee, D.G.; Kang, S.C.; Kim, Y.H. Antibiotic properties of an entomopathogenic fungus, Beauveria bassiana, on Fusarium oxysporum and Botrytis cinerea. Korean J. Plant Pathol. 1996, 12, 245–250. [Google Scholar]
- Renwick, A.; Campbell, R.; Coe, S. Assessment of in vivo screening systems for potential biocontrol agents of Gaeumannomyces graminis. Plant Pathol. 1991, 40, 524–532. [Google Scholar] [CrossRef]
- Parine, N.R.; Pathan, A.K.; Sarayu, B.; Nishanth, V.S.; Bobbarala, V. Antibacterial efficacy of secondary metabolites from entomopathogenic fungi Beauveria bassiana. Int. J. Chem. Anal. Sci. 2010, 1, 94–96. [Google Scholar]
- Sahab, A.F. Antimicrobial efficacy of secondary metabolites of Beauveria bassiana against selected bacteria and phytopathogenic fungi. J. Appl. Sci. Res. 2012, 8, 1441–1444. [Google Scholar]
- Vining, L.C.; Kelleher, W.J.; Schwarting, A.E. Oosporein production by a strain of Beauveria bassiana originally identified as Amanita muscaria. Can. J. Microbiol. 1962, 8, 931–933. [Google Scholar] [CrossRef]
- Hamill, R.L.; Higgens, C.E.; Boaz, H.E.; Gorman, M. The structure of beauvericin, a new depsipeptide antibiotic toxic to Artemia salina. Tetrahedron Lett. 1969, 10, 4255–4258. [Google Scholar] [CrossRef]
- Suzuki, A.; Kanaoka, M.; Isogai, A.; Tamura, S.; Murakoshi, S.; Ichinoe, M. Bassianolide, a new insecticidal cyclodepsipeptide from Beauveria bassiana and Verticillium lecanii. Tetrahedron Lett. 1977, 18, 2167–2170. [Google Scholar] [CrossRef]
- Logrieco, A.; Moretti, A.; Castella, G.; Kostecki, M.; Golinski, P.; Ritieni, A.; Chelkowski, J. Beauvericin production by Fusarium species. Appl. Environ. Microbiol. 1998, 64, 3084–3088. [Google Scholar] [CrossRef] [Green Version]
- Nagaoka, T.; Nakata, K.; Kouno, K. Antifungal activity of oosporein from an antagonistic fungus against Phytophthora infestans. Z. Naturforschung C 2004, 59, 302–304. [Google Scholar] [CrossRef] [Green Version]
- Wang, Q.; Xu, L. Beauvericin, a bioactive compound produced by fungi: A short review. Molecules 2012, 17, 2367–2377. [Google Scholar] [CrossRef]
- Gibson, D.M.; Donzelli, B.G.G.; Krasnoff, S.B.; Keyhani, N.O. Discovering the secondary metabolite potential encoded within entomopathogenic fungi. Nat. Prod. Rep. 2014, 31, 1287–1305. [Google Scholar] [CrossRef]
- El Kichaoui, A.; Elnabris, K.; Shafie, A.; Fayyad, N.; Arafa, M.; El Hindi, M. Development of Beauveria bassiana-Based Bio-Fungicide Against Fusarium Wilt Pathogens for Capsicum annuum, a Promising Approach Toward Vital Biocontrol Industry in Gaza Strip. IUG J. Nat. Stud. 2017, 25, 183–190. [Google Scholar]
- Vinale, F.; Sivasithamparam, K.; Ghisalberti, E.L.; Ruocco, M.; Woo, S.; Lorito, M. Trichoderma secondary metabolites that affect plant metabolism. Nat. Prod. Commun. 2012, 7, 1545–1550. [Google Scholar] [CrossRef] [Green Version]
- Vinale, F.; Manganiello, G.; Nigro, M.; Mazzei, P.; Piccolo, A.; Pascale, A.; Ruocco, M.; Marra, R.; Lombardi, N.; Lanzuise, S.; et al. A novel fungal metabolite with beneficial properties for agricultural applications. Molecules 2014, 19, 9760–9772. [Google Scholar] [CrossRef] [Green Version]
- Lombardi, N.; Salzano, A.M.; Troise, A.D.; Scaloni, A.; Vitaglione, P.; Vinale, F.; Marra, R.; Caira, S.; Lorito, M.; D’Errico, G.; et al. Effect of Trichoderma Bioactive Metabolite Treatments on the Production, Quality, and Protein Profile of Strawberry Fruits. J. Agric. Food Chem. 2020, 68, 7246–7258. [Google Scholar] [CrossRef]
- Marra, R.; Lombardi, N.; D’Errico, G.; Troisi, J.; Scala, G.; Vinale, F.; Woo, S.L.; Bonanomi, G.; Lorito, M. Application of Trichoderma Strains and Metabolites Enhances Soybean Productivity and Nutrient Content. J. Agric. Food Chem. 2019, 67, 1814–1822. [Google Scholar] [CrossRef]
- Lorito, M.; Woo, S.L.; Harman, G.E.; Monte, E. Translational Research on Trichoderma: From ’Omics to the Field. Annu. Rev. Phytopathol. 2010, 48, 395–417. [Google Scholar] [CrossRef] [Green Version]
- El-Deeb, H.M.; Lashin, S.M.; Arab, Y.A.S. Reaction of some tomato cultivars to tomato leaf curl virus and evaluation of the endophytic colonization with Beauveria bassiana on the disease incidence and its vector, Bemisia tabaci. Arch. Phytopathol. Plant Prot. 2012, 45, 1538–1545. [Google Scholar] [CrossRef]
- Di Lelio, I.; Coppola, M.; Comite, E.; Molisso, D.; Lorito, M.; Woo, S.L.; Pennacchio, F.; Rao, R.; Digilio, M.C. Temperature Differentially Influences the Capacity of Trichoderma Species to Induce Plant Defense Responses in Tomato Against Insect Pests. Front. Plant Sci. 2021, 12, 1–15. [Google Scholar] [CrossRef]
- Trizelia; Martinius; Reflinaldon; Liswarni, Y.; Putra, F.S. Colonization of Beauveria bassiana (Bals.) vuill on chili (Capsicum annum) and its effect on populations of Myzus persicae. J. Biopestic. 2020, 13, 40–46. [Google Scholar]
- Lopez, D.C.; Zhu-Salzman, K.; Ek-Ramos, M.J.; Sword, G.A. The entomopathogenic fungal endophytes Purpureocillium lilacinum (formerly Paecilomyces lilacinus) and Beauveria bassiana negatively affect cotton aphid reproduction under both greenhouse and field conditions. PLoS ONE 2014, 9, e103891. [Google Scholar] [CrossRef] [Green Version]
- Mahmood, Z.; Steenberg, T.; Mahmood, K.; Labouriau, R.; Kristensen, M. Endophytic Beauveria bassiana in maize affects survival and fecundity of the aphid Sitobion avenae. Biol. Control 2019, 137, 104017. [Google Scholar] [CrossRef]
- Klieber, J.; Reineke, A. The entomopathogen Beauveria bassiana has epiphytic and endophytic activity against the tomato leaf miner Tuta absoluta. J. Appl. Entomol. 2016, 140, 580–589. [Google Scholar] [CrossRef]
- Jaber, L.R.; Enkerli, J. Fungal entomopathogens as endophytes: Can they promote plant growth? Biocontrol Sci. Technol. 2017, 27, 28–41. [Google Scholar] [CrossRef]
- Jaber, L.R.; Alananbeh, K.M. Fungal entomopathogens as endophytes reduce several species of Fusarium causing crown and root rot in sweet pepper (Capsicum annuum L.). Biol. Control 2018, 126, 117–126. [Google Scholar] [CrossRef]
- Jaber, L.R.; Salem, N.M. Endophytic colonisation of squash by the fungal entomopathogen Beauveria bassiana (Ascomycota: Hypocreales) for managing Zucchini yellow mosaic virus in cucurbits. Biocontrol Sci. Technol. 2014, 24, 1096–1109. [Google Scholar] [CrossRef]
- Rondot, Y.; Reineke, A. Endophytic Beauveria bassiana activates expression of defence genes in grapevine and prevents infections by grapevine downy mildew Plasmopara viticola. Plant Pathol. 2019, 68, 1719–1731. [Google Scholar] [CrossRef] [Green Version]
- Rondot, Y.; Reineke, A. Endophytic Beauveria bassiana in grapevine Vitis vinifera (L.) reduces infestation with piercing-sucking insects. Biol. Control 2018, 116, 82–89. [Google Scholar] [CrossRef]
- Rondot, Y.; Reineke, A. Association of Beauveria bassiana with grapevine plants deters adult black vine weevils, Otiorhynchus sulcatus. Biocontrol Sci. Technol. 2017, 27, 811–820. [Google Scholar] [CrossRef]
- Razinger, J.; Lutz, M.; Schroers, H.J.; Palmisano, M.; Wohler, C.; Urek, G.; Grunder, J. Direct plantlet inoculation with soil or insect-associated fungi may control cabbage root fly maggots. J. Invertebr. Pathol. 2014, 120, 59–66. [Google Scholar] [CrossRef]
- Mantzoukas, S.; Lagogiannis, I. Endophytic colonization of pepper (Capsicum annum) controls aphids (Myzus persicae Sulzer). Appl. Sci. 2019, 9, 2239. [Google Scholar] [CrossRef] [Green Version]
- Jaber, L.R.; Araj, S.E. Interactions among endophytic fungal entomopathogens (Ascomycota: Hypocreales), the green peach aphid Myzus persicae Sulzer (Homoptera: Aphididae), and the aphid endoparasitoid Aphidius colemani Viereck (Hymenoptera: Braconidae). Biol. Control 2018, 116, 53–61. [Google Scholar] [CrossRef]
- Behie, S.W.; Zelisko, P.M.; Bidochka, M.J. Endophytic Insect-Parasitic Fungi Translocate Nitrogen Directly from Insects to Plants. Science 2012, 336, 1576–1578. [Google Scholar] [CrossRef] [Green Version]
- Woo, S.L.; Pepe, O. Microbial consortia: Promising probiotics as plant biostimulants for sustainable agriculture. Front. Plant Sci. 2018, 9, 7–12. [Google Scholar] [CrossRef]
- Humber, R.A. Identification of entomopathogenic fungi. In Manual of Techniques in Invertebrate Pathology, 2nd ed.; Lacey, L.A., Ed.; Academic Press: San Diego, CA, USA, 2012. [Google Scholar]
- Al-hindi, R.R.; Al-najada, A.R.; Mohamed, S.A. Isolation and identification of some fruit spoilage fungi: Screening of plant cell wall degrading enzymes. Afr. J. Microbiol. Res. 2011, 5, 443–448. [Google Scholar] [CrossRef]
- Ruocco, M.; Lanzuise, S.; Lombardi, N.; Woo, S.L.; Vinale, F.; Marra, R.; Varlese, R.; Manganiello, G.; Pascale, A.; Scala, V.; et al. Multiple Roles and Effects of a Novel Trichoderma Hydrophobin. Mol. Plant-Microbe Interact. 2015, 28, 167–179. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coppola, M.; Corrado, G.; Coppola, V.; Cascone, P.; Martinelli, R.; Digilio, M.C.; Pennacchio, F.; Rao, R. Prosystemin Overexpression in Tomato Enhances Resistance to Different Biotic Stresses by Activating Genes of Multiple Signaling Pathways. Plant Mol. Biol. Rep. 2015, 33, 1270–1285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petrini, O.; Fisher, P.J. Fungal endophytes in Salicornia perennis. Trans. Br. Mycol. Soc. 1986, 87, 647–651. [Google Scholar] [CrossRef]
- Sundaramoorthy, S.; Raguchander, T.; Ragupathi, N.; Samiyappan, R. Combinatorial effect of endophytic and plant growth promoting rhizobacteria against wilt disease of Capsicum annum L. caused by Fusarium solani. Biol. Control 2012, 60, 59–67. [Google Scholar] [CrossRef]
- Rueden, C.; Dietz, C.; Horn, M.; Schindelin, J.; Northan, B.; Berthold, M.; Eliceiri, K. ImageJ Ops [Software]. Available online: http://imagej.net/Ops (accessed on 25 May 2020).
- Coppola, M.; Diretto, G.; Digilio, M.C.; Lorito, M.; Rao, R. Transcriptome and Metabolome Reprogramming in Tomato Plants by Trichoderma harzianum strain T22 Primes and Enhances Defense Responses Against Aphids. Front. Physiol. 2019, 10, 745. [Google Scholar] [CrossRef]





| B. bassiana Isolate | Effect on the Plant Growth | Biocontrol of Tomato Pest | Biocontrol of Tomato Pathogens | |
|---|---|---|---|---|
| Macrosiphum euphorbiae | Alternaria alternata | Botrytis cinerea | ||
| Bb74040 | 0 | +++ | +++ | ++ |
| Bb632 | + | +++ | 0 | ++ |
| Bb688 | 0 | +++ | ++ | ++ |
| Bb716 | + | +++ | ++ | ++ |
| Bb762 | 0 | +++ | ++ | +++ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sinno, M.; Ranesi, M.; Di Lelio, I.; Iacomino, G.; Becchimanzi, A.; Barra, E.; Molisso, D.; Pennacchio, F.; Digilio, M.C.; Vitale, S.; et al. Selection of Endophytic Beauveria bassiana as a Dual Biocontrol Agent of Tomato Pathogens and Pests. Pathogens 2021, 10, 1242. https://doi.org/10.3390/pathogens10101242
Sinno M, Ranesi M, Di Lelio I, Iacomino G, Becchimanzi A, Barra E, Molisso D, Pennacchio F, Digilio MC, Vitale S, et al. Selection of Endophytic Beauveria bassiana as a Dual Biocontrol Agent of Tomato Pathogens and Pests. Pathogens. 2021; 10(10):1242. https://doi.org/10.3390/pathogens10101242
Chicago/Turabian StyleSinno, Martina, Marta Ranesi, Ilaria Di Lelio, Giuseppina Iacomino, Andrea Becchimanzi, Eleonora Barra, Donata Molisso, Francesco Pennacchio, Maria Cristina Digilio, Stefania Vitale, and et al. 2021. "Selection of Endophytic Beauveria bassiana as a Dual Biocontrol Agent of Tomato Pathogens and Pests" Pathogens 10, no. 10: 1242. https://doi.org/10.3390/pathogens10101242
APA StyleSinno, M., Ranesi, M., Di Lelio, I., Iacomino, G., Becchimanzi, A., Barra, E., Molisso, D., Pennacchio, F., Digilio, M. C., Vitale, S., Turrà, D., Harizanova, V., Lorito, M., & Woo, S. L. (2021). Selection of Endophytic Beauveria bassiana as a Dual Biocontrol Agent of Tomato Pathogens and Pests. Pathogens, 10(10), 1242. https://doi.org/10.3390/pathogens10101242








