Host–Pathogen Interactions of Chlamydia trachomatis in Porcine Oviduct Epithelial Cells
Abstract
:1. Introduction
2. Results
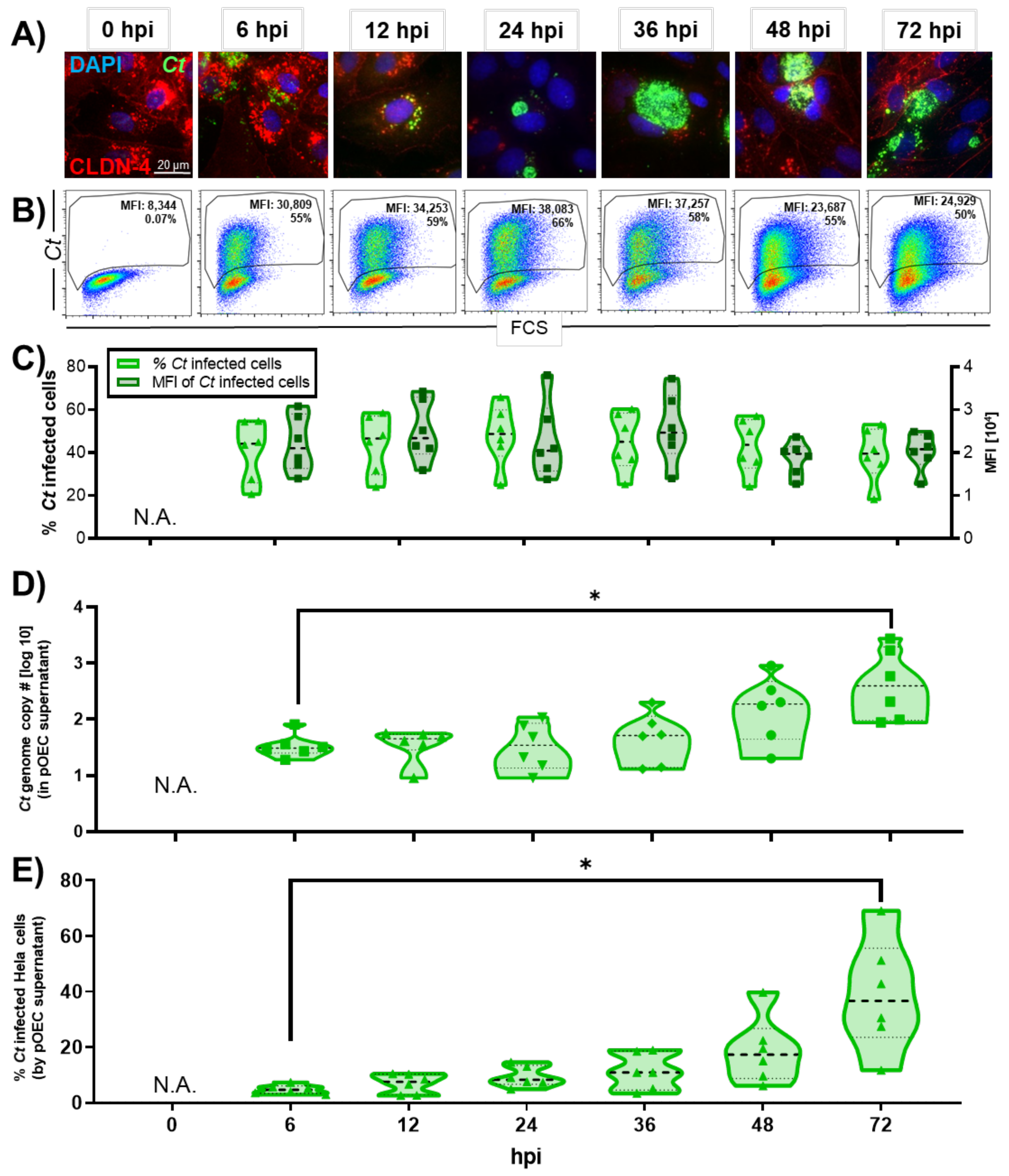
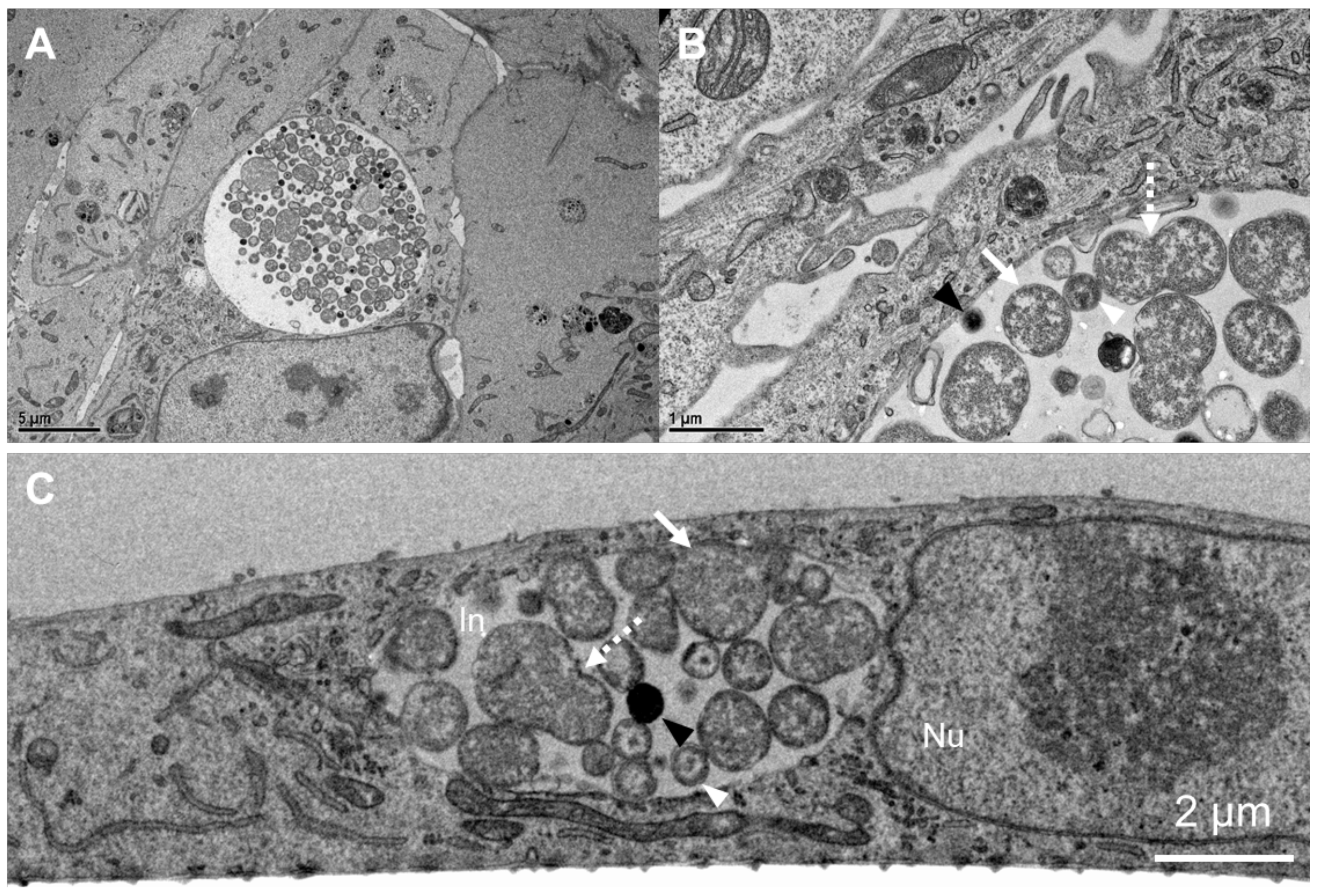
2.1. Chlamydia trachomatis Developmental Cycle in pOECs
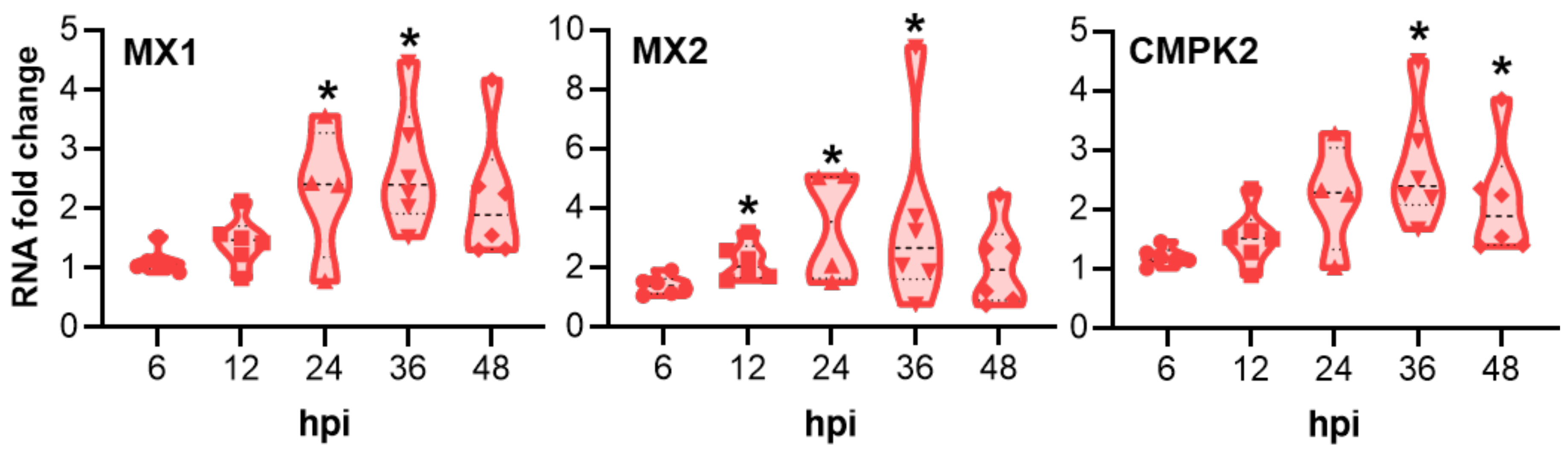
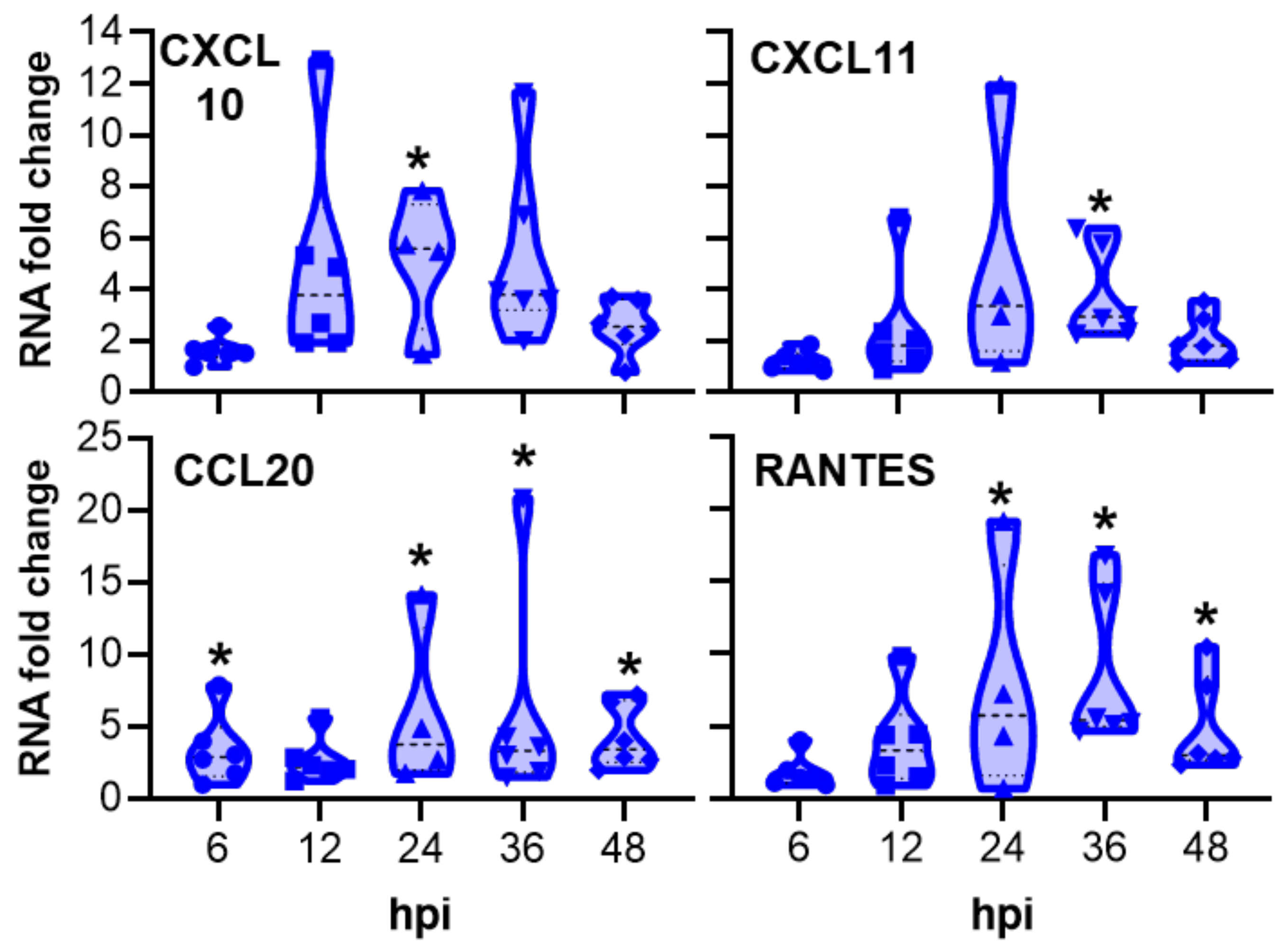
2.2. Innate Immune Response of Porcine Oviduct Epithelial Cells to Chlamydia trachomatis Infection
2.3. Effect of Chlamydia trachomatis Infection on the CLDN-4 mRNA and Protein Expression
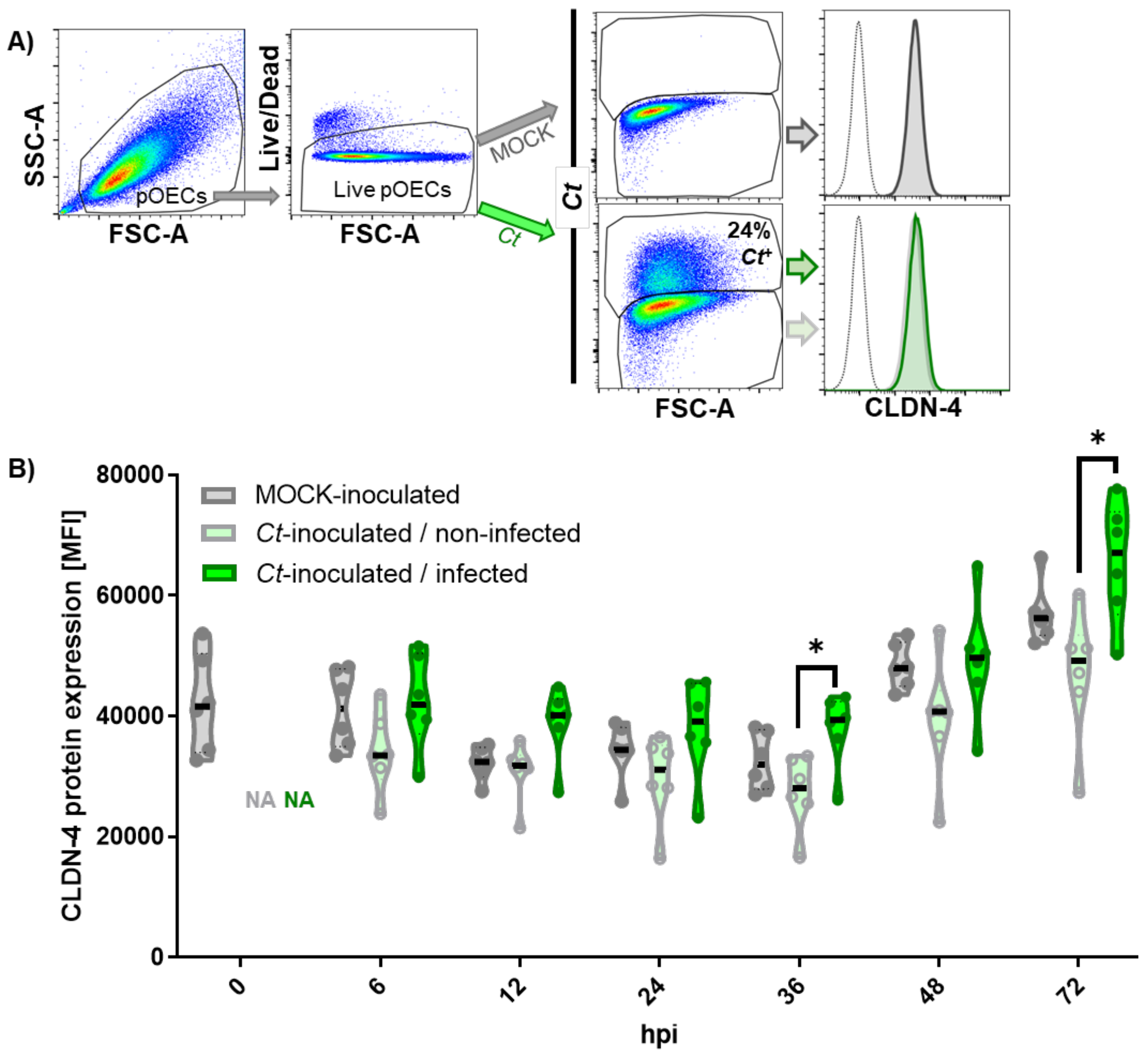
2.3.1. Effect of Chlamydia trachomatis Infection on the Overall CLDN-4 mRNA and Protein Expression
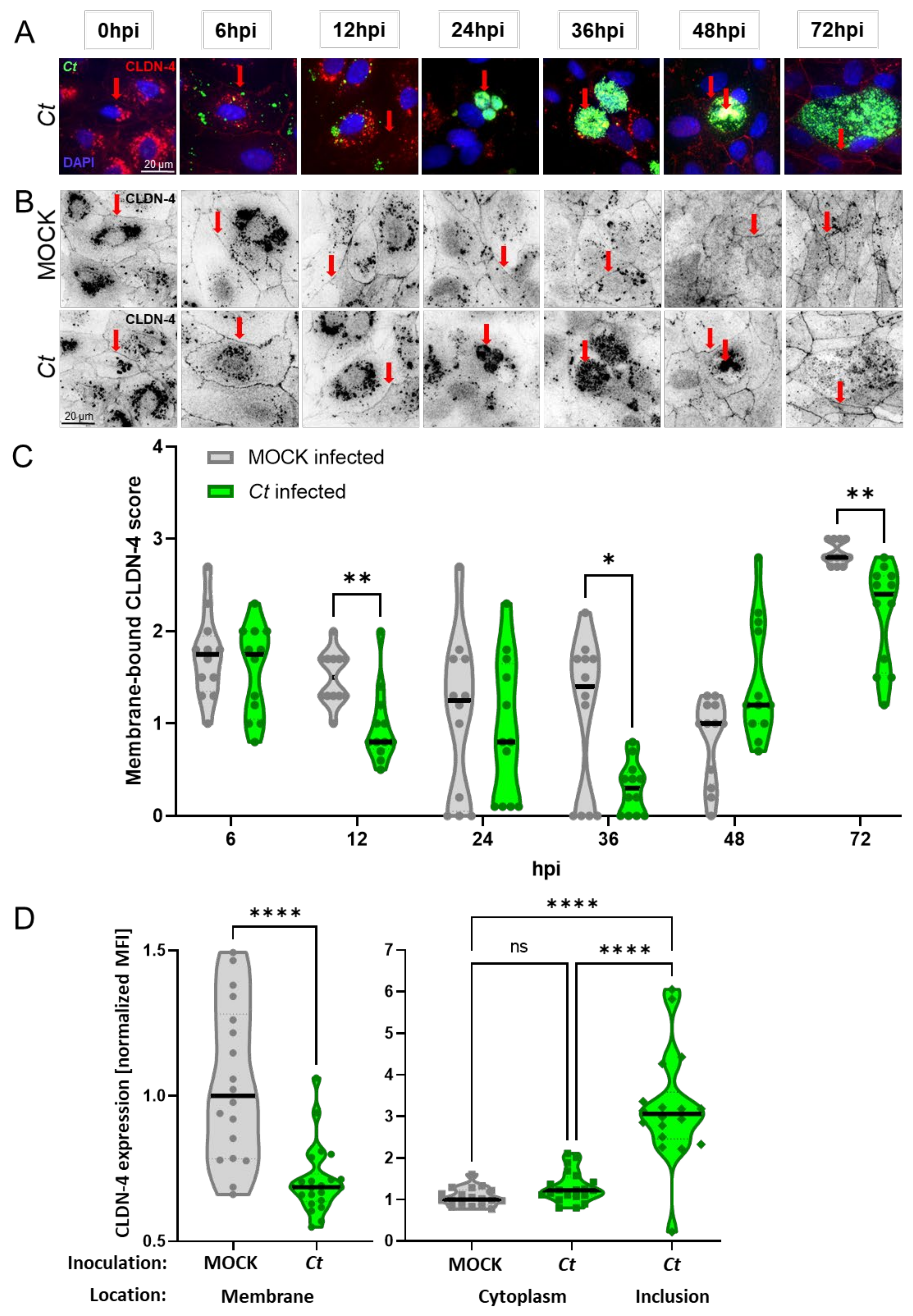
2.3.2. Chlamydia trachomatis Infection of Porcine Oviduct Epithelial Cells Diminishes the Expression of Claudin-4 on the Host Cell Membrane
3. Discussion
3.1. Chlamydia trachomatis Developmental Cycle in Porcine Oviduct Epithelial Cells
3.2. Innate Immune Response of Porcine Oviduct Epithelial Cells to Chlamydia trachomatis Infection
3.3. Chlamydia trachomatis Infection of Porcine Oviduct Epithelial Cells Affects Their Claudin-4 and Tight Junction Expression
4. Materials and Methods
4.1. Porcine Oviduct Epithelial Cells Isolation and Culture
4.2. Chlamydia trachomatis
4.3. Chlamydia trachomatis Infection of Porcine Oviduct Epithelial Cells
4.4. Fluorescence Confocal Microscopy
4.5. Flow Cytometry
4.6. Detection of Chlamydia via qPCR
4.7. NanoString
4.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Newman, L.M.; Rowley, J.T.; Hoorn, S.V.; Wijesooriya, N.S.; Unemo, M.; Low, N.; Stevens, G.; Gottlieb, S.L.; Kiarie, J.; Temmerman, M. Global Estimates of the Prevalence and Incidence of Four Curable Sexually Transmitted Infections in 2012 Based on Systematic Review and Global Reporting. PLoS ONE 2015, 10, e0143304. [Google Scholar] [CrossRef] [Green Version]
- Witkin, S.S.; Minis, E.; Athanasiou, A.; Leizer, J.; Linhares, I. Chlamydia trachomatis: The Persistent Pathogen. Clin. Vaccine Immunol. 2017, 24, e00203-17. [Google Scholar] [CrossRef] [Green Version]
- Darville, T.; Hiltke, T.J. Pathogenesis of Genital Tract Disease Due to Chlamydia trachomatis. J. Infect. Dis. 2010, 201, 114–125. [Google Scholar] [CrossRef] [Green Version]
- Brunham, R.C.; Rey-Ladino, J. Immunology of Chlamydia infection: Implications for a Chlamydia trachomatis vaccine. Nat. Rev. Immunol. 2005, 5, 149–161. [Google Scholar] [CrossRef]
- Lyons, J.M.; Ito, J.I., Jr.; Peña, A.S.; Morré, S.A. Differences in growth characteristics and elementary body associated cytotoxicity between Chlamydia trachomatis oculogenital serovars D and H and Chlamydia muridarum. J. Clin. Pathol. 2005, 58, 397–401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Phillips, D.M.; Burillo, C.A. Ultrastructure of the murine cervix following infection with Chlamydia trachomatis. Tissue Cell 1998, 30, 446–452. [Google Scholar] [CrossRef]
- Belland, R.J.; Zhong, G.; Crane, D.D.; Hogan, D.; Sturdevant, D.; Sharma, J.; Beatty, W.L.; Caldwell, H.D. Genomic transcriptional profiling of the developmental cycle of Chlamydia trachomatis. Proc. Natl. Acad. Sci. USA 2003, 100, 8478–8483. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cortina, M.E.; Ende, R.J.; Bishop, R.C.; Bayne, C.; Derré, I. Chlamydia trachomatis and Chlamydia muridarum spectinomycin resistant vectors and a transcriptional fluorescent reporter to monitor conversion from replicative to infectious bacteria. PLoS ONE 2019, 14, e0217753. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.K.; Enciso, G.A.; Boassa, D.; Chander, C.N.; Lou, T.H.; Pairawan, S.S.; Guo, M.C.; Wan, F.Y.M.; Ellisman, M.H.; Sütterlin, C.; et al. Replication-dependent size reduction precedes differentiation in Chlamydia trachomatis. Nat. Commun. 2018, 9, 45. [Google Scholar] [CrossRef]
- Mathews, S.A.; Volp, K.M.; Timms, P. Development of a quantitative gene expression assay for Chlamydia trachomatis identifed temporal expression of c factors. FEBS Lett. 1999, 458, 354–358. [Google Scholar] [CrossRef] [Green Version]
- Shaw, E.I.; Dooley, C.A.; Fischer, E.R.; Scidmore, M.A.; Fields, K.A.; Hackstadt, T. Three temporal classes of gene expression during the Chlamydia trachomatis developmental cycle. Mol. Microbiol. 2000, 37, 913–925. [Google Scholar] [CrossRef] [PubMed]
- Abdelrahman, Y.M.; Belland, R.J. The chlamydial developmental cycle. FEMS Microbiol. Rev. 2005, 29, 949–959. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bastidas, R.J.; Elwell, C.A.; Engel, J.N.; Valdivia, R.H. Chlamydial Intracellular Survival Strategies. Cold Spring Harb. Perspect. Med. 2013, 3, a010256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elwell, C.; Mirrashidi, K.; Engel, J. Chlamydia cell biology and pathogenesis. Nat. Rev. Genet. 2016, 14, 385–400. [Google Scholar] [CrossRef]
- McQueen, B.E.; Kiatthanapaiboon, A.; Fulcher, M.L.; Lam, M.; Patton, K.; Powell, E.; Kollipara, A.; Madden, V.; Suchland, R.J.; Wyrick, P.; et al. Human Fallopian Tube Epithelial Cell Culture Model to Study Host Responses to Chlamydia trachomatis Infection. Infect. Immun. 2020, 88, 1–16. [Google Scholar] [CrossRef]
- Rank, R.G.; Lacy, H.M.; Goodwin, A.; Sikes, J.; Whittimore, J.; Wyrick, P.B.; Nagarajan, U.M. Host Chemokine and Cytokine Response in the Endocervix within the First Developmental Cycle of Chlamydia muridarum. Infect. Immun. 2010, 78, 536–544. [Google Scholar] [CrossRef] [Green Version]
- Perry, L.L.; Feilzer, K.; Caldwell, H.D. Immunity to Chlamydia trachomatis is mediated by T helper 1 cells through IFN-gamma-dependent and -independent pathways. J. Immunol. 1997, 158, 3344–3352. [Google Scholar]
- Perry, L.L.; Su, H.; Feilzer, K.; Messer, R.; Hughes, S.; Whitmire, W.; Caldwell, H.D. Differential sensitivity of distinct Chlamydia trachomatis isolates to IFN-gamma-mediated inhibition. J. Immunol. 1999, 162, 3541–3548. [Google Scholar]
- Gondek, D.C.; Olive, A.; Stary, G.; Starnbach, M.N. CD4+ T Cells Are Necessary and Sufficient to Confer Protection against Chlamydia trachomatis Infection in the Murine Upper Genital Tract. J. Immunol. 2012, 189, 2441–2449. [Google Scholar] [CrossRef] [Green Version]
- Gondek, D.C.; Roan, N.R.; Starnbach, M.N. T Cell Responses in the Absence of IFN-γ Exacerbate Uterine Infection with Chlamydia trachomatis. J. Immunol. 2009, 183, 1313–1319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Helble, J.D.; Gonzalez, R.J.; von Andrian, U.H.; Starnbach, M.N. Gamma Interferon Is Required for Chlamydia Clearance but Is Dispensable for T Cell Homing to the Genital Tract. mBio 2020, 11, e00191-20. [Google Scholar] [CrossRef] [Green Version]
- Ramsey, K.H.; Miranpuri, G.S.; Poulsen, C.E.; Marthakis, N.B.; Braune, L.M.; Byrne, G.I. Inducible Nitric Oxide Synthase Does Not Affect Resolution of Murine Chlamydial Genital Tract Infections or Eradication of Chlamydiae in Primary Murine Cell Culture. Infect. Immun. 1998, 66, 835–838. [Google Scholar] [CrossRef] [Green Version]
- Beatty, W.L.; Belanger, T.A.; Desai, A.A.; Morrison, R.P.; Byrne, G.I. Tryptophan depletion as a mechanism of gamma interferon-mediated chlamydial persistence. Infect. Immun. 1994, 62, 3705–3711. [Google Scholar] [CrossRef] [Green Version]
- Beatty, W.L.; Morrison, R.P.; Byrne, G.I. Persistent Chlamydiae: From cell culture to a paradigm for chlamydial pathogenesis. Microbiol. Rev. 1994, 58, 686–699. [Google Scholar] [CrossRef] [PubMed]
- Beatty, W.L.; Morrison, R.P.; Byrne, G.I. Reactivation of persistent Chlamydia trachomatis infection in cell culture. Infect. Immun. 1995, 63, 199–205. [Google Scholar] [CrossRef] [Green Version]
- Clark, J.M. The 3Rs in research: A contemporary approach to replacement, reduction and refinement. Br. J. Nutr. 2018, 120, S1–S7. [Google Scholar] [CrossRef] [PubMed]
- Meurens, F.; Summerfield, A.; Nauwynck, H.; Saif, L.; Gerdts, V. The pig: A model for human infectious diseases. Trends Microbiol. 2012, 20, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Lossi, L.; D’Angelo, L.; De Girolamo, P.; Merighi, A. Anatomical features for an adequate choice of experimental animal model in biomedicine: II. Small laboratory rodents, rabbit, and pig. Ann. Anat. 2016, 204, 11–28. [Google Scholar] [CrossRef]
- Lorenzen, E.; Follmann, F.; Jungersen, G.; Agerholm, J.S. A review of the human vs. porcine female genital tract and associated immune system in the perspective of using minipigs as a model of human genital Chlamydia infection. Vet. Res. 2015, 46, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Amaral, A.F.; Rahman, K.S.; Kick, A.R.; Cortes, L.M.; Robertson, J.; Kaltenboeck, B.; Gerdts, V.; O’Connell, C.M.; Poston, T.B.; Zheng, X.; et al. Mucosal Vaccination with UV-Inactivated Chlamydia suis in Pre-Exposed Outbred Pigs Decreases Pathogen Load and Induces CD4 T-Cell Maturation into IFN-γ+ Effector Memory Cells. Vaccines 2020, 8, 353. [Google Scholar] [CrossRef] [PubMed]
- Erneholm, K.; Lorenzen, E.; Bøje, S.; Olsen, A.W.; Jungersen, G.; Jensen, H.E.; Cassidy, J.P.; Andersen, P.; Agerholm, J.S.; Follmann, F. Genital Infiltrations of CD4(+) and CD8(+) T Lymphocytes, IgA(+) and IgG(+) Plasma Cells and Intra-Mucosal Lymphoid Follicles Associate with Protection Against Genital Chlamydia trachomatis Infection in Minipigs Intramuscularly Immunized with UV-Inactivated Bacteria Adjuvanted with CAF01. Front. Microbiol. 2019, 10, 197. [Google Scholar] [CrossRef]
- Käser, T.; Pasternak, J.; Delgado-Ortega, M.; Hamonic, G.; Lai, K.; Erickson, J.; Walker, S.; Dillon, J.; Gerdts, V.; Meurens, F. Chlamydia suis and Chlamydia trachomatis induce multifunctional CD4 T cells in pigs. Vaccine 2017, 35, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Käser, T.; Renois, F.; Wilson, H.L.; Cnudde, T.; Gerdts, V.; Dillon, J.-A.R.; Jungersen, G.; Agerholm, J.S.; Meurens, F. Contribution of the swine model in the study of human sexually transmitted infections. Infect. Genet. Evol. 2018, 66, 346–360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Longbottom, D.; Coulter, L. Animal Chlamydioses and Zoonotic Implications. J. Comp. Pathol. 2003, 128, 217–244. [Google Scholar] [CrossRef] [PubMed]
- Lorenzen, E.; Follmann, F.; Bøje, S.; Erneholm, K.; Olsen, A.W.; Agerholm, J.S.; Jungersen, G.; Andersen, P. Intramuscular Priming and Intranasal Boosting Induce Strong Genital Immunity Through Secretory IgA in Minipigs Infected with Chlamydia trachomatis. Front. Immunol. 2015, 6, 628. [Google Scholar] [CrossRef]
- Schautteet, K.; Stuyven, E.; Cox, E.; Vanrompay, D. Validation of the Chlamydia trachomatis genital challenge pig model for testing recombinant protein vaccines. J. Med. Microbiol. 2011, 60, 117–127. [Google Scholar] [CrossRef] [Green Version]
- Bøje, S.; Olsen, A.W.; Erneholm, K.; Agerholm, J.S.; Jungersen, G.; Andersen, P.; Follmann, F. A multi-subunit Chlamydia vaccine inducing neutralizing antibodies and strong IFN-γ + CMI responses protects against a genital infection in minipigs. Immunol. Cell Biol. 2015, 94, 185–195. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, N.D.N.T.; Olsen, A.W.; Lorenzen, E.; Andersen, P.; Hvid, M.; Follmann, F.; Dietrich, J. Parenteral vaccination protects against transcervical infection with Chlamydia trachomatis and generate tissue-resident T cells post-challenge. NPJ Vaccines 2020, 5, 7. [Google Scholar] [CrossRef] [Green Version]
- Schautteet, K.; De Clercq, E.; Jönsson, Y.; Lagae, S.; Chiers, K.; Cox, E.; Vanrompay, D. Protection of pigs against genital Chlamydia trachomatis challenge by parenteral or mucosal DNA immunization. Vaccine 2012, 30, 2869–2881. [Google Scholar] [CrossRef] [Green Version]
- Schautteet, K.; Stuyven, E.; Beeckman, D.; Van Acker, S.; Carlon, M.; Chiers, K.; Cox, E.; Vanrompay, D. Protection of pigs against Chlamydia trachomatis challenge by administration of a MOMP-based DNA vaccine in the vaginal mucosa. Vaccine 2011, 29, 1399–1407. [Google Scholar] [CrossRef]
- Käser, T.; Cnudde, T.; Hamonic, G.; Rieder, M.; Pasternak, J.A.; Lai, K.; Tikoo, S.K.; Wilson, H.L.; Meurens, F. Porcine retinal cell line VIDO R1 and Chlamydia suis to modelize ocular chlamydiosis. Vet. Immunol. Immunopathol. 2015, 166, 95–107. [Google Scholar] [CrossRef]
- Guseva, N.V.; Knight, S.T.; Whittimore, J.D.; Wyrick, P.B. Primary Cultures of Female Swine Genital Epithelial Cells In Vitro: A New Approach for the Study of Hormonal Modulation of Chlamydia Infection. Infect. Immun. 2003, 71, 4700–4710. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, X.; Shang, X.; Manorek, G.; Howell, S.B. Regulation of the Epithelial-Mesenchymal Transition by Claudin-3 and Claudin-4. PLoS ONE 2013, 8, e67496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Watari, A.; Kodaka, M.; Matsuhisa, K.; Sakamoto, Y.; Hisaie, K.; Kawashita, N.; Takagi, T.; Yamagishi, Y.; Suzuki, H.; Tsujino, H.; et al. Identification of claudin-4 binder that attenuates tight junction barrier function by TR-FRET-based screening assay. Sci. Rep. 2017, 7, 14514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Günzel, D.; Yu, A.S.L. Claudins and the Modulation of Tight Junction Permeability. Physiol. Rev. 2013, 93, 525–569. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fredriksson, K.; Van Itallie, C.M.; Aponte, A.; Gucek, M.; Tietgens, A.J.; Anderson, J.M. Proteomic Analysis of Proteins Surrounding Occludin and Claudin-4 Reveals Their Proximity to Signaling and Trafficking Networks. PLoS ONE 2015, 10, e0117074. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Gong, H.; Liu, L.; Ramos-Solis, N.; Seye, C.I.; Derbigny, W.A. TLR3 deficiency exacerbates the loss of epithelial barrier function during genital tract Chlamydia muridarum infection. PLoS ONE 2019, 14, e0207422. [Google Scholar] [CrossRef] [Green Version]
- Mukura, L.R.; Hickey, D.K.; Rodriguez-Garcia, M.; Fahey, J.V.; Wira, C.R. Chlamydia trachomatis regulates innate immune barrier integrity and mediates cytokine and antimicrobial responses in human uterine ECC-1 epithelial cells. Am. J. Reprod. Immunol. 2017, 78, e12764. [Google Scholar] [CrossRef]
- Galvin, S.R.; Cohen, M.S. The role of sexually transmitted diseases in HIV transmission. Nat. Rev. Genet. 2004, 2, 33–42. [Google Scholar] [CrossRef]
- Ghys, P.D.; Fransen, K.; Diallo, M.O.; Ettiègne-Traoré, V.; Coulibaly, I.; Yeboué, K.M.; Kalish, M.L.; Maurice, C.; Whitaker, J.P.; Greenberg, A.E.; et al. The associations between cervicovaginal HIV shedding, sexually transmitted diseases and immunosuppression in female sex workers in Abidjan, Côte d’Ivoire. AIDS 1997, 11, F85–F93. [Google Scholar] [CrossRef]
- Johnson, L.F.; Lewis, D.A. The Effect of Genital Tract Infections on HIV-1 Shedding in the Genital Tract: A Systematic Review and Meta-Analysis. Sex. Transm. Dis. 2008, 35, 946–959. [Google Scholar] [CrossRef] [PubMed]
- Plummer, F.A.; Simonsen, J.N.; Cameron, D.W.; Ndinya-Achola, J.O.; Kreiss, J.K.; Gakinya, M.N.; Waiyaki, P.; Cheang, M.; Piot, P.; Ronald, A.; et al. Cofactors in Male-Female Sexual Transmission of Human Immunodeficiency Virus Type 1. J. Infect. Dis. 1991, 163, 233–239. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, X.-M.; Huang, Y.; Zhang, K.; Qu, L.-H.; Cong, X.; Su, J.-Z.; Wu, L.-L.; Yu, G.-Y.; Zhang, Y. Expression patterns of tight junction proteins in porcine major salivary glands: A comparison study with human and murine glands. J. Anat. 2018, 233, 167–176. [Google Scholar] [CrossRef] [PubMed]
- Abdelrahman, Y.; Ouellette, S.P.; Belland, R.J.; Cox, J.V. Correction: Polarized Cell Division of Chlamydia trachomatis. PLoS Pathog. 2016, 12, e1005822, Erratum in 2016, 12, e1005866. [Google Scholar] [CrossRef]
- Cox, J.V.; Abdelrahman, Y.M.; Ouellette, S.P. Penicillin-binding proteins regulate multiple steps in the polarized cell division process of Chlamydia. Sci. Rep. 2020, 10, 12588. [Google Scholar] [CrossRef] [PubMed]
- Liechti, G.; Kuru, E.; Packiam, M.; Hsu, Y.-P.; Tekkam, S.; Hall, E.; Rittichier, J.T.; VanNieuwenhze, M.; Brun, Y.V.; Maurelli, A.T. Pathogenic Chlamydia Lack a Classical Sacculus but Synthesize a Narrow, Mid-cell Peptidoglycan Ring, Regulated by MreB, for Cell Division. PLoS Pathog. 2016, 12, e1005590. [Google Scholar] [CrossRef] [Green Version]
- Johnson, R.M.; Kerr, M.S. Modeling the transcriptome of genital tract epithelial cells and macrophages in healthy mucosa versus mucosa inflamed by Chlamydia muridarum infection. FEMS Pathog. Dis. 2015, 73, 100. [Google Scholar] [CrossRef] [Green Version]
- Verhelst, J.; Hulpiau, P.; Saelens, X. Mx Proteins: Antiviral Gatekeepers That Restrain the Uninvited. Microbiol. Mol. Biol. Rev. 2013, 77, 551–566. [Google Scholar] [CrossRef] [Green Version]
- Natividad, A.; Freeman, T.; Jeffries, D.; Burton, M.J.; Mabey, D.C.W.; Bailey, R.L.; Holland, M.J. Human Conjunctival Transcriptome Analysis Reveals the Prominence of Innate Defense in Chlamydia trachomatis Infection. Infect. Immun. 2010, 78, 4895–4911. [Google Scholar] [CrossRef] [Green Version]
- Kumar, R.; Derbigny, W.A. TLR3 Deficiency Leads to a Dysregulation in the Global Gene-Expression Profile in Murine Oviduct Epithelial Cells Infected with Chlamydia muridarum. Int. J. Microbiol. Curr. Res. 2018, 1, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Xu, Y.; Johansson, M.; Karlsson, A. Human UMP-CMP Kinase 2, a Novel Nucleoside Monophosphate Kinase Localized in Mitochondria. J. Biol. Chem. 2008, 283, 1563–1571. [Google Scholar] [CrossRef] [Green Version]
- Lai, J.-H.; Wu, D.-W.; Wu, C.-H.; Hung, L.-F.; Huang, C.-Y.; Ka, S.-M.; Chen, A.; Chang, Z.-F.; Ho, L.-J. Mitochondrial CMPK2 mediates immunomodulatory and antiviral activities through IFN-dependent and IFN-independent pathways. iScience 2021, 24, 102498. [Google Scholar] [CrossRef]
- Groom, J.; Luster, A.D. CXCR3 ligands: Redundant, collaborative and antagonistic functions. Immunol. Cell Biol. 2011, 89, 207–215. [Google Scholar] [CrossRef] [Green Version]
- Lee, A.Y.S.; Eri, R.; Lyons, A.B.; Grimm, M.C.; Korner, H. CC Chemokine Ligand 20 and Its Cognate Receptor CCR6 in Mucosal T Cell Immunology and Inflammatory Bowel Disease: Odd Couple or Axis of Evil? Front. Immunol. 2013, 4, 194. [Google Scholar] [CrossRef] [Green Version]
- Sakthivel, S.K.; Singh, U.P.; Singh, S.; Taub, D.D.; Igietseme, J.U.; Lillard, J.W. CCL5 regulation of mucosal chlamydial immunity and infection. BMC Microbiol. 2008, 8, 136. [Google Scholar] [CrossRef] [Green Version]
- Poston, T.B.; Lee, D.E.; Darville, T.; Zhong, W.; Dong, L.; O’Connell, C.; Wiesenfeld, H.C.; Hillier, S.L.; Sempowski, G.D.; Zheng, X. Cervical Cytokines Associated with Chlamydia trachomatis Susceptibility and Protection. J. Infect. Dis. 2019, 220, 330–339. [Google Scholar] [CrossRef] [PubMed]
- Olive, A.; Gondek, D.C.; Starnbach, M.N. CXCR3 and CCR5 are both required for T cell-mediated protection against C. trachomatis infection in the murine genital mucosa. Mucosal Immunol. 2010, 4, 208–216. [Google Scholar] [CrossRef]
- Bakshi, R.K.; Gupta, K.; Jordan, S.; Chi, X.; Lensing, S.Y.; Press, C.G.; Geisler, W.M. An Adaptive Chlamydia trachomatis-Specific IFN-γ-Producing CD4(+) T Cell Response Is Associated with Protection Against Chlamydia Reinfection in Women. Front. Immunol. 2018, 9, 1981. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morrison, R.P.; Feilzer, K.; Tumas, D.B. Gene knockout mice establish a primary protective role for major histocompatibility complex class II-restricted responses in Chlamydia trachomatis genital tract infection. Infect. Immun. 1995, 63, 4661–4668. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prozialeck, W.C.; Fay, M.J.; Lamar, P.C.; Pearson, C.A.; Sigar, I.; Ramsey, K.H. Chlamydia trachomatis Disrupts N-Cadherin-Dependent Cell-Cell Junctions and Sequesters β-Catenin in Human Cervical Epithelial Cells. Infect. Immun. 2002, 70, 2605–2613. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dolat, L.; Valdivia, R.H. A renewed tool kit to explore Chlamydia pathogenesis: From molecular genetics to new infection models. F1000Research 2019, 8, 935. [Google Scholar] [CrossRef] [PubMed]
- Nunes, A.; Gomes, J.P.; Mead, S.; Florindo, C.; Correia, H.; Borrego, M.J.; Dean, D. Comparative Expression Profiling of the Chlamydia trachomatis pmp Gene Family for Clinical and Reference Strains. PLoS ONE 2007, 2, e878. [Google Scholar] [CrossRef]
- Unemo, M.; Seth-Smith, H.M.B.; Cutcliffe, L.T.; Skilton, R.J.; Barlow, D.; Goulding, D.; Persson, K.; Harris, S.; Kelly, A.; Bjartling, C.; et al. The Swedish new variant of Chlamydia trachomatis: Genome sequence, morphology, cell tropism and phenotypic characterization. Microbiology 2010, 156, 1394–1404. [Google Scholar] [CrossRef] [Green Version]
- Scidmore, M.A. Cultivation and Laboratory Maintenance of Chlamydia trachomatis. Curr. Protoc. Microbiol. 2005, 1, 11A-1. [Google Scholar] [CrossRef]
- Käser, T.; Pasternak, J.A.; Hamonic, G.; Rieder, M.; Lai, K.; Delgado-Ortega, M.; Gerdts, V.; Meurens, F. Flow cytometry as an improved method for the titration of Chlamydia ceae and other intracellular bacteria. Cytom. Part A 2016, 89, 451–460. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]

| Gene | 6 hpi | 12 hpi | 24 hpi | 36 hpi | 48 hpi | Average |
|---|---|---|---|---|---|---|
| ADAMTS9 | 1.83 | 1.42 | 1.73 | 1.12 | −1.37 | 0.95 |
| CCL20 | 2.8 | 2.43 | 5.16 | 3.83 | 3.82 | 3.61 |
| CCL4 | 5.49 | 3.07 | 2.91 | 1.86 | 2.53 | 3.17 |
| CMPK2 | 1.2 | 1.47 | 2.32 | 2.59 | 1.98 | 1.91 |
| CSF3 | 1.6 | 1.93 | 1.64 | 1.37 | 1.26 | 1.56 |
| CXCL10 | 1.59 | 3.86 | 7.95 | 4.5 | 2.3 | 4.04 |
| CXCL11 | 1.18 | 1.97 | 4.81 | 3.45 | 1.9 | 2.66 |
| CXCL8 | 1.18 | −1.01 | 1.12 | 1.5 | 1.99 | 0.96 |
| CXCL9 | −1.15 | −1.28 | 1.11 | −1.2 | 1.02 | −0.3 |
| EBI3 | −1.08 | −1.23 | 1.36 | 1.12 | 1.14 | 0.26 |
| Flt-3L | −1.01 | 1.14 | 1.04 | 1.14 | 1.28 | 0.72 |
| HERC5 | 1.09 | 1.35 | 1.89 | 1.93 | 1.7 | 1.59 |
| ICAM1 | 1.28 | 1.23 | 1.3 | 1.36 | 1.2 | 1.27 |
| IL-1 alpha | 1.36 | 1.21 | 1.24 | 1.18 | 1.44 | 1.29 |
| IL-10 | −1.23 | 1.03 | −1.41 | −1.29 | −1.39 | −0.86 |
| IL-15 | 1.06 | 1.52 | 1.6 | 1.39 | 1.11 | 1.34 |
| IL-16 | −1.03 | 1.22 | −1.02 | 1.14 | 1.05 | 0.27 |
| IL-1RA | 1.02 | 1.39 | 1.49 | 1.03 | 1.29 | 1.24 |
| IL-6 | 1.28 | 1.26 | 1.7 | 1.58 | 1.49 | 1.46 |
| IL-7 | 1.17 | 1.21 | 1.85 | 1.84 | 1.37 | 1.49 |
| MMP-2 | 1.06 | −1.11 | 1.3 | 1.18 | −1.03 | 0.28 |
| MMP-9 | 1.23 | 1.09 | −1.11 | 1.09 | 1.43 | 0.75 |
| MX1 | 1.11 | 1.39 | 2.31 | 2.53 | 1.98 | 1.86 |
| MX2 | 1.38 | 2.12 | 3.49 | 2.66 | 1.74 | 2.28 |
| NEURL3 | −1.06 | −1.19 | −1.08 | −1.36 | 1.12 | −0.71 |
| OAS2 | 1.11 | 1.3 | 1.92 | 2.4 | 1.84 | 1.71 |
| RANTES | 1.6 | 2.88 | 5.42 | 7.42 | 4.08 | 4.28 |
| SAA2 | −2.55 | 1.08 | −1.2 | −1.39 | 1.02 | −0.61 |
| TGF-b | 1.05 | −1.08 | 1.09 | −1.03 | −1.14 | −0.22 |
| TNF-alpha | −1.13 | −1.16 | 1.73 | −1.23 | −1.42 | −0.64 |
| VEGF | 1.08 | 1.06 | 1.21 | 1 | −1.04 | 0.66 |
| Average | 0.8 | 0.95 | 1.75 | 1.43 | 1.15 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amaral, A.F.; McQueen, B.E.; Bellingham-Johnstun, K.; Poston, T.B.; Darville, T.; Nagarajan, U.M.; Laplante, C.; Käser, T. Host–Pathogen Interactions of Chlamydia trachomatis in Porcine Oviduct Epithelial Cells. Pathogens 2021, 10, 1270. https://doi.org/10.3390/pathogens10101270
Amaral AF, McQueen BE, Bellingham-Johnstun K, Poston TB, Darville T, Nagarajan UM, Laplante C, Käser T. Host–Pathogen Interactions of Chlamydia trachomatis in Porcine Oviduct Epithelial Cells. Pathogens. 2021; 10(10):1270. https://doi.org/10.3390/pathogens10101270
Chicago/Turabian StyleAmaral, Amanda F., Bryan E. McQueen, Kimberly Bellingham-Johnstun, Taylor B. Poston, Toni Darville, Uma M. Nagarajan, Caroline Laplante, and Tobias Käser. 2021. "Host–Pathogen Interactions of Chlamydia trachomatis in Porcine Oviduct Epithelial Cells" Pathogens 10, no. 10: 1270. https://doi.org/10.3390/pathogens10101270
APA StyleAmaral, A. F., McQueen, B. E., Bellingham-Johnstun, K., Poston, T. B., Darville, T., Nagarajan, U. M., Laplante, C., & Käser, T. (2021). Host–Pathogen Interactions of Chlamydia trachomatis in Porcine Oviduct Epithelial Cells. Pathogens, 10(10), 1270. https://doi.org/10.3390/pathogens10101270







