Simultaneous Infection of Elaphostrongylus Nematode Species and Parasite Sharing between Sympatrically Occurring Cervids: Moose, Roe Deer, and Red Deer in Poland
Abstract
:1. Introduction
2. Results
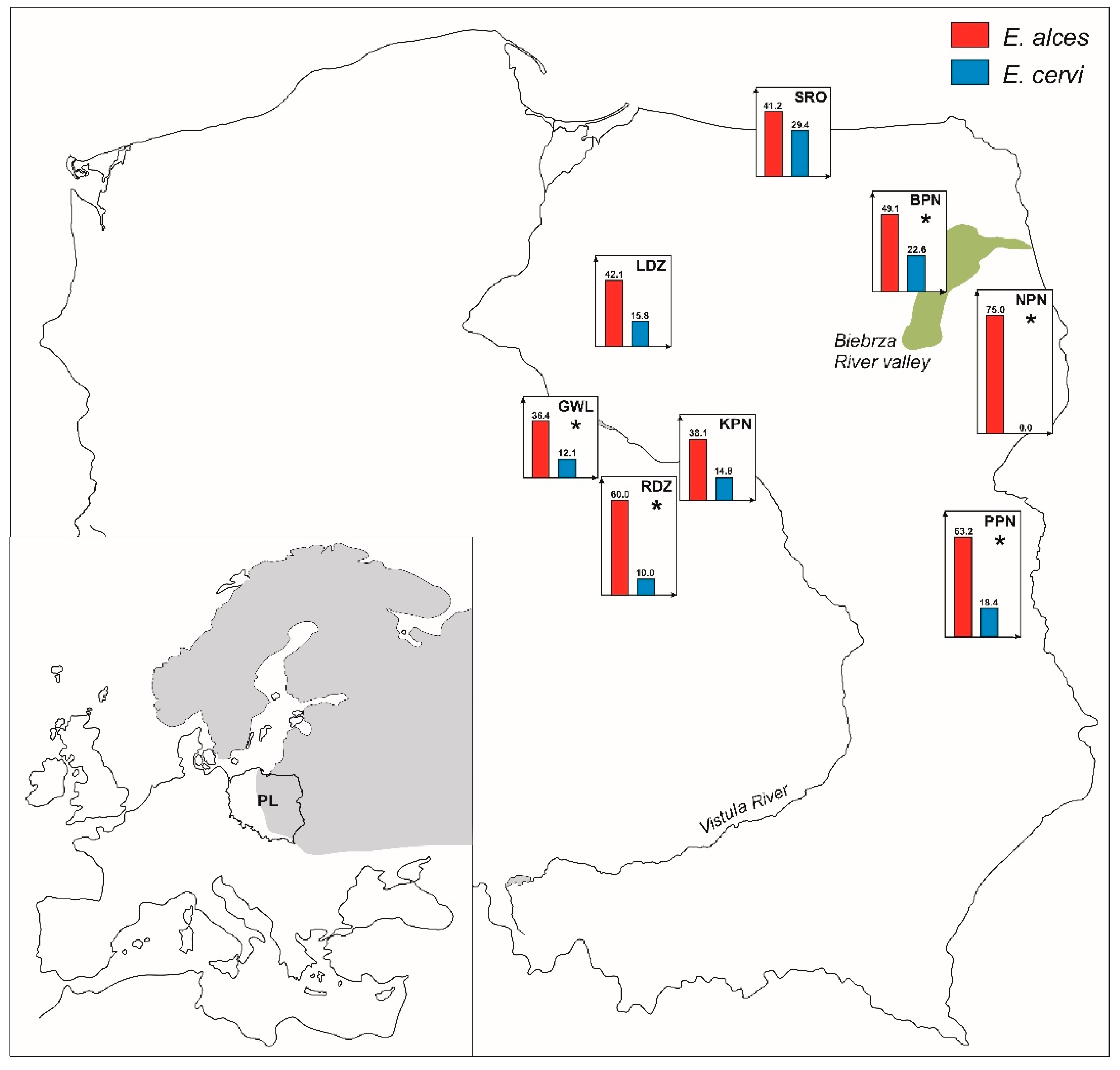
2.1. Occurrence of Elaphostrongylus Species in Moose Populations
2.2. Simultaneous Infection with Elaphostrongylus Species in Sympatrically Occurring Cervids
3. Discussion
4. Materials and Methods
4.1. Data Collection and Genetic Analyses
4.2. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Winter, J.; Rehbein, S.; Joachim, A. Transmission of helminths between species of ruminants in Austria ap-pears more likely to occur than generally assumed. Front. Vet. Sci. 2018, 5, 30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Linnell, J.D.C.; Cretois, B.; Nilsen, E.B.; Rolandsen, C.M.; Solberg, E.J.; Veiberg, V.; Kaczensky, P.; Moorter, B.V.; Panzacchi, M.; Rauset, G.R.; et al. The challenges and opportunities of coexisting with wild ungulates in the human-dominated landscapes of Europe’s Anthropocene. Biol. Conserv. 2020, 244, 108500. [Google Scholar] [CrossRef]
- Horcajada-Sánchez, F.; Navarro-Castilla, Á.; Boadella, M.; Barja, I. Influence of livestock, habitat type, and density of roe deer (Capreolus capreolus) on parasitic larvae abundance and infection seroprevalence in wild populations of roe deer from central Iberian Peninsula. Mammal Res. 2018, 63, 213–222. [Google Scholar] [CrossRef]
- Kołodziej-Sobocińska, M. Factors affecting the spread of parasites in populations of wild European terrestrial mammals. Mammal Res. 2019, 64, 301–318. [Google Scholar] [CrossRef] [Green Version]
- Bordes, F.; Morand, S. The impact of multiple infections on wild animal hosts: A review. Infect. Ecol. Epidemiol. 2011, 1, 7346. [Google Scholar] [CrossRef] [Green Version]
- Walker, J.G.; Morgan, E.R. Generalists at the interface: Nematode transmission between wild and domestic ungulates. Int. J. Parasitol. Parasites Wildl. 2014, 3, 242–250. [Google Scholar] [CrossRef] [Green Version]
- Pugh, D.G.; Baird, A.N.; Edmondson, M.A.; Passler, T. Sheep, Goat, and Cervid Medicine, 3rd ed.; Elsevier Health Science: Philadelphia, PA, USA, 2020; pp. 97–118. [Google Scholar]
- Gibbons, L.M.; Halvorsen, O.; Stuve, G. Revision of the genus Elaphostrongylus Cameron (Nematoda, Metas- trongyloidea) with particular reference to species of the genus occurring in Norwegian cervids. Zool. Scr. 1991, 20, 15–26. [Google Scholar] [CrossRef]
- Stéen, M.; Chabaud, A.G.; Rehbinder, C. Species of the genus Elaphostrongylus parasite of Swedish cervidae. A description of E. alces n. sp. Ann. Parasitol. Hum. Comp. 1989, 64, 134–142. [Google Scholar] [CrossRef] [Green Version]
- Stéen, M.; Warsame, I.; Skorping, A. Experimental infection of reindeer, sheep and goats with Elaphostrongylus spp. (Nematoda, Protostrongylidae) from moose and reindeer. Rangifer 1998, 18, 73–80. [Google Scholar] [CrossRef] [Green Version]
- Mason, P. Elaphostrongylus cervi and its close relatives; a review of protostrongylids (Nematoda, Metastrongyloidea) with spiny-tailed larvae. Surveillance 1995, 22, 19–24. [Google Scholar]
- Handeland, K.; Gibbons, L.M.; Skorping, A. Aspects of the life cycle and pathogenesis of Elaphostrongylus cervi in red deer (Cervus elaphus). J. Parasitol. 2000, 86, 1061–1066. [Google Scholar] [CrossRef]
- Handeland, K.; Gibbons, L.M.; Skorping, A. Experimental Elaphostrongylus cervi infection in sheep and goats. J. Comp. Path. 2000, 123, 248–257. [Google Scholar] [CrossRef]
- Handeland, K.; Stuve, G.; Skorping, A. Experimental Elaphostrongylus alces infection in goats. J. Comp. Path. 2001, 125, 71–75. [Google Scholar] [CrossRef]
- Gilbert, C.; Ropiquet, A.; Hassanin, A. Mitochondrial and nuclear phylogenies of Cervidae (Mammalia, Ruminantia): Systematics, morphology, and biogeography. Mol. Phyl. Evol. 2006, 40, 101–117. [Google Scholar] [CrossRef]
- Raczyński, J. Moose in Poland—A current state and perspectives. In Is There a Place for Moose? Stowarzyszenie “Uroczysko”: Supraśl, Poland, 2006; pp. 24–38, (In Polish with English summary). [Google Scholar]
- Raczyński, J.; Ratkiewicz, M. The functioning of the moose population in Poland. Annals of Warsaw University of Life Sciences—SGGW. Anim. Sci. 2011, 50, 51–56. [Google Scholar]
- Świsłocka, M.; Czajkowska, M.; Duda, N.; Danyłow, J.; Owadowska-Cornil, E.; Ratkiewicz, M. Complex patterns of population genetic structure of moose, Alces alces, after recent spatial expansion in Poland revealed by sex-linked markers. Acta Theriol. 2013, 58, 367–378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gębczyńska, Z.; Raczyński, J. Problems of protection and management of the moose and other game in the Biebrza National Park. Park. Nar. Rezerw. Przyr. 1993, 12, 5–36, (In Polish with English summary). [Google Scholar]
- Kuligowska, I.; Demiaszkiewicz, A.W.; Kowalczyk, R. A new occurrence of Eimeria alces (Apicomplexa: Eimeridae) in elk (Alces alces) in east Poland. Ann. Parasitol. 2014, 60, 277–279. [Google Scholar]
- Filip-Hutsch, K.; Czopowicz, M.; Świsłocka, M.; Ratkiewicz, M.; Borkowska, A.; Kowalczyk, R.; Demiaszkiewicz, A.W. Patterns of parasite eggs, oocysts and larvae shedding by moose in the Biebrza marshland (NE Poland). Int. J. Parasitol. Parasites Wildl. 2020, 11, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Świsłocka, M.; Borkowska, A.; Matosiuk, M.; Czajkowska, M.; Duda, N.; Kowalczyk, R.; Ratkiewicz, M. Sex-biased polyparasitism in moose (Alces alces) based on molecular analysis of faecal samples. Int. J. Parasitol. Parasites Wildl. 2020, 13, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Davidson, R.K.; Ličina, T.; Gorini, L.; Milner, J.M. Endoparasites in a Norwegian moose (Alces alces) population—Faunal diversity, abundance and body condition. Int. J. Parasitol. Parasites Wildl. 2015, 7, 29–36. [Google Scholar] [CrossRef] [Green Version]
- Filip, K.J.; Demiaszkiewicz, A.W. Internal parasitic fauna of elk (Alces alces) in Poland. Acta Parasitol. 2016, 61, 657–664. [Google Scholar] [CrossRef]
- Stéen, M.; Roepstorff, L. Neurological disorder in two moose calves (Alces alces L.) naturally infected with Elaphostrongylus alces. Rangifer 1990, 10, 399–406. [Google Scholar] [CrossRef] [Green Version]
- Stéen, M.; Ressner, I.M.O.; Olsson, B.; Petersson, E. Epizootiology of Elaphostrongylus alces in Swedish moose. Alces 2016, 52, 13–28. [Google Scholar]
- Goliszewska, A.; Demiaszkiewicz, A.W. The first record of Elaphostrongylus alces larvae in moose in Poland and their development to the invasive stage. Ann. Parasitol. 2007, 53, 331–333. [Google Scholar]
- Duneau, D.; Dieter, E. Host sexual dimorphism and parasite adaptation. PLoS Biol. 2012, 10, e1001271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duneau, D.; Dieter, E. The role of moulting in parasite defence. Proc. R. Soc. B 2012, 279, 3049–3054. [Google Scholar] [CrossRef] [PubMed]
- Cozzarolo, C.S.; Sironi, N.; Glaizot, O.; Pigeault, R.; Christe, P. Sex-biased parasitism in vector-borne disease: Vector preference? PLoS ONE 2019, 14, e0218452. [Google Scholar]
- Stuve, G. The prevalence of Elaphostrongylus cervi infection in moose (Alces alces) in southern Norway. Acta Vet. Scand. 1986, 27, 397–409. [Google Scholar] [CrossRef]
- Valente, A.M.; Figueiredo, A.M.; Acevedo, P.; Fonseca, C.; Torres, R.T.; Vicente, J. Long term surveillance reveals nematode Elaphostrongylus cervi as a practical indicator of red deer management. Ecol. Indic. 2021, 123, 107330. [Google Scholar] [CrossRef]
- Stéen, M.; Blackmore, C.G.M.; Skorping, A. Cross-infection of moose (Alces alces) and reindeer (Rangifer tarandus) with Elaphostrongylus alces and Elaphostrongylus rangiferi (Nematoda, Protostrongylidae): Effects on parasite morphology and prepatent period. Vet. Parasitol. 1997, 71, 27–38. [Google Scholar] [CrossRef]
- Tang, X.; Huang, G.; Liu, X.; El-Ashram, S.; Tao, G.; Lu, C.; Suo, J.; Suo, X. An optimized DNA extraction method for molecular identification of coccidian species. Parasitol. Res. 2018, 117, 655–664. [Google Scholar] [CrossRef] [PubMed]
- Handeland, K.; Davidson, R.K.; Viljugrein, H.; Mossing, A.; Meisingset, E.L.; Heum, M.; Strand, O.; Isaksen, K. Elaphostrongylus and Dictyocaulus infections in Norwegian wild reindeer and red deer populations in relation to summer pasture altitude and climate. Int. J. Parasitol. Parasites Wildl. 2019, 10, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Kuligowska, I.; Demiaszkiewicz, A.W. Infection of terrestial snails with larvae of Elaphostrongylus cervi (Nem-atoda, Protostrongylidae) in Białowieża National Park. Helminthologia 2010, 47, 25–28. [Google Scholar] [CrossRef] [Green Version]
- Vicente, J.; Fierro, J.; Gortazar, C. Seasonal dynamics of the fecal excretion of Elaphostrongylus cervi (Nematoda, Metastrongyloidea) first-stage larvae in Iberian red deer (Cervus elaphus hispanicus) from southern Spain. Parasitol. Res. 2005, 95, 60–64. [Google Scholar] [CrossRef]
- Panek, M. Sytuacja zwierząt Łownych w Polsce—Wyniki Monitoringu; Stacja Badawcza PZŁ Czempiń: Czempiń, Poland, 2019; pp. 1–30. (In Polish) [Google Scholar]
- Lloyd-Smith, J.O.; Schreiber, S.J.; Kopp, P.E.; Getz, W.M. Superspreading and the effect of individual variation on disease emergence. Nature 2005, 438, 355. [Google Scholar] [CrossRef]
- Escobar, L.E.; Moen, R.; Craft, M.E.; VanderWaal, K.L. Mapping parasite transmission risk from white-tailed deer to a declining moose population. Eur. J. Wild. Res. 2019, 65, 60. [Google Scholar] [CrossRef]
- Svenning, J.C.; Pedersen, P.B.; Donlan, C.J.; Ejrnæs, R.; Faurby, S.; Galetti, M.; Vera, F.W. Science for a wilder Anthropocene: Synthesis and future directions for trophic rewilding research. Proc. Natl. Acad. Sci. USA 2016, 113, 898–906. [Google Scholar] [CrossRef] [Green Version]
- Świsłocka, M.; Czajkowska, M.; Duda, N.; Ratkiewicz, M. Admixture promotes genetic variation in bottlenecked moose populations in eastern Poland. Mammal Res. 2015, 60, 169–179. [Google Scholar] [CrossRef] [Green Version]
- Kalinowski, S.T.; Taper, M.L.; Marshall, T.C. Revising how the computer program CERVUS accommodates genotyping errors increases success in paternity assignment. Mol. Ecol. 2007, 16, 1099–1106. [Google Scholar] [CrossRef]
- Hall, T.A. BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- Gajadhar, A.; Steeves-Gurnsey, T.; Kendall, J.; Lankester, M.; Stéen, M. Differentiation of dorsal-spined Elaphostrongyline larvae by polymerase chain reaction amplification of ITS-2 of rDNA. J. Wildl. Dis. 2000, 36, 713–722. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Said, Y.; Cabaret, J.; Harmache, A.; Lahmar, S. Identification of first-stage dorsal-spined lungworm larvae of Tunisian barbary red deer: First report of Varestrongylus sagittatus and Elaphostrongylus cervi in Africa. Parasitol. Int. 2018, 67, 386–388. [Google Scholar] [CrossRef] [PubMed]

| Population | No. Males | Males | No. Females | Females | p Value | ||
|---|---|---|---|---|---|---|---|
| No. Positive | % (CI) | No. Positive | % (CI) | ||||
| SRO | 7 | 4 | 57.1 (25.0–60.3) | 10 | 3 | 30.0 (10.8–60.3) | 0.264 |
| LDZ | 8 | 5 | 62.5 (30.6–86.3) | 11 | 3 | 27.3 (9.8–56.6) | 0.125 |
| NPN | 9 | 6 | 66.7 (35.4–87.9) | 7 | 6 | 85.7 (48.7–97.4) | 0.383 |
| KPN | 3 | 1 | 33.3 (6.2–79.2) | 18 | 7 | 38.9 (20.3–61.4) | 0.854 |
| RDZ | 13 | 8 | 61.5 (35.5–82.3) | 7 | 4 | 57.2 (25.0– 84.2) | 0.848 |
| GWL | 13 | 8 | 61.5 (35.5–82.3) | 20 | 4 | 20.0 (8.1–41.6) | 0.015 |
| PPN | 15 | 9 | 60.0 (35.8–80.2) | 23 | 15 | 65.2 (44.9–81.2) | 0.745 |
| BPN * | 26 | 16 | 61.5 (42.5–77.6) | 27 | 10 | 37.04 (21.5–55.8) | 0.075 |
| All | 94 | 57 | 60.6 (50.5–69.9) | 123 | 42 | 34.1 (26.4–42.9) | 0.0001 |
| Population | No. Males | Males | No. Females | Females | p Value | ||
|---|---|---|---|---|---|---|---|
| No. Positive | % (CI) | No. Positive | % (CI) | ||||
| SRO | 7 | 3 | 42.9 (15.8–75.0) | 10 | 2 | 20.0 (5.7–51.0) | 0.311 |
| LDZ | 8 | 2 | 25.0 (7.2–59.2) | 11 | 1 | 9.1 (1.6–37.7) | 0.348 |
| NPN | 9 | 0 | 0 | 7 | 0 | 0 | − |
| KPN | 3 | 0 | 0 | 18 | 3 | 16.7 (5.8–39.2) | 0.459 |
| RDZ | 13 | 2 | 15.4 (4.3–42.2) | 7 | 0 | 0 | 0.275 |
| GWL | 13 | 4 | 30.8 (12.7–57.6) | 20 | 0 | 0 | 0.008 |
| PPN | 15 | 3 | 20.0 (7.0–45.2) | 23 | 4 | 17.4 (7.0–37.1) | 0.839 |
| BPN * | 26 | 7 | 26.9 (13.7–46.1) | 27 | 5 | 18.5 (8.2–36.7) | 0.465 |
| All | 94 | 21 | 22.3 (15.1–31.8) | 123 | 15 | 12.2 (7.5–19.2) | 0.046 |
| Pop. | No. Sample | No Parasite Species | One Parasite Species | Two Parasite Species | |||
|---|---|---|---|---|---|---|---|
| No. Negative | % (CI) | No. Positive | % (CI) | No. Positive | % (CI) | ||
| SRO | 17 | 7 | 41.2 (21.6–64.0) | 8 | 47.0 (26.2–69.0) | 2 | 11.8 (3.3–34.3) |
| LDZ | 19 | 11 | 57.9 (36.3–76.9) | 5 | 26.3 (11.8–48.8) | 3 | 15.8 (5.5–37.6) |
| NPN | 16 | 4 | 25.0 (10.2–49.5) | 12 | 75.0 (50.5–89.8) | 0 | 0 |
| KPN | 21 | 12 | 57.2 (36.6–75.5) | 7 | 33.3 (17.2–54.6) | 2 | 9.5 (2.7–28.9) |
| RDZ | 20 | 7 | 35.0 (18.1–56.7) | 12 | 60.0 (38.7–78.1) | 1 | 5.0 (0.9–28.9) |
| GWL | 33 | 21 | 63.6 (46.6–77.8) | 8 | 24.3 (12.8–41.0) | 4 | 12.1 (4.8–27.3) |
| PPN | 38 | 11 | 29.0 (17.0–44.8) | 23 | 60.5 (44.7–74.4) | 4 | 10.5 (4.2–24.1) |
| BPN * | 53 | 21 | 39.6 (27.6–53.1) | 26 | 49.1 (36.1–62.1) | 6 | 11.3 (5.3–22.6) |
| All | 217 | 94 | 43.4 (36.9–50.0) | 101 | 46.5 (40.0–53.2) | 22 | 10.1 (6.8–14.9) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Świsłocka, M.; Ratkiewicz, M.; Borkowska, A. Simultaneous Infection of Elaphostrongylus Nematode Species and Parasite Sharing between Sympatrically Occurring Cervids: Moose, Roe Deer, and Red Deer in Poland. Pathogens 2021, 10, 1344. https://doi.org/10.3390/pathogens10101344
Świsłocka M, Ratkiewicz M, Borkowska A. Simultaneous Infection of Elaphostrongylus Nematode Species and Parasite Sharing between Sympatrically Occurring Cervids: Moose, Roe Deer, and Red Deer in Poland. Pathogens. 2021; 10(10):1344. https://doi.org/10.3390/pathogens10101344
Chicago/Turabian StyleŚwisłocka, Magdalena, Mirosław Ratkiewicz, and Anetta Borkowska. 2021. "Simultaneous Infection of Elaphostrongylus Nematode Species and Parasite Sharing between Sympatrically Occurring Cervids: Moose, Roe Deer, and Red Deer in Poland" Pathogens 10, no. 10: 1344. https://doi.org/10.3390/pathogens10101344
APA StyleŚwisłocka, M., Ratkiewicz, M., & Borkowska, A. (2021). Simultaneous Infection of Elaphostrongylus Nematode Species and Parasite Sharing between Sympatrically Occurring Cervids: Moose, Roe Deer, and Red Deer in Poland. Pathogens, 10(10), 1344. https://doi.org/10.3390/pathogens10101344






