Next-Generation Computationally Designed Influenza Hemagglutinin Vaccines Protect against H5Nx Virus Infections
Abstract
:1. Introduction
2. Materials and Methods
2.1. Next-Generation Computationally Optimized Broadly Reactive Antigens (COBRA) Design
2.2. Recombinant Protein Production
2.3. Viruses
2.4. Mouse Studies
2.5. Hematoxylin and Eosin (H&E) Staining
2.6. Immunohistochemistry Staining
2.7. Plaque Assays
2.8. Hemagglutination-Inhibition (HAI) Assay
2.9. P-Epitope/P-Sequence Analysis
3. Results
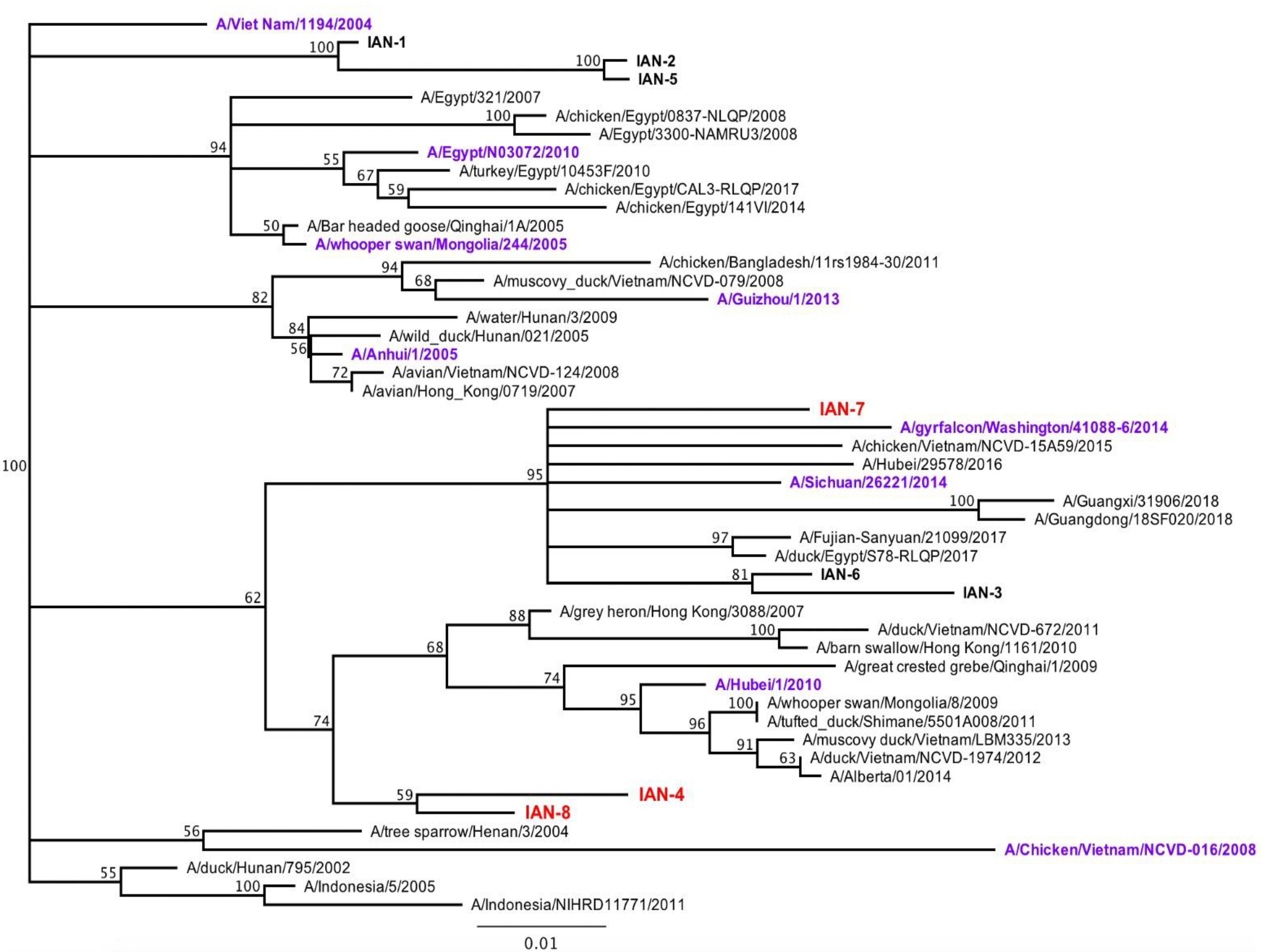
3.1. Design and Characterization of COBRA H5 Hemagglutinin (HA) Vaccines
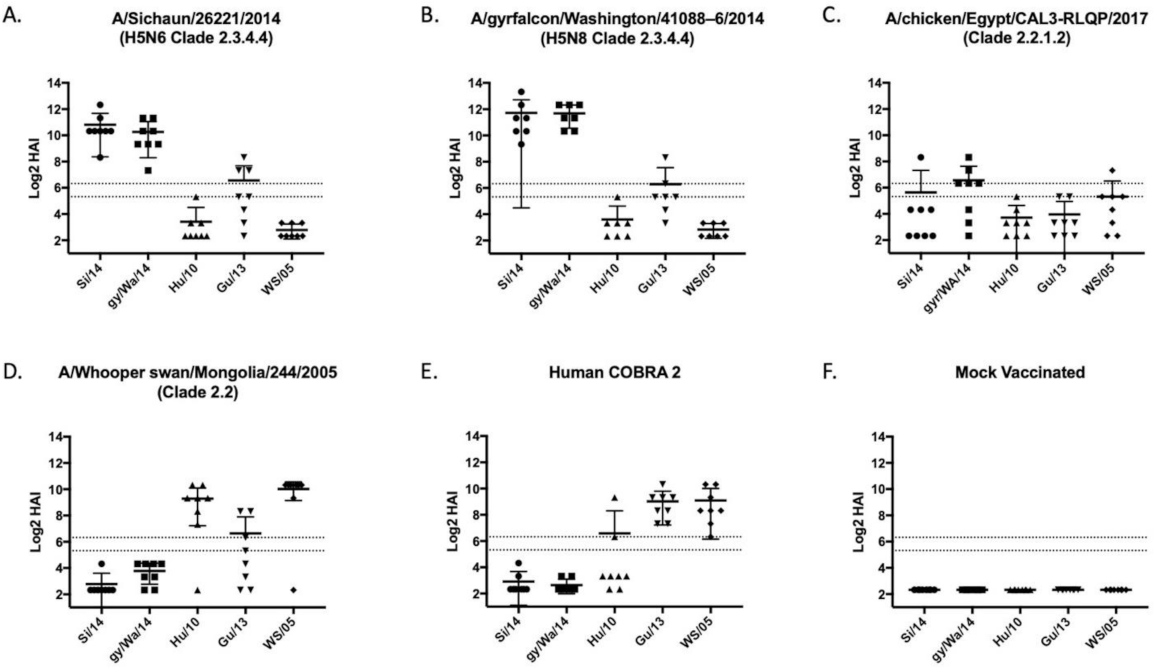
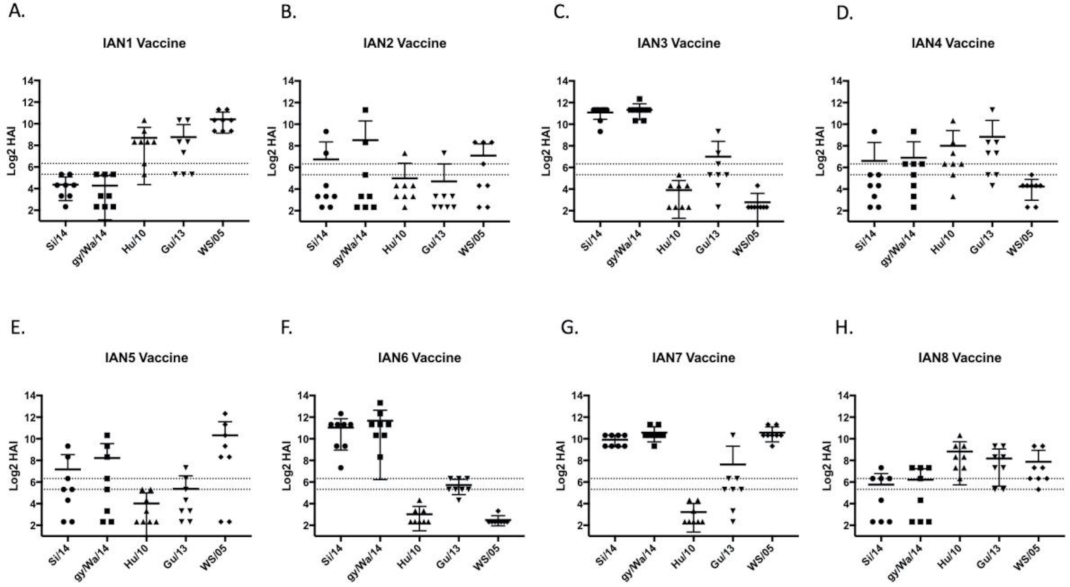
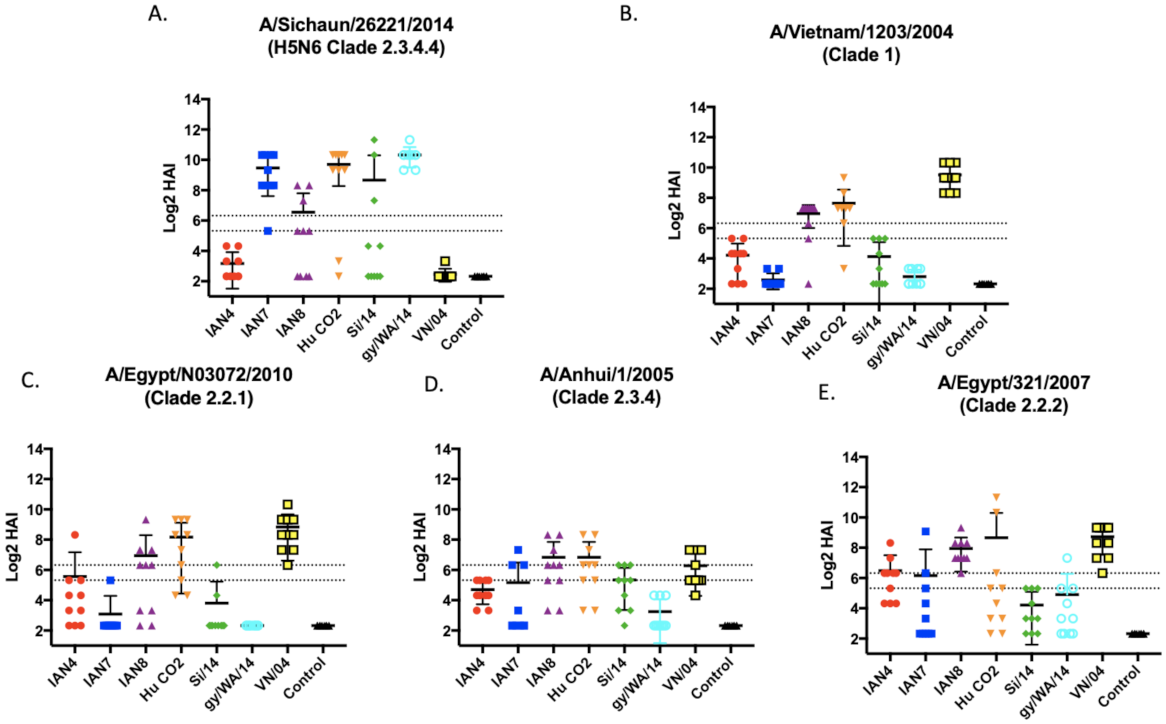
3.2. Vaccines Elicit Antibodies with Hemagglutination-Inhibition Activity
3.3. Viral Challenge
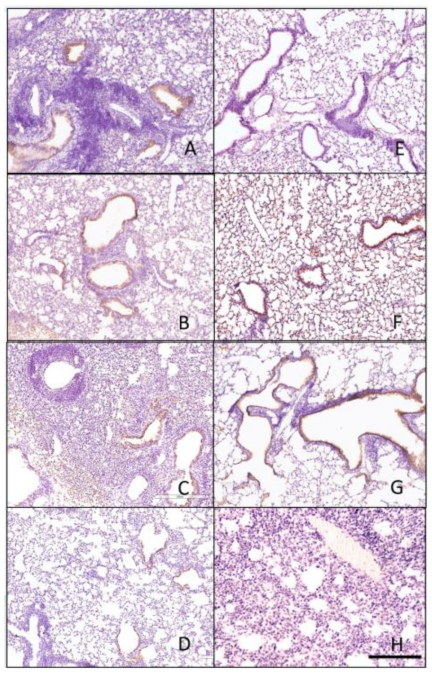
3.4. Histopathology
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Weekly Epidemiological record: Zoonotic influenza viruses: Antigenic and genetic characteristics and development of candidate vaccine viruses for pandemic preparedness. Wkly. Epidemiol. Rec. Relev. Épidémiologique Hebd. 2017, 92, 129–144. [Google Scholar]
- World Health Organization. Antigenic and Genetic Characteristics of Zoonotic Influenza A Viruses and Development of Candidate Vaccine Viruses for Pandemic Preparedness. 2020. Available online: https://www.who.int/influenza/vaccines/virus/characteristics_virus_vaccines/en/ (accessed on 10 June 2020).
- Yang, Z.-F.; Mok, C.K.P.; Peiris, J.S.M.; Zhong, N.-S. Human Infection with a Novel Avian Influenza A(H5N6) Virus. N. Engl. J. Med. 2015, 373, 487–489. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization. Antigenic and Genetic Characteristic of Zoonotic Influenza Viruses and Development of Candiddate Vaccine Viruses for Pandemic Preparedness. 2017. Available online: https://www.who.int/influenza/vaccines/virus/201703_zoonotic_vaccinevirusupdate.pdf/ (accessed on 5 June 2018).
- Ip, H.S.; Torchetti, M.K.; Crespo, R.; Kohrs, P.; DeBruyn, P.; Mansfield, K.G.; Baszler, T.; Badcoe, L.; Bodenstein, B.; Shearn-Bochsler, V. Novel Eurasian highly pathogenic avian influenza A H5 viruses in wild birds, Washington, USA, 2014. Emerg. Infect. Dis. 2015, 21, 886. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.-H.; Torchetti, M.K.; Winker, K.; Ip, H.S.; Song, C.-S.; Swayne, D.E. Intercontinental Spread of Asian-Origin H5N8 to North America through Beringia by Migratory Birds. J. Virol. 2015, 89, 6521–6524. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, L.; Zhu, W.; Li, X.; Bo, H.; Zhang, Y.; Zou, S.; Gao, R.; Dong, J.; Zhao, X.; Chen, W. Genesis and dissemination of highly pathogenic H5N6 avian influenza viruses. J. Virol. 2017, 91, e02199-16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeong, J.; Kang, H.-M.; Lee, E.-K.; Song, B.-M.; Kwon, Y.-K.; Kim, H.-R.; Choi, K.-S.; Kim, J.-Y.; Lee, H.-J.; Moon, O.-K.; et al. Highly pathogenic avian influenza virus (H5N8) in domestic poultry and its relationship with migratory birds in South Korea during 2014. Vet. Microbiol. 2014, 173, 249–257. [Google Scholar] [CrossRef]
- Chutinimitkul, S.; van Riel, D.; Munster, V.J.; van den Brand, J.M.; Rimmelzwaan, G.F.; Kuiken, T.; Osterhaus, A.D.; Fouchier, R.A.; de Wit, E. In vitro assessment of attachment pattern and replication efficiency of H5N1 influenza A viruses with altered receptor specificity. J. Virol. 2010, 84, 6825–6833. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization. Avian Influenza A (H5N8) Infects Humans in Russian Federation. Available online: https://www.euro.who.int/en/health-topics/health-emergencies/pages/news/news/2021/03/avian-influenza-ah5n8-infects-humans-in-russian-federation (accessed on 3 February 2021).
- Zhao, Z.; Guo, Z.; Zhang, C.; Liu, L.; Chen, L.; Zhang, C.; Wang, Z.; Fu, Y.; Li, J.; Shao, H.; et al. Avian Influenza H5N6 Viruses Exhibit Differing Pathogenicities and Transmissibilities in Mammals. Sci. Rep. 2017, 7, 16280. [Google Scholar] [CrossRef] [Green Version]
- Sun, H.; Pu, J.; Hu, J.; Liu, L.; Xu, G.; Gao, G.F.; Liu, X.; Liu, J. Characterization of clade 2.3.4.4 highly pathogenic H5 avian influenza viruses in ducks and chickens. Vet. Microbiol. 2016, 182, 116–122. [Google Scholar] [CrossRef]
- Sun, H.; Pu, J.; Wei, Y.; Sun, Y.; Hu, J.; Liu, L.; Xu, G.; Gao, W.; Li, C.; Zhang, X. Highly pathogenic avian influenza H5N6 viruses exhibit enhanced affinity for human type sialic acid receptor and in-contact transmission in model ferrets. J. Virol. 2016, 90, 6235–6243. [Google Scholar] [CrossRef] [Green Version]
- Herfst, S.; Mok, C.K.; van den Brand, J.M.; van der Vliet, S.; Rosu, M.E.; Spronken, M.I.; Yang, Z.; de Meulder, D.; Lexmond, P.; Bestebroer, T.M. Human clade 2.3. 4.4 A/H5N6 influenza virus lacks mammalian adaptation markers and does not transmit via the airborne route between ferrets. mSphere 2018, 3, e00405-17. [Google Scholar] [CrossRef] [Green Version]
- Smith, G.J.; Donis, R.O.; Health/Food, W.H.O.W.O.f.A.; Group, A.O.H.E.W. Nomenclature updates resulting from the evolution of avian influenza A (H5) virus clades 2.1. 3.2 a, 2.2. 1, and 2.3. 4 during 2013–2014. Influenza Other Respir. Viruses 2015, 9, 271–276. [Google Scholar] [CrossRef]
- Sun, W.; Li, J.; Hu, J.; Jiang, D.; Xing, C.; Zhan, T.; Liu, X. Genetic analysis and biological characteristics of different internal gene origin H5N6 reassortment avian influenza virus in China in 2016. Vet. Microbiol. 2018, 219, 200–211. [Google Scholar] [CrossRef]
- Okamatsu, M.; Ozawa, M.; Soda, K.; Takakuwa, H.; Haga, A.; Hiono, T.; Matsuu, A.; Uchida, Y.; Iwata, R.; Matsuno, K. Characterization of highly pathogenic avian influenza virus A (H5N6), Japan, November 2016. Emerg. Infect. Dis. 2017, 23, 691. [Google Scholar] [CrossRef] [Green Version]
- Bai, R.; Sikkema, R.S.; rong Li, C.; Munnink, B.B.O.; Wu, J.; Zou, L.; Jing, Y.; Lu, J.; Yuan, R.; Liao, M. Antigenic Variation of Avian Influenza A (H5N6) Viruses, Guangdong Province, China, 2014–2018. Emerg. Infect. Dis. 2019, 25, 1932. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- World Health Organization. Antigenic and Genetic Characteristics of Zoonotic Influenza Viruses and Development of Candidate Vaccine Viruses for Pandemic Preparedness. Available online: https://www.who.int/influenza/vaccines/virus/characteristics_virus_vaccines/en/2019 (accessed on 20 October 2020).
- Li, Y.; Shi, J.; Zhong, G.; Deng, G.; Tian, G.; Ge, J.; Zeng, X.; Song, J.; Zhao, D.; Liu, L. Continued evolution of H5N1 influenza viruses in wild birds, domestic poultry, and humans in China from 2004 to 2009. J. Virol. 2010, 84, 8389–8397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Creanga, A.; Thi Nguyen, D.; Gerloff, N.; Thi Do, H.; Balish, A.; Dang Nguyen, H.; Jang, Y.; Thi Dam, V.; Thor, S.; Jones, J.; et al. Emergence of multiple clade 2.3.2.1 influenza A (H5N1) virus subgroups in Vietnam and detection of novel reassortants. Virology 2013, 444, 12–20. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Mullarkey, C.E.; Duty, J.A.; Moran, T.M.; Palese, P.; Miller, M.S. Broadly neutralizing anti-influenza virus antibodies: Enhancement of neutralizing potency in polyclonal mixtures and IgA backbones. J. Virol. 2015, 89, 3610–3618. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bar-Peled, Y.; Huang, J.; Nuñez, I.A.; Pierce, S.R.; Ecker, J.W.; Ross, T.M.; Mousa, J.J. Structural and antigenic characterization of a computationally-optimized H5 hemagglutinin influenza vaccine. Vaccine 2019, 37, 6022–6029. [Google Scholar] [CrossRef]
- WHO Global Influenza Surveillance Network. Manual for the Laboratory Diagnosis and Virological Surveillance of Influenza; World Health Organization: Geneva, Switzerland, 2011. [Google Scholar]
- Giles, B.M.; Ross, T.M. A computationally optimized broadly reactive antigen (COBRA) based H5N1 VLP vaccine elicits broadly reactive antibodies in mice and ferrets. Vaccine 2011, 29, 3043–3054. [Google Scholar] [CrossRef] [Green Version]
- Giles, B.M.; Bissel, S.J.; DeAlmeida, D.R.; Wiley, C.A.; Ross, T.M. Antibody Breadth and Protective Efficacy Are Increased by Vaccination with Computationally Optimized Hemagglutinin but Not with Polyvalent Hemagglutinin-Based H5N1 Virus-Like Particle Vaccines. Clin. Vaccine Immunol. 2012, 19, 128–139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giles, B.M.; Crevar, C.J.; Carter, D.M.; Bissel, S.J.; Schultz-Cherry, S.; Wiley, C.A.; Ross, T.M. A Computationally Optimized Hemagglutinin Virus-Like Particle Vaccine Elicits Broadly Reactive Antibodies that Protect Nonhuman Primates from H5N1 Infection. J. Infect. Dis. 2012, 205, 1562–1570. [Google Scholar] [CrossRef] [PubMed]
- Carter, D.M.; Bloom, C.E.; Nascimento, E.J.; Marques, E.T.; Craigo, J.K.; Cherry, J.L.; Lipman, D.J.; Ross, T.M. Sequential seasonal H1N1 influenza virus infections protect ferrets against novel 2009 H1N1 influenza virus. J. Virol. 2013, 87, 1400–1410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Allen, J.D.; Owino, S.O.; Carter, D.M.; Crevar, C.J.; Reese, V.A.; Fox, C.B.; Coler, R.N.; Reed, S.G.; Baldwin, S.L.; Ross, T.M. Broadened immunity and protective responses with emulsion-adjuvanted H5 COBRA-VLP vaccines. Vaccine 2017, 35, 5209–5216. [Google Scholar] [CrossRef]
- Nuñez, I.A.; Ross, T.M. Human COBRA 2 vaccine contains two major epitopes that are responsible for eliciting neutralizing antibody responses against heterologous clades of viruses. Vaccine 2020, 38, 830–839. [Google Scholar] [CrossRef]
- Allen, J.D.; Ross, T.M. Next generation methodology for updating HA vaccines against emerging human seasonal influenza A(H3N2) viruses. Sci. Rep. 2021, 11, 4554. [Google Scholar] [CrossRef]
- Forrest, H.L.; Khalenkov, A.M.; Govorkova, E.A.; Kim, J.-K.; Del Giudice, G.; Webster, R.G. Single- and multiple-clade influenza A H5N1 vaccines induce cross protection in ferrets. Vaccine 2009, 27, 4187–4195. [Google Scholar] [CrossRef] [Green Version]
- Wong, S.-S.; Duan, S.; DeBeauchamp, J.; Zanin, M.; Kercher, L.; Sonnberg, S.; Fabrizio, T.; Jeevan, T.; Crumpton, J.-C.; Oshansky, C.; et al. The immune correlates of protection for an avian influenza H5N1 vaccine in the ferret model using oil-in-water adjuvants. Sci. Rep. 2017, 7, 44727. [Google Scholar] [CrossRef] [Green Version]
- Krammer, F.; Hai, R.; Yondola, M.; Tan, G.S.; Leyva-Grado, V.H.; Ryder, A.B.; Miller, M.S.; Rose, J.K.; Palese, P.; García-Sastre, A. Assessment of influenza virus hemagglutinin stalk-based immunity in ferrets. J. Virol. 2014, 88, 3432–3442. [Google Scholar] [CrossRef] [Green Version]
- Rockman, S.; Brown, L.E.; Barr, I.G.; Gilbertson, B.; Lowther, S.; Kachurin, A.; Kachurina, O.; Klippel, J.; Bodle, J.; Pearse, M. Neuraminidase-inhibiting antibody is a correlate of cross-protection against lethal H5N1 influenza in ferrets immunised with seasonal influenza vaccine. J. Virol. 2013, 87, 3053–3061. [Google Scholar] [CrossRef] [Green Version]
- Ko, E.-J.; Lee, Y.-T.; Kim, K.-H.; Jung, Y.-J.; Lee, Y.; Denning, T.L.; Kang, S.-M. Effects of MF59 Adjuvant on Induction of Isotype-Switched IgG Antibodies and Protection after Immunization with T-Dependent Influenza Virus Vaccine in the Absence of CD4+ T Cells. J. Virol. 2016, 90, 6976–6988. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wack, A.; Baudner, B.C.; Hilbert, A.K.; Manini, I.; Nuti, S.; Tavarini, S.; Scheffczik, H.; Ugozzoli, M.; Singh, M.; Kazzaz, J.; et al. Combination adjuvants for the induction of potent, long-lasting antibody and T-cell responses to influenza vaccine in mice. Vaccine 2008, 26, 552–561. [Google Scholar] [CrossRef] [PubMed]
- Velkov, T.; Ong, C.; Baker, M.A.; Kim, H.; Li, J.; Nation, R.L.; Huang, J.X.; Cooper, M.A.; Rockman, S. The antigenic architecture of the hemagglutinin of influenza H5N1 viruses. Mol. Immunol. 2013, 56, 705–719. [Google Scholar] [CrossRef]
- Wu, W.L.; Chen, Y.; Wang, P.; Song, W.; Lau, S.-Y.; Rayner, J.M.; Smith, G.J.D.; Webster, R.G.; Peiris, J.S.M.; Lin, T.; et al. Antigenic Profile of Avian H5N1 Viruses in Asia from 2002 to 2007. J. Virol. 2008, 82, 1798–1807. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cai, Z.; Ducatez, M.F.; Yang, J.; Zhang, T.; Long, L.-P.; Boon, A.C.; Webby, R.J.; Wan, X.-F. Identifying antigenicity associated sites in highly pathogenic H5N1 influenza virus hemagglutinin by using sparse learning. J. Mol. Biol. 2012, 422, 145–155. [Google Scholar] [CrossRef] [Green Version]
- Pan, Y.; Deem, M.W. Prediction of influenza B vaccine effectiveness from sequence data. Vaccine 2016, 34, 4610–4617. [Google Scholar] [CrossRef] [Green Version]
- Pan, K.; Subieta, K.C.; Deem, M.W. A novel sequence-based antigenic distance measure for H1N1, with application to vaccine effectiveness and the selection of vaccine strains. Protein Eng. Des. Sel. 2011, 24, 291–299. [Google Scholar] [CrossRef]
- Li, X.; Deem, M.W. Influenza evolution and H3N2 vaccine effectiveness, with application to the 2014/2015 season. Protein Eng. Des. Sel. 2016, 29, 309–315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muñoz, E.T.; Deem, M.W. Epitope analysis for influenza vaccine design. Vaccine 2005, 23, 1144–1148. [Google Scholar] [CrossRef] [Green Version]





| Group | Vaccine | Mice |
|---|---|---|
| 1 | IAN-1 | 8 |
| 2 | IAN-2 | 8 |
| 3 | IAN-3 | 8 |
| 4 | IAN-4 | 8 |
| 5 | IAN-5 | 8 |
| 6 | IAN-6 | 8 |
| 7 | IAN-7 | 8 |
| 8 | IAN-8 | 8 |
| 9 | Human COBRA 2 | 8 |
| 10 | A/Sichuan/26211/2014 | 8 |
| 11 | A/GYLFALCON/Washington/41088-6/2014 | 8 |
| 12 | A/chivken/Egypt/CAL3-RLQP/2017 | 8 |
| 13 | a/whooper swan/Mongolia/244/2005 | 8 |
| 14 | Mock | 8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nuñez, I.A.; Huang, Y.; Ross, T.M. Next-Generation Computationally Designed Influenza Hemagglutinin Vaccines Protect against H5Nx Virus Infections. Pathogens 2021, 10, 1352. https://doi.org/10.3390/pathogens10111352
Nuñez IA, Huang Y, Ross TM. Next-Generation Computationally Designed Influenza Hemagglutinin Vaccines Protect against H5Nx Virus Infections. Pathogens. 2021; 10(11):1352. https://doi.org/10.3390/pathogens10111352
Chicago/Turabian StyleNuñez, Ivette A., Ying Huang, and Ted M. Ross. 2021. "Next-Generation Computationally Designed Influenza Hemagglutinin Vaccines Protect against H5Nx Virus Infections" Pathogens 10, no. 11: 1352. https://doi.org/10.3390/pathogens10111352
APA StyleNuñez, I. A., Huang, Y., & Ross, T. M. (2021). Next-Generation Computationally Designed Influenza Hemagglutinin Vaccines Protect against H5Nx Virus Infections. Pathogens, 10(11), 1352. https://doi.org/10.3390/pathogens10111352






