An Updated View on the Antiviral Therapy of Hepatitis C in Chronic Kidney Disease
Abstract
:1. Introduction
2. Information Sources and Search Strategy
3. Current Epidemiology of HCV in Chronic Kidney Disease
4. Natural History of HCV in Chronic Kidney Disease
5. Antiviral Therapy of HCV and Its Aim
6. Antiviral Therapy of HCV in Dialysis Patients (IFN-Based Therapy)
7. Antiviral Therapy of HCV in Patients with Advanced CKD (DAAs)
8. PI Containing SOF-Free DAAs (Clinical Trials)
9. PI-Containing SOF-Free DAAs (Real World Studies)
10. SOF-Based DAAs (Clinical Trials)
11. SOF-Based DAAs (Real World Studies)
12. SOF-Based DAAs and Kidney Impairment
13. Conclusions and Personal Views
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fabrizi, F.; Dixit, V.; Messa, P. Hepatitis C virus and mortality among patients on dialysis: A systematic review and meta-analysis. Clin. Res. Hepatol. Gastroenterol. 2019, 43, 244–254. [Google Scholar] [CrossRef]
- Jadoul, M.; Bieber, B.; Martin, P.; Akiba, T.; Nwankwo, C.; Arduino, J.; Goodkin, D.; Pisoni, R. Prevalence, incidence, and risk factors for hepatitis C virus infection in haemodialysis patients. Kidney Int. 2019, 95, 939–947. [Google Scholar] [CrossRef]
- Bruchfeld, A.; Lindahl, K. Direct-acting anti-viral medications for hepatitis C: Clinical trials in patients with advanced chronic kidney disease. Semin. Dial. 2019, 32, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Kidney Disease: Improving Global Outcomes (KDIGO) Hepatitis C Work Group. KDIGO 2018 clinical practice guideline for the Prevention, Diagnosis, Evaluation and Treatment of hepatitis C in chronic kidney disease. Kidney Int. 2018, 8, 91–165. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fissell, R.; Bragg-Gresham, J.; Woods, J.; Jadoul, M.; Gillespie, B.; Hedderwick, S.; Rayner, H.; Greenwood, R.; Akiba, T.; Young, E. Patterns of hepatitis C prevalence and seroconversion in hemodialysis units from three continents: The DOPPS. Kidney Int. 2004, 65, 2335–2342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fabrizi, F.; Martin, P. Health care-associated transmission of hepatitis B and C viruses in hemodialysis units. Clin. Liver Dis. 2010, 14, 49–60. [Google Scholar] [CrossRef] [PubMed]
- Mukolomov, S.; Trifonova, G.; Levakova, I.; Bolsun, D.; Kriganova, E. Hepatitis C in the Russian Federation: Challenges and future directions. Hepatic Med. Evid. Res. 2016, 8, 51–60. [Google Scholar]
- Rinonce, H.; Yano, Y.; Utsumi, T.; Heriyanto, D.; Angorowati, N.; Widasari, D.; Lusida, M.; Prasanto, H.; Hotta, H.; Hayashi, Y. Hepatitis B and C virus infection among hemodialysis patients in Yogyakarta, Indonesia: Prevalence and molecular evidence for nosocomial transmission. J. Med. Virol. 2016, 85, 1348–1361. [Google Scholar] [CrossRef]
- Jakupi, X.; Mlakar, J.; Lunar, M.; Seme, K.; Rudhani, I.; Raka, L.; Vince, A.; Poljak, M. A very high prevalence of hepatitis C virus infection among patients undergoing hemodialysis in Kosovo: A nationwide study. BMC Nephrol. 2018, 19, 304. [Google Scholar] [CrossRef]
- Kataruka, M.; Gupta, S.; Ramchandran, R.; Singh, M.; Dhiman, R.; Lal Gupta, K. Incidence and risk factors for hepatitis C virus and hepatitis B virus seroconversion in end-stage renal failure patients on maintenance hemodialysis. J. Clin. Exp. Hepat. 2020, 10, 316–321. [Google Scholar]
- Lodhi, A.; Sajad, A.; Mehmood, K.; Lodhi, A.; Rizwan, S.; Ubaid, A.; Baloch, K.; Ahmed, S.; Ud Din, M.; Mehmood, Z. Profile and predictors of hepatitis and HIV infection in patients on hemodialysis of Quetta, Pakistan. Drug Discov. Ther. 2019, 13, 274–279. [Google Scholar] [CrossRef] [Green Version]
- Madhavan, A.; Sachu, A.; Balakrishnan, A.; Vasudevan, A.; Balakrishnan, S.; Vasudevapanicker, J. Prevalence of hepatitis C among haemodialysis patients in a tertiary care hospital in south India. Iran. J. Microbiol. 2020, 12, 644–649. [Google Scholar] [PubMed]
- Timofte, D.; Dragos, D.; Balcangiu-Stroescu, A.; Tanasescu, M.; Balan, D.; Avino, A.; Tulin, A.; Stiru, O.; Ionescu, D. Infection with hepatitis C virus in haemodialysis patients: An overview of the diagnosis and prevention rules within a haemodialysis center (review). Exp. Ther. Med. 2020, 20, 109–116. [Google Scholar]
- Kalita, D.; Deka, S.; Chamuah, K. Circulation of an atypical hepatitis C virus (HCV) strain at a dialysis unit in northeast India. Microbiol. Open 2021, 10, e1147. [Google Scholar] [CrossRef] [PubMed]
- Mahupe, P.; Molefe-Baikai, O.; Saleshando, G.; Rwegerera, G. Prevalence and risk factors for hepatitis B and C among end-stage renal disease patients on haemodialysis in Gaborone, Botswana. Niger. J. Clin. Pract. 2021, 24, 81–88. [Google Scholar]
- Fabrizi, F.; Martin, P.; Dixit, V.; Brezina, M.; Cole, M.; Gerosa, S.; Vinson, S.; Mousa, M.; Gitnick, G. Quantitative assessment of HCV load in chronic hemodialysis patients: A cross-sectional survey. Nephron 1998, 80, 428–433. [Google Scholar] [CrossRef]
- Pol, S.; Parlati, L.; Jadoul, M. Hepatitis C virus and the kidney. Nat. Rev. Nephrol. 2019, 15, 73–86. [Google Scholar] [CrossRef]
- Liu, C.; Huang, C.; Liu, C.; Dai, C.; Liang, C.; Huang, F.; Hung, P.; Tsai, H.; Tsai, M.; Chen, S.; et al. Pegylated interferon-alpha 2a with or without low-dose ribavirin for treatment –naïve patients with hepatitis C virus genotype 1 receiving hemodialysis: A randomized trial. Ann. Intern. Med. 2013, 159, 729–738. [Google Scholar] [CrossRef]
- Liu, C.; Liu, C.; Huang, C.; Lin, J.; Dai, C.; Liang, C.; Huang, J.; Hung, P.; Tsai, H.; Tsai, M.; et al. Peginterferon alfa-2a with or without low-dose ribavirin for treatmend –naïve patients with hepatitis C virus genotype 2 receiving haemodialysis: A randomized trial. Gut 2015, 64, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Fabrizi, F.; Dixit, V.; Messa, P.G.; Martin, P. Pegylated interferon monotherapy of chronic hepatitis C in the dialysis population: Systematic review and meta-analysis. Apher. Dial. 2015, 19, 611–621. [Google Scholar] [CrossRef]
- Fabrizi, F.; Dixit, V.; Messa, P.; Martin, P. Antiviral therapy (pegylated interferon and ribavirin) of hepatitis C in dialysis patients: Meta-analysis of clinical studies. J. Viral. Hepat. 2014, 21, 681–689. [Google Scholar] [CrossRef]
- Goodkin, D.; Bieber, B.; Jadoul, M.; Martin, P.; Kanda, E.; Pisoni, R. Mortality, hospitalization, and quality of life among patients with hepatitis C infection on hemodialysis. Clin. J. Am. Soc. Nephrol. 2017, 12, 287–297. [Google Scholar] [CrossRef] [Green Version]
- Roth, D.; Nelson, D.; Bruchfeld, A.; Liapakis, A.; Silva, M.; Monsour, H.; Martin, P.; Pol, S.; Londono, M.; Hassanein, T.; et al. Grazoprevir plus elbasvir in treatment naïve and treatment experienced patients with hepatitis C virus genotype infection and stage 4–5 chronic kidney disease (the C –SURFER study): A combination phase 3 study. Lancet 2015, 386, 1537–1545. [Google Scholar] [CrossRef]
- Bruchfeld, A.; Roth, D.; Martin, P.; Nelson, D.; Pol, S.; Londono, M.; Monsour, H.; Silva, M.; Hwang, P.; Arduino, J.; et al. Elbasvir plus grazoprevir in patients with hepatitis C virus infection and stage 4–5 chronic kidney disease: Clinical, virological, and health-related quality of life outcomes from a phase 3, multicenter, randomized, double-blind, placebo-controlled trial. Lancet Gastroenterol. Hepatol. 2017, 2, 585–594. [Google Scholar] [CrossRef]
- Gane, E.; Lawitz, E.; Pugatch, D.; Papatheodoridis, G.; Brau, N.; Brown, A.; Pol, S.; Leroy, V.; Persico, M.; Moreno, C.; et al. Glecaprevir and pibrentasvir in patients with HCV and severe renal impairment. N. Engl. J. Med. 2017, 377, 1448–1455. [Google Scholar] [CrossRef]
- Lawitz, E.; Flisiak, R.; Abunimeh, M.; Sise, M.; Park, J.; Kaskas, M.; Bruchfeld, A.; Worns, A.; Aglitti, A.; Zamor, P.; et al. Efficacy and safety of glecaprevir/pibrentasvir in renally impaired patients with chronic HCV infection. Liver Int. 2020, 40, 1031–1041. [Google Scholar] [CrossRef] [PubMed]
- Pockros, P.; Reddy, R.; Mantry, P.; Cohen, E.; Bennett, M.; Sulkowski, M.; Bernstein, D.; Cohen, D.; Shulman, N.; Wang, D.; et al. Efficacy of direct-acting antiviral combinations for patients with hepatitis C virus genotype 1 infection and severe renal impairment or end-stage renal disease. Gastroenterology 2016, 150, 1590–1598. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vierling, J.; Lawitz, E.; Reddy, R.; Cohen, E.; Kemmer, N.; Morelli, G.; Zamor, P.; Bennett, M.; Bernstein, D.; Kowdley, K.; et al. RUBY-I, Safety and efficacy of ombitasvir/paritaprevir/ritonavir and dasabuvir with or without ribavirin in adults with genotype 1 chronic hepatitis C virus infection with severe renalm impairment or end stage renal disease. In Proceedings of the AASLD 67th Annual Meeting, Boston, MA, USA, 11–15 November 2016. [Google Scholar]
- Gane, E.; Solà, R.; Cohen, E.; Roberts, S.; George, J.; Skoien, R.; Riordan, S.; Mobashery, N.; Abunimeh, M.; Cohen, D.; et al. RUBY-II study: Ombitasvir/paritaprevir/ritonavir±dasabuvir for HCV genotype 1a or 4 with severe renal impairment. In Proceedings of the AASLD 67th Annual Meeting, Boston, MA, USA, 11–15 November 2016. [Google Scholar]
- Lexchin, J. Sponsorship bias in clinical research. Int. J. Risk Saf. Med. 2012, 24, 233–242. [Google Scholar] [CrossRef] [PubMed]
- Flamm, S.; Bacon, B.; Curry, M.; Milligan, S.; Nwankwo, C.; Tsai, N.; Younossi, Z.; Afdhal, N. Real-world use of elbasvir-grazoprevir in patients with chronic hepatitis C: Retrospective analyses from the TRIO network. Aliment. Pharm. 2018, 47, 1511–1522. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garcia-Agudo, R.; Aoufi-Rabih, S.; Salgueira-Lazo, M.; Gonzalez-Corvillo, C.; Fabrizi, F. ‘Real life’ experience with direct-acting antiviral agents for hepatitis C virus in end stage renal disease. Int. J. Artif. Organs 2018, 41, 363–370. [Google Scholar] [CrossRef]
- Ogawa, E.; Furusyo, N.; Azuma, K.; Nakamuta, M.; Nomura, H.; Dohmen, K.; Satoh, T.; Kawano, A.; Koyanagi, T.; Ooho, A.; et al. Kyushu University Liver Disease Study (KULDS) Group. Elbasvir plus grazoprevir for patients with chronic hepatitis C genotype 1: A multicenter, real –world cohort study focusing on chronic kidney disease. Antivir. Res. 2018, 159, 143–152. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.; Woo, H.; Heo, J.; Park, S.; Hong, Y.; Yoon, K.; Kim, D.; Kim, G.; Kim, H.; Song, G.; et al. Real-life effectiveness and safety of glecaprevir/pibrentasvir for Korean patients with chronic hepatitis C at a single institution. Gut Liver 2021, 15, 440–450. [Google Scholar] [CrossRef]
- Stein, K.; Albrecht, S.; Hartwig, K.; Gerlinde, T.; Uwe, N.; Christine, J.; Heyne, R.; Serfert, Y.; Niedereau, C.; Zeuzem, S.; et al. German Hepatitis C-Registry. Hepatitis C therapy with grazoprevir/elbasvir and glecaprevir/pibrentasvir in patients with advanced chronic kidney disease; data from the German Hepatitis C-Registry (DHC-R). Eur. J. Gastroenterol. Hepatol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Munoz-Gomez, R.; Rincon, D.; Ahumada, A.; Hernandez, E.; Devesa, M.; Izquierdo, S.; Ortiz, M.; Hernandez-Albujar, A.; Fernandez-Rodriguez, C.; Calvo, M.; et al. Therapy with ombitasvir/paritaprevir/ritonavir plus dasabuvir is effective and safe for the treatment of genotypes 1 and 4 hepatitis C virus (HCV) infection in patients with severe renal impairment: A multicenter experience. J. Viral Hepat. 2017, 24, 464–471. [Google Scholar] [CrossRef]
- Liu, C.; Shih, Y.; Yang, S.; Lin, C.; Fang, Y.; Cheng, P.; Chen, C.; Peng, C.; Hsieh, T.; Chiu, Y.; et al. Paritaprevir/ritonavir, ombitasvir plus dasabuvir for East Asian non-cirrhotic hepatitis C virus genotype 1b patients receiving hemodialysis. J. Gastroenterol. Hepatol. 2019, 34, 1977–1983. [Google Scholar] [CrossRef] [PubMed]
- Suda, G.; Hasebe, C.; Abe, M.; Kurosaki, M.; Itakura, J.; Izumi, N.; Uchida, Y.; Mochida, S.; Haga, H.; Ueno, Y.; et al. NORTE Study Group. Safety and efficacy of glecaprevir and pibrentasvir in Japanese hemodialysis patients with genotype 2 hepatitis C virus infection. J. Gastroenterol. 2019, 54, 641–649. [Google Scholar] [CrossRef]
- Atsukawa, M.; Tsubota, A.; Toyoda, H.; Takaguchi, K.; Kondo, C.; Okubo, T.; Hiraoka, A.; Michitaka, K.; Fujioka, S.; Uojima, H.; et al. Efficacy and safety of elbasvir/grazoprevir for Japanese patients with genotype 1b chronic hepatitis C complicated by chronic kidney disease, including those undergoing hemodialysis: A post hoc analysis of a multicenter study. J. Gastroenterol. Hepatol. 2019, 34, 364–369. [Google Scholar] [CrossRef]
- Alric, L.; Ollivier-Hourmand, I.; Berard, E.; Hillaire, S.; Guillaume, M.; Vallet-Pichard, A.; Bernard-Cabert, B.; Loustaud-Ratti, V.; Bourliere, M.; de Ledinghen, V.; et al. Grazoprevir plus elbasvir in HCV genotype -1 or -4 infected patients with stage 4/5 severe chronic kidney disease is safe and effective. Kidney Int. 2018, 94, 206–213. [Google Scholar] [CrossRef]
- Liu, C.; Peng, C.; Fang, Y.; Kao, W.; Yang, S.; Lin, C.; Lai, H.; Su, W.; Fang, S.; Chang, C.; et al. Elbasvir/grazoprevir for hepatitis C virus genotype 1b East—Asian patients receiving hemodialysis. Sci. Rep. 2020, 10, 9180. [Google Scholar] [CrossRef] [PubMed]
- Atsukawa, M.; Tsubota, A.; Toyoda, H.; Takaguchi, K.; Nakamuta, M.; Watanabe, T.; Michitaka, K.; Ikegami, T.; Nozaki, A.; Uojima, H.; et al. The efficacy and safety of glecaprevir plus pibrentasvir in 141 patients with severe renal impairment: A prospective multicenter study. Aliment. Pharm. 2019, 49, 1230–1241. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Yang, S.; Peng, C.; Lin, W.; Liu, C.; Su, T.; Tseng, T.; Chen, P.; Chen, D.; Kao, J. Glecaprevir/pibrentasvir for patients with chronic hepatitis C virus infection and severe renal impairment. J. Viral Hepat. 2020, 27, 568–575. [Google Scholar] [CrossRef]
- Yen, H.; Su, P.; Zeng, Y.; Liu, I.; Huang, S.; Hsu, Y.; Chen, Y.; Yang, C.; Wu, S.; Chou, K. Glecaprevir-pibrentasvir for chronic hepatitis C; comparing treatment effect in patients with and without end-stage renal disease in a real world setting. PLoS ONE 2020, 15, e237582. [Google Scholar] [CrossRef]
- Suda, G.; Kurosaki, M.; Itakura, J.; Izumi, N.; Uchida, Y.; Mochida, S.; Hasebe, C.; Abe, M.; Haga, H.; Ueno, Y.; et al. NORTE Study Group. Safety and efficacy of elbasvir and grazoprevir in Japanese hemodialysis patients with genotype 1 hepatitis C virus infection. J. Gastroenterol. 2019, 54, 78–86. [Google Scholar]
- Borgia, S.; Dearden, J.; Yoshida, E.; Shafran, S.; Brown, A.; Ben-Ari, Z.; Cramp, M.; Cooper, C.; Foxton, M.; Rodriguez, C.; et al. Sofosbuvir/velpatasvir for 12 weeks in hepatitis C virus-infected patients with end-stage renal disease undergoing dialysis. J. Hepatol. 2019, 71, 660–665. [Google Scholar] [CrossRef] [Green Version]
- Lawitz, E.; Landis, C.; Flamm, S.; Bonacini, M.; Ortiz-Lasanta, G.; Huang, J.; Zhang, J.; Kirby, B.; De-Oertel, S.; Hyland, R.; et al. Sofosbuvir plus ribavirin and sofosbuvir plus ledipasvir in patients with genotype 1 or 3 hepatitis C virus and severe renal impairment: A multicenter, phase 2b, non-randomised, open-label study. Lancet Gastroenterol. Hepatol. 2020, 5, 918–926. [Google Scholar] [CrossRef]
- Chuang, W.; Hu, T.; Buggisch, P.; Moreno, C.; Su, W.; Biancone, L.; Camargo, M.; Hyland, R.; Lu, S.; Kirby, B.; et al. Ledipasvir/sofosbuvir for 8, 12, or 24 weeks in hepatitis C patients undergoing dialysis for end-stage renal disease. Am. J. Gastroenterol. 2021, 116, 1924–1928. [Google Scholar] [CrossRef] [PubMed]
- Kirby, B.; Symonds, W.; Kearney, B.; Mathias, A. Pharmacokinetic, pharmacodynamic, and drug-interaction profile of the hepatitis C virus NS5B polymerase inhibitor sofosbuvir. Clin. Pharm. 2015, 54, 677–690. [Google Scholar] [CrossRef]
- Fabrizi, F.; Cerutti, R.; Dixit, V.; Ridruejo, E. Sofosbuvir-based regimens for HCV in stage 4–5 chronic kidney disease: A systematic review with meta-analysis. Nefrologia 2021, 41, 578–589. [Google Scholar] [CrossRef]
- Li, M.; Chen, J.; Fang, Z.; Lin, Q. Sofosbuvir-based regimen is safe and effective for hepatitis C infected patients with stage 4–5 chronic kidney disease: A systematic review and meta-analysis. Virol. J. 2019, 16, 34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaur, N.; Malhotra, V.; Agrawal, D.; Singh, S.; Beniwal, P.; Sharma, S.; Jhorawal, R.; Rathore, V.; Joshi, H. Sofosbuvir-velpatasvir fixed drug combination for the treatment of chronic hepatitis C infection in patients with end-stage renal disease and kidney transplantation. J. Clin. Exp. Hepatol. 2020, 10, 189–193. [Google Scholar] [CrossRef] [PubMed]
- Butt, A.; Ren, Y.; Puenpatom, A.; Arduino, J.; Kumar, R.; Abou-Samra, A. Effectiveness, treatment completion and safety of sofosbuvir/ledipasvir and paritaprevir/ritonavir/ombitasvir+dasabuvir in patients with chronic kidney disease: An ERCHIVES study. Aliment. Pharm. 2018, 48, 35–43. [Google Scholar] [CrossRef] [Green Version]
- Bhamidimarri, K.; Czul, F.; Peyton, A.; Levy, C.; Hernandez, M.; Jeffers, L.; Roth, D.; Schiff, E.; O’Brien, C.; Martin, P. Safety, efficacy and tolerability of half-dose sofosbuvir plus simeprevir in treatment of hepatitis C in patients with end stage renal disease. J. Hepatol. 2015, 63, 763–765. [Google Scholar]
- Singh, A.; Kumari, S.; Kumar, P.; De, A.; Singh, V. Sofosbuvir with NS5a inhibitors in hepatitis C virus infection with severe renal insufficiency. J. Viral Hepat. 2018, 25, 1501–1526. [Google Scholar] [CrossRef]
- Eletreby, R.; El-Serafy, M.; Anees, M.; Kasem, G.; Salama, M.; Elkhouly, M.; Hamdy, M.; Haleem, H.; Kamal, E.; Abdel-Razek, V.; et al. Sofosbuvir-containing regimens are safe and effective in the treatment of HCV patients with moderate to severe renal impairment. Liver Int. 2020, 40, 797–805. [Google Scholar] [CrossRef]
- Okubo, T.; Atsukawa, M.; Tsubota, A.; Toyoda, H.; Shimada, N.; Abe, H.; Kato, K.; Hayama, K.; Arai, T.; Nagakawa-Igashita, A.; et al. Efficacy and safety of ledipasvir/sofosbuvir for genotype 1b chronic hepatitis C patients with moderate renal impairment. Hepatol. Int. 2018, 12, 133–142. [Google Scholar] [CrossRef]
- Taneja, S.; Duseja, A.; Mehta, M.; De, A.; Verma, N.; Premkumar, M.; Dhiman, R.; Singh, V.; Singh, M.; Ratho, R.; et al. Sofosbuvir and velpatasvir combination is safe and effective in treating chronic hepatitis C in end-stage renal disease on maintenance hemodialysis. Liver Int. 2021, 41, 705–709. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Chen, C.; Su, W.; Tseng, K.; Lo, C.; Liu, C.; Chen, J.; Peng, C.; Shih, Y.; Yang, S.; et al. Sofosbuvir/velpatasvir with or without low-dose ribavirin for patients with chronic hepatitis C virus infection and severe renal impairment. Gut 2021. [Google Scholar] [CrossRef] [PubMed]
- Compropst, M.; Denning, J.; Clemens, D.; Marbury, T.; Alcorn, H.; Smith, W.; Sale, M.; Fang, L.; Berrey, M.; Symonds, W. The effect of renal impairment and end stage renal disease on the single dose pharmacokinetics of PSI-7977. J. Hepatol. 2012, 56, S433. [Google Scholar] [CrossRef]
- Desnoyer, A.; Pospai, D.; Le, M.; Gervais, A.; Heurgue-Berlot, A.; Laradi, A.; Harent, S.; Pinto, A.; Salmon, D.; Hillaire, S.; et al. Pharmacokinetics, safety and efficacy of a full dose sofosbuvir-based regimen given daily in hemodialysis patients with chronic hepatitis C. J. Hepatol. 2016, 65, 40–47. [Google Scholar] [CrossRef] [PubMed]
- AASLD/IDSA. HCV Guidance: Recommendations for Testing, Managing, and Treating Hepatitis C. Patients with Renal Impairment. Available online: https://www.hcvguidelines.org (accessed on 29 September 2021).
- Hu, T.; Su, W.; Yang, C.; Yang, C.; Kuo, H.; Chen, Y.; Yeh, Y.; Chen, S.; Tsao, Y.; Chen, K.; et al. Elimination of hepatitis C virus in a dialysis population: A collaborative care model in Taiwan. Am. J. Kidney Dis. 2021, 78, 511–519. [Google Scholar] [CrossRef]
- WHO. Guidelines for the Screening Care and Treatment of Persons with Chronic Hepatitis C Infection. 2018. Available online: https://apps.who.int/iris/bitstream/handle/10665/273174/9789241550345-eng.pdf (accessed on 27 July 2021).
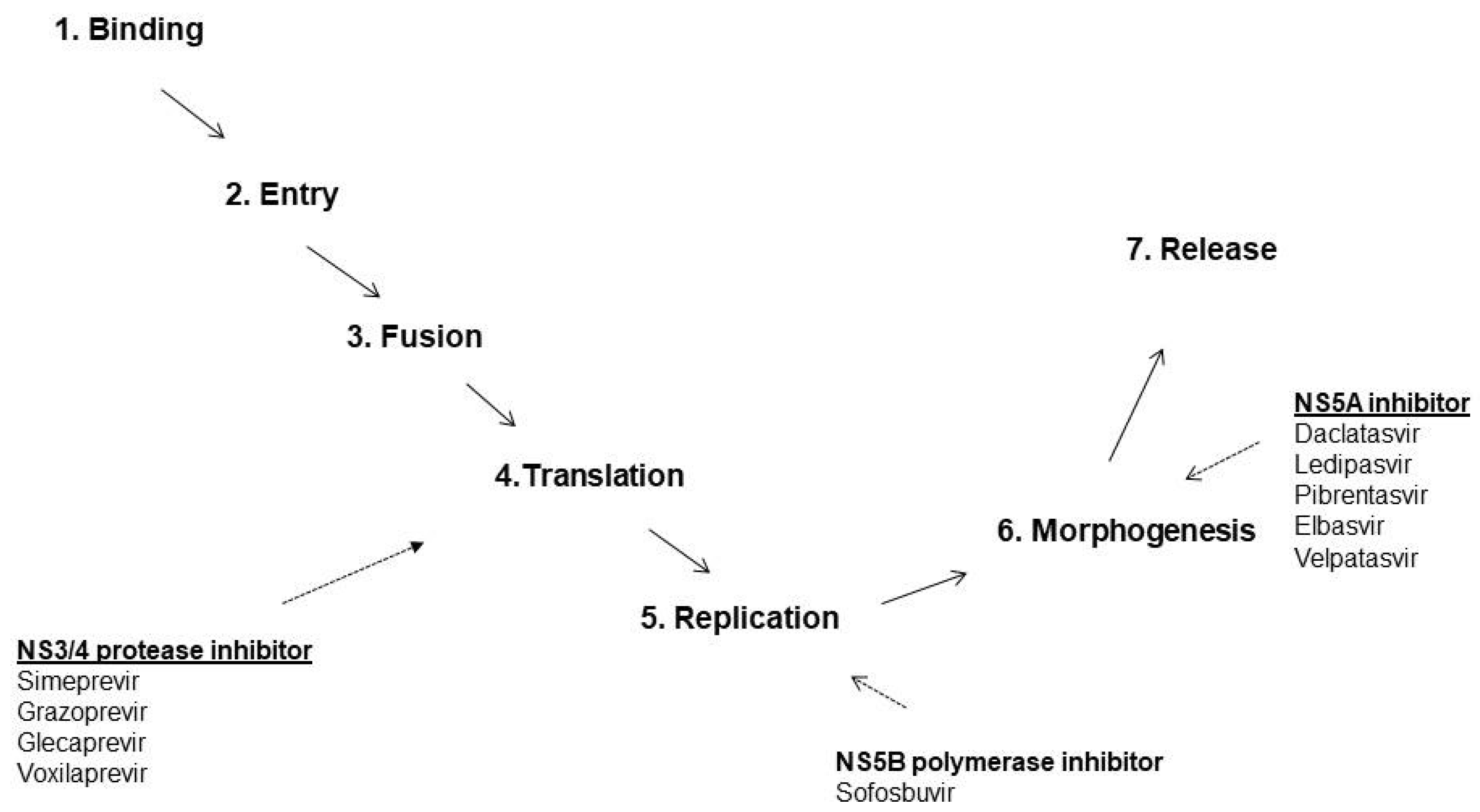

| Group | Target | Drug | Others |
|---|---|---|---|
| Protease inhibitors | NS3/NS4A | Simeprevir Paritaprevir Grazoprevir Voxilaprevir Glecaprevir | No dose adjustment in patients with stage 4–5 CKD |
| Non-nucleoside polymerase inhibitors | NS5B | Dasabuvir Beclabuvir | No dose adjustment in patients with stage 4–5 CKD |
| Nucleoside polymerase inhibitors | NS5B | Sofosbuvir | Licensed for patients with stage 4–5 CKD (since Nov 2019) |
| NS5A inhibitors | NS5A | Daclatasvir Elbasvir Ombitasvir Velpatasvir Ledipasvir | No dose adjustment in patients with stage 4–5 CKD |
| DAA Regimen | Dose | HCV Genotype | ClinicalTrials.gov Number (Gov Identifier) |
|---|---|---|---|
| Elbasvir/Grazoprevir | Daily fixed-dose combination (50 mg/100 mg) for 12 weeks | 1, and 4 | [C-SURFER] NCT 02092350 |
| Glecaprevir/Pibrentasvir | Daily fixed-dose combination (100 mg/40 mg × 3) for 12 or 8 weeks | 1, 2, 3, 4, 5, and 6 | [EXPEDITION-4] NCT 02651194 [EXPEDITION-5] NCT 03069365 |
| Ledipasvir/Sofosbuvir | Daily fixed-dose combination (90 mg/400 mg) for 12 weeks | 1, 4, 5, and 6 | NCT 03036852 |
| Sofosbuvir/Velpatasvir | Daily fixed-dose combination (400 mg/100 mg) for 12 weeks | 1, 2, 3, 4, 5, and 6 | NCT 01958281 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fabrizi, F.; Cerutti, R.; Messa, P. An Updated View on the Antiviral Therapy of Hepatitis C in Chronic Kidney Disease. Pathogens 2021, 10, 1381. https://doi.org/10.3390/pathogens10111381
Fabrizi F, Cerutti R, Messa P. An Updated View on the Antiviral Therapy of Hepatitis C in Chronic Kidney Disease. Pathogens. 2021; 10(11):1381. https://doi.org/10.3390/pathogens10111381
Chicago/Turabian StyleFabrizi, Fabrizio, Roberta Cerutti, and Piergiorgio Messa. 2021. "An Updated View on the Antiviral Therapy of Hepatitis C in Chronic Kidney Disease" Pathogens 10, no. 11: 1381. https://doi.org/10.3390/pathogens10111381
APA StyleFabrizi, F., Cerutti, R., & Messa, P. (2021). An Updated View on the Antiviral Therapy of Hepatitis C in Chronic Kidney Disease. Pathogens, 10(11), 1381. https://doi.org/10.3390/pathogens10111381






