Post COVID-19 Syndrome in Patients with Asymptomatic/Mild Form
Abstract
:1. Introduction
2. Methods
3. Results
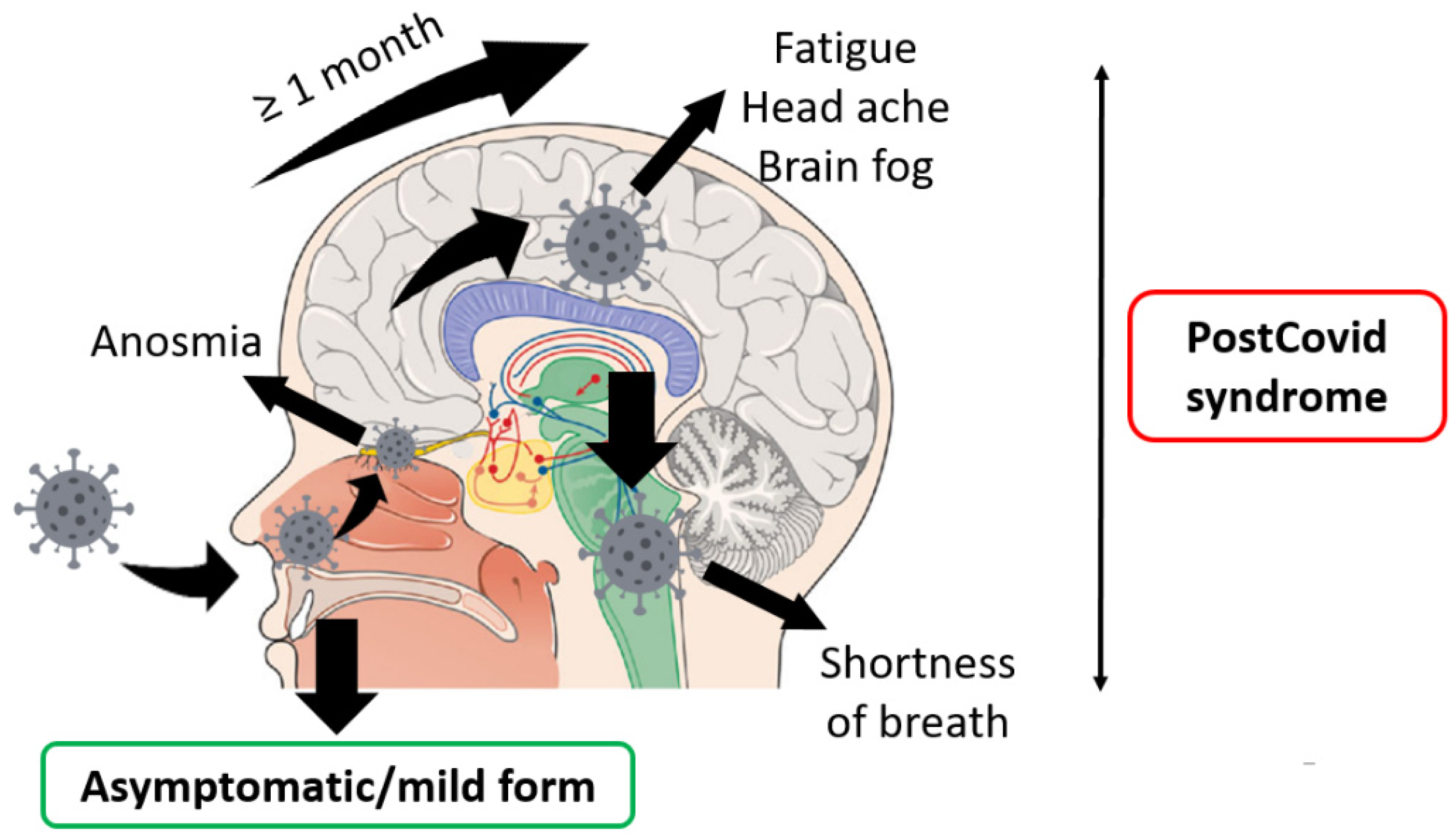
- PCS developed among 30–60% of patients with asymptomatic or mild forms of COVID-19 on average.
- The most common symptoms were fatigue, shortness of breath, cough, anosmia, and ageusia. Headaches, brain fog, and other symptoms of central nervous system damage were also reported.
- Most common PCS occurs among women (on average 60%).
4. Discussion
4.1. Pathogenesis of the Described Complications
4.1.1. Fatigue
4.1.2. Shortness of Breath, Cough
4.1.3. Anosmia
4.1.4. Headaches, Brain, and Other CNS Symptoms
4.2. The Hypothesis
- -
- -
- -
- higher number of activated and terminally differentiated T cell populations (CD38 and HLA-DR-positive activated T cells) among women [75];
- -
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ni, W.; Yang, X.; Yang, D.; Bao, J.; Li, R.; Xiao, Y.; Hou, C.; Wang, H.; Liu, J.; Yang, D.; et al. Role of angiotensin-converting enzyme 2 (ACE2) in COVID-19. Crit. Care 2020, 24, 422. [Google Scholar] [CrossRef]
- Zhang, H.; Li, H.B.; Lyu, J.R.; Lei, X.M.; Li, W.; Wu, G.; Lyu, J.; Dai, Z.M. Specific ACE2 expression in small intestinal enterocytes may cause gastrointestinal symptoms and injury after 2019-nCoV infection. Int. J. Infect. Dis. 2020, 96, 19–24. [Google Scholar] [CrossRef]
- Ojo, A.S.; Balogun, S.A.; Williams, O.T.; Ojo, O.S. Pulmonary Fibrosis in COVID-19 Survivors: Predictive Factors and Risk Reduction Strategies. Pulm. Med. 2020, 2020, 6175964. [Google Scholar] [CrossRef]
- Gudima, G.O.; Khaitov, R.M.; Kudlay, D.A.; Khaitov, M.R. Molecular immunological aspects of diagnostics, prevention and treatment of coronavirus infection. Immunologiya 2021, 42, 198–210. [Google Scholar] [CrossRef]
- Amenta, E.M.; Spallone, A.; Rodriguez-Barradas, M.C.; El Sahly, H.M.; Atmar, R.L.; Kulkarni, P.A. Postacute COVID-19: An overview and approach to classification. In Open Forum Infectious Diseases; Oxford University Press: Oxford, UK, 2020; Volume 7. [Google Scholar]
- Ceravolo, M.G.; Arienti, C.; De Sire, A.; Andrenelli, E.; Negrini, F.; Lazzarini, S.G.; Patrini, M.; Negrini, S. Rehabilitation and COVID-19: The Cochrane Rehabilitation 2020 rapid living systematic review. Eur. J. Phys. Rehabil. Med. 2020, 56, 642–651. [Google Scholar] [CrossRef]
- Post-COVID Conditions | CDC. Available online: https://www.cdc.gov/coronavirus/2019-ncov/long-term-effects/index.html (accessed on 26 October 2021).
- Carod-Artal, F.J. Post-COVID-19 syndrome: Epidemiology, diagnostic criteria and pathogenic mechanisms involved. Rev. Neurol. 2021, 72, 384–396. [Google Scholar] [CrossRef] [PubMed]
- Ladds, E.; Rushforth, A.; Wieringa, S.; Taylor, S.; Rayner, C.; Husain, L.; Greenhalgh, T. Persistent symptoms after COVID-19: Qualitative study of 114 “long Covid” patients and draft quality principles for services. BMC Health Serv. Res. 2020, 20, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Coronavirus (COVID-19) Infection Survey, UK Statistical Bulletins—Office for National Statistics. Available online: https://www.ons.gov.uk/peoplepopulationandcommunity/healthandsocialcare/conditionsanddiseases/bulletins/coronaviruscovid19infectionsurveypilot/previousReleases (accessed on 26 October 2021).
- Living with COVID-19—Second Review. 2021. Available online: https://evidence.nihr.ac.uk/themedreview/living-with-covid19-second-review/ (accessed on 26 October 2021).
- Augustin, M.; Schommers, P.; Stecher, M.; Dewald, F.; Gieselmann, L.; Gruell, H.; Horn, C.; Vanshylla, K.; Di Cristanziano, V.; Osebold, L.; et al. Post-COVID syndrome in non-hospitalised patients with COVID-19: A longitudinal prospective cohort study. Lancet Reg. Health Eur. 2021, 6, 100122. [Google Scholar] [CrossRef] [PubMed]
- Tenforde, M.W.; Kim, S.S.; Lindsell, C.J.; Rose, E.B.; Shapiro, N.I.; Files, D.C.; Gibbs, K.W.; Erickson, H.L.; Steingrub, J.S.; Smithline, H.A.; et al. Symptom Duration and Risk Factors for Delayed Return to Usual Health Among Outpatients with COVID-19 in a Multistate Health Care Systems Network—United States, March–June 2020. Morb. Mortal. Wkly. Rep. 2020, 69, 993–998. [Google Scholar] [CrossRef]
- Carvalho-Schneider, C.; Laurent, E.; Lemaignen, A.; Beaufils, E.; Bourbao-Tournois, C.; Laribi, S.; Flament, T.; Ferreira-Maldent, N.; Bruyère, F.; Stefic, K.; et al. Follow-up of adults with noncritical COVID-19 two months after symptom onset. Clin. Microbiol. Infect. 2021, 27, 258–263. [Google Scholar] [CrossRef]
- Huang, Y.; Pinto, M.D.; Borelli, J.L.; Mehrabadi, M.A.; Abrihim, H.; Dutt, N.; Lambert, N.; Nurmi, E.L.; Chakraborty, R.; Rahmani, A.M.; et al. COVID Symptoms, Symptom Clusters, and Predictors for Becoming a Long-Hauler: Looking for Clarity in the Haze of the Pandemic. medRxiv 2021. medRxiv:21252086. [Google Scholar]
- Tabacof, L.; Tosto-Mancuso, J.; Wood, J.; Cortes, M.; Kontorovich, A.; McCarthy, D.; Rizk, D.; Nasr, L.; Breyman, E.; Mohammadi, N.; et al. Post-acute COVID-19 syndrome negatively impacts health and wellbeing despite less severe acute infection. medRxiv 2020. medRxiv:20226126. [Google Scholar]
- Logue, J.K.; Franko, N.M.; McCulloch, D.J.; McDonald, D.; Magedson, A.; Wolf, C.R.; Chu, H.Y. Sequelae in Adults at 6 Months after COVID-19 Infection. JAMA Netw. Open 2021, 4, e210830. [Google Scholar] [CrossRef]
- Bell, M.L.; Catalfamo, C.J.; Farland, L.V.; Ernst, K.C.; Jacobs, E.T.; Klimentidis, Y.C.; Jehn, M.; Pogreba-Brown, K. Post-acute sequelae of COVID-19 in a non-hospitalized cohort: Results from the Arizona CoVHORT. PLoS ONE 2021, 16, e0254347. [Google Scholar] [CrossRef] [PubMed]
- Havervall, S.; Rosell, A.; Phillipson, M.; Mangsbo, S.M.; Nilsson, P.; Hober, S.; Thålin, C. Symptoms and Functional Impairment Assessed 8 Months after Mild COVID-19 among Health Care Workers. JAMA—J. Am. Med Assoc. 2021, 325, 2015–2016. [Google Scholar] [CrossRef] [PubMed]
- Bliddal, S.; Banasik, K.; Pedersen, O.B.; Nissen, J.; Cantwell, L.; Schwinn, M.; Tulstrup, M.; Westergaard, D.; Ullum, H.; Brunak, S.; et al. Acute and persistent symptoms in non-hospitalized PCR-confirmed COVID-19 patients. Sci. Rep. 2021, 11, 13153. [Google Scholar] [CrossRef]
- Fernández-De-Las-Peñas, C.; Palacios-Ceña, D.; Gómez-Mayordomo, V.; Florencio, L.L.; Cuadrado, M.L.; Plaza-Manzano, G.; Navarro-Santana, M. Prevalence of post-COVID-19 symptoms in hospitalized and non-hospitalized COVID-19 survivors: A systematic review and meta-analysis. Eur. J. Intern. Med. 2021, 92, 55–70. [Google Scholar] [CrossRef]
- Andrews, P.J.; Pendolino, A.L.; Ottaviano, G.; Scarpa, B.; Grant, J.; Gaudioso, P.; Bordin, A.; Marchese-Ragona, R.; Leoni, D.; Cattelan, A.; et al. Olfactory and taste dysfunction among mild-to-moderate symptomatic COVID-19 positive health care workers: An international survey. Laryngoscope Investig. Otolaryngol. 2020, 5, 1019–1028. [Google Scholar] [CrossRef]
- Nalbandian, A.; Sehgal, K.; Gupta, A.; Madhavan, M.V.; McGroder, C.; Stevens, J.S.; Cook, J.R.; Nordvig, A.S.; Shalev, D.; Sehrawat, T.S.; et al. Post-acute COVID-19 syndrome. Nat. Med. 2021, 27, 601–615. [Google Scholar] [CrossRef]
- Nauen, D.W.; Hooper, J.E.; Stewart, C.M.; Solomon, I.H. Assessing Brain Capillaries in Coronavirus Disease 2019. JAMA Neurol. 2021, 78, 760–762. [Google Scholar] [CrossRef]
- Miglis, M.G.; Prieto, T.; Shaik, R.; Muppidi, S.; Sinn, D.I.; Jaradeh, S. A case report of postural tachycardia syndrome after COVID-19. Clin. Auton. Research. Nat. Publ. Group 2020, 30, 449–451. [Google Scholar] [CrossRef] [PubMed]
- Lau, S.T.; Yu, W.C.; Mok, N.S.; Tsui, P.T.; Tong, W.L.; Stella, W.C. Tachycardia amongst subjects recovering from severe acute respiratory syndrome (SARS). Int. J. Cardiol. 2005, 100, 167–169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dani, M.; Dirksen, A.; Taraborrelli, P.; Torocastro, M.; Panagopoulos, D.; Sutton, R.; Lim, P.B. Autonomic dysfunction in ‘long COVID’: Rationale, physiology and management strategies. Clinical Medicine. J. R. Coll. Physicians Lond. 2021, 21, E63. [Google Scholar]
- Halpert, G.; Watad, A.; Tsur, A.M.; Dotan, A.; Quiros-Lim, H.E.; Heidecke, H.; Gilburd, B.; Haik, J.; Levy, Y.; Blank, M.; et al. Autoimmune dysautonomia in women with silicone breast implants. J. Autoimmun. 2021, 120, 102631. [Google Scholar] [CrossRef] [PubMed]
- Schofield, J.R.; Blitshteyn, S.; Shoenfeld, Y.; Hughes, G.R.V. Postural tachycardia syndrome (POTS) and other autonomic disorders in antiphospholipid (Hughes) syndrome (APS). Lupus 2014, 23, 697–702. [Google Scholar] [CrossRef]
- Yu, X.; Stavrakis, S.; Hill, M.A.; Huang, S.; Reim, S.; Li, H.; Khan, M.; Hamlett, S.; Cunningham, M.W.; Kem, D.C. Autoantibody activation of beta-adrenergic and muscarinic receptors contributes to an “autoimmune” orthostatic hypotension. J. Am. Soc. Hypertens. 2012, 6, 40–47. [Google Scholar] [CrossRef] [Green Version]
- Townsend, L.; Moloney, D.; Finucane, C.; McCarthy, K.; Bergin, C.; Bannan, C.; Kenny, R.-A. Fatigue following COVID-19 infection is not associated with autonomic dysfunction. PLoS ONE 2021, 16, e0247280. [Google Scholar] [CrossRef]
- Mackay, A. A Paradigm for Post-COVID-19 Fatigue Syndrome Analogous to ME/CFS. Front. Neurol. 2021, 12, 1334. [Google Scholar] [CrossRef]
- Ryabkova, V.A.; Churilov, L.P.; Shoenfeld, Y. Neuroimmunology: What role for autoimmunity, neuroinflammation, and small fiber neuropathy in fibromyalgia, chronic fatigue syndrome, and adverse events after human papillomavirus vaccination? Int. J. Mol. Sci. 2019, 20, 5164. [Google Scholar] [CrossRef] [Green Version]
- Cortes Rivera, M.; Mastronardi, C.; Silva-Aldana, C.T.; Arcos-Burgos, M.; Lidbury, B.A. Myalgic encephalomyelitis/chronic fatigue syndrome: A comprehensive review. Diagnostics. Diagn. 2019, 9, 91. [Google Scholar] [CrossRef] [Green Version]
- Mackay, A. A neuro-inflammatory model can explain the onset, symptoms and flare-ups of myalgic encephalomyelitis/chronic fatigue syndrome. J. Prim. Health Care 2019, 11, 300–307. [Google Scholar] [CrossRef]
- Mackay, A.; Tate, W.P. A compromised paraventricular nucleus within a dysfunctional hypothalamus: A novel neuroinflammatory paradigm for ME/CFS. Int. J. Immunopathol. Pharmacol. 2018, 32, 2058738418812342. [Google Scholar] [CrossRef] [Green Version]
- Petracek, L.S.; Suskauer, S.J.; Vickers, R.F.; Patel, N.R.; Violand, R.L.; Swope, R.L.; Rowe, P.C. Adolescent and Young Adult ME/CFS After Confirmed or Probable COVID-19. Front. Med. 2021, 8, 668944. [Google Scholar] [CrossRef]
- Lim, E.J.; Ahn, Y.C.; Jang, E.S.; Lee, S.W.; Lee, S.H.; Son, C.G. Systematic review and meta-analysis of the prevalence of chronic fatigue syndrome/myalgic encephalomyelitis (CFS/ME). J. Transl. Medicine. BioMed Cent. 2020, 18, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Townsend, L.; Dowds, J.; O’Brien, K.; Sheill, G.; Dyer, A.H.; O’Kelly, B.; Hynes, J.P.; Mooney, A.; Dunne, J.; Ni Cheallaigh, C.; et al. Persistent poor health after COVID-19 is not associated with respiratory complications or initial disease severity. Ann. Am. Thorac. Soc. 2021, 18, 997–1003. [Google Scholar] [CrossRef]
- Townsend, A. Autoimmunity to ACE2 as a possible cause of tissue inflammation in COVID-19. Med. Hypotheses 2020, 144, 110043. [Google Scholar] [CrossRef] [PubMed]
- Kanduc, D.; Shoenfeld, Y. On the molecular determinants of the SARS-CoV-2 attack. Clin. Immunol. 2020, 215, 108426. [Google Scholar] [CrossRef]
- Cappello, F.; Marino Gammazza, A.; Dieli, F.; Conway de Macario, E.; Macario, A.J. Does sars-cov-2 trigger stress-induced autoimmunity by molecular mimicry? A hypothesis. J. Clin. Med. 2020, 9, 2038. [Google Scholar] [CrossRef] [PubMed]
- Gammazza, A.M.; Légaré, S.; Bosco, G.L.; Fucarino, A.; Angileri, F.; de Macario, E.C.; Macario, A.J.; Cappello, F. Human molecular chaperones share with SARS-CoV-2 antigenic epitopes potentially capable of eliciting autoimmunity against endothelial cells: Possible role of molecular mimicry in COVID-19. Cell Stress Chaperones 2020, 25, 737–741. [Google Scholar] [CrossRef]
- Cappello, F. Is COVID-19 a proteiform disease inducing also molecular mimicry phenomena?. Cell Stress and Chaperones. Cell Stress Chaperones 2020, 25, 381–382. [Google Scholar] [CrossRef] [PubMed]
- Perrin, R.; Riste, L.; Hann, M.; Walther, A.; Mukherjee, A.; Heald, A. Into the looking glass: Post-viral syndrome post COVID-19. Med. Hypotheses 2020, 144, 110055. [Google Scholar] [CrossRef] [PubMed]
- Morbini, P.; Benazzo, M.; Verga, L.; Pagella, F.G.; Mojoli, F.; Bruno, R.; Marena, C. Ultrastructural evidence of direct viral damage to the olfactory complex in patients testing positive for SARS-COV-2. JAMA Otolaryngol.—Head Neck Surg. 2020, 146, 972–973. [Google Scholar] [CrossRef] [PubMed]
- Meinhardt, J.; Radke, J.; Dittmayer, C.; Franz, J.; Thomas, C.; Mothes, R.; Laue, M.; Schneider, J.; Brünink, S.; Greuel, S.; et al. Olfactory transmucosal SARS-CoV-2 invasion as a port of central nervous system entry in individuals with COVID-19. Nat. Neurosci. 2021, 24, 168–175. [Google Scholar] [CrossRef]
- Gordon, D.E.; Jang, G.M.; Bouhaddou, M.; Xu, J.; Obernier, K.; White, K.M.; O’Meara, M.J.; Rezelj, V.V.; Guo, J.Z.; Swaney, D.L.; et al. A SARS-CoV-2 protein interaction map reveals targets for drug repurposing. Nature 2020, 583, 459–468. [Google Scholar] [CrossRef]
- Lee, Y.; Min, P.; Lee, S.; Kim, S.-W. Prevalence and duration of acute loss of smell or taste in COVID-19 patients. J. Korean Med. Sci. 2020, 35, e174. [Google Scholar] [CrossRef]
- Romero-Sánchez, C.M.; Díaz-Maroto, I.; Fernández-Díaz, E.; Sánchez-Larsen, Á.; Layos-Romero, A.; García-García, J.; González, E.; Redondo-Peñas, I.; Perona-Moratalla, A.B.; Del Valle-Pérez, J.A.; et al. Neurologic manifestations in hospitalized patients with COVID-19: The ALBACOVID registry. Neurology 2020, 95, e1060–e1070. [Google Scholar] [CrossRef]
- Reichard, R.R.; Kashani, K.B.; Boire, N.A.; Constantopoulos, E.; Guo, Y.; Lucchinetti, C.F. Neuropathology of COVID-19: A spectrum of vascular and acute disseminated encephalomyelitis (ADEM)-like pathology. Acta Neuropathol. 2020, 140, 1–6. [Google Scholar] [CrossRef]
- Bortolato, B.; Carvalho, A.F.; Soczynska, J.K.; Perini, G.I.; McIntyre, R.S. The Involvement of TNF-α in Cognitive Dysfunction Associated with Major Depressive Disorder: An Opportunity for Domain Specific Treatments. Curr. Neuropharmacol. 2015, 13, 558–576. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aiello, A.; Farzaneh, F.; Candore, G.; Caruso, C.; Davinelli, S.; Gambino, C.M.; Ligotti, M.E.; Zareian, N.; Accardi, G. Immunosenescence and its hallmarks: How to oppose aging strategically? A review of potential options for therapeutic intervention. Front. Immunol. 2019, 10, 2247. [Google Scholar] [CrossRef] [Green Version]
- Moriguchi, T.; Harii, N.; Goto, J.; Harada, D.; Sugawara, H.; Takamino, J.; Ueno, M.; Sakata, H.; Kondo, K.; Myose, N.; et al. A first case of meningitis/encephalitis associated with SARS-Coronavirus-2. Int. J. Infect. Dis. 2020, 94, 55–58. [Google Scholar] [CrossRef]
- Khan, S.; Gomes, J. Neuropathogenesis of SARS-CoV-2 infection. Elife 2020, 9, e59136. [Google Scholar] [CrossRef] [PubMed]
- Brann, D.H.; Tsukahara, T.; Weinreb, C.; Lipovsek, M.; Van den Berge, K.; Gong, B.; Chance, R.; Macaulay, I.C.; Chou, H.J.; Fletcher, R.B.; et al. Non-neuronal expression of SARS-CoV-2 entry genes in the olfactory system suggests mechanisms underlying COVID-19-associated anosmia. Sci. Adv. 2020, 6, eabc5801. [Google Scholar] [CrossRef] [PubMed]
- Wallukat, G.; Hohberger, B.; Wenzel, K.; Fürst, J.; Schulze-Rothe, S.; Wallukat, A.; Hönicke, A.-S.; Müller, J. Functional autoantibodies against G-protein coupled receptors in patients with persistent Long-COVID-19 symptoms. J. Transl. Autoimmun. 2021, 4, 100100. [Google Scholar] [CrossRef]
- Kharraziha, I.; Axelsson, J.; Ricci, F.; Di Martino, G.; Persson, M.; Sutton, R.; Fedorowski, A.; Hamrefors, V. Serum activity against g protein–coupled receptors and severity of orthostatic symptoms in postural orthostatic tachycardia syndrome. J. Am. Heart Assoc. 2020, 9, e015989. [Google Scholar] [CrossRef] [PubMed]
- Shuwa, H.A.; Shaw, T.N.; Knight, S.B.; Wemyss, K.; McClure, F.A.; Pearmain, L.; Prise, I.; Jagger, C.; Morgan, D.J.; Khan, S.; et al. Alterations in T and B cell function persist in convalescent COVID-19 patients. Med [Letter] 2021, 2, 720–735.e4. [Google Scholar] [CrossRef]
- Wu, J.; Tang, L.; Ma, Y.; Li, Y.; Zhang, D.; Li, Q.; Mei, H.; Hu, Y. Immunological Profiling of COVID-19 Patients with Pulmonary Sequelae. mBio 2021, 12, e0159921. [Google Scholar] [CrossRef]
- Orologas-Stavrou, N.; Politou, M.; Rousakis, P.; Kostopoulos, I.V.; Ntanasis-Stathopoulos, I.; Jahaj, E.; Tsiligkeridou, E.; Gavriatopoulou, M.; Kastritis, E.; Kotanidou, A.; et al. Peripheral blood immune profiling of convalescent plasma donors reveals alterations in specific immune subpopulations even at 2 months post sars-cov-2 infection. Viruses 2021, 13, 26. [Google Scholar] [CrossRef]
- Knochelmann, H.M.; Dwyer, C.J.; Bailey, S.R.; Amaya, S.M.; Elston, D.M.; Mazza-McCrann, J.M.; Paulos, C.M. When worlds collide: Th17 and Treg cells in cancer and autoimmunity. Cell. Mol. Immunol. 2018, 15, 458–469. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gong, F.; Dai, Y.; Zheng, T.; Cheng, L.; Zhao, D.; Wang, H.; Liu, M.; Pei, H.; Jin, T.; Yu, D.; et al. Peripheral CD4+ T cell subsets and antibody response in COVID-19 convalescent individuals. J. Clin. Investig. 2020, 130, 6588–6599. [Google Scholar] [CrossRef]
- Tangye, S.G.; Ma, C.S.; Brink, R.; Deenick, E.K. The good, the bad and the ugly-T FH cells in human health and disease. Nat. Rev. Immunol. 2013, 13, 412–426. [Google Scholar] [CrossRef]
- Kurata, I.; Matsumoto, I.; Sumida, T. T follicular helper cell subsets: A potential key player in autoimmunity. Immunol. Med. 2021, 44, 1–9. [Google Scholar] [CrossRef]
- Collier, J.L.; Weiss, S.A.; Pauken, K.E.; Sen, D.R.; Sharpe, A.H. Not-so-opposite ends of the spectrum: CD8+ T cell dysfunction across chronic infection, cancer and autoimmunity. Nat. Immunol. 2021, 22, 809–819. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Yu, B.; Yang, Y.; Huang, J.; Liang, Y.; Zhou, J.; Li, L.; Peng, X.; Cheng, B.; Lin, Y. Immunological and inflammatory profiles during acute and convalescent phases of severe/ critically ill COVID-19 patients. Int. Immunopharmacol. 2021, 97, 107685. [Google Scholar] [CrossRef]
- Sudre, C.H.; Murray, B.; Varsavsky, T.; Graham, M.S.; Penfold, R.S.; Bowyer, R.C.; Pujol, J.C.; Klaser, K.; Antonelli, M.; Canas, L.S.; et al. Attributes and predictors of long COVID. Nat. Med. 2021, 27, 626–631. [Google Scholar] [CrossRef]
- Malkova, A.; Kudlay, D.; Kudryavtsev, I.; Starshinova, A.; Yablonskiy, P.; Shoenfeld, Y. Immunogenetic predictors of severe COVID-19. Vaccines 2021, 9, 211. [Google Scholar] [CrossRef]
- Furman, D.; Hejblum, B.P.; Simon, N.; Jojic, V.; Dekker, C.L.; Thiébaut, R.; Tibshirani, R.J.; Davis, M.M. Systems analysis of sex differences reveals an immunosuppressive role for testosterone in the response to influenza vaccination. Proc. Natl. Acad. Sci. USA 2014, 111, 869–874. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taneja, V. Sex hormones determine immune response. Front. Immunol. 2018, 9, 1931. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhao, Z.; Wang, Y.; Zhou, Y.; Ma, Y.; Zuo, W. Single-Cell RNA Expression Profiling of ACE2, the Receptor of SARS-CoV-2. Am. J. Respir. Crit. Care Med. 2020, 202, 756. [Google Scholar] [CrossRef] [PubMed]
- Hou, Y.; Zhao, J.; Martin, W.; Kallianpur, A.; Chung, M.K.; Jehi, L.; Sharifi, N.; Erzurum, S.; Eng, C.; Cheng, F. New insights into genetic susceptibility of COVID-19: An ACE2 and TMPRSS2 polymorphism analysis. BMC Med. 2020, 18, 216. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Li, L.; Feng, Z.; Wan, S.; Huang, P.; Sun, X.; Wen, F.; Huang, X.; Ning, G.; Wang, W. Comparative genetic analysis of the novel coronavirus (2019-nCoV/SARS-CoV-2) receptor ACE2 in different populations. Cell Discov. 2020, 6, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takahashi, T.; Ellingson, M.K.; Wong, P.; Israelow, B.; Lucas, C.; Klein, J.; Silva, J.; Mao, T.; Oh, J.E.; Tokuyama, M.; et al. Sex differences in immune responses that underlie COVID-19 disease outcomes. Nature 2020, 588, 315–320. [Google Scholar] [CrossRef]
- The Novel Coronavirus Pneumonia Emergency Response Epidemiology Team. The Epidemiological Characteristics of an Outbreak of 2019 Novel Coronavirus Diseases (COVID-19). China CDC Wkly. 2020, 2, 113–122. [Google Scholar] [CrossRef]
- Zeng, F.; Dai, C.; Cai, P.; Wang, J.; Xu, L.; Li, J.; Hu, G.; Wang, Z.; Zheng, F.; Wang, L. A comparison study of SARS-CoV-2 IgG antibody between male and female COVID-19 patients: A possible reason underlying different outcome between sex. J. Med. Virol. 2020, 92, 2050–2054. [Google Scholar] [CrossRef]
- Angum, F.; Khan, T.; Kaler, J.; Siddiqui, L.; Hussain, A. The Prevalence of Autoimmune Disorders in Women: A Narrative Review. Cureus 2020, 12, e8094. [Google Scholar]
- Wostyn, P. Anosmia as a predictor for post-COVID-19 fatigue syndrome. Lancet Reg. Health—Eur. 2021, 7, 100162. [Google Scholar] [CrossRef]
- Shkurnikov, M.; Nersisyan, S.; Jankevic, T.; Galatenko, A.; Gordeev, I.; Vechorko, V.; Tonevitsky, A. Association of HLA Class I Genotypes with Severity of Coronavirus Disease-19. Front. Immunol. 2021, 12, 423. [Google Scholar] [CrossRef]
- Langton, D.J.; Bourke, S.C.; Lie, B.A.; Reiff, G.; Natu, S.; Darlay, R.; Burn, J.; Echevarria, C. The influence of HLA genotype on the severity of COVID-19 infection. HLA 2021, 98, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Joseph, J.W.; Roberts, J.C.; Weaver, C.N.; Anderson, J.S.; Wong, M.L. Patients with Mild COVID-19 Symptoms and Coincident Pulmonary Embolism: A Case Series. Clin. Pract. Cases Emerg. Med. 2020, 4, 295–298. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Hu, B.; Hu, C.; Zhu, F.; Liu, X.; Zhang, J.; Wang, B.; Xiang, H.; Cheng, Z.; Xiong, Y.; et al. Clinical Characteristics of 138 Hospitalized Patients with 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA J. Am. Med Assoc. 2020, 323, 1061–1069. [Google Scholar] [CrossRef] [PubMed]
- Sartoretti, E.; Sartoretti, T.; Imoberdorf, R.; Dracklé, J.; Sartoretti-Schefer, S. Long-segment arterial cerebral vessel thrombosis after mild COVID-19. BMJ Case Rep. 2020, 13, 236571. [Google Scholar] [CrossRef] [PubMed]

| Amenta EM et al. [5] | Ceravolo MG et al. [6] | Centers for Disease Control and Prevention [7] |
|---|---|---|
| residual symptoms that persist after recovery from acute SARS-CoV-2 infection | symptoms persisting from the acute phase and their treatment | persistent COVID series of symptoms last for weeks or months |
| symptoms due to single or multiple organ dysfunction that persists after initial recovery | symptoms associated with a new disease | symptoms resulting from damage to multiple organs, such as the heart, lung, kidneys, skin, and nervous system |
| novel symptoms or syndromes that arise after mild or asymptomatic infection | late-onset symptoms resulting from COVID-19 arising at the end of the acute phase | consequences of COVID-19 treatment or prolonged hospitalisation |
| impact on a previous pathology or disability |
| Authors | Sample | Period of Observation | Symptoms (%) | PCS (%) |
|---|---|---|---|---|
| Augustin M et al. [12] | 958 SARS-CoV-2-convalescent patients, the majority initially presented with absent to mild symptoms | 4 months | anosmia (12.4), ageusia (11.1), fatigue (9.7), and shortness of breath (8.6) | 27.8 |
| 7 months | 34.8 | |||
| Tenforde MW et al. [13] | 292 young patients (mean age: 42.5 years) with mild COVID-19 16 days after diagnosis | 2–3 weeks | cough, fatigue, and dyspnea | 35.0 |
| Carvalho-Schneider C et al. [14] | 150 patients with mild COVID–19 | 2 months | asthenia (40.0), dyspnoea (30.0) anosmia/ageusia (23.0) | 66.7 |
| Yong Huang et al. [15] | 1407 records No hospitalized | 60 days | shortness of breath, chest pain, cough, or abdominal pain | 27.0 |
| Tabacof L et al. [16] | 84 less severe acute infection with PCS | 151 (54 to 255) days | fatigue (92.0%), loss of concentration/memory (74.0), weakness (68.0), headache (65.0), and dizziness (64.0) | All 84 were with PCS |
| Logue JK et al. [17] | 177 11 (6.2%) were asymptomatic, 150 (84.7%) were outpatients with mild illness, and 16 (9.0%) had moderate or severe disease requiring hospitalization | 9 months | fatigue (13.6) and loss of sense of smell or taste (13.6). 23 patients (13.0%) reported other symptoms, including brain fog (2.3) | 32.7 |
| Melanie L. Bell et al. [18] | 303 no hospitalized | 30–59 days post-diagnosis | fatigue (37.5), shortness of breath (37.5), brain fog (30.8), and stress/anxiety (30.8) | 68.7 |
| ≥60 days | 73.0 | |||
| Havervall S et al. [19] | 323 (94%) seropositive | 2 months 8 months | anosmia, fatigue, ageusia, and dyspnea | 26.0 15.0 |
| and 1072 (84%) seronegative participants | 2 months 8 months | 9.0 3.0 | ||
| Bliddal S et al. [20] | 445 Danish non-hospitalized Completely asymptomatic COVID-19 was reported by 34% | ≥4 weeks | fatigue (16.0), concentration or memory difficulties (13.0), reduced sense of smell (10.0), and shortness of breath (10.0) | 36.0 |
| ≥12 weeks | fatigue (16.0) and concentration difficulties (13.0) | 40.0 | ||
| Fernández-de-Las-Peñas C et al. [21] | 9011 non-hospitalized patients | ≥one post-COVID-19 symptom at 30, 60, or ≥90days after onset | Fatigue and dyspnea 35.0–60.0 cough (20.0–25.0), anosmia (10.0–20.0), ageusia (15.0–20.0), or joint pain (15.0–20.0) | 45.9 |
| Andrews PJ et al. [22] | Mild to moderate Health Care Workers 114 | 52 days | olfactory 73.1 taste alteration 69.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malkova, A.; Kudryavtsev, I.; Starshinova, A.; Kudlay, D.; Zinchenko, Y.; Glushkova, A.; Yablonskiy, P.; Shoenfeld, Y. Post COVID-19 Syndrome in Patients with Asymptomatic/Mild Form. Pathogens 2021, 10, 1408. https://doi.org/10.3390/pathogens10111408
Malkova A, Kudryavtsev I, Starshinova A, Kudlay D, Zinchenko Y, Glushkova A, Yablonskiy P, Shoenfeld Y. Post COVID-19 Syndrome in Patients with Asymptomatic/Mild Form. Pathogens. 2021; 10(11):1408. https://doi.org/10.3390/pathogens10111408
Chicago/Turabian StyleMalkova, Annа, Igor Kudryavtsev, Anna Starshinova, Dmitry Kudlay, Yulia Zinchenko, Anzhela Glushkova, Piotr Yablonskiy, and Yehuda Shoenfeld. 2021. "Post COVID-19 Syndrome in Patients with Asymptomatic/Mild Form" Pathogens 10, no. 11: 1408. https://doi.org/10.3390/pathogens10111408
APA StyleMalkova, A., Kudryavtsev, I., Starshinova, A., Kudlay, D., Zinchenko, Y., Glushkova, A., Yablonskiy, P., & Shoenfeld, Y. (2021). Post COVID-19 Syndrome in Patients with Asymptomatic/Mild Form. Pathogens, 10(11), 1408. https://doi.org/10.3390/pathogens10111408









