Rapid Detection of Equine Piroplasms Using Multiplex PCR and First Genetic Characterization of Theileria haneyi in Egypt
Abstract
:1. Introduction
2. Results
2.1. Molecular Detection of Equine Piroplasmosis
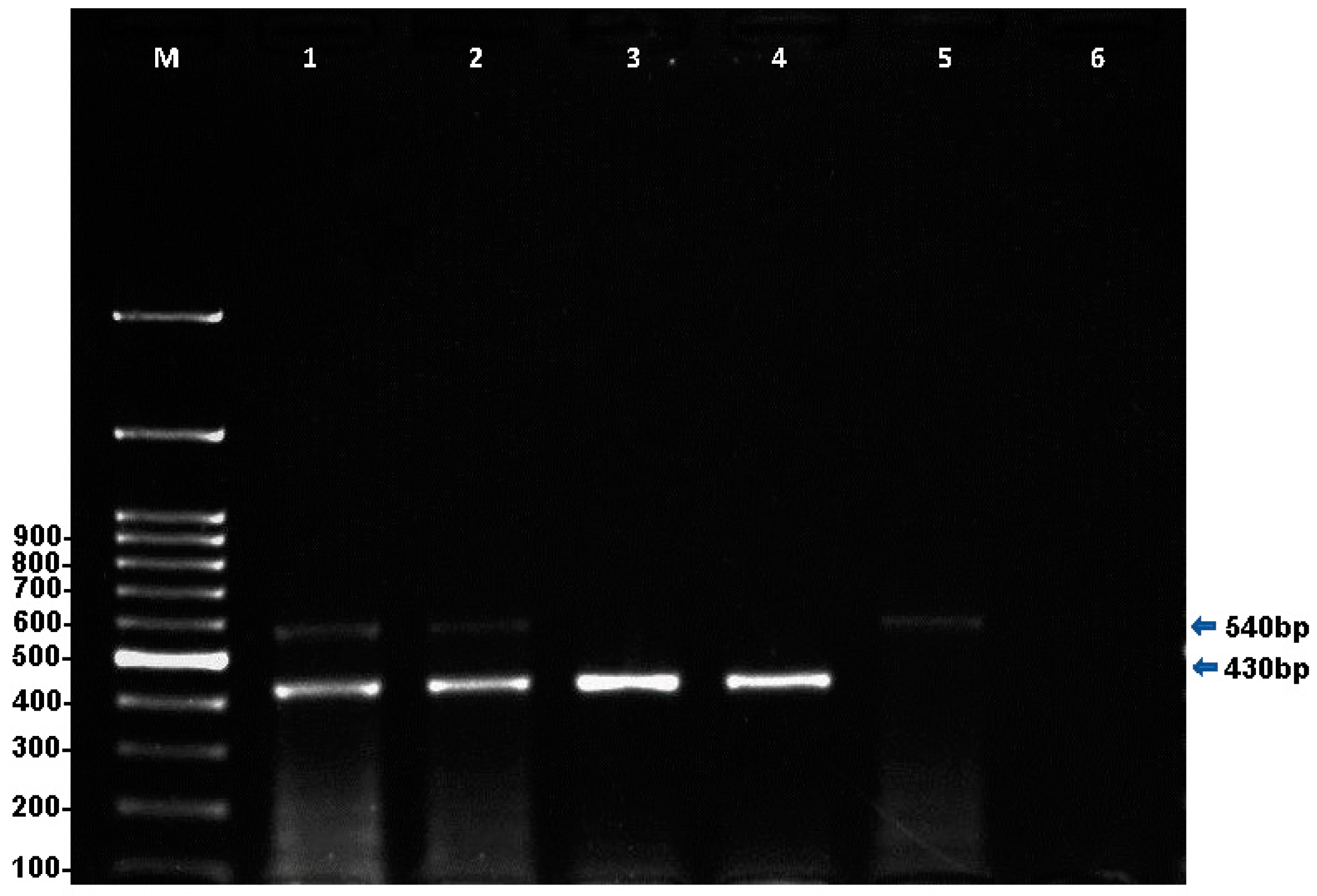
2.1.1. Multiplex PCR for the Simultaneous Detection of T. equi and B. caballi
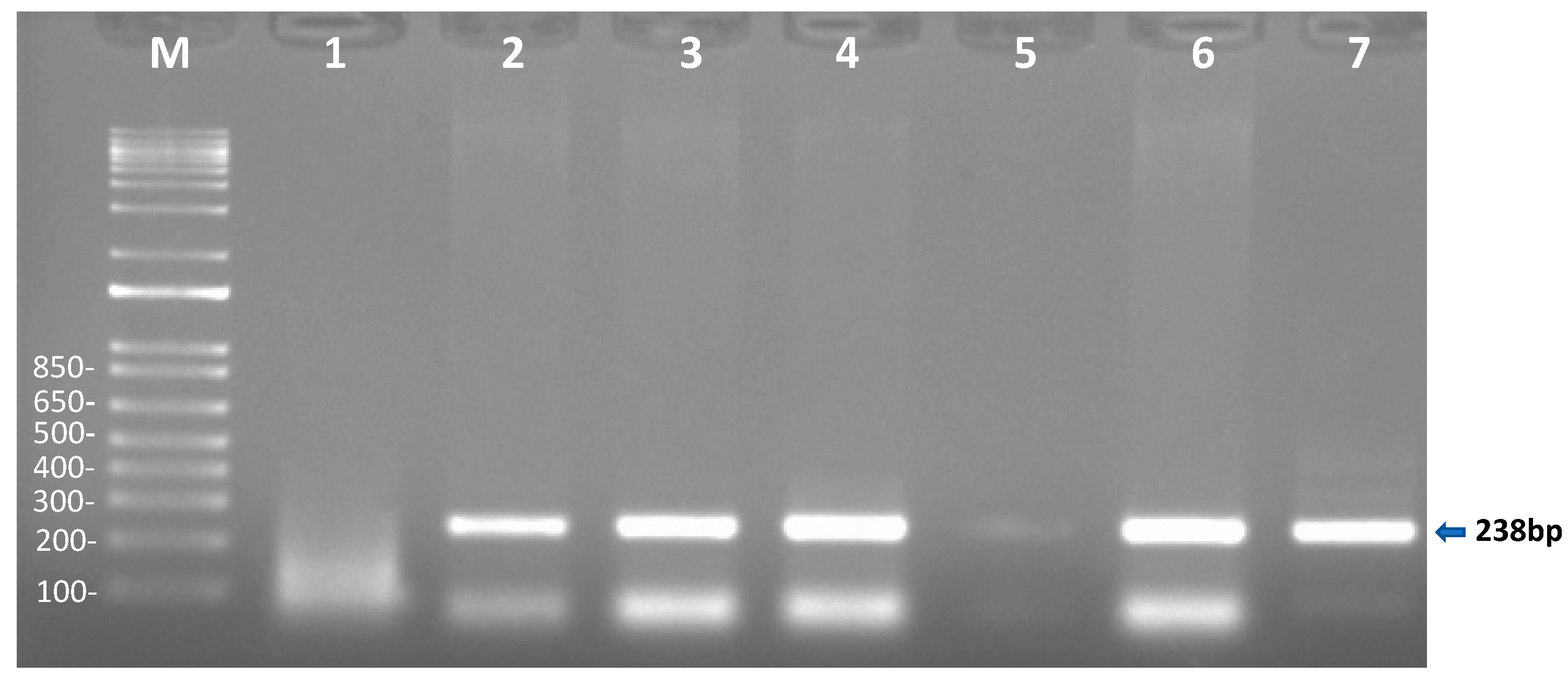
2.1.2. Conventional PCR Analysis for the Detection of T. haneyi in Egyptian Equids
2.1.3. Coinfections with T. haneyi (cPCR), T. equi, and B. caballi (mPCR)
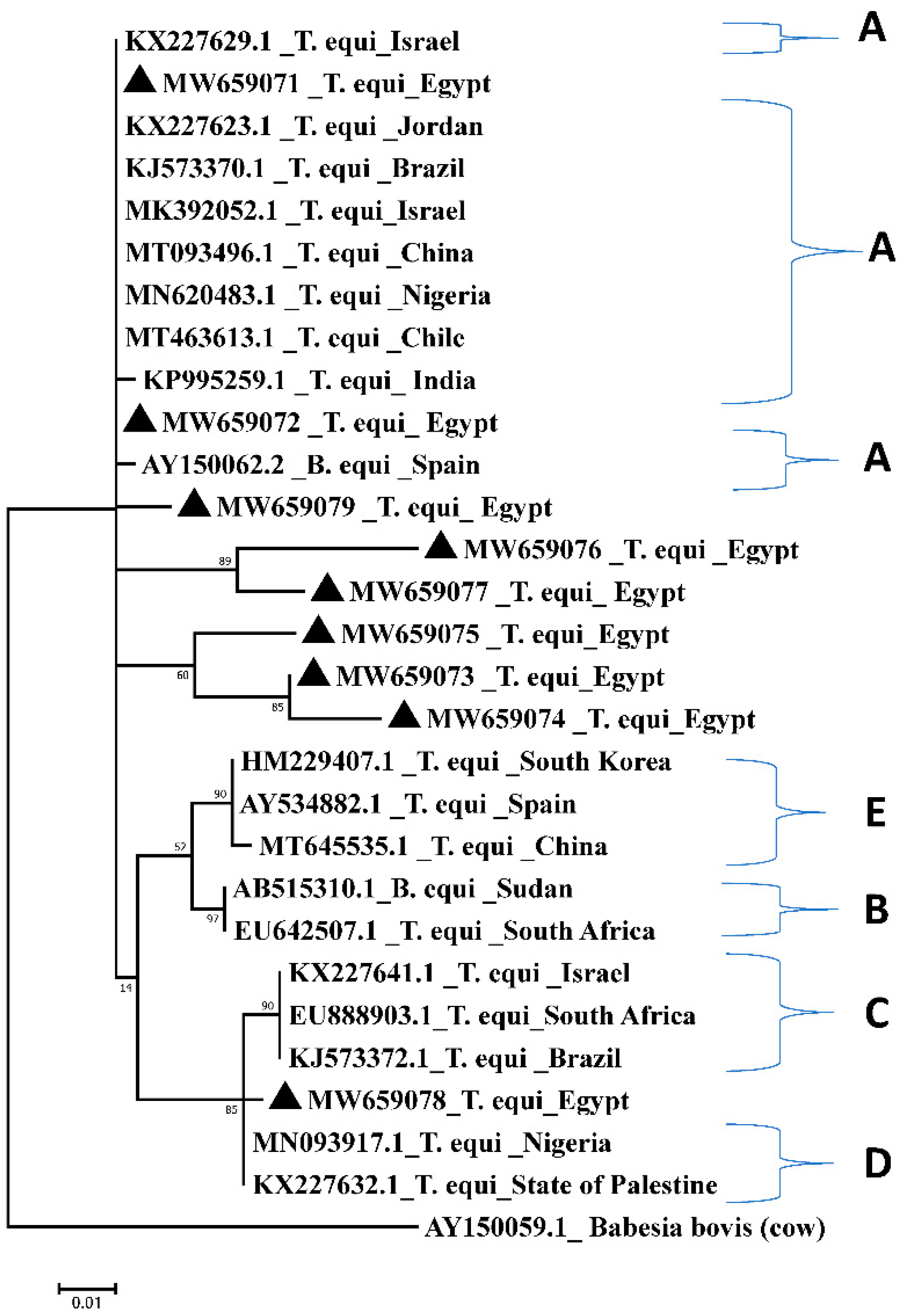
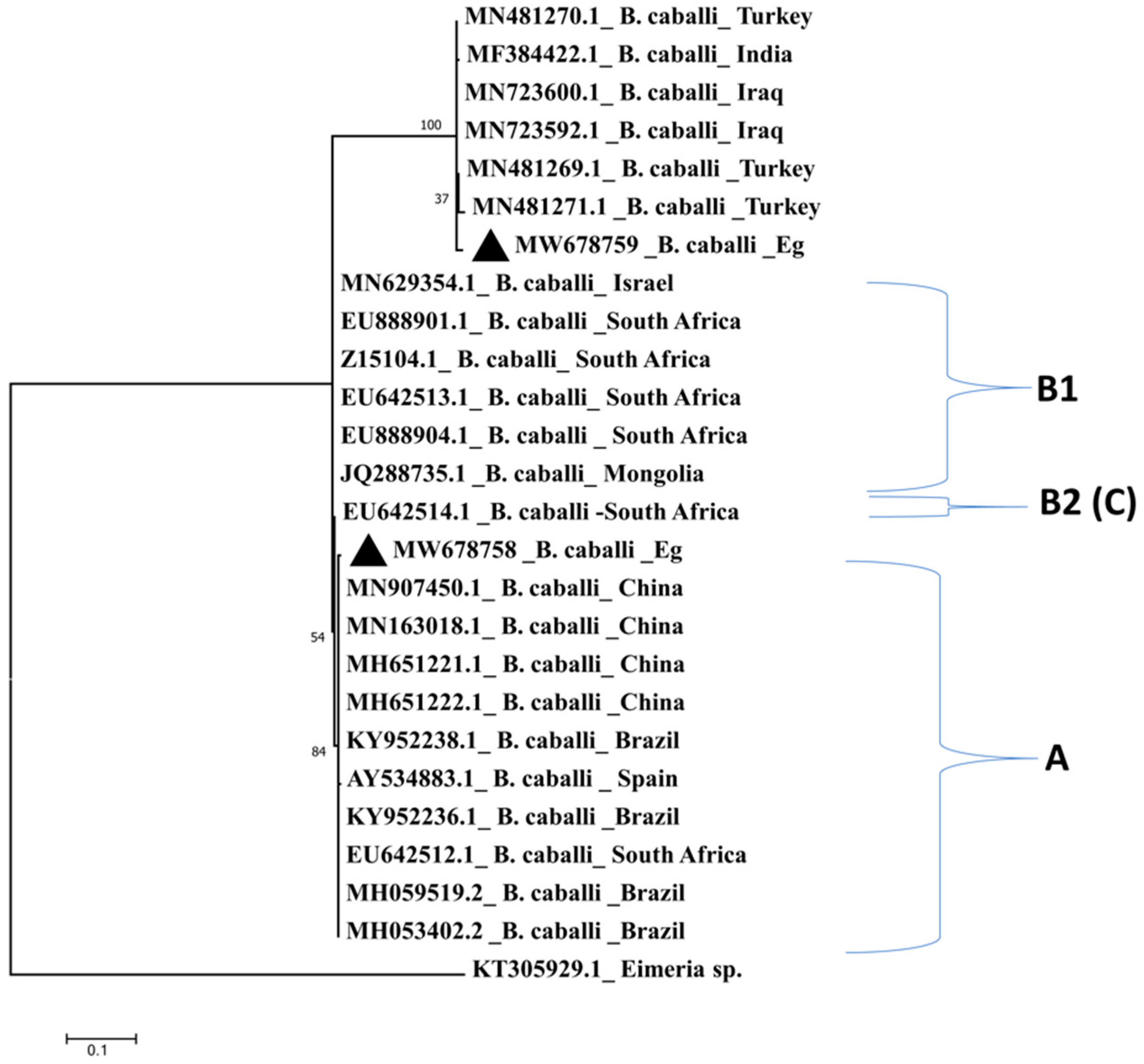
2.2. Comparative Analysis and Sequence Conservation of the 18S rRNA Amplicons among Different Isolates
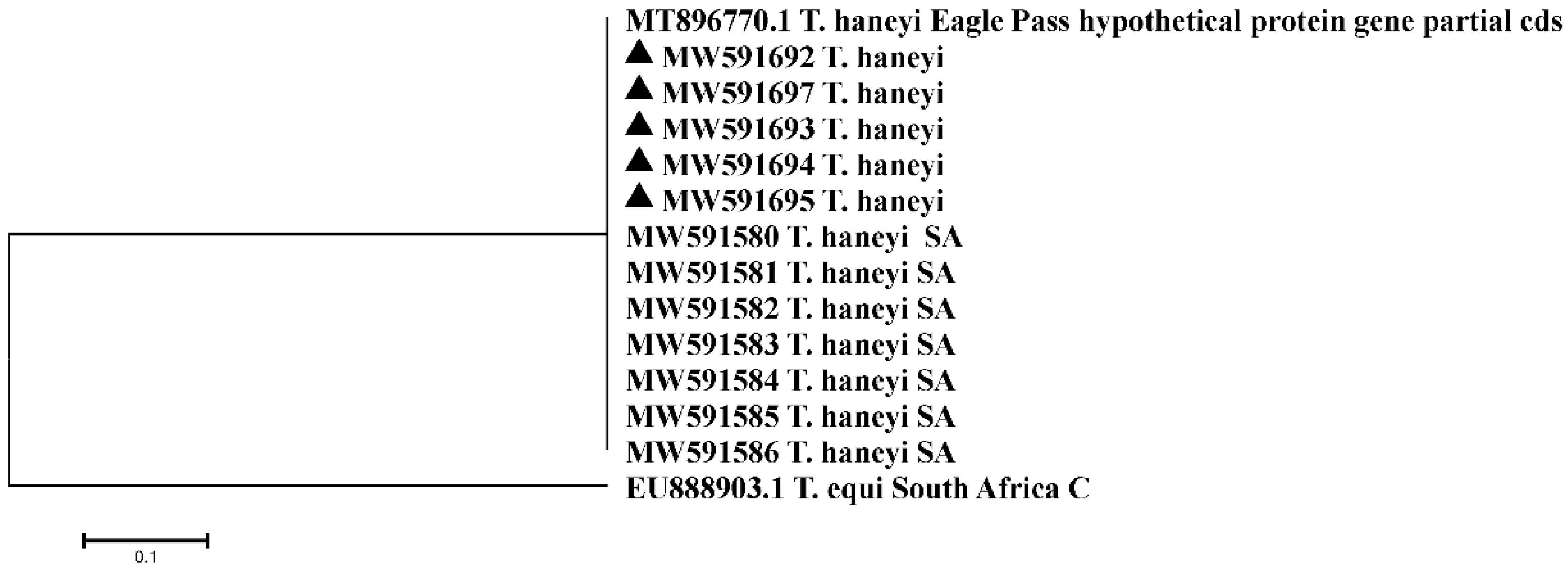
2.3. Sequencing Analysis of a T. haneyi Hypothetical-Protein-Coding Gene
3. Discussion
4. Materials and Methods
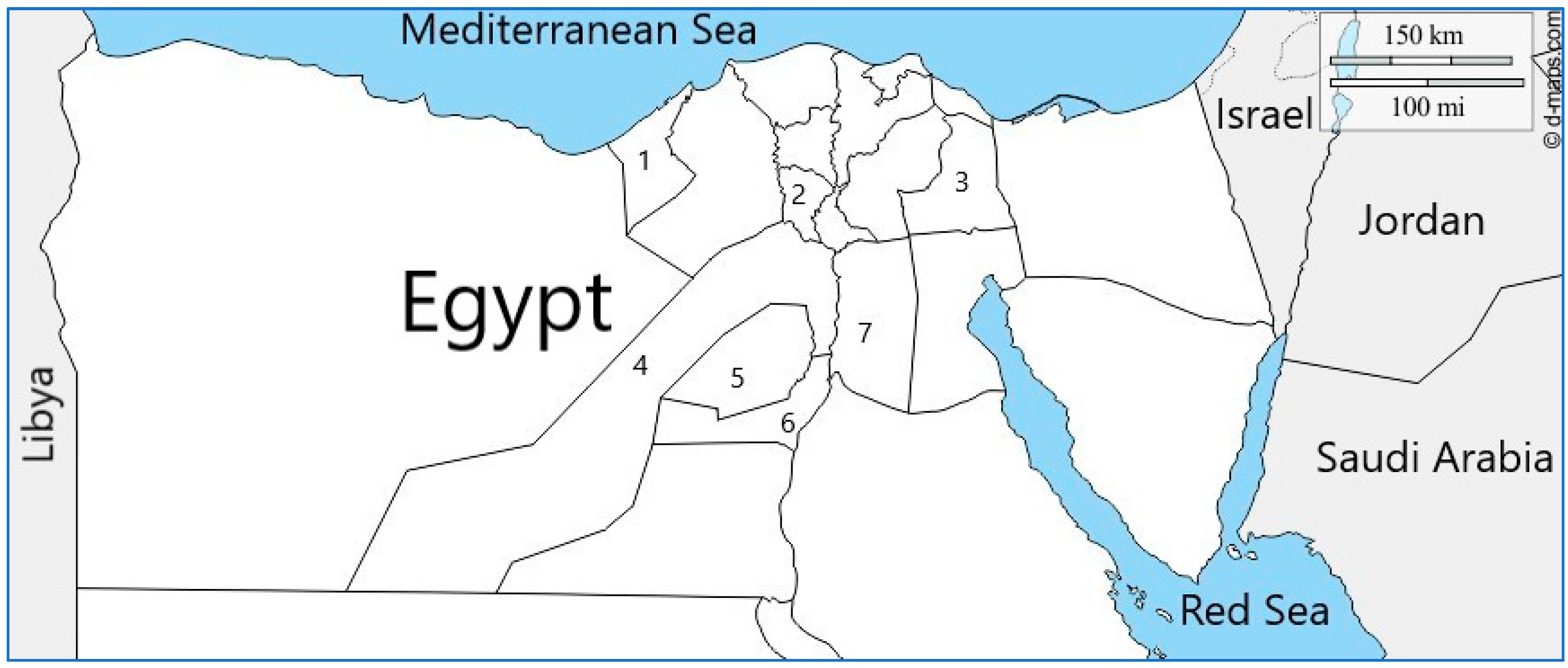
4.1. Collection of Field Samples
4.2. DNA Extraction
4.3. Molecular Detection of Equine Piroplasmosis by Three PCR Approaches
4.3.1. Multiplex PCR (mPCR) for the Detection of T. equi and B. caballi
4.3.2. Uniplex PCR (uPCR) for Confirmation of the mPCR Results for the Detection of T. equi and B. caballi
4.3.3. Detection of T. haneyi
4.4. Sequencing and Sequence Analysis
4.5. Comparative Analysis
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Valette, D. Invisible Workers. The Economic Contributions of Working Donkeys, Horses and Mules to Livelihoods; The Brooke: Louisville, KY, USA, 2015; pp. 1–23. Available online: https://www.thebrooke.org/sites/default/files/Advocacy-and-policy/Invisible-workers-report.pdf (accessed on 20 May 2021).
- Mahmoud, M.S.; Kandil, O.M.; Abu Ezz, N.T.E.; Hendawy, S.H.M.; Elsawy, B.S.M.; Knowles, D.P.; Bastos, R.G.; Kappmeyer, L.S.; Laughery, J.M.; Alzan, H.F.; et al. Identification and Antigenicity of the Babesia caballi Spherical Body Protein 4 (SBP4). Parasit. Vectors 2020, 13, 369. [Google Scholar] [CrossRef]
- Church, S. BEASTS of Burden Targeting Disease in Africa’s Working Donkeys and Horses. Available online: https://thehorse.com/features/beasts-of-burden-africas-working-horses-and-donkeys/ (accessed on 17 May 2021).
- Romero-Salas, D.; Solis-Cortés, M.; Zazueta-Islas, H.M.; Flores-Vásquez, F.; Cruz-Romero, A.; Aguilar-Domínguez, M.; Salguero-Romero, J.L.; de León, A.P.; Fernández-Figueroa, E.A.; Lammoglia-Villagómez, M.Á.; et al. Molecular Detection of Theileria equi in Horses from Veracruz, Mexico. Ticks Tick-Borne Dis. 2021, 12, 101671. [Google Scholar] [CrossRef] [PubMed]
- Knowles, D.P.; Kappmeyer, L.S.; Haney, D.; Herndon, D.R.; Fry, L.M.; Munro, J.B.; Sears, K.; Ueti, M.W.; Wise, L.N.; Silva, M.; et al. Discovery of a novel species, Theileria haneyi n. sp. infective to equids, highlights exceptional genomic diversity within the genus Theileria: Implications for apicomplexan parasite surveillance. Int. J. Parasitol. 2018, 48, 679–690. [Google Scholar] [CrossRef]
- Scoles, G.A.; Ueti, M.W. Vector Ecology of Equine Piroplasmosis. Annu. Rev. Entomol. 2015, 60, 561–580. [Google Scholar] [CrossRef]
- Okely, M.; Anan, R.; Gadallah, S.; Samy, A. Hard ticks (Acari: Ixodidae) infesting domestic animals in Egypt: Diagnostic characters and a Taxonomic key to the collected species. Med. Vet. Entomol. 2021, 10, 111. [Google Scholar]
- Idoko, I.S.; Edeh, R.E.; Adamu, A.M.; Machunga-mambula, S.; Okubanjo, O.O.; Balogun, E.O.; Adamu, S.; Johnson, W.; Kappmeyer, L.; Mousel, M.; et al. Molecular and Serological Detection of Piroplasms in Horses from Nigeria. Pathogen 2021, 10, 508. [Google Scholar] [CrossRef] [PubMed]
- Mshelia, P.W.; Kappmeyer, L.; Johnson, W.C.; Kudi, C.A.; Oluyinka, O.O.; Balogun, E.O.; Richard, E.E.; Onoja, E.; Sears, K.P.; Ueti, M.W. Molecular detection of Theileria species and Babesia caballi from horses in Nigeria. Parasitol. Res. 2020, 119, 2955–2963. [Google Scholar] [CrossRef]
- 1Sears, K.P.; Kappmeyer, L.S.; Wise, L.N.; Silva, M.; Ueti, M.W.; White, S.; Reif, K.E.; Knowles, D.P. Infection dynamics of Theileria equi and Theileria haneyi, a newly discovered apicomplexan of the horse. Vet. Parasitol. 2019, 271, 68–75. [Google Scholar]
- Ueti, M.W.; Palmer, G.H.; Scoles, G.A.; Kappmeyer, L.S.; Knowles, D.P. Persistently infected horses are reservoirs for intrastadial tick-borne transmission of the apicomplexan parasite Babesia equi. Infect. Immun. 2008, 76, 3525–3529. [Google Scholar] [CrossRef] [Green Version]
- Bhoora, R.; Franssen, L.; Oosthuizen, M.C.; Guthrie, A.J.; Zweygarth, E.; Penzhorn, B.L.; Jongejan, F.; Collins, N.E. Sequence Heterogeneity in the 18S rRNA Gene within Theileria equi and Babesia caballi from horses in South Africa. Vet. Parasitol. 2009, 159, 112–120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tirosh-Levy, S.; Steinman, A.; Levy, H.; Katz, Y.; Shtilman, M.; Gottlieb, Y. Parasite load and genotype are associated with clinical outcome of piroplasm—Infected equines in Israel. Parasit. Vectors 2020, 13, 267. [Google Scholar] [CrossRef]
- Qablan, M.A.; Oborník, M.; Petrželková, K.J.; Sloboda, M.; Shudiefat, M.F.; Hořín, P.; Lukeš, J.; Modrý, D. Infections by Babesia caballi and Theileria equi in Jordanian equids: Epidemiology and genetic diversity. Parasitology 2013, 140, 1096–1103. [Google Scholar] [CrossRef]
- Camino, E.; Cruz-lopez, F.; Juan, L.D.; Dominguez, L.; Shiels, B.; Coultous, R.M. Phylogenetic analysis and geographical distribution of Theileria equi and Babesia caballi sequences from horses residing in Spain. Ticks Tick-Borne Dis. 2020, 11, 101521. [Google Scholar] [CrossRef]
- Wang, J.; Liu, J.; Yang, J.; Wang, X.; Li, Z.; Jianlin, X.; Li, X.; Xiang, Q.; Li, Y.; Liu, Z.; et al. The First molecular detection and genetic diversity of Babesia caballi and Theileria equi in horses of Gansu Province, China. Ticks Tick-Borne Dis. 2019, 10, 528–532. [Google Scholar] [CrossRef]
- Ueti, M.W.; Tan, Y.; Broschat, S.L.; Ortiz, E.J.C.; Camacho-Nuez, M.; Mosqueda, J.J.; Scoles, G.A.; Grimes, M.; Brayton, K.A.; Palmerc, G.H. Expansion of variant diversity associated with a high prevalence of pathogen strain superinfection under conditions of natural transmission. Infect. Immun. 2012, 80, 2354–2360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tirosh-Levy, S.; Gottlieb, Y.; Fry, L.M.; Knowles, D.P.; Steinman, A. Twenty years of equine piroplasmosis research: Global distribution, molecular diagnosis, and Phylogeny. Pathogens 2020, 9, 926. [Google Scholar] [CrossRef]
- Salib, F.A.; Youssef, R.R.; Rizk, L.G.; Said, S.F. Epidemiology, diagnosis and therapy of Theileria equi infection in Giza, Egypt. Vet. World 2013, 6, 76–82. [Google Scholar] [CrossRef]
- Selim, A.; Khater, H. Seroprevalence and risk factors associated with equine piroplasmosis in North Egypt. Comp. Immunol. Microbiol. Infect. Dis. 2020, 73, 101549. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Sánchez, A.A.; Pires, M.S.; Estrada, C.Y.; Cañizares, E.V.; Del Castillo Domínguez, S.L.; Cabezas-Cruz, A.; Rivero, E.L.; da Fonseca, A.H.; Massard, C.L.; Corona-González, B. First molecular evidence of Babesia caballi and Theileria equi Infections in horses in Cuba. Parasitol. Res. 2018, 117, 3109–3118. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, A.K.; Gamil, I.S.; Abd-El baky, A.A.; Hussein, M.M.; Tohamy, A.A. Comparative molecular and conventional detection methods of Babesia equi (B. equi) in Egyptian equine. Glob. Vet. 2011, 7, 201–210. [Google Scholar]
- Mahmoud, M.S.; Abu El-Ezz, N.T.; Abdel-Shafy, S.; Nassar, S.A.; Namaky, A.H.E.; Khalil, W.K.B.; Knowles, D.; Kappmeyer, L.; Silva, M.G.; Suarez, C.E. Assessment of Theileria equi and Babesia caballi infections in equine populations in Egypt by molecular, serological and hematological approaches. Parasit. Vectors 2016, 9, 260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahdy, O.A.; Nassar, A.S.; Mohamed, B.S.; Mahmoud, M.S. Comparative diagnosis utilizing molecular and serological techniques of Theileria equi in distinct equine poupulation in Egypt. Int. J. ChemTech Res. 2016, 9, 185–197. [Google Scholar]
- El-seify, M.A.; City, K.E.; Helmy, N.; Mahmoud, A.; Soliman, M. Use molecular techniques as an alternative tool for diagnosis and characterization of Theileria equi. Iraqi J. Vet. Sci. 2018, 32, 5–11. [Google Scholar]
- Rothschild, C.M. Equine Piroplasmosis. J. Equine Vet. Sci. 2013, 33, 497–508. [Google Scholar] [CrossRef]
- El-Ashker, M.; Hotzel, H.; Gwida, M.; El-Beskawy, M.; Silaghi, C.; Tomaso, H. Molecular biological identification of Babesia, Theileria, and Anaplasma species in cattle in Egypt using PCR Assays, Gene sequence analysis and a novel DNA microarray. Vet. Parasitol. 2015, 207, 329–334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bilgiç, H.B.; Karagenç, T.; Simuunza, M.; Shiels, B.; Tait, A.; Eren, H.; Weir, W. Development of a multiplex PCR assay for simultaneous detection of Theileria annulata, Babesia bovis and Anaplasma marginale in cattle. Exp. Parasitol. 2013, 133, 222–229. [Google Scholar] [CrossRef] [Green Version]
- Georges, K.; Loria, G.R.; Riili, S.; Greco, A.; Caracappa, S.; Jongejan, F.; Sparagano, O. Detection of haemoparasites in cattle by reverse line blot hybridisation with a note on the distribution of ticks in Sicily. Vet. Parasitol. 2001, 99, 273–286. [Google Scholar] [CrossRef]
- Markoulatos, P.; Siafakas, N.; Moncany, M. Multiplex polymerase chain reaction: A practical approach. J. Clin. Lab. Anal. 2002, 16, 47–51. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Wang, H.; Zhang, S.; Xie, S.; Li, H.; Zhang, X.; Jia, L. First report of genetic diversity and risk Factor analysis of equine piroplasm Infection in equids in Jilin, China. Parasit. Vectors 2020, 13, 459. [Google Scholar] [CrossRef]
- Ketter-ratzon, D.; Tirosh-levy, S.; Nachum-biala, Y.; Saar, T.; Qura’n, L.; Zivotofsky, D.; Abdeen, Z.; Baneth, G.; Steinman, A. Characterization of Theileria equi genotypes in horses in Israel, the Palestinian Authority and Jordan. Ticks Tick-Borne Dis. 2017, 8, 499–505. [Google Scholar] [CrossRef]
- Onyiche, T.E.; Taioe, M.O.; Ogo, N.I.; Sivakumar, T.; Biu, A.A.; Mbaya, A.W.; Xuan, X.; Yokoyama, N.; Thekisoe, O. Molecular evidence of Babesia caballi and Theileria equi in equines and ticks in Nigeria: Prevalence and risk factors analysis. Parasitology 2020, 147, 1238–1248. [Google Scholar] [CrossRef] [PubMed]
- Idoko, S.I.; Tirosh-Levy, S.; Leszkowicz Mazuz, M.; Mohammed Adam, B.; Sikiti Garba, B.; Wesley Nafarnda, D.; Steinman, A. Genetic characterization of piroplasms in donkeys and horses from Nigeria. Animals 2020, 10, 324. [Google Scholar] [CrossRef] [Green Version]
- Costa, S.; Braga, D.O.; Costa, F.N.; Regina, D.; Gomes, M.; Xavier, D.R.; André, M.R.; Gonçalves, L.R.; Freschi, C.R.; Machado, R.Z. Genetic diversity of piroplasmids species in equids from Island of São Luís, Northeastern Brazil. Rev. Bras. Parasitol. Vet. 2017, 26, 331–339. [Google Scholar]
- Bhoora, R.V.; Collins, N.E.; Schnittger, L.; Troskie, C.; Marumo, R.; Labuschagne, K.; Smith, R.M.; Dalton, D.L.; Mbizeni, S. Molecular genotyping and epidemiology of equine piroplasmids in South Africa. Ticks Tick-Borne Dis. 2020, 11, 101358. [Google Scholar] [CrossRef]
- Wise, L.N.; Kappmeyer, L.S.; Mealey, R.H.; Knowles, D.P. Review of equine piroplasmosis. J. Vet. Intern. Med. 2013, 27, 1334–1346. [Google Scholar] [CrossRef]
- Abedi, V.; Razmi, G.; Seifi, H.; Naghibi, A. Molecular and serological detection of Theileria equi and Babesia caballi Infection in horses and Ixodid ticks in Iran. Ticks Tick-Borne Dis. 2014, 5, 239–244. [Google Scholar] [CrossRef]
- Brüning, A. Equine piroplasmosis an update on diagnosis, treatment and prevention. Br. Vet. J. 1996, 152, 139–151. [Google Scholar] [CrossRef]
- Alhassan, A.; Pumidonming, W.; Okamura, M.; Hirata, H.; Battsetseg, B.; Fujisaki, K.; Yokoyama, N.; Igarashi, I. Development of a single-round and multiplex PCR method for the simultaneous detection of Babesia caballi and Babesia equi in horse blood. Vet. Parasitol. 2005, 129, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Aziz, K.J.; Mohammed, Z.A.; Naqid, I.A. Molecular identification and phylogenetic analysis of Theileria equi and Babesia caballi infections in equids from Erbil Province, North of Iraq. Adv. Anim. Vet. Sci. 2019, 7, 1060–1066. [Google Scholar] [CrossRef]
- Bishop, R.P.; Kappmeyer, L.S.; Onzere, C.K.; Odongo, D.O.; Githaka, N.; Sears, K.P.; Knowles, D.P.; Fry, L.M. Equid Infective Theileria Cluster in Distinct 18S RRNA Gene Clades Comprising Multiple Taxa with Unusually Broad Mammalian Host Ranges. Parasit. Vectors 2020, 13, 261. [Google Scholar] [CrossRef]
- Sumbria, D.; Singla, L.D.; Sharma, A.; Kumar, S.; Bal, M.S. Comparative sensitivity and specificity of PCR assays for the detection of Theileria equi coupled with three DNA template extraction methods. J. Equine Vet. Sci. 2016, 38, 87–93. [Google Scholar] [CrossRef]
- Sharma, S. A Comparative study between blood Smear, whole Blood PCR and FTA card PCR for diagnosis of Theileria Annulata and Theileria Orientalis in Cattle. J. Anim. Res. 2019, 9, 585–588. [Google Scholar] [CrossRef]
- Allsopp, M.T.E.P.; Cavalier-Smith, T.; Waal, D.T.D.; Allsopp, B.A. Phylogeny and evolution of the piroplasms. Parasitology 1994, 108, 147–152. [Google Scholar] [CrossRef]
- Criado-Fornelio, A.; Martinez-Marcos, A.; Buling-Saraña, A.; Barba-Carretero, J.C. Molecular studies on Babesia, Theileria and Hepatozoon in Southern Europe: Part I. Epizootiological Aspects. Vet. Parasitol. 2003, 113, 189–201. [Google Scholar] [CrossRef]
- Kumar, S.; Sudan, V.; Shanker, D.; Devi, A. Babesia (Theileria) equi genotype A among Indian equine population. Vet. Parasitol. Reg. Stud. Rep. 2020, 19, 100367. [Google Scholar] [CrossRef] [PubMed]
- Peckle, M.; Pires, M.S.; Silva, C.B.D.; Costa, R.L.D.; Vitari, G.L.V.; Senra, M.V.X.; Dias, R.J.P.; Santos, H.A.; Massard, C.L. Molecular characterization of Theileria equi in horses from the State of Rio de Janeiro, Brazil. Ticks Tick-Borne Dis. 2018, 9, 349–353. [Google Scholar] [CrossRef]
- Salim, B.; Bakheit, M.A.; Kamau, J.; Nakamura, I.; Sugimoto, C. Nucleotide sequence heterogeneity in the small subunit ribosomal RNA gene within Theileria equi from horses in Sudan. Parasitol. Res. 2009, 106, 493–498. [Google Scholar] [CrossRef]
- Seo, M.-G.; Yun, S.-H.; Choi, S.-K.; Cho, G.-J.; Park, Y.-S.; Cho, K.-H.; Kwon, O.-D.; Kwak, D. Molecular and hylogenetic analysis of equine piroplasms in the republic of Korea. Res. Vet. Sci. 2013, 94, 579–583. [Google Scholar] [CrossRef]
- Nagore, D.; García-Sanmartín, J.; García-Pérez, A.L.; Juste, R.A.; Hurtado, A. Detection and identification of equine Theileria and Babesia species by reverse line blotting: Epidemiological survey and phylogenetic analysis. Vet. Parasitol. 2004, 123, 41–54. [Google Scholar] [CrossRef] [PubMed]
- Al-Habsi, K.; Yang, R.; Ryan, U.; Miller, D.W.; Jacobson, C. Morphological and molecular characterization of three Eimeria species from Captured Rangeland goats in Western Australia. Vet. Parasitol. Reg. Stud. Rep. 2017, 9, 75–83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valente, J.D.M.; Mongruel, A.C.B.; Machado, C.A.L.; Chiyo, L.; Leandro, A.S.; Britto, A.S.; Martins, T.F.; Barros-Filho, I.R.; Biondo, A.W.; Perotta, J.H.; et al. Tick-borne pathogens in carthorses from Foz do Iguaçu City, Paraná State, Southern Brazil: A Tri-border area of Brazil, Paraguay and Argentina. Vet. Parasitol. 2019, 273, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Narankhajid, M.; Yeruult, C.; Gurbadam, A.; Battsetseg, J.; Aberle, S.W.; Bayartogtokh, B.; Joachim, A.; Duscher, G.G. Some aspects on tick species in Mongolia and their potential role in the transmission of equine piroplasms, Anaplasma phagocytophilum and Borrelia burgdorferi L. Parasitol. Res. 2018, 117, 3557–3566. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, S.; Stecher, G.; Tamura, K. Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Criado-fornelio, A.; Martinez-marcos, A.; Buling-Saraña, A.; Barba-Carretero, J.C. Molecular studies on Babesia, Theileria and Hepatozoon in Southern Europe Part II. Phylogenetic Analysis and evolutionary history. Vet. Parasitol. 2003, 114, 173–194. [Google Scholar] [CrossRef]
- Snedecor, G.W.; Cochran, W.G. Statistical Methods, 8th ed.; Iowa State University Press: Ames, IA, USA, 1989; pp. 217–235. [Google Scholar]
| Tested Animal | No. | Positive mPCR | |||
|---|---|---|---|---|---|
| EP | Single Infection | Co-Infection (T. equi and B. caballi) | |||
| T. equi | B. caballi | ||||
| No. (%, 95% CI) | No. (%, 95% CI) | No. (%, 95% CI) | No. (%, 95% CI) | ||
| Horses | 79 | 19 (24.1%, 14.7–33.5%) | 16 (20.3%, 11.1–29.1%) | 1(1.2%,0.1–3.6) | 2 (2.5%, 0–5.9%) |
| Donkeys | 76 | 11 (14.4%, 6.5–22.2%) | 10 (13.1%, 5.5–20.6%) | 0 | 1 (1.3%, 0.1–3.8%) |
| Total equine | 155 | 30 (19.3%, 13.1–25.5%) | 26 (16.7%, 10.1–22.5%) | 1(0.6%, 0–1.8%) | 3 (1.9%, 0–4.0) |
| Animal | No. of Tested Animals | Positive | ||
|---|---|---|---|---|
| T. haneyi | T. haneyi and T. equi | T. haneyi, T. equi and B. caballi | ||
| No. (%, 95% CI) | No. (%, 95% CI) | No. (%, 95% CI) | ||
| Horses | 79 | 42 (53.1%, 40.4–62.1%) | 3 (4.5%, 0–9.0%) | 2 (2.5%, 0–5.9%) |
| Donkeys | 76 | 29 (38.1%, 27.2–49.0%) | 18 (26.8%, 16.1–36.7%) | 1 (1.3%, 0.1–3.8%) |
| Total equine | 155 | 71 (45.8%, 37.3–53.6%) | 21(13.5%, 8.8–18.8%) | 3 (1.9%, 0–4.0%) |
| Parasite | Primer Name | Gene Name | PCR Type | Amplicon Size | Primer Forward | Primer Reverse | Reference |
|---|---|---|---|---|---|---|---|
| B. caballi | B. caballi (diagnosis and sequencing) | 18S rRNA | mPCR | 540 bp | Bec-UF2 5-TCG AAG ACG ATC AGA TAC CGT CG-3 | Cab-R 5-CTCGTTCATGATTTAGAATTG CT-3 | [38,40] |
| T. equi | T. equi 1 (diagnosis) | 18S rRNA | mPCR | 430 bp | Equi-R 5-TGCCTTAAACTTCCTTGCGAT-3 | ||
| T. equi 2 (sequencing) | 18SrRNA | uPCR | 360 bp | TBM 5′-CTTCAGCACCTTGAGAGAAATC-3′ | Equi-R 5′-TGCCTTAAACTTCCTTGCGAT-3 | [14] | |
| T. haneyi | Th int. (diagnosis and sequencing) | hypothetical protein gene of unknown function | cPCR | 238 bp | Than_intfor 5′-GACAACAGAGAGGTGATT-3 | Than_intrev 5′-CGTTGAATGTAATGGGAAC-3 | [5] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elsawy, B.S.M.; Nassar, A.M.; Alzan, H.F.; Bhoora, R.V.; Ozubek, S.; Mahmoud, M.S.; Kandil, O.M.; Mahdy, O.A. Rapid Detection of Equine Piroplasms Using Multiplex PCR and First Genetic Characterization of Theileria haneyi in Egypt. Pathogens 2021, 10, 1414. https://doi.org/10.3390/pathogens10111414
Elsawy BSM, Nassar AM, Alzan HF, Bhoora RV, Ozubek S, Mahmoud MS, Kandil OM, Mahdy OA. Rapid Detection of Equine Piroplasms Using Multiplex PCR and First Genetic Characterization of Theileria haneyi in Egypt. Pathogens. 2021; 10(11):1414. https://doi.org/10.3390/pathogens10111414
Chicago/Turabian StyleElsawy, Bassma S. M., Ahmed M. Nassar, Heba F. Alzan, Raksha V. Bhoora, Sezayi Ozubek, Mona S. Mahmoud, Omnia M. Kandil, and Olfat A. Mahdy. 2021. "Rapid Detection of Equine Piroplasms Using Multiplex PCR and First Genetic Characterization of Theileria haneyi in Egypt" Pathogens 10, no. 11: 1414. https://doi.org/10.3390/pathogens10111414
APA StyleElsawy, B. S. M., Nassar, A. M., Alzan, H. F., Bhoora, R. V., Ozubek, S., Mahmoud, M. S., Kandil, O. M., & Mahdy, O. A. (2021). Rapid Detection of Equine Piroplasms Using Multiplex PCR and First Genetic Characterization of Theileria haneyi in Egypt. Pathogens, 10(11), 1414. https://doi.org/10.3390/pathogens10111414







