Sustained Antiviral and Liver Protection by a Nasal Therapeutic Vaccine (NASVAC, Containing Both HBsAg and HBcAg) in Patients with Chronic Hepatitis B: 2-Year Follow-Up of Phase III Clinical Trial
Abstract
1. Introduction
2. Results
2.1. Parameters of Safety
2.2. Follow Up of Patients with CHB for 2 Years after EOT
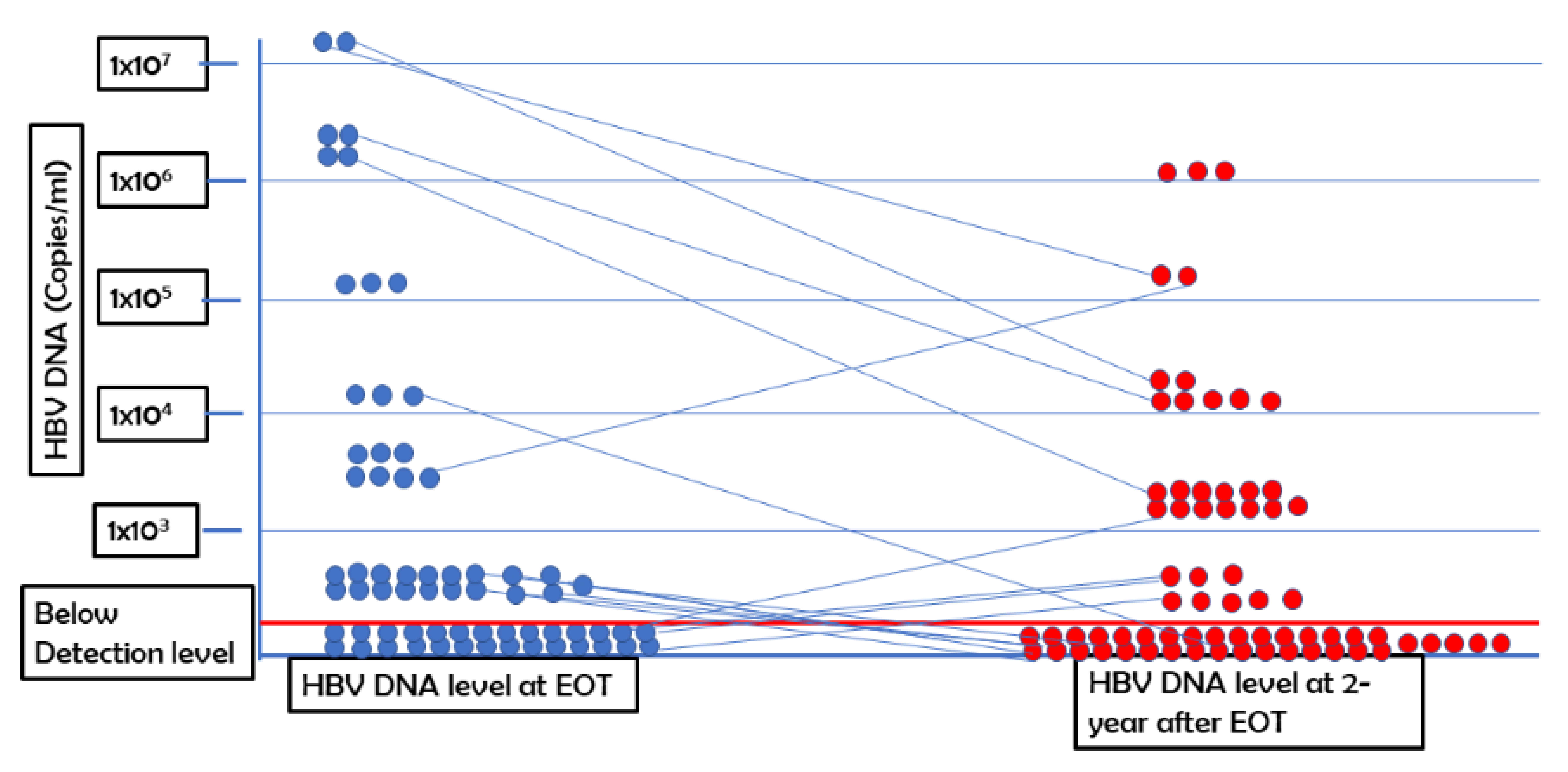
2.3. Sustained Control of HBV DNA by NASVAC in CHB Patients 2 Years after EOT
2.4. Kinetics of ALT in Patients Receiving NASVAC
2.5. Role of NASVAC in HBeAg Positivity
2.6. Anti-Fibrotic Effects of NASVAC
3. Discussion
4. Materials and Methods
4.1. Therapeutic Vaccine: NASVAC
4.2. Phase III Clinical Trial with NASVAC in CHB Patients
4.3. Design of Long-Term Follow-Up of NASVAC-Recipient CHB Patients
4.4. Assessment of HBV DNA, ALT, and Hepatitis B e Antigen (HBeAg) and Abdominal Ultrasonography
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Akbar, S.M.F.; Al Mahtab, M.; Begum, F.; Hossain, S.A.S.; Sarker, S.; Shrestha, A.; Khan, M.S.I.; Yoshida, O.; Hiasa, Y. Implication of birth-dose vaccination against hepatitis B virus in South-east Asia. Vaccines 2021, 9, 374. [Google Scholar] [CrossRef]
- WHO. Global Hepatitis Report 2017; World Health Organization: Geneva, Switzerland, 2017; Available online: http://apps.who.int/iris/bitstream/10665/255016/1/9789241565455-eng.pdf (accessed on 1 November 2021).
- Fattovich, G.; Bertoletti, F.; Donato, F. Natural history of chronic hepatitis B: Special emphasis on disease progression and prognostic factors. J. Hepatol. 2008, 48, 335–352. [Google Scholar] [CrossRef] [PubMed]
- MacLachlan, J.H.; Cowie, B.C. Hepatitis B virus epidemiology. Cold Spring Harb. Perspect. Med. 2015, 5, a021410. [Google Scholar] [CrossRef]
- Terrault, N.A.; Bzowej, N.H.; Chang, K.M.; Hwang, J.P.; Jonas, M.M.; Murad, M.; American Association for the Study of Liver Diseases. AASLD guidelines for treatment of chronic hepatitis B. Hepatology 2016, 63, 261–283. [Google Scholar] [CrossRef] [PubMed]
- European Association for the Study of the Liver. EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J. Hepatol. 2017, 67, 370–398. [Google Scholar]
- Sarin, S.K.; Kumar, M.; Lau, G.K.; Abbas, Z.; Chan, H.L.Y.; Chen, D.D.; Chen, H.L.; Chen, P.J.; Chien, R.N.; Dockmeci, A.K.; et al. Asian-Pacific clinical practice guidelines on the management of hepatitis B: A 2015 update. Hepatol. Int. 2016, 10, 1–98. [Google Scholar] [PubMed]
- Ozaras, R.; Khodor, H.; Yetim, N.; Unal, U.K.; Demirhan, Y.E.; Gultekin, G.; Isal, B. Monotherapy for hepatitis B infection: A review of treatment options. Expert Rev. Anti-Infect. Ther. 2015, 13, 1457–1468. [Google Scholar] [CrossRef] [PubMed]
- Bourlière, M.; Rabiega, P.; Ganne-Carrie, N.; Serfaty, L.; Marcellin, P.; Barthe, Y.; Thabut, D.; Guyader, D.; Hezode, C.; Picon, M.; et al. Effect on HBs antigen clearance of addition of pegylated interferon alfa-2a to nucleos(t)ide analogue therapy versus nucleos(t)ide analogue therapy alone in patients with HBe antigen-negative chronic hepatitis B and sustained undetectable plasma hepatitis B virus DNA: A randomised, controlled, open-label trial. Lancet Gastroenterol. Hepatol. 2017, 2, 177–188. [Google Scholar]
- Liem, K.S.; Fung, S.; Wong, D.K.; Yim, C.; Noureldin, S.; Chen, J.; Feld, J.J.; Hansen, B.E.; Janseen, H.L. Limited sustained response after stopping nucleos(t)ide analogues in patients with chronic hepatitis B: Results from a randomised controlled trial (Toronto STOP study). Gut 2019, 68, 2206–2213. [Google Scholar] [CrossRef]
- Wu, H.M.; Li, J.; Yang, X.; Yang, D. Immunopathogenesis of HBV Infection. Adv. Exp. Med. Biol. 2020, 1179, 71–107. [Google Scholar]
- Stasi, C.; Silvestri, C.; Voller, F. Hepatitis B vaccination and immunotherapies: An update. Clin. Exp. Vaccine Res. 2020, 9, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Akbar, S.M.; Chen, S.; Al-Mahtab, M.; Abe, M.; Hiasa, Y.; Onji, M. Strong and multi-antigen specific immunity by hepatitis B core antigen (HBcAg)-based vaccines in a murine model of chronic hepatitis B: HBcAg is a candidate for a therapeutic vaccine against hepatitis B virus. Antiviral Res. 2012, 96, 59–64. [Google Scholar]
- Betancourt, A.A.; Delgado, C.A.; Estévez, Z.C.; Martinez, J.C.; Rios, G.V.; Aureoles-Rosello, S.R.M.; Guzman, M.A.; Baile, N.F.; Reyes, P.A.D.; Ruano, L.O. Phase I clinical trial in healthy adults of a nasal vaccine candidate containing recombinant hepatitis B surface and core antigens. Int. J. Infect. Dis. 2007, 11, 394–401. [Google Scholar] [CrossRef]
- Al-Mahtab, M.; Akbar, S.M.; Aguilar, J.C.; Uddin, M.H.; Khan, M.S.; Rahman, S. Therapeutic potential of a combined hepatitis B virus surface and core antigen vaccine in patients with chronic hepatitis B. Hepatol. Int. 2013, 7, 981–989. [Google Scholar] [CrossRef] [PubMed]
- Al Mahtab, M.; Akbar, S.M.F.; Aguilar, J.C.; Guillen, G.; Penton, E.; Tuero, A.; Yoshida, O.; Hiasa, Y.; Onji, M. Treatment of chronic hepatitis B naïve patients with a therapeutic vaccine containing HBs and HBc antigens (a randomized, open and treatment-controlled phase III clinical trial). PLoS ONE 2018, 13, e0201236. [Google Scholar]
- Zoulim, F.; Durantel, D. Antiviral therapies and prospects for a cure of chronic hepatitis B. Cold Spring Harb. Perspect. Med. 2015, 5, a021501. [Google Scholar] [CrossRef]
- Li, T.; Liang, Y.; Zhang, M.; Liu, F.; Zhang, L.; Yang, B.; Wang, L. Nucleoside/nucleotide analog consolidation therapy in hepatitis B e-antigen-postive chronic hepatitis B patients: Three years should be preferred. Hepatol. Res. 2021, 51, 633–640. [Google Scholar] [CrossRef]
- Chong, C.H.; Lim, S.G. When can we stop nucleoside analogues in patients with chronic hepatitis B? Liver Int. 2017, 37 (Suppl. 1), 52–58. [Google Scholar] [CrossRef][Green Version]
- Pol, S.; Driss, F.; Michel, M.L.; Nalpas, B.; Berthelot, P.; Brechot, C. Specific vaccine therapy in chronic hepatitis B infection. Lancet 1994, 344, 342. [Google Scholar] [CrossRef]
- Boni, C.; Barili, V.; Acerbi, G.; Rossi, M.; Vecchi, A.; Laccabue, D.; Penna, A.; Missale, G.; Ferrari, C.; Fisicaro, P. HBV immune-therapy: From molecular mechanisms to clinical applications. Int. J. Mol. Sci. 2019, 20, 2754. [Google Scholar]
- Xu, D.Z.; Zhao, K.; Guo, L.M.; Li, A.-L.; Xie, Q.; Ren, H.; Zhang, J.-M.; Xu, M.; Wang, H.-F.; Huang, W.-X.; et al. A randomized controlled phase IIb trial of antigen-antibody immunogenic complex therapeutic vaccine in chronic hepatitis B patients. PLoS ONE 2008, 3, e2565. [Google Scholar]
- Yoshida, O.; Imai, Y.; Shiraishi, K.; Tokumoto, Y.; Sanada, T.; Tsukiyama-Kohara, K.; Miyazaki, T.; Kamishita, T.; Aguilar, J.C.; Guillen, G.E. HBsAg Reduction by nasal administration of a therapeutic vaccine containing HBsAg and HBcAg (NASVAC) in patients with chronic HBV Infection: The results of 18 months follow-up. Hepatology 2020, 72 (Suppl. 1), 60A. [Google Scholar]
- Chianese, A.; Santella, B.; Ambrosino, A.; Stelitano, D.; Rinaldi, L.; Galdiero, M.; Zannella, C.; Franci, G. Oncolytic viruses in combination therapeutic approaches with epigenetic modulators: Past, present, and future perspectives. Cancers 2021, 13, 2761. [Google Scholar] [PubMed]

| Variables | Basal Level | EOT | 2-Year after EOT |
|---|---|---|---|
| White Blood (Counts/mm3) | 9.0 (5.3–10) | 9.0 (5.4–11) | 9.0 (5.8–10.0) |
| Bilirubin (mg/dL) | 0.63 (0.2–1.26) | 0.7 (0.4–1.16) | 0.79 (0.56–0.97) |
| Albumin (gm/dL) | 3.7 (3.4–4.2) | 4.1 (3.6–4.6) | 3.6 (3.2–4.1) |
| Creatinine (mg/dL) | 0.99 (0.46–1.8) | 1.0 (0.62–1.42) | 0.89 (0.54–1.27) |
| Hemoglobulin (gm/dL) | 13.2 (12.6–14.2) | 12.4 (11.3–15.2) | 12.5 (10.6–13.2) |
| Platelets count | 230,000 (190,000–270,000) | 210,000 (195,000–245,000) | 205,000 (195,000–320,000) |
| (A) Kinetics of HBV DNA | |||
| Number of Patients | HBV DNA-Negative (<250 copies/mL) | HBV DNA Reduced from Basal Levels | HBV DNA Increased from Basal Levels |
| 66 | 33 | 30 | 3 |
| (B) Kinetics of ALT | |||
| Number of Patients | Alanine Aminotransferase (ALT) below upper Level of Normal (ULN) (0–42 IU/L) | Alanine Aminotransferase (ALT) above ULN and2×ULN(43–84 IU/L) | Alanine Aminotransferase (ALT) above 86 IU/L |
| 66 | 37 | 29 | 0 |
| (C) HBeAg Negativity | |||
| Number of Patients | HBeAg-Negative | HBeAg-Positive | |
| 12 | 8 | 4 | |
| Variables | Values | |
|---|---|---|
| Total | 66 | |
| Male: Female | 59:7 | |
| Age (Years) | 20 (18–50) | |
| HBV DNA (copies/mL) | 4.33 × 103 (range: 1.5 × 103–1.0 × 1013) | |
| ALT (IU/L) * | 30 (10–262) * | |
| HBeAg-positive | 12 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akbar, S.M.F.; Al Mahtab, M.; Aguilar, J.C.; Yoshida, O.; Penton, E.; Gerardo, G.N.; Hiasa, Y. Sustained Antiviral and Liver Protection by a Nasal Therapeutic Vaccine (NASVAC, Containing Both HBsAg and HBcAg) in Patients with Chronic Hepatitis B: 2-Year Follow-Up of Phase III Clinical Trial. Pathogens 2021, 10, 1440. https://doi.org/10.3390/pathogens10111440
Akbar SMF, Al Mahtab M, Aguilar JC, Yoshida O, Penton E, Gerardo GN, Hiasa Y. Sustained Antiviral and Liver Protection by a Nasal Therapeutic Vaccine (NASVAC, Containing Both HBsAg and HBcAg) in Patients with Chronic Hepatitis B: 2-Year Follow-Up of Phase III Clinical Trial. Pathogens. 2021; 10(11):1440. https://doi.org/10.3390/pathogens10111440
Chicago/Turabian StyleAkbar, Sheikh Mohammad Fazle, Mamun Al Mahtab, Julio Cesar Aguilar, Osamu Yoshida, Eduardo Penton, Guillen Nieto Gerardo, and Yoichi Hiasa. 2021. "Sustained Antiviral and Liver Protection by a Nasal Therapeutic Vaccine (NASVAC, Containing Both HBsAg and HBcAg) in Patients with Chronic Hepatitis B: 2-Year Follow-Up of Phase III Clinical Trial" Pathogens 10, no. 11: 1440. https://doi.org/10.3390/pathogens10111440
APA StyleAkbar, S. M. F., Al Mahtab, M., Aguilar, J. C., Yoshida, O., Penton, E., Gerardo, G. N., & Hiasa, Y. (2021). Sustained Antiviral and Liver Protection by a Nasal Therapeutic Vaccine (NASVAC, Containing Both HBsAg and HBcAg) in Patients with Chronic Hepatitis B: 2-Year Follow-Up of Phase III Clinical Trial. Pathogens, 10(11), 1440. https://doi.org/10.3390/pathogens10111440






