Transplacental Antibody Transfer of Respiratory Syncytial Virus Specific IgG in Non-Human Primate Mother-Infant Pairs
Abstract
:1. Introduction
2. Results
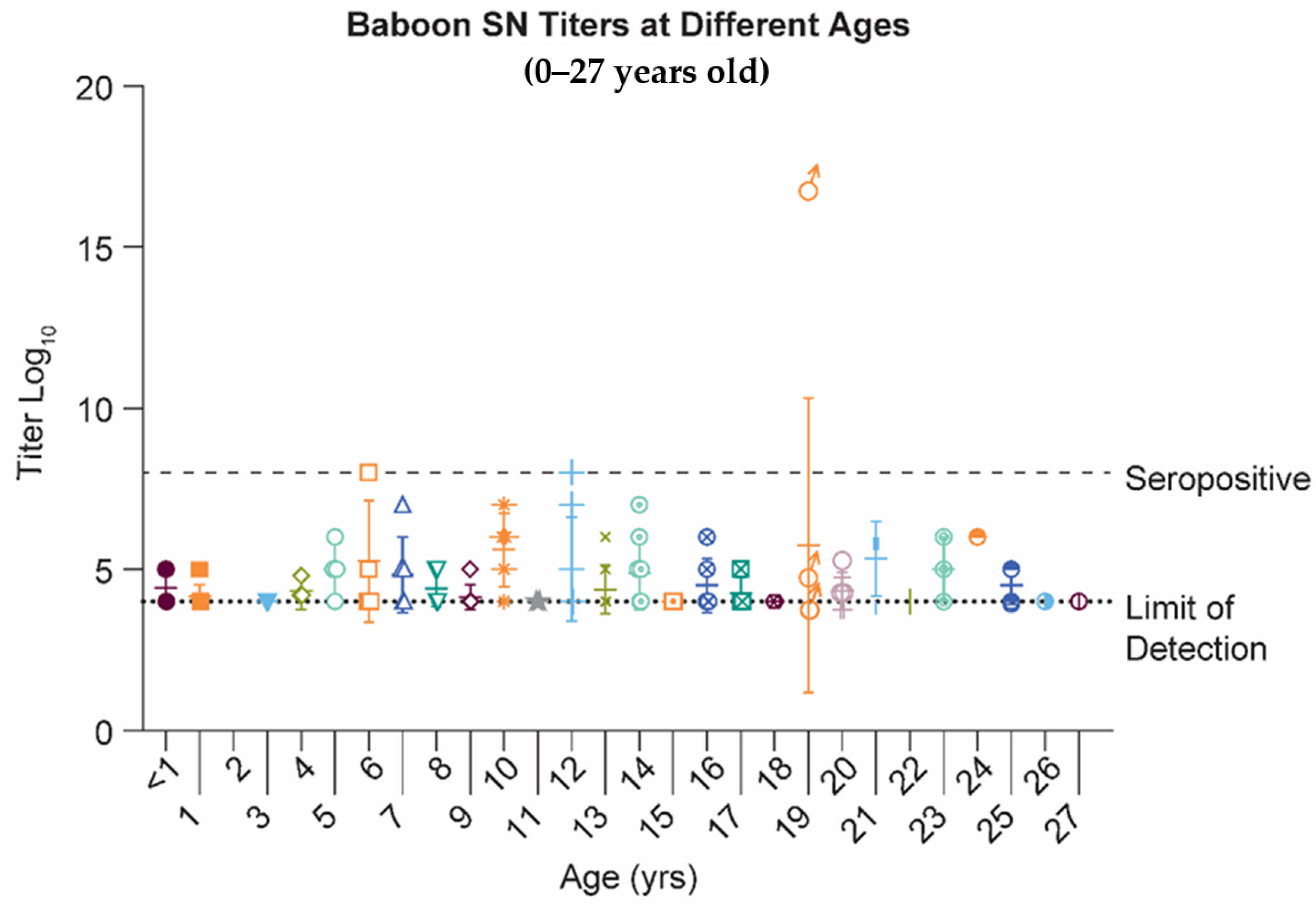
2.1. Papio Hamadryas Baboons Lack Robust Antibody Levels against Natural RSV Infection, Independent of Age
2.2. African Green Monkeys Demonstrate Antibody Levels against Natural and Experimental RSV Infection That Make Them Suitable to Evaluate Maternal Immunization for RSV Vaccines
2.3. Amplitude of Infant Serostatus Is Independent of the Manner of How Animals Seroconverted
2.4. No Relationship Exists between the RSV Serostatus of New-Born Monkeys and Seasonality of Birth Date
2.5. Maternal Immunization of Pregnant Monkeys Results in Offspring with Significant Increase of Level and Durability of Anti = RSV Antibody
3. Discussion
4. Materials and Methods
4.1. Animal Experiments
4.2. Serology, Immunizations, and Viral Challenge
4.3. Detection of Sera IgG (H+L), IgG (Fc), and IgM, Antibody Titers by ELISA
4.4. Determination of RSV Sera Neutralizing Titers (Microneutralization Assay)
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Shay, D.K.; Holman, R.C.; Roosevelt, G.E.; Clarke, M.J.; Anderson, L.J. Bronchiolitis-associated mortality and estimates of respiratory syncytial virus-associated deaths among US children, 1979–1997. J. Infect. Dis. 2001, 183, 16–22. [Google Scholar] [CrossRef]
- Thompson, W.W.; Shay, D.K.; Weintraub, E.; Brammer, L.; Cox, N.; Anderson, L.J.; Fukuda, K. Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA 2003, 289, 179–186. [Google Scholar] [CrossRef]
- Shi, T.; McAllister, D.A.; O’Brien, K.L.; Simoes, E.A.F.; Madhi, S.A.; Gessner, B.D.; Polack, F.P.; Balsells, E.; Acacio, S.; Aguayo, C.; et al. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in young children in 2015: A systematic review and modelling study. Lancet 2017, 390, 946–958. [Google Scholar] [CrossRef] [Green Version]
- Nair, H.; Nokes, D.J.; Gessner, B.D.; Dherani, M.; Madhi, S.A.; Singleton, R.J.; O’Brien, K.L.; Roca, A.; Wright, P.F.; Bruce, N.; et al. Global burden of acute lower respiratory infections due to respiratory syncytial virus in young children: A systematic review and meta-analysis. Lancet 2010, 375, 1545–1555. [Google Scholar] [CrossRef] [Green Version]
- Jorquera, P.A.; Tripp, R.A. Respiratory syncytial virus: Prospects for new and emerging therapeutics. Expert Rev. Respir. Med. 2017, 11, 609–615. [Google Scholar] [CrossRef]
- Higgins, D.; Trujillo, C.; Keech, C. Advances in RSV vaccine research and development-A global agenda. Vaccine 2016, 34, 2870–2875. [Google Scholar] [CrossRef] [PubMed]
- Murphy, B.R.; Hall, S.L.; Kulkarni, A.B.; Crowe, J.E., Jr.; Collins, P.L.; Connors, M.; Karron, R.A.; Chanock, R.M. An update on approaches to the development of respiratory syncytial virus (RSV) and parainfluenza virus type 3 (PIV3) vaccines. Virus Res. 1994, 32, 13–36. [Google Scholar] [CrossRef]
- Nyiro, J.U.; Kombe, I.K.; Sande, C.J.; Kipkoech, J.; Kiyuka, P.K.; Onyango, C.O.; Munywoki, P.K.; Kinyanjui, T.M.; Nokes, D.J. Defining the vaccination window for respiratory syncytial virus (RSV) using age-seroprevalence data for children in Kilifi, Kenya. PLoS ONE 2017, 12, e0177803. [Google Scholar] [CrossRef] [PubMed]
- Trento, A.; Rodriguez-Fernandez, R.; Gonzalez-Sanchez, M.I.; Gonzalez-Martinez, F.; Mas, V.; Vazquez, M.; Palomo, C.; Melero, J.A. The complexity of antibody responses elicited against the respiratory syncytial virus glycoproteins in hospitalized children younger than 2 Years. Front. Microbiol. 2017, 8, 2301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chin, J.; Magoffin, R.L.; Shearer, L.A.; Schieble, J.H.; Lennette, E.H. Field evaluation of a respiratory syncytial virus vaccine and a trivalent parainfluenza virus vaccine in a pediatric population. Am. J. Epidemiol. 1969, 89, 449–463. [Google Scholar] [CrossRef]
- Kim, H.W.; Canchola, J.G.; Brandt, C.D.; Pyles, G.; Chanock, R.M.; Jensen, K.; Parrott, R.H. Respiratory syncytial virus disease in infants despite prior administration of antigenic inactivated vaccine. Am. J. Epidemiol. 1969, 89, 422–434. [Google Scholar] [CrossRef]
- Scheltema, N.M.; Kavelaars, X.M.; Thorburn, K.; Hennus, M.P.; van Woensel, J.B.; van der Ent, C.K.; Borghans, J.A.M.; Bont, L.J.; Drylewicz, J. Potential impact of maternal vaccination on life-threatening respiratory syncytial virus infection during infancy. Vaccine 2018, 36, 4693–4700. [Google Scholar] [CrossRef]
- Hause, A.M.; Avadhanula, V.; Maccato, M.L.; Pinell, P.M.; Bond, N.; Santarcangelo, P.; Ferlic-Stark, L.; Ye, X.; Iwuchukwu, O.; Maurer, L.; et al. Clinical characteristics and outcomes of respiratory syncytial virus infection in pregnant women. Vaccine 2019, 37, 3464–3471. [Google Scholar] [CrossRef]
- Madhi, S.A.; Cutland, C.L.; Downs, S.; Jones, S.; van Niekerk, N.; Simoes, E.A.F.; Nunes, M.C. Burden of respiratory syncytial virus infection in South African Human Immunodeficiency Virus (HIV)-infected and HIV-uninfected pregnant and postpartum women: A longitudinal cohort study. Clin. Infect. Dis. 2018, 66, 1658–1665. [Google Scholar] [CrossRef] [Green Version]
- Walsh, E.E.; Wang, L.; Falsey, A.R.; Qiu, X.; Corbett, A.; Holden-Wiltse, J.; Mariani, T.J.; Topham, D.J.; Caserta, M.T. Virus-specific antibody, viral load, and disease severity in respiratory syncytial virus infection. J. Infect. Dis. 2018, 218, 208–217. [Google Scholar] [CrossRef] [PubMed]
- Crowe, J.E., Jr. Influence of maternal antibodies on neonatal immunization against respiratory viruses. Clin. Infect. Dis. 2001, 33, 1720–1727. [Google Scholar] [CrossRef] [PubMed]
- Chu, H.Y.; Newman, K.L.; Englund, J.A.; Cho, S.; Bull, C.; Lacombe, K.; Carlin, K.; Bulkow, L.R.; Rudolph, K.; DeByle, C.; et al. Transplacental respiratory syncytial virus and influenza virus antibody transfer in Alaska native and Seattle mother-infant pairs. J. Pediatr. Infect. Dis. Soc. 2021, 10, 230–236. [Google Scholar] [CrossRef]
- Giles, M.L.; Krishnaswamy, S.; Wallace, E.M. Maternal immunisation: What have been the gains? Where are the gaps? What does the future hold? F1000Research 2018, 7, 1733. [Google Scholar] [CrossRef]
- Larson, H.J. Maternal immunization: The new “normal” (or it should be). Vaccine 2015, 33, 6374–6375. [Google Scholar] [CrossRef] [PubMed]
- Bardaji, A.; MacDonald, N.E.; Omer, S.B.; Aguado, T. Maternal immunization: A call to accelerate progress. Vaccine 2019, 37, 2882–2883. [Google Scholar] [CrossRef]
- Cromer, D.; van Hoek, A.J.; Newall, A.T.; Pollard, A.J.; Jit, M. Burden of paediatric respiratory syncytial virus disease and potential effect of different immunisation strategies: A modelling and cost-effectiveness analysis for England. Lancet Publ. Health 2017, 2, e367–e374. [Google Scholar] [CrossRef] [Green Version]
- Chu, H.Y.; Englund, J.A. Maternal immunization. Birth Defects Res. 2017, 109, 379–386. [Google Scholar] [CrossRef] [Green Version]
- Munoz, F.M.; Jamieson, D.J. Maternal Immunization. Obstet. Gynecol. 2019, 133, 739–753. [Google Scholar] [CrossRef]
- Zaman, K.; Roy, E.; Arifeen, S.E.; Rahman, M.; Raqib, R.; Wilson, E.; Omer, S.B.; Shahid, N.S.; Breiman, R.F.; Steinhoff, M.C. Effectiveness of maternal influenza immunization in mothers and infants. N. Engl. J. Med. 2008, 359, 1555–1564. [Google Scholar] [CrossRef] [Green Version]
- Madhi, S.A.; Cutland, C.L.; Kuwanda, L.; Weinberg, A.; Hugo, A.; Jones, S.; Adrian, P.V.; van Niekerk, N.; Treurnicht, F.; Ortiz, J.R.; et al. Influenza vaccination of pregnant women and protection of their infants. N. Engl. J. Med. 2014, 371, 918–931. [Google Scholar] [CrossRef] [Green Version]
- Manske, J.M. Efficacy and effectiveness of maternal influenza vaccination during pregnancy: A review of the evidence. Matern. Child Health J. 2014, 18, 1599–1609. [Google Scholar] [CrossRef]
- Steinhoff, M.C.; Katz, J.; Englund, J.A.; Khatry, S.K.; Shrestha, L.; Kuypers, J.; Stewart, L.; Mullany, L.C.; Chu, H.Y.; LeClerq, S.C.; et al. Year-round influenza immunisation during pregnancy in Nepal: A phase 4, randomised, placebo-controlled trial. Lancet Infect. Dis. 2017, 17, 981–989. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vojtek, I.; Dieussaert, I.; Doherty, T.M.; Franck, V.; Hanssens, L.; Miller, J.; Bekkat-Berkani, R.; Kandeil, W.; Prado-Cohrs, D.; Vyse, A. Maternal immunization: Where are we now and how to move forward? Ann. Med. 2018, 50, 193–208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blanco, J.C.G.; Boukhvalova, M.S.; Morrison, T.G.; Vogel, S.N. A multifaceted approach to RSV vaccination. Hum. Vaccines Immunother. 2018, 14, 1734–1745. [Google Scholar] [CrossRef]
- Vekemans, J.; Moorthy, V.; Giersing, B.; Friede, M.; Hombach, J.; Arora, N.; Modjarrad, K.; Smith, P.G.; Karron, R.; Graham, B.; et al. Respiratory syncytial virus vaccine research and development: World Health Organization technological roadmap and preferred product characteristics. Vaccine 2019, 37, 7394–7395. [Google Scholar] [CrossRef] [PubMed]
- August, A.; Glenn, G.M.; Kpamegan, E.; Hickman, S.P.; Jani, D.; Lu, H.; Thomas, D.N.; Wen, J.; Piedra, P.A.; Fries, L.F. A Phase 2 randomized, observer-blind, placebo-controlled, dose-ranging trial of aluminum-adjuvanted respiratory syncytial virus F particle vaccine formulations in healthy women of childbearing age. Vaccine 2017, 35, 3749–3759. [Google Scholar] [CrossRef]
- Blanco, J.C.G.; Pletneva, L.M.; McGinnes-Cullen, L.; Otoa, R.O.; Patel, M.C.; Fernando, L.R.; Boukhvalova, M.S.; Morrison, T.G. Efficacy of a respiratory syncytial virus vaccine candidate in a maternal immunization model. Nat. Commun. 2018, 9, 1904. [Google Scholar] [CrossRef]
- Blanco, J.C.G.; Fernando, L.R.; Zhang, W.; Kamali, A.; Boukhvalova, M.S.; McGinnes-Cullen, L.; Morrison, T.G. Alternative virus-like particle-associated prefusion F proteins as maternal vaccines for respiratory syncytial virus. J. Virol. 2019, 93, e00914-19. [Google Scholar] [CrossRef] [PubMed]
- Jelonek, M.T.; Maskrey, J.L.; Steimer, K.S.; Potts, B.J.; Higgins, K.W.; Keller, M.A. Inhibition of the offspring anti-recombinant gp120 antibody response to a human immunodeficiency virus vaccine by maternal immunization in a murine model. J. Infect. Dis. 1995, 172, 539–542. [Google Scholar] [CrossRef] [PubMed]
- Pentsuk, N.; van der Laan, J.W. An interspecies comparison of placental antibody transfer: New insights into developmental toxicity testing of monoclonal antibodies. Birth Defects Res. B Dev. Reprod. Toxicol. 2009, 86, 328–344. [Google Scholar] [CrossRef] [PubMed]
- Abdiche, Y.N.; Yeung, Y.A.; Chaparro-Riggers, J.; Barman, I.; Strop, P.; Chin, S.M.; Pham, A.; Bolton, G.; McDonough, D.; Lindquist, K.; et al. The neonatal Fc receptor (FcRn) binds independently to both sites of the IgG homodimer with identical affinity. MAbs 2015, 7, 331–343. [Google Scholar] [CrossRef] [Green Version]
- Grigsby, P.L. Animal models to study placental development and function throughout normal and dysfunctional human pregnancy. Semin. Reprod. Med. 2016, 34, 11–16. [Google Scholar] [CrossRef] [Green Version]
- Carter, A.M. Animal models of human placentation—A review. Placenta 2007, 28 (Suppl. A), S41–S47. [Google Scholar] [CrossRef]
- Warfel, J.M.; Beren, J.; Kelly, V.K.; Lee, G.; Merkel, T.J. Nonhuman primate model of pertussis. Infect. Immun. 2012, 80, 1530–1536. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Warfel, J.M.; Zimmerman, L.I.; Merkel, T.J. Acellular pertussis vaccines protect against disease but fail to prevent infection and transmission in a nonhuman primate model. Proc. Natl. Acad. Sci. USA 2014, 111, 787–792. [Google Scholar] [CrossRef] [Green Version]
- Warfel, J.M.; Zimmerman, L.I.; Merkel, T.J. Comparison of three whole-cell pertussis vaccines in the baboon model of pertussis. Clin. Vaccine Immunol. 2016, 23, 47–54. [Google Scholar] [CrossRef] [Green Version]
- Shearer, M.H.; Lucas, A.H.; Anderson, P.W.; Carey, K.D.; Jenson, H.B.; Chanh, T.C.; Stanley, J.R.; Kennedy, R.C. The baboon as a nonhuman primate model for assessing the effects of maternal immunization with Haemophilus influenzae type B polysaccharide vaccines. Infect. Immun. 1997, 65, 3267–3270. [Google Scholar] [CrossRef] [Green Version]
- Wang, D.; Phan, S.; DiStefano, D.J.; Citron, M.P.; Callahan, C.L.; Indrawati, L.; Dubey, S.A.; Heidecker, G.J.; Govindarajan, D.; Liang, X.; et al. A single-dose recombinant parainfluenza virus 5-vectored vaccine expressing respiratory syncytial virus (RSV) F or G protein protected cotton rats and African Green Monkeys from RSV challenge. J. Virol. 2017, 91, e00066-17. [Google Scholar] [CrossRef] [Green Version]
- Phan, S.I.; Adam, C.M.; Chen, Z.; Citron, M.; Liang, X.; Espeseth, A.S.; Wang, D.; He, B. Genetic stability of parainfluenza virus 5-vectored human respiratory syncytial virus vaccine candidates after in vitro and in vivo passage. J. Virol. 2017, 91, e00559-17. [Google Scholar] [CrossRef] [Green Version]
- Kamigaki, T.; Chaw, L.; Tan, A.G.; Tamaki, R.; Alday, P.P.; Javier, J.B.; Olveda, R.M.; Oshitani, H.; Tallo, V.L. Seasonality of influenza and respiratory syncytial viruses and the effect of climate factors in subtropical-tropical Asia using influenza-like illness surveillance data, 2010–2012. PLoS ONE 2016, 11, e0167712. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stensballe, L.G.; Ravn, H.; Kristensen, K.; Meakins, T.; Aaby, P.; Simoes, E.A. Seasonal variation of maternally derived respiratory syncytial virus antibodies and association with infant hospitalizations for respiratory syncytial virus. J. Pediatr. 2009, 154, 296–298. [Google Scholar] [CrossRef] [PubMed]
- Madhi, S.A.; Polack, F.P.; Piedra, P.A.; Munoz, F.M.; Trenholme, A.A.; Simoes, E.A.F.; Swamy, G.K.; Agrawal, S.; Ahmed, K.; August, A.; et al. Respiratory syncytial virus vaccination during pregnancy and effects in infants. N. Engl. J. Med. 2020, 383, 426–439. [Google Scholar] [CrossRef] [PubMed]
- Chu, H.Y.; Steinhoff, M.C.; Magaret, A.; Zaman, K.; Roy, E.; Langdon, G.; Formica, M.A.; Walsh, E.E.; Englund, J.A. Respiratory syncytial virus transplacental antibody transfer and kinetics in mother-infant pairs in Bangladesh. J. Infect. Dis. 2014, 210, 1582–1589. [Google Scholar] [CrossRef] [Green Version]
- Chu, H.Y.; Tielsch, J.; Katz, J.; Magaret, A.S.; Khatry, S.; LeClerq, S.C.; Shrestha, L.; Kuypers, J.; Steinhoff, M.C.; Englund, J.A. Transplacental transfer of maternal respiratory syncytial virus (RSV) antibody and protection against RSV disease in infants in rural Nepal. J. Clin. Virol. 2017, 95, 90–95. [Google Scholar] [CrossRef] [PubMed]
- De Rijk, E.P.C.T.; Van Esch, E. The macaque placenta-a mini-review. Toxicol. Pathol. 2008, 36, 108s–118s. [Google Scholar] [CrossRef] [Green Version]
- Polack, F.P. The changing landscape of respiratory syncytial virus. Vaccine 2015, 33, 6473–6478. [Google Scholar] [CrossRef] [Green Version]
- Polack, F.P. Respiratory syncytial virus during pregnancy. Clin. Infect. Dis. 2018, 66, 1666–1667. [Google Scholar] [CrossRef] [Green Version]
- Lindsey, B.; Kampmann, B.; Jones, C. Maternal immunization as a strategy to decrease susceptibility to infection in newborn infants. Curr. Opin. Infect. Dis. 2013, 26, 248–253. [Google Scholar] [CrossRef]
- Saso, A.; Kampmann, B. Vaccination against respiratory syncytial virus in pregnancy: A suitable tool to combat global infant morbidity and mortality? Lancet Infect. Dis. 2016, 16, e153–e163. [Google Scholar] [CrossRef]
- Saso, A.; Kampmann, B. Vaccine responses in newborns. Semin. Immunopathol. 2017, 39, 627–642. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saso, A.; Kampmann, B. Maternal immunization: Nature meets nurture. Front. Microbiol. 2020, 11, 1499. [Google Scholar] [CrossRef] [PubMed]
- Switzer, C.; D’Heilly, C.; Macina, D. Immunological and clinical benefits of maternal immunization against pertussis: A Systematic review. Infect. Dis. Ther. 2019, 8, 499–541. [Google Scholar] [CrossRef] [Green Version]
- Jarvis, J.R.; Dorey, R.B.; Warricker, F.D.M.; Alwan, N.A.; Jones, C.E. The effectiveness of influenza vaccination in pregnancy in relation to child health outcomes: Systematic review and meta-analysis. Vaccine 2020, 38, 1601–1613. [Google Scholar] [CrossRef] [PubMed]
- Schlaudecker, E.P.; Steinhoff, M.C.; Omer, S.B.; McNeal, M.M.; Roy, E.; Arifeen, S.E.; Dodd, C.N.; Raqib, R.; Breiman, R.F.; Zaman, K. IgA and neutralizing antibodies to influenza a virus in human milk: A randomized trial of antenatal influenza immunization. PLoS ONE 2013, 8, e70867. [Google Scholar] [CrossRef]
- Schlaudecker, E.P.; Steinhoff, M.C.; Omer, S.B.; Roy, E.; Arifeen, S.E.; Dodd, C.N.; Altaye, M.; Raqib, R.; Breiman, R.F.; Zaman, K. Antibody persistence in mothers one year after pneumococcal immunization in pregnancy. Vaccine 2012, 30, 5063–5066. [Google Scholar] [CrossRef] [Green Version]
- Maertens, K.; De Schutter, S.; Braeckman, T.; Baerts, L.; Van Damme, P.; De Meester, I.; Leuridan, E. Breastfeeding after maternal immunisation during pregnancy: Providing immunological protection to the newborn: A review. Vaccine 2014, 32, 1786–1792. [Google Scholar] [CrossRef] [PubMed]
- Munoz, F.M.; Swamy, G.K.; Hickman, S.P.; Agrawal, S.; Piedra, P.A.; Glenn, G.M.; Patel, N.; August, A.M.; Cho, I.; Fries, L. Safety and immunogenicity of a respiratory syncytial virus fusion (F) protein nanoparticle vaccine in healthy third-trimester pregnant women and their infants. J. Infect. Dis. 2019, 220, 1802–1815. [Google Scholar] [CrossRef]
- Boonyaratanakornkit, J.; Chu, H.Y. Why should we advocate maternal immunization? Pediatr. Infect. Dis. J. 2019, 38, S28–S32. [Google Scholar] [CrossRef] [PubMed]
- DiTosto, J.D.; Weiss, R.E.; Yee, L.M.; Badreldin, N. Association of Tdap vaccine guidelines with vaccine uptake during pregnancy. PLoS ONE 2021, 16, e0254863. [Google Scholar] [CrossRef] [PubMed]
- Khodr, Z.G.; Bukowinski, A.T.; Gumbs, G.R.; Conlin, A.M.S. Tetanus, diphtheria, and acellular pertussis vaccination during pregnancy and reduced risk of infant acute respiratory infections. Vaccine 2017, 35, 5603–5610. [Google Scholar] [CrossRef]
- Schlaudecker, E.P.; Englund, J.A.; Kimberlin, D.W. Update from the advisory committee on immunization practices. J. Pediatr. Infect. Dis. Soc. 2013, 2, 97–99. [Google Scholar] [CrossRef] [Green Version]
- Schlaudecker, E.P.; Sawyer, M.H.; Kimberlin, D.W. Update from the advisory committee on immunization practices. J. Pediatr. Infect. Dis. Soc. 2014, 3, 1–3. [Google Scholar] [CrossRef] [Green Version]
- Abu Raya, B.; Edwards, K.M.; Scheifele, D.W.; Halperin, S.A. Pertussis and influenza immunisation during pregnancy: A landscape review. Lancet Infect. Dis. 2017, 17, e209–e222. [Google Scholar] [CrossRef]
- Edwards, K.M. Maternal immunisation in pregnancy to protect newborn infants. Arch. Dis. Child 2019, 104, 316–319. [Google Scholar] [CrossRef]
- Schuster, J.E.; O’Leary, S.; Kimberlin, D.W. Update from the advisory committee on immunization practices. J. Pediatr. Infect. Dis. Soc. 2016, 5, 349–355. [Google Scholar] [CrossRef] [Green Version]
- Keller-Stanislawski, B.; Englund, J.A.; Kang, G.; Mangtani, P.; Neuzil, K.; Nohynek, H.; Pless, R.; Lambach, P.; Zuber, P. Safety of immunization during pregnancy: A review of the evidence of selected inactivated and live attenuated vaccines. Vaccine 2014, 32, 7057–7064. [Google Scholar] [CrossRef]
- Abu-Raya, B.; Maertens, K.; Edwards, K.M.; Omer, S.B.; Englund, J.A.; Flanagan, K.L.; Snape, M.D.; Amirthalingam, G.; Leuridan, E.; Damme, P.V.; et al. Global perspectives on immunization during pregnancy and priorities for future research and development: An international consensus statement. Front. Immunol. 2020, 11, 1282. [Google Scholar] [CrossRef]
- Madhi, S.A.; Dangor, Z. Prospects for preventing infant invasive GBS disease through maternal vaccination. Vaccine 2017, 35, 4457–4460. [Google Scholar] [CrossRef]
- Gill, L.; Jones, C.W. Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) antibodies in neonatal cord blood after vaccination in pregnancy. Obstet. Gynecol. 2021, 137, 894–896. [Google Scholar] [CrossRef]
- Abu-Raya, B. Vaccination of pregnant women against COVID-19. Neoreviews 2021, 22, e570–e573. [Google Scholar] [CrossRef]
- Abu Raya, B.; Giles, M.L.; Sadarangani, M. Vertical transmission of Severe Acute Respiratory Syndrome Coronavirus 2 from the mother to the infant. JAMA Pediatr. 2020, 174, 1007–1008. [Google Scholar] [CrossRef]
- Beharier, O.; Plitman Mayo, R.; Raz, T.; Nahum Sacks, K.; Schreiber, L.; Suissa-Cohen, Y.; Chen, R.; Gomez-Tolub, R.; Hadar, E.; Gabbay-Benziv, R.; et al. Efficient maternal to neonatal transfer of antibodies against SARS-CoV-2 and BNT162b2 mRNA COVID-19 vaccine. J. Clin. Investig. 2021, 131. [Google Scholar] [CrossRef] [PubMed]
- Taleb, S.A.; Al Thani, A.A.; Al Ansari, K.; Yassine, H.M. Human respiratory syncytial virus: Pathogenesis, immune responses, and current vaccine approaches. Eur. J. Clin. Microbiol. Infect. Dis. 2018, 37, 1817–1827. [Google Scholar] [CrossRef] [PubMed]
- Munoz, F.M.; Piedra, P.A.; Glezen, W.P. Safety and immunogenicity of respiratory syncytial virus purified fusion protein-2 vaccine in pregnant women. Vaccine 2003, 21, 3465–3467. [Google Scholar] [CrossRef]
- McLellan, J.S.; Chen, M.; Joyce, M.G.; Sastry, M.; Stewart-Jones, G.B.; Yang, Y.; Zhang, B.; Chen, L.; Srivatsan, S.; Zheng, A.; et al. Structure-based design of a fusion glycoprotein vaccine for respiratory syncytial virus. Science 2013, 342, 592–598. [Google Scholar] [CrossRef] [Green Version]
- Leroux-Roels, G.; De Boever, F.; Maes, C.; Nguyen, T.L.; Baker, S.; Gonzalez Lopez, A. Safety and immunogenicity of a respiratory syncytial virus fusion glycoprotein F subunit vaccine in healthy adults: Results of a phase 1, randomized, observer-blind, controlled, dosage-escalation study. Vaccine 2019, 37, 2694–2703. [Google Scholar] [CrossRef]
- Brandt, C.; Power, U.F.; Plotnicky-Gilquin, H.; Huss, T.; Nguyen, T.; Lambert, P.H.; Binz, H.; Siegrist, C.A. Protective immunity against respiratory syncytial virus in early life after murine maternal or neonatal vaccination with the recombinant G fusion protein BBG2Na. J. Infect. Dis. 1997, 176, 884–891. [Google Scholar] [CrossRef] [Green Version]
- Noh, Y.; Shim, B.S.; Cheon, I.S.; Rho, S.; Kim, H.J.; Choi, Y.; Kang, C.Y.; Chang, J.; Song, M.K.; Kim, J.O. Neonatal immunization with respiratory syncytial virus glycoprotein fragment induces protective immunity in the presence of maternal antibodies in mice. Viral Immunol. 2013, 26, 268–276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shao, H.Y.; Chen, Y.C.; Chung, N.H.; Lu, Y.J.; Chang, C.K.; Yu, S.L.; Liu, C.C.; Chow, Y.H. Maternal immunization with a recombinant adenovirus-expressing fusion protein protects neonatal cotton rats from respiratory syncytia virus infection by transferring antibodies via breast milk and placenta. Virology 2018, 521, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Taylor, G. Animal models of respiratory syncytial virus infection. Vaccine 2017, 35, 469–480. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hagglund, S.; Hu, K.; Blodorn, K.; Makabi-Panzu, B.; Gaillard, A.L.; Ellencrona, K.; Chevret, D.; Hellman, L.; Bengtsson, K.L.; Riffault, S.; et al. Characterization of an experimental vaccine for bovine respiratory syncytial virus. Clin. Vaccine Immunol. 2014, 21, 997–1004. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garg, R.; Latimer, L.; Wang, Y.; Simko, E.; Gerdts, V.; Potter, A.; van Drunen Littel-van den Hurk, S. Maternal immunization with respiratory syncytial virus fusion protein formulated with a novel combination adjuvant provides protection from RSV in newborn lambs. Vaccine 2016, 34, 261–269. [Google Scholar] [CrossRef]
- Hogan, A.B.; Campbell, P.T.; Blyth, C.C.; Lim, F.J.; Fathima, P.; Davis, S.; Moore, H.C.; Glass, K. Potential impact of a maternal vaccine for RSV: A mathematical modelling study. Vaccine 2017, 35, 6172–6179. [Google Scholar] [CrossRef]
- Blanco, J.C.G.; Pletneva, L.M.; Otoa, R.O.; Patel, M.C.; Vogel, S.N.; Boukhvalova, M.S. Preclinical assessment of safety of maternal vaccination against respiratory syncytial virus (RSV) in cotton rats. Vaccine 2017, 35, 3951–3958. [Google Scholar] [CrossRef]
- Miller, D.W.; Fraser, H.M.; Brooks, A.N. Suppression of fetal gonadotrophin concentrations by maternal passive immunization to GnRH in sheep. J. Reprod. Fertil. 1998, 113, 69–73. [Google Scholar] [CrossRef] [Green Version]
- Orendi, K.; Klein, K.; Krampl-Bettelheim, E.; Nuk, M.; Holzapfel-Bauer, M.; Magnet, E.; Griesbacher, A.; Lang, U.; Pertl, B. SRY-specific cell free fetal DNA in maternal plasma in twin pregnancies throughout gestation. Placenta 2011, 32, 611–615. [Google Scholar] [CrossRef]
- Stensballe, L.G.; Ravn, H.; Kristensen, K.; Agerskov, K.; Meakins, T.; Aaby, P.; Simoes, E.A. Respiratory syncytial virus neutralizing antibodies in cord blood, respiratory syncytial virus hospitalization, and recurrent wheeze. J. Allergy Clin. Immunol. 2009, 123, 398–403. [Google Scholar] [CrossRef] [PubMed]
- Glezen, W.P.; Paredes, A.; Allison, J.E.; Taber, L.H.; Frank, A.L. Risk of respiratory syncytial virus infection for infants from low-income families in relationship to age, sex, ethnic group, and maternal antibody level. J. Pediatr. 1981, 98, 708–715. [Google Scholar] [CrossRef]
- Sharma, A.; Wendland, R.; Sung, B.; Wu, W.; Grunwald, T.; Worgall, S. Maternal immunization with chimpanzee adenovirus expressing RSV fusion protein protects against neonatal RSV pulmonary infection. Vaccine 2014, 32, 5761–5768. [Google Scholar] [CrossRef] [Green Version]
- Prince, G.A.; Horswood, R.L.; Camargo, E.; Suffin, S.C.; Chanock, R.M. Parenteral immunization with live respiratory syncytial virus is blocked in seropositive cotton rats. Infect. Immun. 1982, 37, 1074–1078. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blanco, J.C.G.; Pletneva, L.M.; Oue, R.O.; Patel, M.C.; Boukhvalova, M.S. Maternal transfer of RSV immunity in cotton rats vaccinated during pregnancy. Vaccine 2015, 33, 5371–5379. [Google Scholar] [CrossRef] [Green Version]
- Steff, A.M.; Monroe, J.; Friedrich, K.; Chandramouli, S.; Nguyen, T.L.; Tian, S.; Vandepaer, S.; Toussaint, J.F.; Carfi, A. Pre-fusion RSV F strongly boosts pre-fusion specific neutralizing responses in cattle pre-exposed to bovine RSV. Nat. Commun. 2017, 8, 1085. [Google Scholar] [CrossRef] [Green Version]
- Hodgins, D.C.; Shewen, P.E. Vaccination of neonates: Problem and issues. Vaccine 2012, 30, 1541–1559. [Google Scholar] [CrossRef] [PubMed]
- Borghi, S.; Bournazos, S.; Thulin, N.K.; Li, C.; Gajewski, A.; Sherwood, R.W.; Zhang, S.; Harris, E.; Jagannathan, P.; Wang, L.X.; et al. FcRn, but not FcgammaRs, drives maternal-fetal transplacental transport of human IgG antibodies. Proc. Natl. Acad. Sci. USA 2020, 117, 12943–12951. [Google Scholar] [CrossRef] [PubMed]
- Carter, A.M.; Enders, A.C.; Pijnenborg, R. The role of invasive trophoblast in implantation and placentation of primates. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2015, 370, 20140070. [Google Scholar] [CrossRef]
- Baintner, K. Transmission of antibodies from mother to young: Evolutionary strategies in a proteolytic environment. Vet. Immunol. Immunopathol. 2007, 117, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Carter, A.M. Animal models of human pregnancy and placentation: Alternatives to the mouse. Reproduction 2020, 160, R129–R143. [Google Scholar] [CrossRef] [PubMed]
- Fujimoto, K.; Terao, K.; Cho, F.; Honjo, S. The placental transfer of IgG in the cynomolgus monkey. Jpn. J. Med. Sci. Biol. 1983, 36, 171–176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen, S.M.; Antony, K.M.; Dudley, D.M.; Kohn, S.; Simmons, H.A.; Wolfe, B.; Salamat, M.S.; Teixeira, L.B.C.; Wiepz, G.J.; Thoong, T.H.; et al. Highly efficient maternal-fetal Zika virus transmission in pregnant Rhesus macaques. PLoS Pathog. 2017, 13, e1006378. [Google Scholar] [CrossRef] [Green Version]
- Barry, P.A.; Lockridge, K.M.; Salamat, S.; Tinling, S.P.; Yue, Y.; Zhou, S.S.; Gospe, S.M., Jr.; Britt, W.J.; Tarantal, A.F. Nonhuman primate models of intrauterine cytomegalovirus infection. ILAR J. 2006, 47, 49–64. [Google Scholar] [CrossRef] [Green Version]
- Itell, H.L.; Kaur, A.; Deere, J.D.; Barry, P.A.; Permar, S.R. Rhesus monkeys for a nonhuman primate model of cytomegalovirus infections. Curr. Opin. Virol. 2017, 25, 126–133. [Google Scholar] [CrossRef] [Green Version]
- Bialas, K.M.; Tanaka, T.; Tran, D.; Varner, V.; Cisneros De La Rosa, E.; Chiuppesi, F.; Wussow, F.; Kattenhorn, L.; Macri, S.; Kunz, E.L.; et al. Maternal CD4+ T cells protect against severe congenital cytomegalovirus disease in a novel nonhuman primate model of placental cytomegalovirus transmission. Proc. Natl. Acad. Sci. USA 2015, 112, 13645–13650. [Google Scholar] [CrossRef] [Green Version]
- Maertens, K.; Orije, M.R.P.; Van Damme, P.; Leuridan, E. Vaccination during pregnancy: Current and possible future recommendations. Eur. J. Pediatr. 2020, 179, 235–242. [Google Scholar] [CrossRef]
- Itell, H.L.; Nelson, C.S.; Martinez, D.R.; Permar, S.R. Maternal immune correlates of protection against placental transmission of cytomegalovirus. Placenta 2017, 60 (Suppl. S1), S73–S79. [Google Scholar] [CrossRef]
- Nelson, C.S.; Cruz, D.V.; Tran, D.; Bialas, K.M.; Stamper, L.; Wu, H.; Gilbert, M.; Blair, R.; Alvarez, X.; Itell, H.; et al. Preexisting antibodies can protect against congenital cytomegalovirus infection in monkeys. JCI Insight 2017, 2, e94002. [Google Scholar] [CrossRef] [Green Version]
- Papin, J.F.; Wolf, R.F.; Kosanke, S.D.; Jenkins, J.D.; Moore, S.N.; Anderson, M.P.; Welliver, R.C., Sr. Infant baboons infected with respiratory syncytial virus develop clinical and pathological changes that parallel those of human infants. Am. J. Physiol. Lung Cell. Mol. Physiol. 2013, 304, L530–L539. [Google Scholar] [CrossRef] [Green Version]
- Messaoudi, I.; Estep, R.; Robinson, B.; Wong, S.W. Nonhuman primate models of human immunology. Antioxid. Redox Signal. 2011, 14, 261–273. [Google Scholar] [CrossRef] [Green Version]
- Olmsted, R.A.; Buller, R.M.; Collins, P.L.; London, W.T.; Beeler, J.A.; Prince, G.A.; Chanock, R.M.; Murphy, B.R. Evaluation in non-human primates of the safety, immunogenicity and efficacy of recombinant vaccinia viruses expressing the F or G glycoprotein of respiratory syncytial virus. Vaccine 1988, 6, 519–524. [Google Scholar] [CrossRef]
- Vaughan, K.; Rhodes, G.H.; Gershwin, L.J. DNA immunization against respiratory syncytial virus (RSV) in infant Rhesus monkeys. Vaccine 2005, 23, 2928–2942. [Google Scholar] [CrossRef]
- Warfel, J.M.; Merkel, T.J. The baboon model of pertussis: Effective use and lessons for pertussis vaccines. Expert Rev. Vaccines 2014, 13, 1241–1252. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Warfel, J.M.; Papin, J.F.; Wolf, R.F.; Zimmerman, L.I.; Merkel, T.J. Maternal and neonatal vaccination protects newborn baboons from pertussis infection. J. Infect. Dis. 2014, 210, 604–610. [Google Scholar] [CrossRef] [Green Version]
- Byrd, L.G.; Prince, G.A. Animal models of respiratory syncytial virus infection. Clin. Infect. Dis. 1997, 25, 1363–1368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Janet, S.; Broad, J.; Snape, M.D. Respiratory syncytial virus seasonality and its implications on prevention strategies. Hum. Vaccines Immunother. 2018, 14, 234–244. [Google Scholar] [CrossRef] [Green Version]
- Fujimoto, K.; Terao, K.; Cho, F.; Nakamura, F.; Honjo, S. Age-related immunoglobulin levels in cynomolgus monkeys. Jpn. J. Med. Sci. Biol. 1982, 35, 17–23. [Google Scholar] [CrossRef] [Green Version]
- Mankarious, S.; Lee, M.; Fischer, S.; Pyun, K.H.; Ochs, H.D.; Oxelius, V.A.; Wedgwood, R.J. The half-lives of IgG subclasses and specific antibodies in patients with primary immunodeficiency who are receiving intravenously administered immunoglobulin. J. Lab. Clin. Med. 1988, 112, 634–640. [Google Scholar] [PubMed]
- Wang, W.; Wang, E.Q.; Balthasar, J.P. Monoclonal antibody pharmacokinetics and pharmacodynamics. Clin. Pharmacol. Ther. 2008, 84, 548–558. [Google Scholar] [CrossRef]
- Challacombe, S.J.; Russell, M.W. Estimation of the intravascular half-lives of normal Rhesus monkey IgG, IgA and IgM. Immunology 1979, 36, 331–338. [Google Scholar]
- Oguti, B.; Assad, A.; Andrews, N.; Barug, D.; Dang, D.A.; Halperin, S.; Hoang, H.; Holder, B.; Kampmann, B.; Kazi, M.; et al. The Half-Life of Maternal Transplacental Antibodies in infants from mothers vaccinated with diphtheria, tetanus and pertussis: An individual participant data meta-analysis. Access Microbiol. 2020, 2, 239. [Google Scholar] [CrossRef]
- Tam, S.H.; McCarthy, S.G.; Brosnan, K.; Goldberg, K.M.; Scallon, B.J. Correlations between pharmacokinetics of IgG antibodies in primates vs. FcRn-transgenic mice reveal a rodent model with predictive capabilities. MAbs 2013, 5, 397–405. [Google Scholar] [CrossRef] [Green Version]
- Welliver, R.C.; Papin, J.F.; Preno, A.; Ivanov, V.; Tian, J.H.; Lu, H.; Guebre-Xabier, M.; Flyer, D.; Massare, M.J.; Glenn, G.; et al. Maternal immunization with RSV fusion glycoprotein vaccine and substantial protection of neonatal baboons against respiratory syncytial virus pulmonary challenge. Vaccine 2020, 38, 1258–1270. [Google Scholar] [CrossRef] [PubMed]
- Collins, P.L.; Crowe, J.E. Respiratory syncytial virus and metapneumovirus. In Fiedls Virology, 5th ed.; Lippincott Williams and Wilkins: Philadelphia, PA, USA, 2007. [Google Scholar]
- Flynn, J.A.; Durr, E.; Swoyer, R.; Cejas, P.J.; Horton, M.S.; Galli, J.D.; Cosmi, S.A.; Espeseth, A.S.; Bett, A.J.; Zhang, L. Stability characterization of a vaccine antigen based on the respiratory syncytial virus fusion glycoprotein. PLoS ONE 2016, 11, e0164789. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Callahan, C.; Citron, M.; Wen, Z.; Touch, S.; Monslow, M.A.; Cox, K.S.; DiStefano, D.J.; Vora, K.A.; Bett, A.; et al. Respiratory syncytial virus elicits enriched CD8+ T lymphocyte responses in lung compared with blood in African green monkeys. PLoS ONE 2017, 12, e0187642. [Google Scholar] [CrossRef] [Green Version]
- Citron, M.P.; Patel, M.; Purcell, M.; Lin, S.A.; Rubins, D.J.; McQuade, P.; Callahan, C.; Gleason, A.; Petrescu, I.; Knapp, W.; et al. A novel method for strict intranasal delivery of non-replicating RSV vaccines in cotton rats and non-human primates. Vaccine 2018, 36, 2876–2885. [Google Scholar] [CrossRef] [PubMed]
- Espeseth, A.S.; Cejas, P.J.; Citron, M.P.; Wang, D.; DiStefano, D.J.; Callahan, C.; Donnell, G.O.; Galli, J.D.; Swoyer, R.; Touch, S.; et al. Modified mRNA/lipid nanoparticle-based vaccines expressing respiratory syncytial virus F protein variants are immunogenic and protective in rodent models of RSV infection. NPJ Vaccines 2020, 5, 16. [Google Scholar] [CrossRef] [Green Version]


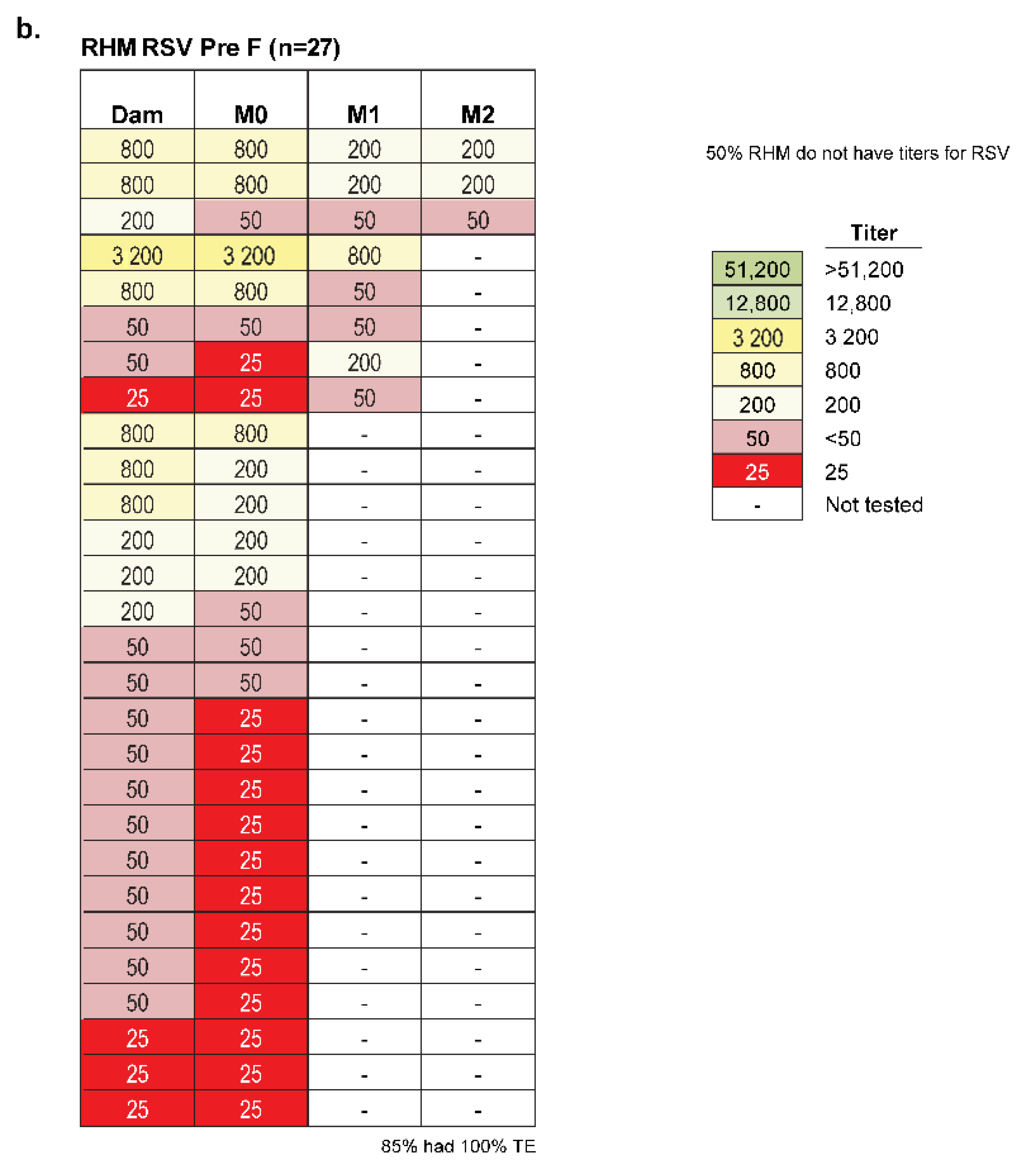

| Dam | Vaccine | Baseline | Day 28 Post Vax | Infant | Birth | 1 Month old | Days Prior Birth |
|---|---|---|---|---|---|---|---|
| A8M011 | None | 2 × 102 | 2 × 102 | A18M070 | 2 × 102 | 5 × 101 | No Vaccine |
| 1932 | None | 8 × 102 | 8 × 102 | A18U011 | 8 × 102 | 2 × 102 | No Vaccine |
| A13M046 | None | 8 × 102 | 2 × 102 | A18M057 | 2 × 102 | 2 × 102 | No Vaccine |
| 99M007 | None | 8 × 102 | 2 × 102 | A18M067 | 2 × 102 | 5 × 101 | No Vaccine |
| 1799 | None | 3 × 101 | 3 × 101 | A18U009 | 3 × 101 | 3 × 101 | No Vaccine |
| A9M003 | None | 8 × 102 | 2 × 102 | A18M073 | 2 × 102 | 5 × 101 | No Vaccine |
| A7M063 | None | 8 × 102 | 8 × 102 | A18M076 | 8 × 102 | 8 × 102 | No Vaccine |
| A7M058 | PreF | 8 × 102 | 5 × 104 | A18M071 | 5 × 104 | 1 × 104 | 14 |
| A8M026 | PreF | 8 × 102 | 5 × 104 | A18M075 | 1 × 104 | 3 × 103 | 21 |
| 1941 | PreF | 5 × 104 | 2 × 105 | A18U010 | 2 × 105 | 5 × 104 | 28 |
| A6S002 | PreF | 1 × 104 | 2 × 105 | A18M068 | 3 × 103 | 8 × 102 | 2 days |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Citron, M.P.; McAnulty, J.; Callahan, C.; Knapp, W.; Fontenot, J.; Morales, P.; Flynn, J.A.; Douglas, C.M.; Espeseth, A.S. Transplacental Antibody Transfer of Respiratory Syncytial Virus Specific IgG in Non-Human Primate Mother-Infant Pairs. Pathogens 2021, 10, 1441. https://doi.org/10.3390/pathogens10111441
Citron MP, McAnulty J, Callahan C, Knapp W, Fontenot J, Morales P, Flynn JA, Douglas CM, Espeseth AS. Transplacental Antibody Transfer of Respiratory Syncytial Virus Specific IgG in Non-Human Primate Mother-Infant Pairs. Pathogens. 2021; 10(11):1441. https://doi.org/10.3390/pathogens10111441
Chicago/Turabian StyleCitron, Michael P., Jessica McAnulty, Cheryl Callahan, Walter Knapp, Jane Fontenot, Pablo Morales, Jessica A. Flynn, Cameron M. Douglas, and Amy S. Espeseth. 2021. "Transplacental Antibody Transfer of Respiratory Syncytial Virus Specific IgG in Non-Human Primate Mother-Infant Pairs" Pathogens 10, no. 11: 1441. https://doi.org/10.3390/pathogens10111441
APA StyleCitron, M. P., McAnulty, J., Callahan, C., Knapp, W., Fontenot, J., Morales, P., Flynn, J. A., Douglas, C. M., & Espeseth, A. S. (2021). Transplacental Antibody Transfer of Respiratory Syncytial Virus Specific IgG in Non-Human Primate Mother-Infant Pairs. Pathogens, 10(11), 1441. https://doi.org/10.3390/pathogens10111441







