Abstract
The emergence of the COVID-19 pandemic highlighted the importance of studying newly emerging viruses that cause respiratory illnesses. Human bocavirus (HBoV) is one of the relatively newly discovered viruses that has been detected worldwide and causes respiratory and gastrointestinal infections, mainly in pediatric patients. However, little is known about the pathogenicity and evolution of HBoV. This systematic review was initiated to clarify the prevalence and circulating genotypes of HBoV in both respiratory and stool samples from patients of all age groups in the Middle East and North Africa (MENA) from 2005 to February 2021. We performed an electronic search through Science Direct, Scopus, PubMed, Mendeley and Cochrane Library databases. We included all studies reporting the detection rate of HBoV in the MENA region. Data were extracted, and the quality of the included articles was assessed. We included articles containing data on HBoV only or with other respiratory or gastrointestinal viral infections. Review articles, case studies, and animal and environmental studies were excluded. The final number of articles included in this study was 65 articles. The results showed that the HBoV prevalence in children was the lowest in Iran (0%) and the highest in Egypt (56.8%). In adults, the lowest and the highest prevalence were reported in Iran, with values of 0% and 6.6%, respectively. Regarding the respiratory cases, our findings revealed no significant difference between HBoV prevalence among the tested categories (p-value = 0.998). The present study has shown that HBoV is common in children and adults in the MENA region. This systematic review highlights the need for more data on the role of coinfection of HBoV and other viruses, for instance, SARS-CoV-2 in children with acute bronchiolitis.
1. Introduction
Human bocavirus (HBoV) is a parvovirus reported for the first time in 2005 [1]. Since then, an increasing number of reports have emerged indicating the common presence of the virus in the respiratory and gastrointestinal samples. HBoV is known to cause viral respiratory and gastrointestinal tract infections [1,2]. However, the pathogenicity of the virus is not fully understood [3,4]. As with other viruses that cause respiratory tract infections, HBoV can occur during any time of the year, with the highest incidence rate during winter and spring [5,6]. Although HBoV has been found in individuals of all ages, it was mainly reported in infants aged 6–24 months [4,5].
HBoV is a small non-enveloped single-stranded DNA virus with a genome size of 5300 nucleotides. The name Bocavirus was derived after the phylogenetic analysis of the HBoV genome, which showed a close relation to bovine parvovirus (BPV1) and minute virus of canines (MVC). HBoV belongs to the family Parvoviridae, subfamily Parvovirinae and genus Bocavirus. There are four genotypes that belong to the Bocavirus genus. The first genotype was named HBoV1, and was predominantly reported in respiratory samples [7]. The others, named HBoV2, 3 and 4, were reported in the stool samples of gastroenteritis patients [8].
Globally, the total prevalence of HBoV was estimated at around 6.0% [3]. Death cases due to HBoV infections have been reported [9,10,11]. However, there is no definite death rate. The Middle East and North Africa (MENA) region is a term that represents a group of twenty-one countries found in Asia (Bahrain, Iran, Iraq, Israel, Jordan, Kuwait, Lebanon, Oman, Qatar, Saudi Arabia, Syria, Turkey, United Arab Emirates, Palestine and Yemen) and Africa (Algeria, Egypt, Libya, Morocco, Sudan and Tunisia) (https://istizada.com/mena-region/, accessed on 16 January 2021) (Figure 1). Only 14 countries reported the prevalence of HBoV in the MENA region. On the other hand, a lack of reported data was noticed in several countries (Bahrain, Syria, Palestine, Yemen, Algeria, Libya and Morocco) due to lack of knowledge, awareness and attitude of physicians, wars, conflicts, civil revolutions and low scientific research output. The aim of this systematic review was to investigate the prevalence of HBoV and its distribution in the MENA region. Data included patients of all age groups, mainly children, with acute respiratory and gastrointestinal infections, including pilgrims returning from Hajj and Umrah and suffering from acquired acute respiratory tract illness (ARI).
Figure 1.
The Middle East and North Africa region (MENA) (https://istizada.com/mena-region/ (assessed on 16 January 2021).
2. Methods
2.1. Search Strategy and Selection Criteria
This systematic literature review involves all published journal articles and preprints that reported HBoV prevalence and genotypes in the Middle East and North Africa (MENA) region between 2005 and February 2021. Five databases were searched (Science Direct, Scopus, PubMed, Mendeley and Cochrane Library) by using (“boca*” OR “bocavirus” OR “boca virus”) AND (“gastro*” OR “genotype” OR “epidemiology” OR “resp*” OR “prevalence” OR “type”) AND (“The Middle East” OR “North Africa” OR “The Middle East and North Africa” OR “The Middle East & North Africa” OR “MENA” OR “Algeria” OR “Bahrain” OR “Djibouti” OR “Egypt” OR “Iran” OR “Iraq” OR “Jordan” OR “Kuwait” OR “Lebanon” OR “Libya” OR “Morocco” OR “Occupied Palestinian Territories” OR “Oman” OR “Palestine” OR “Qatar” OR “Saudi Arabia” OR “KSA” OR “Somalia” OR “Sudan” OR “Syria” OR “Tunisia” OR “UAE” OR “The United Arab Emirates” OR “Yemen”) as a search strategy. The eligible articles were screened for both the titles and abstracts. The studies involved in this systematic review were selected based on the following criteria: (1) the published articles contain data on HBoV only or with other respiratory or gastrointestinal viral infections from 2005 to February 2021, (2) the studied population in the article is patients residing in, or having acquired infection from, the MENA region. Review articles, case studies, and animal and environmental studies were excluded.
2.2. Data Collection and Data Adjustment
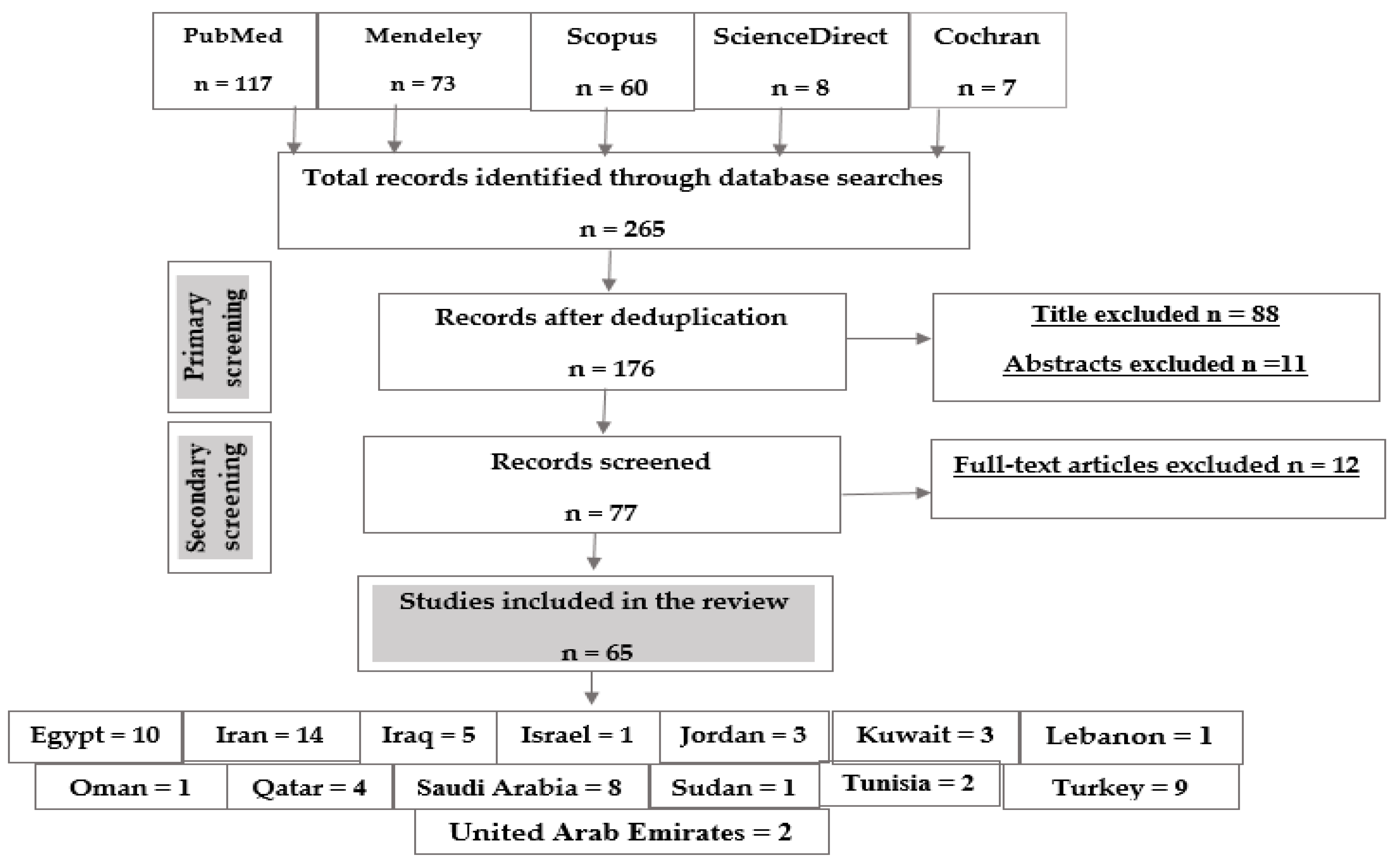
Following the research strategy, a total of 265 articles were identified as follows: 117 articles from PubMed, 73 from Mendeley, 60 from Scopus, eight from Science Direct and seven articles from Cochran. The number of records after deduplication was 175, 88 articles were excluded due to their titles, 11 articles were excluded due to their abstracts and 12 articles were excluded after full-text article screening. The final number of articles included in this study was 65 articles (Figure 2).
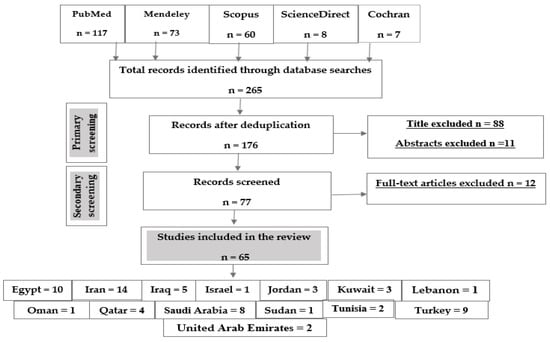
Figure 2.
Flow chart of the study selection protocol.
The data collection sheet was designed to extract data from the selected articles at a 95% confidence interval. The prevalence data were extracted and arranged according to the country and year of sample collection and were reported as percentages. Data of respiratory records were compared by Fisher’s exact test, and p-values were calculated in IBM SPSS statistics version 28 by using the Chi-square test to identify associations.
The summary of individual study parameters was prepared using Microsoft Excel. A mean percentage prevalence was taken if more than one prevalence study was reported from the same country. Prevalence charts were produced for both respiratory and gastrointestinal samples.
3. Results
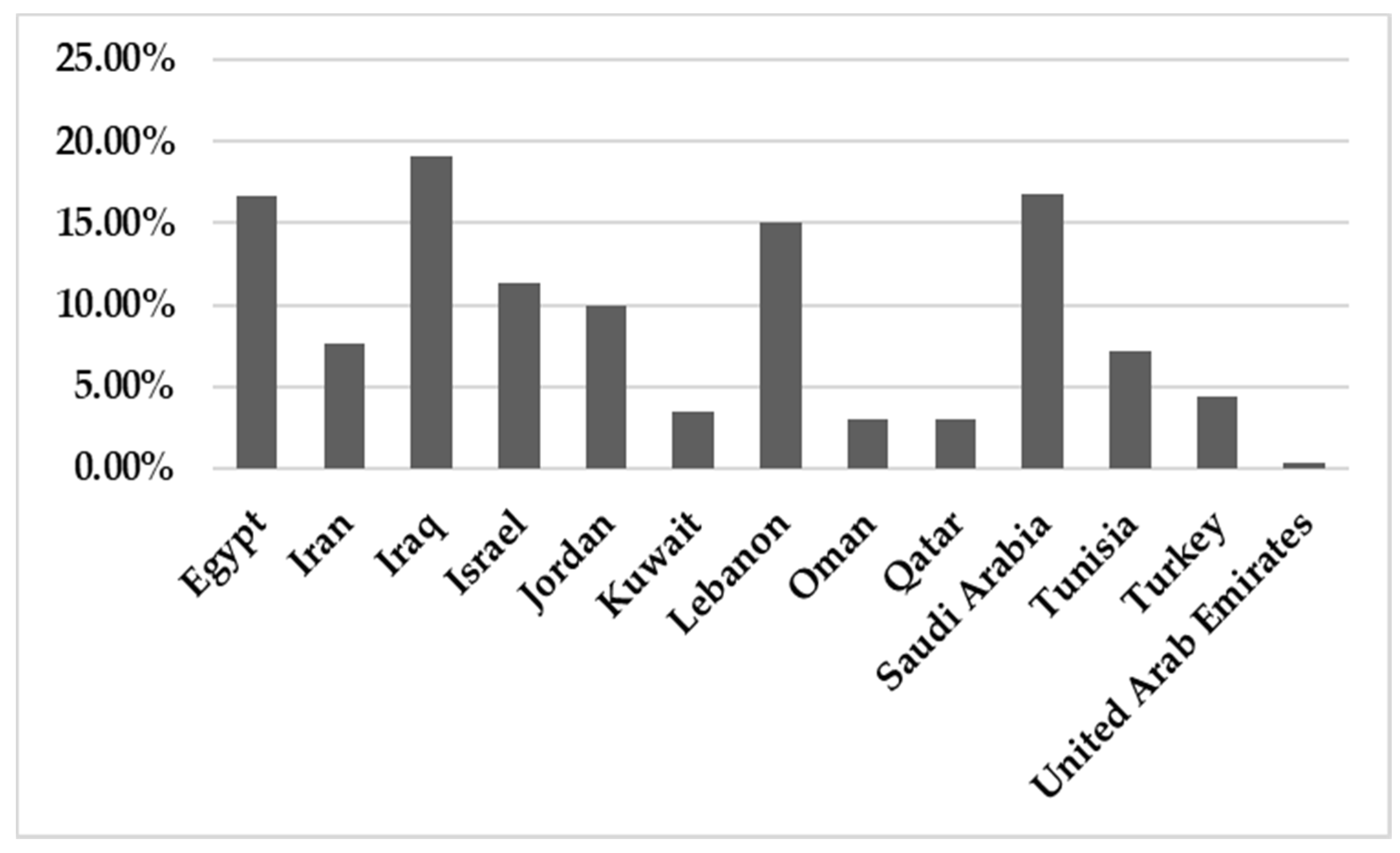
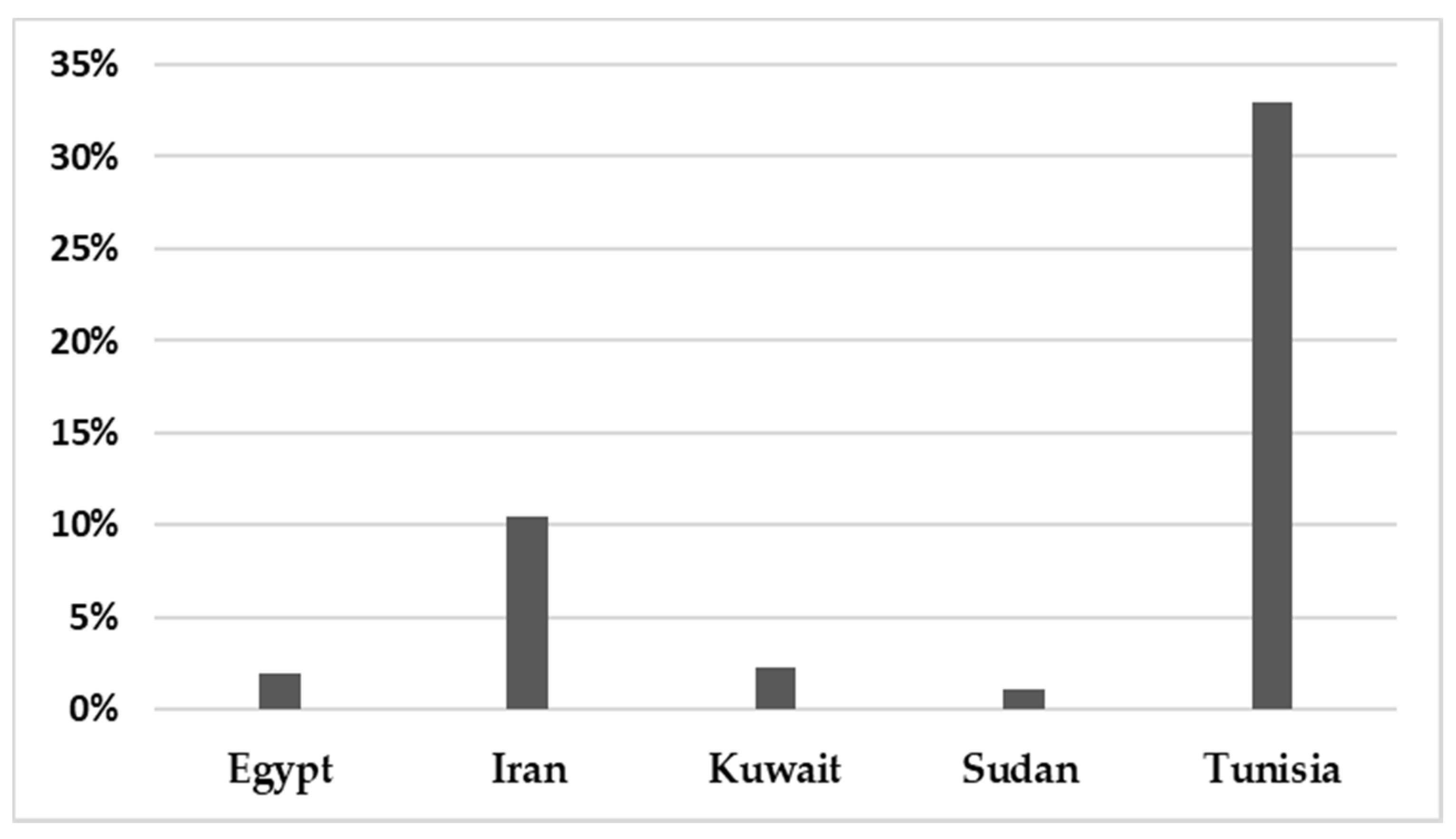
In total, 142,748 patients were reported in sixty-five studies, and 5622 (3.94%) were positive for infection. All those studies reported the prevalence of HBoV in the MENA from 2005 to February 2021 (Table 1). A mean percentage prevalence was calculated for each country for both respiratory and gastrointestinal samples. The prevalence charts were constructed for both respiratory (Figure 3) and gastrointestinal samples (Figure 4).

Table 1.
The prevalence of HBoV in 14 countries of the MENA region.
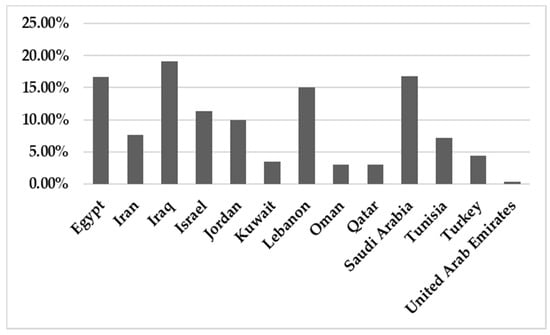
Figure 3.
The prevalence of HBoV in respiratory samples in the MENA.
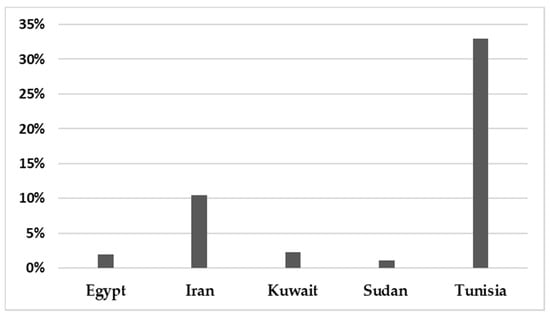
Figure 4.
The prevalence of HBoV in gastrointestinal samples in the MENA.
This systematic review reports the prevalence of HBoV in the MENA region among different tested categories including various age groups (pediatric, children, adults and elderly), COVID-19 cases, pilgrims, health care providers, blood donors and patients with colorectal cancer. Concerning the respiratory cases, our findings revealed no significant differences between HBoV prevalence values among the tested categories (p-value = 0.998).
The study design for almost all of the included studies was a cross-sectional study that aligns with the prevalence determination. In addition, a pilot study, case-control and cohort studies were included in this systematic review. The different study designs can explain the heterogeneity of the sample size.
All included studies used valid assay procedures for the detection of HBoV. The most commonly used method is real-time polymerase chain reaction (RT-PCR). Samples from the upper (nasopharyngeal aspirates, nasopharyngeal swabs or oropharyngeal swab), middle (tracheal aspirate) and lower respiratory tract (Broncho alveolar lavage) were examined for patients with respiratory tract infection. Stool was the specimen of choice for patients with gastroenteritis.
Surgically excised specimens were used to screen human bocavirus in colorectal cancer patients. Whole blood samples from blood donors were screened for HBoV to investigate the possibility of parenteral transmission.
4. Discussion
In the MENA Region, several reports studied the prevalence of HBoV among hospitalized children and adults suffering respiratory tract infections and whether HBoV was the causal agent [27,41]. At the same time, others investigated the HBoV prevalence in patients with gastroenteritis [14,24].
The results showed that the prevalence of HBoV varied from one country to another. The HBoV prevalence, in cases of respiratory tract infection in children, ranged from 0% in Iran to 56.8% in Egypt [16,30]. In adults, the highest prevalence (6.6%) was observed in Iran [27]. Few studies have focused on HBoV isolated from stool specimens to recognize the role of HBoV in gastroenteritis. Only nine studies were found in the MENA, five of them from Iran, and the others were conducted on populations in Egypt, Kuwait, Sudan and Tunisia. Among these studies, the lowest prevalence was reported in Sudan in 2018 (1.10%) [61], while the highest prevalence (33%) was reported in Tunisia [62]. Several factors affect the variations in the prevalence of the virus in these populations, including the geographical location of the country, the clinical diagnosis of the studied population, the type of sample, the method used for detection of the virus, the age group of the examined population and the outbreak season of the virus.
Abdel-Moneim et al. (2016) used newly developed primers to increase the sensitivity of the PCR test for HBoV detection. Using these novel primers, the prevalence of HBoV was 56.8%, which significantly differs from previous and further studies conducted in Egypt, which found prevalence values of 22%, 10% and 18.2% respectively [12,13,17]. Abdel-Moneim et al. explained that the high rate of prevalence of HBoV-1 was reported because of a potential nosocomial pathogen among pediatric care units. This explanation was verified by Cabral et al. in (2021) after he demonstrated that bocavirus is one of the airborne respiratory conditions transmitted during the analysis of the air in pediatric emergency department waiting rooms [75]. Therefore, early diagnosis of HBoV infection in the initial hospitalization time may decrease the spread of the viral infection, especially in pediatric units [47]. Moreover, unlike other respiratory viruses, HBoV can be detected in the serum and whole blood samples of patients suffering from viremia [56].
In Egypt, Abdel-Moneim et al. (2016) studied the presence of HBoV in colorectal cancer patients and found that among one hundred and one patients, twenty-four of them (23.8%) were positive for HBoV [15]. Moreover, Niya et al. (2018) used a case versus control population to detect the presence of the HBoV genome in colorectal cancer patients’ tissue and compared the result with matched healthy control group tissue; HBoV was detected in one patient from each group, with a total prevalence of 1.3% [31].
Several studies have reported the spreading of HBoV among pilgrims during Hajj and Umrah, as mass gathering aids in the transmission of respiratory diseases. The studies concluded that raising awareness among pilgrims of the importance of following public health precautions, such as wearing masks and undergoing vaccination, significantly reduces the transmission of respiratory pathogens [57,60,70].
Currently, four genotypes have been identified worldwide (HBoV1, HBoV2, HBoV3 and HBoV4). In the MENA Region, HBoV1 is the most prominent reported genotype and is mainly associated with respiratory diseases [20,28]. However, HBoV1 was rarely detected in stool samples [25]. Genotypes 2, 3 and 4 were reported in cases of acute gastroenteritis [25,62].
HBoV is detected more frequently with other viruses in the respiratory and gastrointestinal tract (Table 2). HBoV co-infection is present at a high rate among the tested samples, especially with respiratory syncytial virus (RSV) [13,32,41], which is the most prominent virus that causes respiratory illness.

Table 2.
HBoV and other viruses detected in patients with viral co-infection.
However, there is a conflict regarding the role of HBoV in cases of co-infection. Some studies reported no differences in clinical severity between patients hospitalized with a single infection (sole virus) and those with viral co-infection [13,47]. Others proved that more disease severity was associated with a high viral load detected in a single infection [18,76].
5. Conclusions
This systematic review provides a clear summary of the existing knowledge about the prevalence of HBoV infection in the MENA region. The data presented show that HBoV infection is common in children admitted to hospitals and should be screened for as a part of the standard diagnostic panels. This systematic review also highlights the importance of studying the presence of this virus alone or in association with other viruses and stresses the need for further research on the pathogenicity and genomic variation of HBoV.
Author Contributions
Supervision: L.A.; Data collection: R.A. and H.H.; Data analysis and interpretation: R.A., H.H. and L.A.; Prepared tables: R.A. and H.H.; Figure preparation: R.A. and H.H.; Statistical analysis: H.H.; Writing—original draft: R.A., H.H. and L.A.; Writing—review and editing: all authors. All authors have read and agreed to the published version of the manuscript.
Funding
This study did not receive any funding.
Conflicts of Interest
We declare that we have no competing interests nor conflicts of interest.
References
- Allander, T.; Tammi, M.T.; Eriksson, M.; Bjerkner, A.; Tiveljung-Lindell, A.; Andersson, B. Cloning of a human parvovirus by molecular screening of respiratory tract samples. Proc. Natl. Acad. Sci. USA 2005, 102, 12891–12896. [Google Scholar] [CrossRef] [PubMed]
- Albuquerque, M.C.M.; Rocha, L.N.; Benati, F.J.; Soares, C.C.; Maranhão, A.G.; Ramírez, M.L.; Erdman, D.; Santos, N. Human bocavirus infection in children with gastroenteritis, Brazil. Emerg. Infect. Dis. 2007, 13, 1756. [Google Scholar] [CrossRef]
- Guido, M.; Tumolo, M.R.; Verri, T.; Romano, A.; Serio, F.; De Giorgi, M.; De Donno, A.; Bagordo, F.; Zizza, A. Human bocavirus: Current knowledge and future challenges. World J. Gastroenterol. 2016, 22, 8684. [Google Scholar] [CrossRef]
- Jartti, T.; Hedman, K.; Jartti, L.; Ruuskanen, O.; Allander, T.; Söderlund-Venermo, M. Human bocavirus—The first 5 years. Rev. Med. Virol. 2012, 22, 46–64. [Google Scholar] [CrossRef] [PubMed]
- Kesebir, D.; Vazquez, M.; Weibel, C.; Shapiro, E.D.; Ferguson, D.; Landry, M.L.; Kahn, J.S. Human bocavirus infection in young children in the United States: Molecular epidemiological profile and clinical characteristics of a newly emerging respiratory virus. J. Infect. Dis. 2006, 194, 1276–1282. [Google Scholar] [CrossRef]
- Kumar, A.; Filippone, C.; Lahtinen, A.; Hedman, L.; Söderlund-Venermo, M.; Hedman, K.; Franssila, R. Comparison of Th-cell Immunity against Human Bocavirus and Parvovirus B19: Proliferation and Cytokine Responses are Similar in Magnitude but More Closely Interrelated with Human Bocavirus. Scand. J. Immunol. 2011, 73, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Zheng, S.; Xiao, Q.; Ren, L.; Xie, X.; Luo, J.; Wang, L.; Huang, A.; Liu, W.; Liu, E. Single detection of human bocavirus 1 with a high viral load in severe respiratory tract infections in previously healthy children. BMC Infect. Dis. 2014, 14, 1–8. [Google Scholar] [CrossRef]
- Arthur, J.L.; Higgins, G.D.; Davidson, G.P.; Givney, R.C.; Ratcliff, R.M. A novel bocavirus associated with acute gastroenteritis in Australian children. PLoS Pathog. 2009, 5, e1000391. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, M.; Kantola, K.; Finnegan, D.P.; McCaughey, C.; Hedman, L.; Söderlund-Venermo, M.; Hedman, K. Possible involvement of human bocavirus 1 in the death of a middle-aged immunosuppressed patient. J. Clin. Microbiol. 2013, 51, 3461–3463. [Google Scholar] [CrossRef] [PubMed]
- Ziyade, N.; Şirin, G.; Elgörmüş, N.; Daş, T. Detection of human bocavirus DNA by multiplex PCR analysis: Postmortem case report. Balk. Med. J. 2015, 32, 226. [Google Scholar] [CrossRef]
- Choi, S.-H.; Huh, J.W.; Hong, S.-B.; Jung, J.; Kim, M.J.; Chong, Y.P.; Kim, S.; Sung, H.; Chae, E.; Do, K.-H. Severe Human Bocavirus–Associated Pneumonia in Adults at a Referral Hospital, Seoul, South Korea. Emerg. Infect. Dis. 2021, 27, 226. [Google Scholar] [CrossRef] [PubMed]
- Zaghloul, M.Z. Human bocavirus (HBoV) in children with respiratory tract infection by enzyme linked immunosorbent assay (ELISA) and qualitative polymerase chain reaction (PCR). Virol. J. 2011, 8, 239. [Google Scholar] [CrossRef] [PubMed]
- Tabl, H.A.E.-M.; Emam, S.M.; Assar, E.H. Human Bocavirus among Viral Causes of Pediatric Respiratory Tract Infections at Benha University Hospital. Egypt. J. Med. Microbiol. 2012, 38, 1–18. [Google Scholar] [CrossRef]
- El-Mosallamy, W.A.; Awadallah, M.G.; El-Fattah, A.; Diaa, M.; Aboelazm, A.A.; Seif El-Melouk, M. Human bocavirus among viral causes of infantile gastroenteritis. Egypt. J. Med. Microbiol. 2015, 38, 1–7. [Google Scholar] [CrossRef]
- Abdel-Moneim, A.S.; El-Fol, H.A.; Kamel, M.M.; Soliman, A.S.A.; Mahdi, E.A.; El-Gammal, A.S.; Mahran, T.Z.M. Screening of human bocavirus in surgically excised cancer specimens. Arch. Virol. 2016, 161, 2095–2102. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Moneim, A.S.; Kamel, M.M.; Hamed, D.H.; Hassan, S.S.; Soliman, M.S.; Al-Quraishy, S.A.; El Kholy, A.A. A novel primer set for improved direct gene sequencing of human bocavirus genotype-1 from clinical samples. J. Virol. Methods 2016, 228, 108–113. [Google Scholar] [CrossRef] [PubMed]
- Meligy, B.; Sayed, A.; Ismail, D.K.; Kamal, D.; Abdel-Latif, W.; Erfan, D.M. Detection of viral acute lower respiratory tract infection in hospitalized infants using real-time PCR. Egypt. Pediatric Assoc. Gaz. 2016, 64, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Amr, G.E.; Atef, D.M.; Elbehedy, R. Detection of Human Bocavirus in Egyptian Children Suffering from Acute Lower Respiratory Tract Infection. Int. J. Curr. Microbiol. App. Sci. 2017, 6, 809–816. [Google Scholar] [CrossRef]
- Hatem, A.; Mohamed, S.; Elhassan, U.E.A.; Ismael, E.A.; Rizk, M.S.; El-Kholy, A.; El-Harras, M. Clinical characteristics and outcomes of patients with severe acute respiratory infections (SARI): Results from the Egyptian surveillance study 2010–2014. Multidiscip. Respir. Med. 2019, 14, 11. [Google Scholar] [CrossRef]
- Abozahra, R.; Abdelhamid, S.M.; Khairy, K.; Baraka, K. Detection and phylogenetic analysis of Human bocavirus in children diagnosed with acute respiratory tract infection. J. Med. Microbiol. 2020, 69, 1197–1202. [Google Scholar] [CrossRef] [PubMed]
- Roshdy, W.H.; Naguib, A.M.; Elsayed, I.; Shamikh, Y.I. Circulating pattern of respiratory viruses, in Egypt, Season 2013–2014. Bull. Pharm. Sci. Assiut 2020, 43, 237–253. [Google Scholar] [CrossRef]
- Naghipour, M.; Cuevas, L.E.; Bakhshinejad, T.; Dove, W.; Hart, C.A. Human bocavirus in Iranian children with acute respiratory infections. J. Med. Virol. 2007, 79, 539–543. [Google Scholar] [CrossRef] [PubMed]
- Nadji, S.A.; Poos-Ashkan, L.; Khalilzadeh, S.; Baghaie, N.; Shiraghaei, M.J.; Hassanzad, M.; Bolursaz, M.R. Phylogenetic analysis of human bocavirus isolated from children with acute respiratory illnesses and gastroenteritis in Iran. Scand. J. Infect. Dis. 2010, 42, 598–603. [Google Scholar] [CrossRef]
- Monavari, S.H.; Noorbakhsh, S.; Mollaie, H.; Fazlalipour, M.; Kiasari, B.A. Human Bocavirus in Iranian children with acute gastroenteritis. Med. J. Islamic Repub. Iran 2013, 27, 127. [Google Scholar]
- Romani, S.; Mohebbi, S.R.; Khanyaghma, M.; Azimzadeh, P.; Bozorgi, S.M.; Damavand, B.; Jadali, F. Detection of human Bocavirus 1, 2 and 3 from patients with acute gastroenteritis. Gastroenterol. Hepatol. Bed Bench 2013, 6 (Suppl. S1), S77. [Google Scholar] [PubMed]
- Shokrollahi, M.R.; Noorbakhsh, S.; Monavari, H.R.; Darestani, S.G.; Motlagh, A.V.; Nia, S.J. Acute nonbacterial gastroenteritis in hospitalized children: A cross sectional study. Jundishapur J. Microbiol. 2014, 7, e11840. [Google Scholar] [CrossRef] [PubMed]
- Mortazavi, H.S.; Monavari, S.H.; Molaee, H.; Keyvani, H.; Pirkooh, A.A. Human bocavirus in hospitalized Iranian adults with respiratory tract infections during January–June 2014. J. Pure Appl. Microbiol. 2015, 9, 483–488. [Google Scholar]
- Tabasi, M.; Mokhtari-Azad, T.; Eshraghian, M.R.; Shadab, A.; Shatizadeh, S.; Shafiei-Jandaghi, N.Z.; Yavarian, J. Human bocavirus infections among children less than two years old in Iran during fall and winter 2012–2013. Iran. J. Microbiol. 2016, 8, 80. [Google Scholar] [PubMed]
- Moradi, P.; Keyvani, H.; Mousavi, S.-A.J.; Niya, M.H.K.; Esghaei, M.; Bokharaei-Salim, F.; Ataei-Pirkooh, A.; Monavari, S.H. Investigation of viral infection in idiopathic pulmonary fibrosis among Iranian patients in Tehran. Microb. Pathog. 2017, 104, 171–174. [Google Scholar] [CrossRef]
- Shatizadeh Malekshahi, S.; Shafiei-Jandaghi, N.Z.; Yavarian, J.; Shadab, A.; Naseri, M.; Mokhtari Azad, T. Detection of respiratory co-infections in children less than five years with adenovirus infection. Arch. Pediatric Infect. Dis. 2017, 5, e36953. [Google Scholar] [CrossRef]
- Niya, M.H.K.; Ajdarkosh, H.; Tameshkel, F.S.; Panahi, M.; Tabasi, M.; Bouzari, B.; Alemrajabi, M.; Keyvani, H. The molecular detection of human bocavirus (HBoV) in colorectal tissue with malignant and non-malignant lesions. Asian Pac. J. Cancer Prev. APJCP 2018, 19, 3295. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, M.; Yavarian, J.; Karbasizade, V.; Moghim, S.; Esfahani, B.N.; Hosseini, N.S. Phylogenetic analysis of human bocavirus in children with acute respiratory infections in Iran. Acta Microbiol. Immunol. Hung. 2019, 66, 485–497. [Google Scholar] [CrossRef]
- Mohammadi, M.; Armin, S.; Yazdanpour, Z. Human bocavirus infections and co-infections with respiratory syncytial virus and Rotavirus in children with acute respiratory or gastrointestinal disease. Braz. J. Microbiol. 2020, 51, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Hashemi, S.A.; Safamanesh, S.; Ghasemzadeh-moghaddam, H.; Ghafouri, M.; Azimian, A. High prevalence of SARS-CoV-2 and influenza A virus (H1N1) coinfection in dead patients in Northeastern Iran. J. Med. Virol. 2021, 93, 1008–1012. [Google Scholar] [CrossRef]
- Atyah, N.S.; Fadhil, H.Y.; Auffi, I.; Al-Hamadani, F.G. First identification of human bocavirus (HBoV) in Iraqi children with respiratory complications. J. Pharm. Biol. Sci. 2017, 12, 15–20. [Google Scholar]
- Al-Mayah, Q.S.; Hussein, A.A.; Hasan, D.A. Detection of Human Bocavirus Infection in Children with Lower Respiratory. Rafidain J. Sci. 2018, 27, 64–74. [Google Scholar] [CrossRef]
- Shamiran, A.R. Detection of Human Bocavirus amongst Kids Tormented by Respiratory Tract Infections in Hilla Town. Scopus Ijphrd Cit. Score 2019, 10, 960. [Google Scholar] [CrossRef]
- Hasan, D.A.; Hussein, A.A.; Al-Mayah, Q.S.; Aufi, I.M. Phylogenetic Analysis of Human Bocavirus Isolated from Children with Lower Respiratory Tract Infections in Baghdad, Iraq. Ann. Trop. Med. Health 2020, 23, 250–259. [Google Scholar] [CrossRef]
- Yaseen, Z.T.; Khudair, M.K.; Naseef, A.S.; Alezzi, J.I. Detection of Human Bocavirus in Children Suffering from Respiratory Tract Infection in Diyala Province. Diyala J. Med. 2020, 18, 121–129. [Google Scholar] [CrossRef]
- Hindiyeh, M.Y.; Keller, N.; Mandelboim, M.; Ram, D.; Rubinov, J.; Regev, L.; Levy, V.; Orzitzer, S.; Shaharabani, H.; Azar, R. High rate of human bocavirus and adenovirus coinfection in hospitalized Israeli children. J. Clin. Microbiol. 2008, 46, 334–337. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kaplan, N.M.; Dove, W.; Abu-Zeid, A.F.; Shamoon, H.E.; Abd-Eldayem, S.A.; Hart, C.A. Human bocavirus infection among children, Jordan. Emerg. Infect. Dis. 2006, 12, 1418–1420. [Google Scholar] [CrossRef] [PubMed]
- Al-Rousan, H.O.; Meqdam, M.M.; Alkhateeb, A.; Al-Shorman, A.; Qaisy, L.M.; Al-Moqbel, M.S. Human bocavirus in Jordan: Prevalence and clinical symptoms in hospitalised paediatric patients and molecular virus characterisation. Singap. Med. J. 2011, 52, 365–369. [Google Scholar]
- Awad, S.; Khader, Y.; Moa’th Mansi, D.Y.; Alawadin, S.; Qudah, W.; Khasawneh, R. Viral surveillance of children with acute respiratory infection in two main hospitals in Northern Jordan, Irbid, during winter of 2016. J. Pediatric Infect. Dis. 2020, 15, 1. [Google Scholar] [CrossRef] [PubMed]
- Essa, S.; Owayed, A.; Altawalah, H.; Khadadah, M.; Behbehani, N.; Al-Nakib, W. The prevalence of human bocavirus, human coronavirus-NL63, human metapneumovirus, human polyomavirus KI and WU in respiratory tract infections in Kuwait. Med. Princ. Pract. 2015, 24, 382–387. [Google Scholar] [CrossRef]
- Madi, N.M.; Al-Adwani, A. Human bocavirus (HBoV) in Kuwait: Molecular epidemiology and clinical outcome of the virus among patients with respiratory diseases. J. Med. Microbiol. 2020, 69, 1005. [Google Scholar] [CrossRef]
- Mohammad, H.A.; Madi, N.M.; Al-Nakib, W. Analysis of viral diversity in stool samples from infants and children with acute gastroenteritis in Kuwait using Metagenomics approach. Virol. J. 2020, 17, 10. [Google Scholar] [CrossRef]
- Finianos, M.; Issa, R.; Curran, M.D.; Afif, C.; Rajab, M.; Irani, J.; Hakimeh, N.; Naous, A.; Hajj, M.-J.; Hajj, P. Etiology, seasonality, and clinical characterization of viral respiratory infections among hospitalized children in Beirut, Lebanon. J. Med. Virol. 2016, 88, 1874–1881. [Google Scholar] [CrossRef]
- Khamis, F.; Al-Kobaisi, M.; Al-Areimi, W.; Al-Kindi, H.; Al-Zakwani, I. Epidemiology of respiratory virus infections among infants and young children admitted to hospital in Oman. J. Med. Virol. 2012, 84, 1323–1329. [Google Scholar] [CrossRef]
- Janahi, I.; Abdulkayoum, A.; Almeshwesh, F.; Alkuwari, M.; Alameri, M. Viral aetiology of bronchiolitis in hospitalised children in Qatar. BMC Infect. Dis. 2017, 17, 1–11. [Google Scholar] [CrossRef]
- Al-Romaihi, H.E.; Smatti, M.K.; Ganesan, N.; Nadeem, S.; Farag, E.; Coyle, P.V.; Nader, J.D.; Al-Khatib, H.A.; Elmagboul, E.B.; Al Dhahry, S. Epidemiology of respiratory infections among adults in Qatar (2012–2017). PLoS ONE 2019, 14, e0218097. [Google Scholar] [CrossRef]
- Al-Romaihi, H.E.; Smatti, M.K.; Al-Khatib, H.A.; Coyle, P.V.; Ganesan, N.; Nadeem, S.; Farag, E.A.; Al Thani, A.A.; Al Khal, A.; Al Ansari, K.M. Molecular epidemiology of influenza, RSV, and other respiratory infections among children in Qatar: A six years report (2012–2017). Int. J. Infect. Dis. 2020, 95, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Nadeem, S.; Al Romaihi, H.; Ganesan, N.; Elberdiny, A. Multiple viral etiologies in patients with influenza-like illness and severe acute respiratory infection in Qatar, 2013–2016. J. Infect. Public Health 2020, 13, 466. [Google Scholar] [CrossRef]
- Memish, Z.A.; Assiri, A.M.; Alshehri, M.; Hussain, R.; Alomar, I. The prevalance of respiratory viruses among healthcare workers serving pilgrims in Makkah during the 2009 influenza A (H1N1) pandemic. Travel Med. Infect. Dis. 2012, 10, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Moneim, A.S.; Kamel, M.M.; Al-Ghamdi, A.S.; Al-Malky, M.I. Detection of bocavirus in children suffering from acute respiratory tract infections in Saudi Arabia. PLoS ONE 2013, 8, e55500. [Google Scholar]
- Al-Ayed, M.S.; Asaad, A.M.; Qureshi, M.A.; Ameen, M.S. Viral etiology of respiratory infections in children in southwestern Saudi Arabia using multiplex reverse-transcriptase polymerase chain reaction. Saudi Med. J. 2014, 35, 1348. [Google Scholar] [PubMed]
- Bubshait, D.K.; Albuali, W.H.; Yousef, A.A.; Obeid, O.E.; Alkharsah, K.R.; Hassan, M.I.; Vatte, C.; Alzahrani, A.J.; Bukhari, H. Clinical description of human bocavirus viremia in children with LRTI, Eastern Province, Saudi Arabia. Ann. Thorac. Med. 2015, 10, 146. [Google Scholar] [CrossRef]
- Memish, Z.A.; Assiri, A.; Turkestani, A.; Yezli, S.; al Masri, M.; Charrel, R.; Drali, T.; Gaudart, J.; Edouard, S.; Parola, P. Mass gathering and globalization of respiratory pathogens during the 2013 Hajj. Clin. Microbiol. Infect. 2015, 21, 571.e1–571.e8. [Google Scholar] [CrossRef]
- Eifan, S.A.; Hanif, A.; AlJohani, S.M.; Atif, M. Respiratory tract viral infections and coinfections identified by Anyplex™ II RV16 detection kit in pediatric patients at a riyadh tertiary care hospital. BioMed Res. Int. 2017, 2017, 1928795. [Google Scholar] [CrossRef]
- Abdel-Moneim, A.S.; Mahfouz, M.E.; Zytouni, D.M. Detection of human bocavirus in Saudi healthy blood donors. PLoS ONE 2018, 13, e0193594. [Google Scholar] [CrossRef]
- Koul, P.A.; Mir, H.; Saha, S.; Chadha, M.S.; Potdar, V.; Widdowson, M.-A.; Lal, R.B.; Krishnan, A. Respiratory viruses in returning Hajj & Umrah pilgrims with acute respiratory illness in 2014-2015. Indian J. Med. Res. 2018, 148, 329. [Google Scholar]
- Adam, M.A.; Wang, J.; Enan, K.-A.; Shen, H.; Wang, H.; El Hussein, A.R.; Musa, A.B.; Khidir, I.M.; Ma, X. Molecular survey of viral and bacterial causes of childhood diarrhea in Khartoum state, Sudan. Front. Microbiol. 2018, 9, 112. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, A.; Simmonds, P.; Slikas, E.; Li, L.; Bodhidatta, L.; Sethabutr, O.; Triki, H.; Bahri, O.; Oderinde, B.S.; Baba, M.M. Human bocaviruses are highly diverse, dispersed, recombination prone, and prevalent in enteric infections. J. Infect. Dis. 2010, 201, 1633–1643. [Google Scholar] [CrossRef] [PubMed]
- Brini Khalifa, I.; Hannachi, N.; Guerrero, A.; Orth-Höller, D.; Bhiri, S.; Bougila, J.; Boughamoura, L.; Merchaoui, S.N.; Sboui, H.; Mahdhaoui, N. Demographic and seasonal characteristics of respiratory pathogens in neonates and infants aged 0 to 12 months in the Central-East region of Tunisia. J. Med. Virol. 2019, 91, 570–581. [Google Scholar] [CrossRef] [PubMed]
- Midilli, K.; Yılmaz, G.; Türkoğlu, S.; Iskanova, B.; Ergin, S.; Yarımcam, F.; Ozdamar, M.; Gürol, Y.; Taştan, Y.; Altaş, K. Detection of human bocavirus DNA by polymerase chain reaction in children and adults with acute respiratory tract infections. Mikrobiyoloji Bul. 2010, 44, 405–413. [Google Scholar]
- Azkur, D.; Özaydın, E.; Dibek-Mısırlıoğlu, E.; Vezir, E.; Tombuloğlu, D.; Köse, G.; Kocabaş, C.N. Viral etiology in infants hospitalized for acute bronchiolitis. Turk. J. Pediatr. 2014, 56, 592. [Google Scholar]
- Uyar, M.; Kuyucu, N.; Tezcan, S.; Aslan, G.; Tasdelen, B. Determination of the frequency of human bocavirus and other respiratory viruses among 0–2 years age group children diagnosed as acute bronchiolitis. Mikrobiyoloji Bul. 2014, 48, 242–258. [Google Scholar] [CrossRef][Green Version]
- Akturk, H.; Sık, G.; Salman, N.; Sutcu, M.; Tatli, B.; Akcay Ciblak, M.; Bulent Erol, O.; Hancerli Torun, S.; Citak, A.; Somer, A. Atypical presentation of human bocavirus: Severe respiratory tract infection complicated with encephalopathy. J. Med. Virol. 2015, 87, 1831–1838. [Google Scholar] [CrossRef]
- Çiçek, C.; Arslan, A.; Karakuş, H.S.; Yalaz, M.; Saz, E.U.; Pullukçu, H.; Çok, G. Prevalence and seasonal distribution of respiratory viruses in patients with acute respiratory tract infections, 2002–2014. Mikrobiyoloji Bul. 2015, 49, 188–200. [Google Scholar] [CrossRef] [PubMed]
- Demirci, P.; Akin, Y.; Midilli, K.; Karaaslan, A.; CAMCIOĞLU, Y. Human Bocavirus Infection Inistanbul; Çukurova Üniversitesi Tıp Fakültesi Dergisi: Adana, Turkey, 2016; Volume 41, pp. 762–766. [Google Scholar]
- Erdem, H.; Ak, O.; Elaldi, N.; Demirdal, T.; Hargreaves, S.; Nemli, S.; Cag, Y.; Ulug, M.; Naz, H.; Gunal, O. Infections in travellers returning to Turkey from the Arabian peninsula: A retrospective cross-sectional multicenter study. Eur. J. Clin. Microbiol. Infect. Dis. 2016, 35, 903–910. [Google Scholar] [CrossRef]
- Goktas, S.; Sirin, M.C. Prevalence and seasonal distribution of respiratory viruses during the 2014–2015 season in Istanbul. Jundishapur J. Microbiol. 2016, 9, e39132. [Google Scholar] [CrossRef]
- Bakir, A.; Karabulut, N.; Alacam, S.; Mese, S.; Somer, A.; Agacfidan, A. Investigation of human bocavirus in pediatric patients with respiratory tract infection. J. Infect. Dev. Ctries. 2020, 14, 1191–1196. [Google Scholar] [CrossRef]
- Alsuwaidi, A.R.; Alkalbani, A.M.; Alblooshi, A.; George, J.; Albadi, G.; Kamal, S.M.; Narchi, H.; Souid, A.-K. Nasopharyngeal isolates and their clinical impact on young children with asthma: A pilot study. J. Asthma Allergy 2018, 11, 233. [Google Scholar] [CrossRef]
- Jeon, J.H.; Han, M.; Chang, H.E.; Park, S.S.; Lee, J.W.; Ahn, Y.J.; Hong, D.J. Incidence and seasonality of respiratory viruses causing acute respiratory infections in the Northern United Arab Emirates. J. Med. Virol. 2019, 91, 1378–1384. [Google Scholar] [CrossRef] [PubMed]
- Cabral, C.Z.; Guaragna, J.B.D.A.; Amantéa, F.C.; Lopes, P.G.M.; Pasqualotto, A.C.; Rhoden, C.R.; Amantéa, S.L. Distribution of airborne respiratory pathogens in pediatric emergency department waiting room. Pediatric Pulmonol. 2021, 56, 2724–2728. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Yu, X.; Wang, C.; Teng, Z.; Wang, C.; Shen, J.; Gao, Y.; Zhu, Z.; Wang, J.; Yuan, Z. High human bocavirus viral load is associated with disease severity in children under five years of age. PLoS ONE 2013, 8, e62318. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).