Informing Stewardship Measures in Canadian Food Animal Species through Integrated Reporting of Antimicrobial Use and Antimicrobial Resistance Surveillance Data—Part I, Methodology Development
Abstract
:1. Introduction
2. Methodology Development for Species-Level AMU–AMR Data Integration
2.1. General Surveillance Methods
2.1.1. Study Design, Data and Sample Collection
- Broiler chickens: during the first surveillance year (2013), 100 flocks were allocated nationally and distributed across the five major broiler chicken producing provinces where the number of flocks allocated per province was based on the provinces’ relative contribution to national chicken production. However, in subsequent years, the number of flocks per province was adjusted to satisfy a requirement of collaborative surveillance system, FoodNet Canada, to have a minimum of 30 flocks within a FoodNet Canada Sentinel site [34]. This adjustment resulted in >135 flocks being sampled per year. Quantitative AMU methodology was developed within the first three years of surveillance (i.e., mg/PCU); these AMU estimates enabled validation of national flock allocations. From the 2705 (national three-year (2013–2015) mean) broiler chicken producers in Canada [35], approximately 133 broiler chicken flocks were required per year based on the three-year (2013–2015) mean mg/PCU of 147 [36], an accepted error of 5% and a 90% confidence interval (CI).
- Turkeys: 56 flocks from a national total of 548 turkey producers (2010 data) [37] were required per year based on the first three-years (2013–2015 pilot project) mean mg/PCU of 67 [30], accepted error of 5% and a 95% CI. These flocks were allocated in the four major turkey producing provinces. Similar to broiler chickens, additional flocks were added in FoodNet Canada sentinel sites which increased the total number of flocks to greater than 90 per year.
- GF pigs: herds were distributed across the five major pork producing provinces in proportion to each province’s contribution to the number of GF operations nationally. Participating veterinarians were directed to enroll herds that were representative of the demographics of their swine practice with respect to size and management type. Unless they withdrew from the program, enrolled herds participated in subsequent years. Between 2006 and 2019, the number of herds participating nationally ranged from 85 to 108. In 2017, questionnaire and database refinements yielded more complete quantitative AMU data (i.e., inclusion of water and parenteral AMU in addition to feed), thereby allowing total AMU to be calculated and validation of enrolled herd numbers to be performed. Based on the three-years mean of 101 mg/PCU and mean of 7973 swine herds nationally [38] with an accepted error of 5% and 95% CI, 96 herds were required per year.
2.1.2. Statistical Analysis
2.2. AMR Data Component
2.2.1. Bacterial Isolation and Susceptibility Testing
2.2.2. AMR Indicator Analysis
2.2.3. Temporal Changes in AMR
2.2.4. AMR Outcomes for Integration and Rationale for Selection
2.2.4.1. Homologous or Single Resistance Outcomes
2.2.4.2. Additional Composite AMR Outcomes
| AMR Outcome | About This AMR Outcome |
|---|---|
| Ceftriaxone resistance (CRO-R) | Organism: E. coli Indicator for resistance to: 3rd generation cephalosporins * Prevalence levels **: Low to moderate Rationale for inclusion: monitoring of Step 1 of the AMU reduction strategy in the poultry industry [49,50] ***. Closely monitored by other integrated surveillance system because of its public health significance [31,32,46,47]. |
| Ciprofloxacin (CIP-R) | Organism: Campylobacter spp. Indicator for resistance to: fluoroquinolones * Prevalence: Moderate to high Rationale for inclusion: the detection of CIP-R E. coli was relatively very rare in the CIPARS farm AMR dataset. Therefore, CIP-R Campylobacter is used in routine monitoring and AMU–AMR data integration. Used for monitoring of the AMU reduction strategy in the poultry industry [49,50]. Closely monitored by other integrated surveillance system because of its public health significance [31,32,46,47]. Initial contamination of CIP-R could lead to a self-perpetuating cycle of CIP-R within farms [51]. |
| Gentamicin resistance (GEN-R) | Organism: E. coli Indicator for resistance to: Aminoglycosides and aminocyclitols Prevalence: Low to high Rationale for inclusion: A compensatory increase in GEN-R because of aminoglycosides/aminocyclitol use in poultry was noticed following the voluntary elimination of the preventive use of ceftiofur in poultry in Canada. Flocks that used these antimicrobials were catgorized as high users of antimicrobials [24]. This antimicrobial class is included as part of a voluntary AMU reduction strategy in the poultry industry [49,50]. |
| Trimethoprim and sulfamethoxazole (SXT-R) | Organism: E. coli Indicator for resistance to: trimethoprim and sulfonamides combination (inhibitors of folate synthesis) *. Prevalence: Low to moderate. Rationale for inclusion: flocks that used antimicrobials belonging to this antimicrobial class combination were categorized as high users of antimicrobials [24]. Resistance is monitored because the antimicrobial class is deemed as extra-label use in poultry [5,6]. |
| Tetracycline resistance (TET-R) | Organism: E. coli Indicator for resistance to: tetracyclines Prevalence: High to very high Rationale for inclusion: commonly used in poultry and swine; flocks that used antimicrobials belonging to this class were categorized as high users of antimicrobials [24]. |
| Azithromycin resistance (AZM-R) | Organism: Campylobacter spp. Indicator for resistance to: macrolides (plus lincosamides and streptogramin B). Prevalence: Low to very high Rationale for inclusion: correlates with erythromycin resistance. Macrolides are also part of the voluntary AMU reduction strategy in the Canadian poultry industry [49,50]. |
| AMR Outcome | About This Composite AMR Outcome |
|---|---|
| Susceptible E. coli * | This includes isolates that exhibited susceptibility to all of the 14 antimicrobials included in the NARMS CMV4AGNF panel. For brevity, the term ”susceptible” is used to refer to these isolates. Rationale for inclusion: used for monitoring the progress of regulatory and voluntary changes in AMU in the food animal sector. As described in the literature, susceptible E. coli are an indicator used to assess the development of AMR in relation to total AMU in food-producing animals and are reflective of the overall AMR situation, including the status of E. coli carrying plasmid-mediated AMR genes [46]. |
| Multiclass resistant E. coli (≥3 multiclass resistance) | Isolate resistant to individual antimicrobial classes was summed to provide the total number of classes that the isolate was resistant to. For brevity, the term “multiclass resistance” is used to refer to these isolates and represents isolates with resistance to ≥3 antimicrobial classes. Rationale for inclusion: complementary to the indicator above. As described in the literature, multiclass resistance is reflective of simultaneous actions of multiple antimicrobials on the indicator organism, therefore this outcome is also equally informative in detecting emerging AMR issues and is routinely used for AMR reporting in surveillance systems [31,32,46,47,52]. |
2.3. AMU Data Component
2.3.1. AMU Data Preparation
- Count-based: the percentage of use was determined (number of flocks or herds using an antimicrobial divided by the total flocks or herds multiplied by 100). The count-based AMU results were examined in relation to animal health data.
- Quantitative: the numerator and denominator input parameters are summarized in Appendix A and the formulae can be found in Appendix D. Throughout this manuscript, relevant descriptive statistics were used in the analyses and exploratory exercises. Description of the five candidate AMU indicators are summarized in Table 4.
2.3.2. Temporal Analysis
- Count based: The first step involved visual inspection and the relative changes between two time points similar to AMR findings (i.e., percentage of flocks medicated with antimicrobial A in 2019 minus the % of flocks medicated with antimicrobial A in 2015) were determined. The second step involved temporal analysis using logistic regression models developed with AMU (Yes/No) as the binary outcome variable with year as a categorical independent variable and using p ≤ 0.05 for significance as previously described [28].
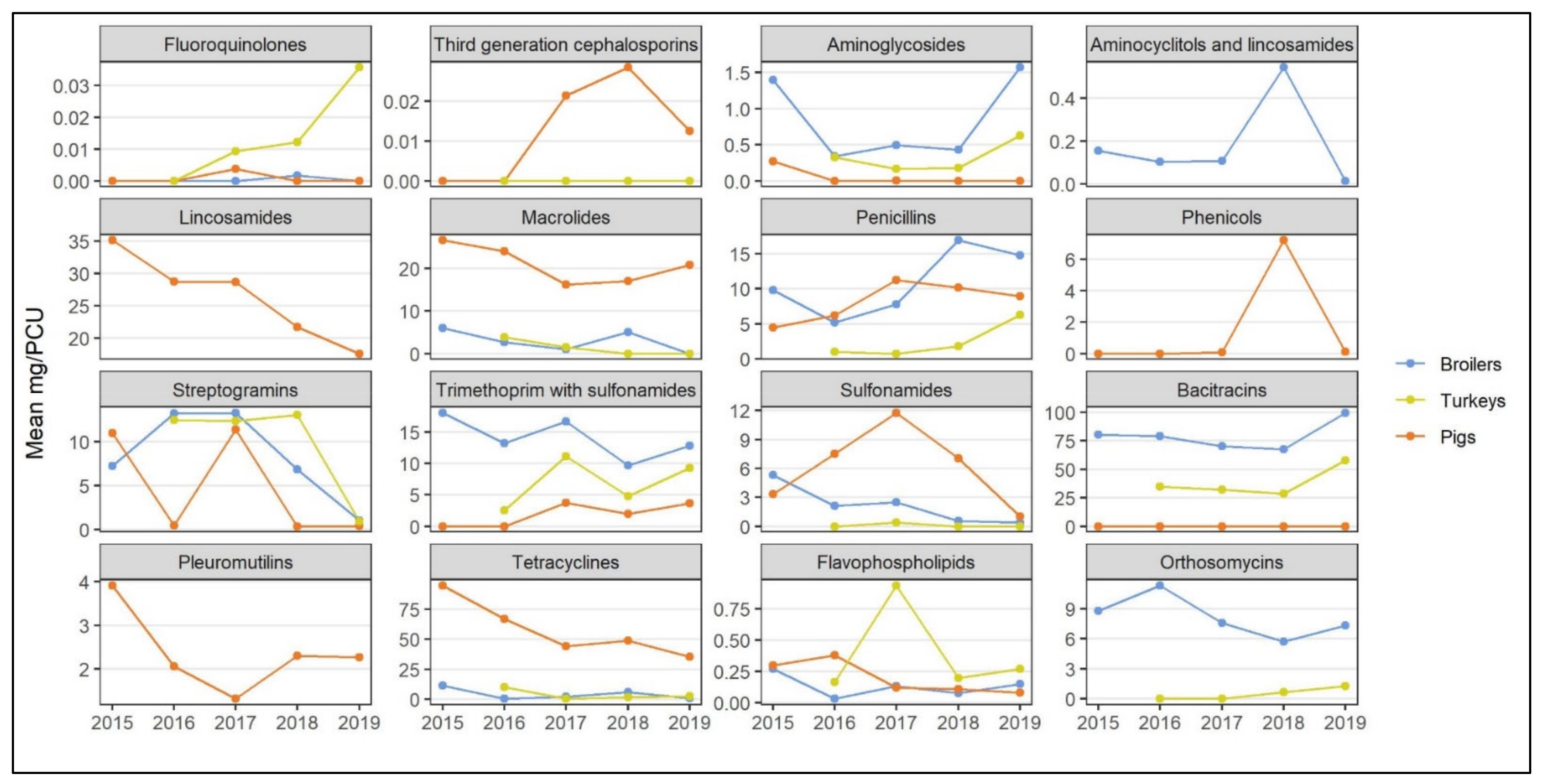
- Quantitative: trends in national-level AMU in the three species studied using the general AMU indicator used by CIPARS, mg/PCU, were visually inspected for levels of use and summarized in Appendix A Figure A1, organized by antimicrobial class. This provided an overall picture of the diversity of antimicrobial classes used and variations in temporal trends and quantity of use by antimicrobial class between the animal species in Canada. Relative changes in AMU quantity between two time points were descriptively assessed and summarized in Table 1 (directionality of the shift between 2015 and 2019).
2.3.3. AMU Outcomes Selected for Integration and Rationale
2.3.4. AMU Indicator Selection
2.3.4.1. How the AMU Indicators Relate to Each Other
2.3.4.2. AMU Indicator for Comparison of Use between Species
2.3.4.3. AMU Indicator for Studying the Relationship between AMU–AMR
- (1)
- For simultaneous evaluation of AMU levels (and trends) between species:Recommended: unit of measurement/s comparable between species (11) *; dose-based (11) **.Assessment: Descriptive statistics by species (mean, 95% CI).Candidate AMU indicators:
- 1)
- nDDDvetCA/kg animal biomass
- 2)
- nDDDvetCA/PCU
- (2)
- For evaluating relationships between AMU and AMRRecommended: unit of measurement reflective of the level and duration of exposure; dose-based.Assessment: mixed effect logistic regression model, evaluation of effect estimates (OR’s, 95% CI) by AMU indicator across species and forest plots visualization.Candidate AMU indicators:
- 1)
- nDDDvetCA/kg animal biomass
- 2)
- DDDvetCA/PCU
- (3)
- nDDDvetCA/1000 animal-days at riskOption selected for 1 and 2: nDDDvetCA/kg animal biomassOther considerations for using this AMU indicator:
- (a)
- Reduced analytic burden in cases where input parameters such as the days at risk and average treatment weight (Option 3) are unavailable.
- (b)
- The denominator, average pre-slaughter weight, can be accessed from farm records or production summary reports. Alternate denominator such as processing weight and final birds/pigs slaughtered can be obtained from processing plant records.
- (c)
- Preferred for ease of communication with stakeholders (familiarity with the contextualizing denominator, e.g., kg live weight vs. PCU).
- *
- adjusts for number of animals treated and weight ofanimals.
- **
- adjusts for average daily dose per kg per day.
2.4. Development of Analytic Methods for AMU and AMR Integration
- Option 1. Generalized linear model. The outcome of interest was the flock or herd level AMR for the antimicrobial and organism of interest. The strength of the association was evaluated using a generalized linear model (i.e., GLM procedure in Stata/SE V16.1; family–binomial distribution), with the flock- or herd-level AMR as the outcome variable (number of resistant isolates per flock and herd, accounting for the total isolates recovered per flock or herd), and the flock- or herd-level nDDDvetCA/kg animal biomass as the continuous predictor variable. The models were fitted for each animal species and adjusted for year (categorical variable).
- Option 2. Mixed effect logistic regression analysis. The second approach used the isolate-level AMR data matched with the corresponding flock or herd level AMU indicator, nDDDvetCA/kg animal biomass, as above. The models were fit for each animal species. For these data combinations, the strength of association was determined using mixed effect logistic regression (i.e., MELOGIT procedure in Stata/SE V16.1) adjusted for year, with a random intercept to account for the correlation of resistance within the same flock or herd.
- (1)
- Visual inspection, descriptive analysis of the percentages and directionality of the shift in AMU and AMR, followed by temporal analysis
- Simple logistic regression model: AMR percentages, count-based AMU and animal health indicators
- Nonparametric, Wilcoxon rank sum test: quantitative AMU indicators.
- (2)
- Identify the AMU-AMR pairs:
- public health importance
- animal health significance
- relevance to broader AMU stewardship and monitoring of AMU reduction efforts or other interventions
- (3)
- Determine the strength of AMU-AMR association
- mixed effect logistic regression approach
- (4)
- Visualize the trends in AMU and AMR.
- (5)
- Examine animal health data for context (are trends in reported disease syndromes reflective of the trends in AMU?).
2.5. Animal Health Context
2.6. Summary, Species-Level Integration of Farm Surveillance Data
- AMU indicator/s to use: there is no single AMU indicator that can encompass every analysis objective, in particular for comparison of AMU between species (Section 2.3.4.2) and for studying AMU and AMR relationships (Section 2.3.4.3) However, the high-resolution CIPARS farm-level data enabled exploration of the utility of various AMU indicators for these various analysis objectives. In additi.on to being able to investigate various methodologies, the other advantage of the CIPARS farm surveillance design is that the collection of AMU and AMR information is from the same epidemiological unit (same flocks or herds). This is different than the situation in other countries, where data are collected at the national level and only ecological associations between AMU and AMR can be explored.
- Animal health context: the syndromic data collected from CIPARS farms also helps provide insights on the potential implications of changing AMU practices on the health of flocks/herds. More detailed analysis could be conducted in the future to assess the impact of the shifts in AMU on mortality and flock or herd production performance parameters, as well as the susceptibility profiles of the clinical pathogens.
- AMU–AMR associations: the analytic options presented here (e.g., GLM and mixed effects logistic regression models) were appropriate for use in routine annual analysis and reporting (adjustments to various levels of clustering such as within flocks/herds). It is important to note that there are many analytic methodologies described elsewhere, ranging from simple logistic regression analysis to more advanced methodologies where other relevant variables could also be included in the model (e.g., multilevel analysis accounting for other sources of variations in the data). Emerging methodologies could be continuously explored to refine the approach used here.
3. Methodology Development for Multispecies AMU–AMR Data Integration
3.1. AMR Summarized Indicator
- % Resistance, multispecies: Overall % resistance in E. coli and Campylobacter, adjusted for clustering at the species level to account for similarities in AMR within animal species, was determined. Logistic regression analysis as described previously (i.e., isolate-level AMR as binary outcome variable and year as independent categorical predictor variable, and p ≤ 0.05 for significance) was used to detect temporal changes in overall % resistance.
- Animal biomass adjusted % resistance: The summarized AMR indicator developed in the EU/EEA, known as the AMR Indicator Index or Key Outcome Indicator [31,46,70], was determined. This AMR indicator was previously explored in another study using poultry data from CIPARS/FoodNet Canada [30], which was modified in this current study to also include AMR data from GF pigs and using kg animal biomass instead of PCU in the denominator. As shown in Equation (2), this AMR indicator accounts for the species-specific AMR results (total resistant isolates/total isolates per species) and fluctuations in animal biomass over time, such as when new herds or flocks are added to the sampling frame. Relative changes in the biomass adjusted AMR indicators were determined between two time points.
3.2. AMU Summarized Indicators
3.3. AMU–AMR Association, Multispecies
3.4. Summary, Multispecies-Level Integration of Farm Surveillance Data and Limitations
- AMU: in terms of comprehensiveness, the data included three food animal species, but other important commodities in Canada, such as beef and dairy cattle and chicken egg layers, were not included, as these data were not yet available at the time of the study.
- AMR: data were limited to Gram-negative indicator organisms for many of the analyses. Also, AMR of clinical pathogen data were not available.
- AMU–AMR analysis: other models (i.e., multivariate analyses) evaluating the full range of potential risk factors for AMR by species were also not completed as they were beyond the scope of this study; however, they could be explored in future research.
- Animal health: the addition of other production performance parameters that could substantiate stewardship efforts, such as feed conversion rates and final kg of meat produced (slaughter data), are not collected by CIPARS, but will be useful to include to provide a full understanding of the impact of AMU reduction efforts in the health, welfare and sustainability of the food animal sectors in Canada.
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AMU | Antimicrobial use |
| AMR | Antimicrobial resistance |
| CIPARS | Canadian Integrated Program for Antimicrobial Resistance Surveillance |
| DDDvetCA | defined daily doses using Canadian standards |
| ESVAC | European Surveillance for Veterinary Antimicrobial Consumption |
| OIE | World Organization for Animal Health |
| PCU | population correction unit |
| nDDDvetCA | number of defined daily doses using Canadian standards |
| TI | Treatment Incidence |
| UK VARSS | United Kingdom Veterinary Antibiotic Resistance and Sales Report |
| AMC | Amoxicillin-clavulanic acid |
| GEN | Gentamicin |
| AMP | Ampicillin |
| MEM | Meropenem |
| AZM | Azithromycin |
| NAL | Nalidixic acid |
| CHL | Chloramphenicol |
| SSS | Sulfisoxazole |
| CIP | Ciprofloxacin |
| STR | Streptomycin |
| CLI | Clindamycin |
| SXT | Trimethoprim-sulfamethoxazole |
| CRO | Ceftriaxone |
| TEL | Telithromycin |
| ERY | Erythromycin |
| TET | Tetracycline |
| FLR | Florfenicol |
| TIO | Ceftiofur |
| FOX | Cefoxitin |
| 3GC’s | third generation cephalosporins |
| AMCL | aminocyclitol |
| AMGL | aminoglycosides |
| BAC | bacitracins |
| FQ | fluoroquinolones |
| LINC | lincosamides |
| MACR | macrolides |
| MLSB | macrolides, lincosamides and streptogramin B |
| PEN | penicillins |
| STRE | streptogramins |
| TET | tetracyclines |
| TMPS | trimethoprim and sulfonamides combination |
Appendix A
| 2015 | 2016 | 2017 | 2018 | 2019 | Mean | SD | |
|---|---|---|---|---|---|---|---|
| Broiler chickens | |||||||
| Number of flocks | 135 | 136 | 137 | 141 | 147 | 139 | 4 |
| Numerator parameters (AMU quantity) | |||||||
| Total kg | 448 | 396 | 407 | 469 | 495 | 443 | 34 |
| Total nDDDvetCA’s ('000) | 56,254 | 59,195 | 58,248 | 63,290 | 55,707 | 58,539 | 2461 |
| Denominator parameters | |||||||
| Population, n birds | 3,035,442 | 3,052,498 | 3,212,784 | 3,794,167 | 3,474,669 | 3,313,912 | 262,233 |
| PCU (n birds × 1 kg) | 3,035,442 | 3,052,498 | 3,212,784 | 3,794,167 | 3,474,669 | 3,313,912 | 262,233 |
| Average pre-slaughter live weight, kg | 2 | 2 | 2 | 2 | 2 | 2 | 0 |
| Total broiler chicken biomass, kg | 6,302,129 | 6,006,219 | 6,464,738 | 7,563,080 | 7,239,121 | 6,715,057 | 536,875 |
| Average days at risk | 35 | 34 | 34 | 34 | 35 | 35 | 0 |
| Number of samples | 544 | 544 | 548 | 560 | 588 | 557 | 17 |
| Number of isolates | |||||||
| Escherichia coli | 539 | 543 | 539 | 547 | 571 | 548 | 12 |
| Campylobacter | 117 | 93 | 122 | 122 | 142 | 119 | 16 |
| Grower-finisher pigs | |||||||
| Number of herds | 85 | 91 | 82 | 97 | 107 | 92 | 8 |
| Numerator parameters (AMU quantity) | |||||||
| Total kg | 1704 | 1110 | 984 | 1235 | 1197 | 1246 | 223 |
| Total nDDDvetCA’s ('000) | 292,705 | 214,175 | 169,554 | 183,335 | 196,013 | 211,156 | 39,567 |
| Denominator parameters | |||||||
| Population, n pigs | 148,696 | 147,795 | 130,829 | 149,693 | 169,894 | 149,381 | 11,314 |
| PCU (n pigs × 65 kg) | 9,665,240 | 9,606,675 | 8,503,885 | 9,730,045 | 11,043,110 | 9,709,791 | 735,411 |
| Average pre-slaughter live weight, kg | 124 | 125 | 126 | 127 | 126 | 126 | 1 |
| Total GF-pigs biomass, kg | 18,126,786 | 18,235,934 | 16,293,023 | 18,609,944 | 21,314,478 | 18,516,033 | 1,472,631 |
| Average days at risk | 113 | 114 | 114 | 114 | 115 | 114 | 0 |
| Number of samples | 510 | 552 | 492 | 594 | 641 | 558 | 55 |
| Number of isolates | |||||||
| Escherichia coli | 500 | 544 | 484 | 585 | 628 | 548 | 53 |
| Campylobacter | 369 | 483 | 447 | 433 | 48 | ||
| Turkeys | |||||||
| Number of flocks | 72 | 74 | 95 | 98 | 85 | 11 | |
| Numerator parameters (AMU quantity) | |||||||
| Total kg | 219 | 210 | 211 | 374 | 253 | 62 | |
| Total nDDDvetCA’s ('000) | 31,705 | 32,973 | 36,719 | 37,883 | 34,820 | 2285 | |
| Denominator parameters | |||||||
| Population, n birds | 558,396 | 550,587 | 608,994 | 687,360 | 601,334 | 48,740 | |
| PCU (n birds × 6.5 kg) | 3,629,571 | 3,578,812 | 3,958,461 | 4,467,840 | 3,908,671 | 316,813 | |
| Average pre-slaughter live weight, kg | 10 | 10 | 10 | 10 | 10 | 0 | |
| Total turkey biomass, kg | 5,011,422 | 5,070,026 | 5,582,249 | 6,353,551 | 5,504,312 | 481,420 | |
| Average days at risk | 90 | 89 | 87 | 89 | 89 | 1 | |
| Number of samples | 280 | 292 | 371 | 399 | 336 | 51 | |
| Number of isolates | |||||||
| Escherichia coli | 277 | 287 | 367 | 393 | 331 | 50 | |
| Campylobacter | 171 | 157 | 191 | 214 | 183 | 21 | |
| Combined total | |||||||
| Number of sentinel flocks and herds | 220 | 299 | 293 | 333 | 352 | 299 | 41 |
| Numerator parameters (AMU quantity) | |||||||
| Total kg | 2151 | 1725 | 1600 | 1916 | 2066 | 1891 | 188 |
| Total nDDDvetCA’s ('000) | 348,959 | 305,075 | 260,776 | 283,343 | 289,603 | 297,551 | 26,823 |
| Denominator parameters | |||||||
| Population, n animals | 3,184,138 | 3,758,689 | 3,894,200 | 4,552,854 | 4,331,923 | 3,944,360 | 435,009 |
| PCU, total | 85 | 31,796 | 33,055 | 36,816 | 37,990 | 27,948 | 12,889 |
| Total animal biomass, kg | 1,704 | 559,505 | 551,570 | 610,229 | 688,557 | 482,313 | 245,210 |
| Number of samples | 1054 | 1376 | 1332 | 1525 | 1628 | 1383 | 195 |
| Number of isolates | |||||||
| Escherichia coli | 1039 | 1364 | 1310 | 1499 | 1592 | 1361 | 189 |
| Campylobacter | 117 | 264 | 648 | 796 | 803 | 526 | 283 |
Appendix B. Minimum Inhibitory Concentration
| Panel Type: CMV4AGNF | |||
|---|---|---|---|
| Antimicrobial | Susceptible | Intermediate | Resistant |
| Amoxicillin/Clavulanic Acid 1 | ≤8 | 16 | ≥32 |
| Ampicillin 1 | ≤8 | 16 | ≥32 |
| Azithromycin 2 | ≤16 | - | ≥32 |
| Cefoxitin 1 | ≤8 | 16 | ≥32 |
| Ceftriaxone 1 | ≤1 | 2 | ≥4 |
| Chloramphenicol 1 | ≤8 | 16 | ≥32 |
| Ciprofloxacin 1 | ≤0.06 | 0.12–0.5 | ≥1 |
| Gentamicin 1 | ≤4 | 8 | ≥16 |
| Meropenem 1 | ≤1 | 2 | ≥4 |
| Nalidixic Acid 1 | ≤16 | - | ≥32 |
| Sulfisoxazole 1 | ≤256 | - | ≥512 |
| Tetracycline 1 | ≤4 | 8 | ≥16 |
| Trimethoprim/Sulphamethoxazole 1 | ≤2 | - | ≥4 |
| Panel Type: Cmvcampy | |||
|---|---|---|---|
| Antimicrobial | Susceptible | Intermediate | Resistant |
| Gentamicin 2 | ≤2 | 4 | ≥8 |
| Clindamycin 2 | ≤2 | 4 | ≥8 |
| Azithromycin 2 | ≤2 | 4 | ≥8 |
| Erythromycin 1 | ≤8 | 16 | ≥32 |
| Florfenicol 2 | ≤4 | - | - |
| Ciprofloxacin 1 | ≤1 | 2 | ≥4 |
| Meropenem 2 | ≤1 | 2 | ≥4 |
| Nalidixic Acid 2 | ≤16 | 32 | ≥64 |
| Tetracycline 1 | ≤4 | 8 | ≥16 |
Appendix C. Antimicrobial Resistance Summaries
| Year | Isolate Level Analysis (Adjusted for Clustering) | Flock or Herd-Level Analysis (Mean Prevalence) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 2015 | 2016 | 2017 | 2018 | 2019 | 2015 | 2016 | 2017 | 2018 | 2019 | |
| Broilers | ||||||||||
| Number of isolates/flocks | 539 | 543 | 539 | 547 | 571 | 134 | 136 | 137 | 140 | 147 |
| Ampicillin | 42% | 40% | 38% | 32% | 32% | 47% | 48% | 50% | 46% | 40% |
| Ceftriaxone | 12% | 9% | 10% | 7% | 7% | 12% | 9% | 10% | 7% | 7% |
| Gentamicin | 19% | 21% | 20% | 20% | 17% | 19% | 21% | 20% | 21% | 17% |
| Nalidixic acid | 6% | 5% | 5% | 10% | 8% | 6% | 5% | 6% | 10% | 8% |
| Streptomycin | 46% | 48% | 50% | 46% | 40% | 47% | 48% | 50% | 46% | 40% |
| Tetracycline | 54% | 48% | 48% | 42% | 39% | 54% | 48% | 47% | 42% | 39% |
| Trimethoprim-sulfamethoxazole | 16% | 16% | 17% | 12% | 15% | 16% | 16% | 18% | 12% | 14% |
| Susceptible isolates | 22% | 24% | 28% | 32% | 34% | 22% | 24% | 28% | 33% | 34% |
| Multiclass resistance | 40% | 37% | 39% | 33% | 31% | 40% | 37% | 39% | 34% | 31% |
| GF pigs | ||||||||||
| Number of isolates/herds | 500 | 544 | 484 | 585 | 628 | 85 | 92 | 80 | 99 | 107 |
| Ampicillin | 30% | 33% | 28% | 29% | 29% | 30% | 33% | 28% | 28% | 28% |
| Ceftriaxone | 2% | 2% | 0% | 2% | 2% | 2% | 2% | 0% | 2% | 2% |
| Gentamicin | 1% | 1% | 1% | 2% | 1% | 1% | 1% | 1% | 2% | 1% |
| Nalidixic acid | 0% | 0% | 0% | 1% | 0% | 0% | 0% | 0% | 1% | 0% |
| Streptomycin | 45% | 42% | 42% | 43% | 38% | 45% | 42% | 42% | 43% | 39% |
| Tetracycline | 67% | 70% | 68% | 67% | 65% | 67% | 70% | 68% | 67% | 64% |
| Trimethoprim-sulfamethoxazole | 12% | 13% | 15% | 12% | 12% | 13% | 13% | 15% | 12% | 12% |
| Susceptible isolates | 23% | 21% | 23% | 22% | 22% | 22% | 21% | 24% | 22% | 22% |
| Multiclass resistance | 38% | 39% | 38% | 36% | 31% | 39% | 39% | 37% | 36% | 30% |
| Turkeys | ||||||||||
| Number of isolates/herds | 277 | 287 | 367 | 393 | 70 | 73 | 93 | 100 | ||
| Ampicillin | 30% | 37% | 29% | 29% | 30% | 38% | 29% | 29% | ||
| Ceftriaxone | 1% | 1% | 1% | 2% | 1% | 1% | 1% | 2% | ||
| Gentamicin | 20% | 24% | 14% | 11% | 20% | 24% | 14% | 11% | ||
| Nalidixic acid | 1% | 2% | 1% | 2% | 1% | 2% | 1% | 2% | ||
| Streptomycin | 48% | 51% | 38% | 39% | 48% | 51% | 38% | 39% | ||
| Tetracycline | 69% | 63% | 56% | 61% | 69% | 62% | 56% | 62% | ||
| Trimethoprim-sulfamethoxazole | 9% | 9% | 11% | 10% | 9% | 9% | 10% | 10% | ||
| Susceptible isolates | 25% | 25% | 31% | 28% | 25% | 26% | 31% | 28% | ||
| Multiclass resistance | 36% | 39% | 26% | 28% | 36% | 39% | 26% | 28% | ||
| Isolate Level Analysis (Adjusted for Clustering) | Flock or Herd-Level Analysis (Mean Prevalence) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 2015 | 2016 | 2017 | 2018 | 2019 | 2015 | 2016 | 2017 | 2018 | 2019 | |
| Broilers | ||||||||||
| Number of isolates | 117 | 93 | 122 | 122 | 142 | |||||
| Azithromycin | 18% | 0% | 5% | 0% | 1% | 18% | 0% | 6% | 0% | 1% |
| Ciprofloxacin | 16% | 13% | 18% | 12% | 24% | 16% | 13% | 18% | 12% | 24% |
| Tetracycline | 56% | 22% | 40% | 27% | 27% | 56% | 22% | 40% | 27% | 27% |
| GF pigs | ||||||||||
| Number of isolates | 369 | 483 | 447 | |||||||
| Azithromycin | 44% | 40% | 41% | 43% | 40% | 40% | ||||
| Ciprofloxacin | 8% | 11% | 12% | 9% | 12% | 13% | ||||
| Tetracycline | 67% | 64% | 63% | 67% | 64% | 63% | ||||
| Turkeys | ||||||||||
| Number of isolates | 171 | 157 | 191 | 214 | ||||||
| Azithromycin | 1% | 16% | 8% | 5% | 1% | 16% | 8% | 6% | ||
| Ciprofloxacin | 22% | 30% | 38% | 37% | 21% | 31% | 38% | 36% | ||
| Tetracycline | 43% | 49% | 41% | 43% | 43% | 46% | 41% | 43% | ||
Appendix D. Methods Used to Calculate Antimicrobial Use for Surveillance Data Collected from Farms
| Measurements | Description and Reference | Numerator | Denominator |
| Count-Based Indicator, National-Level Values | |||
| Yes, No data from the questionnaire. | No. of flocks or herds using antimicrobials | Total no. of flocks or herds surveyed |
| Other count-based measures: disease syndromes reported, vaccines, preventive health measures | |||
| Weight-based indicators, flock or herd level | |||
| Generic AMU measurement developed by ESVAC [14], used by CIPARS [24,28] in reporting the farm and national sales/distribution data and research studies | Total flock or herd: mg of all classes By class: mg antimicrobial class | Broilers: Birds at risk × 1 kg ESVAC average weight at treatment GF pigs: Pigs at risk × 65 kg ESVAC average weight at treatment Turkeys: Birds at risk × 6.5 kg ESVAC average weight at treatment |
| CIPARS exploratory analysis: used by OIE for reporting the global OIE AMU database [15]. Actual pre-slaughter weights collected on farm at close to market were used in the study. | Total flock or herd: mg of all classes, as in #2 By class: mg antimicrobial class | Broilers: Birds at risk × kg broiler biomass GF pigs: Pigs at risk × kg GF pigs biomass Turkeys: Turkeys at risk × kg turkey biomass |
| Dose-based indicators, flock or herd level | |||
| Also known as treatment incidence (TI1000,) when values are divided by 100, it is called TI100; routine dose-based AMU indicator for reporting the CIPARS farm data and research studies | Total flock: nDDDvetCA’s of all classes By class: nDDDvetCAs, antimicrobial class | Broilers: Birds at risk × 1 kg ESVAC average weight at treatment × days at risk GF pigs: Pigs at risk × 65 kg ESVAC average weight at treatment × days at risk Turkeys: Birds at risk × 1 kg ESVAC average weight at treatment × days at risk |
| Values were multiplied by 1000 or 100 for TI100 [12,13] | |||
| Used in past CIPARS research and reports [24,30,34] and in research studies and found to be highly correlated to the indicator in 4 above. | As in indicator 4 above | As in indicator 2 above. |
| Explored previously [24]: used actual kg live pre-slaughter weight in the denominator instead of PCU | As in indicator 4 above | As in indicator 3 above. |
| nDDDvetCA-number of defined daily doses for animals using Canadian standards; this is the mg active ingredient divided by the DDDvetCA standard developed for each species and antimicrobial active ingredient [53]. ESVAC-European Surveillance for Veterinary Antimicrobial Consumption. PCU-population correction unit. | |||
| mg/PCU | mg/kg Animal Biomass | TI1000 | nDDDvetCA/PCU | nDDDvetCA/kg Animal Biomass | |
|---|---|---|---|---|---|
| Broiler chickens | |||||
| mg/PCU | 1 | ||||
| mg/kg animal biomass | 0.9115 * | 1 | |||
| TI1000 | 0.7959 * | 0.7933 * | 1 | ||
| nDDDvetCA/PCU | 0.8320 * | 0.7529 * | 0.9758 * | 1 | |
| nDDDvetCA/kg animal biomass | 0.7380 * | 0.8126 * | 0.9741 * | 0.9211 * | 1 |
| GF pigs | |||||
| mg/PCU | 1 | ||||
| mg/kg animal biomass | 0.9976 * | 1 | |||
| TI1000 | 0.8943 * | 0.8921 * | 1 | ||
| nDDDvetCA/PCU | 0.8976 * | 0.8943 * | 0.9904 * | 1 | |
| nDDDvetCA/kg animal biomass | 0.8970 * | 0.8982 * | 0.9889 * | 0.9976 * | 1 |
| Turkeys | |||||
| mg/PCU | 1 | ||||
| mg/kg animal biomass | 0.8592 * | 1 | |||
| TI1000 | 0.7981 * | 0.7507 * | 1 | ||
| nDDDvetCA/PCU | 0.8310 * | 0.6859 * | 0.9729 * | 1 | |
| nDDDvetCA/kg animal biomass | 0.6589 * | 0.7815 * | 0.9263 * | 0.8318 * | 1 |
| AMR Outcome | Year | % Resistance | % Resistance, Biomass Adjusted | Difference Between AMR Adjusted and Unadjusted Values |
|---|---|---|---|---|
| Susceptible | 2015 | 22% | 23% | 1% |
| 2016 | 23% | 22% | −1% | |
| 2017 | 25% | 25% | −1% | |
| 2018 | 28% | 26% | −2% | |
| 2019 | 28% | 26% | −2% | |
| Multiclass resistance | 2015 | 39% | 36% | −3% |
| 2016 | 38% | 38% | 1% | |
| 2017 | 39% | 38% | 0% | |
| 2018 | 33% | 34% | 1% | |
| 2019 | 30% | 32% | 1% | |
| Ceftriaxone | 2015 | 7% | 5% | −3% |
| 2016 | 5% | 3% | −1% | |
| 2017 | 4% | 3% | −2% | |
| 2018 | 3% | 3% | 0% | |
| 2019 | 4% | 3% | −1% | |
| Gentamicin | 2015 | 10% | 6% | −4% |
| 2016 | 13% | 9% | −4% | |
| 2017 | 14% | 10% | −4% | |
| 2018 | 11% | 8% | −3% | |
| 2019 | 9% | 6% | −3% | |
| Trimethoprim–sulfamethoxazole | 2015 | 14% | 13% | −1% |
| 2016 | 13% | 13% | 0% | |
| 2017 | 15% | 14% | 0% | |
| 2018 | 12% | 12% | 0% | |
| 2019 | 13% | 12% | 0% | |
| Tetracycline | 2015 | 60% | 63% | 3% |
| 2016 | 61% | 65% | 4% | |
| 2017 | 59% | 62% | 4% | |
| 2018 | 55% | 59% | 4% | |
| 2019 | 55% | 59% | 4% | |
| Ciprofloxacin | 2017 | 14% | 14% | 1% |
| 2018 | 11% | 16% | 5% | |
| 2019 | 13% | 19% | 6% | |
| Azithromycin | 2017 | 22% | 30% | 8% |
| 2018 | 24% | 25% | 1% | |
| 2019 | 34% | 26% | −7% |
References
- WeCAHN. Western Canadian Animal Health Network. Available online: https://www.wecahn.ca/ (accessed on 6 July 2021).
- OAHN. Ontario Animal Health Network. Available online: https://www.oahn.ca/news-post/ (accessed on 1 June 2021).
- RAIZO. Alert Network and Information Animal Health. Available online: https://www.mapaq.gouv.qc.ca/fr/Productions/santeanimale/maladies/RAIZO/Pages/raizo.aspx (accessed on 1 June 2021).
- Canadian Swine Health Intelligence Network. Available online: https://www.cshin.ca/doku.php?id=en:domum (accessed on 22 August 2021).
- Animalytix. Compendium of Veterinary Products, Canada Edition. Available online: https://bam.cvpservice.com/ (accessed on 1 June 2021).
- Government of Canada. Compendium of Medicating Ingredient Brochure. Available online: https://inspection.canada.ca/animal-health/livestock-feeds/medicating-ingredients/eng/1300212600464/1320602461227 (accessed on 1 June 2021).
- World Health Organization. WHO Global Action Plan on Antimicrobial Resistance. Available online: http://apps.who.int/iris/bitstream/10665/193736/1/9789241509763_eng.pdf?ua=1 (accessed on 23 December 2015).
- Food and Agriculture Organization of the United Nations. The FAO Action Plan on Antimicrobial Resistance, 2016–2020. Available online: http://www.fao.org/antimicrobial-resistance/en/ (accessed on 2 July 2018).
- World Organization for Animal Health (OIE). The OIE Strategy on Antimicrobial Resistance and the Prudent Use of Antimicrobials. Available online: http://www.oie.int/fileadmin/Home/eng/Media_Center/docs/pdf/PortailAMR/EN_OIE-AMRstrategy.pdf (accessed on 23 September 2018).
- Weese, J.S.; Page, S.W.; Prescott, J.F. Antimicrobial Stewardship in Animals. In Antimicrobial Therapy in Veterinary Medicine; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2013; pp. 117–132. [Google Scholar]
- Collineau, L.; Belloc, C.; Stärk, K.D.C.; Hémonic, A.; Postma, M.; Dewulf, J.; Chauvin, C. Guidance on the Selection of Appropriate Indicators for Quantification of Antimicrobial Usage in Humans and Animals. Zoonoses Public Health 2016, 64, 165–184. [Google Scholar] [CrossRef] [Green Version]
- AACTING. Guidelines for Collection, Analysis and Reporting of Farm-Level Antimicrobial Use, in the Scope of Antimicrobial Stewardship. Available online: https://aacting.org/aacting-guidelines/ (accessed on 1 June 2018).
- Sanders, P.; Vanderhaeghen, W.; Fertner, M.; Fuchs, K.; Obritzhauser, W.; Agunos, A.; Carson, C.; Høg, B.B.; Andersen, V.D.; Chauvin, C.; et al. Monitoring of Farm-Level Antimicrobial Use to Guide Stewardship: Overview of Existing Systems and Analysis of Key Components and Processes. Front. Veter. Sci. 2020, 7, 540. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency. Sales of Veterinary Antimicrobial Agents in 31 European Countries in 2018. Trends from 2010 to 2018. Tenth ESVAC Report. Available online: https://www.ema.europa.eu/en/veterinary-regulatory/overview/antimicrobial-resistance/european-surveillance-veterinary-antimicrobial-consumption-esvac (accessed on 1 June 2021).
- World Organization for Animal Health (OIE). Fifth OIE Annual Report on Antimicrobial Agents Intended for Use in Animals. Available online: https://www.oie.int/en/document/fifth-oie-annual-report-on-antimicrobial-agents-intended-for-use-in-animals/ (accessed on 1 June 2021).
- European Medicines Agency. Defined Daily Doses for Animals (DDDvet) and Defined Course Doses for Animals (DCDvet). European Surveillance of Veterinary Antimicrobial Consumption (ESVAC). Available online: https://www.ema.europa.eu/en/documents/other/defined-daily-doses-animals-dddvet-defined-course-doses-animals-dcdvet-european-surveillance_en.pdf (accessed on 16 February 2021).
- O’Neill, L.; Da Costa, M.R.; Leonard, F.; Gibbons, J.; Díaz, J.A.C.; McCutcheon, G.; Manzanilla, E.G. Does the Use of Different Indicators to Benchmark Antimicrobial Use Affect Farm Ranking? Front. Veter. Sci. 2020, 7, 558793. [Google Scholar] [CrossRef]
- Kuemmerlen, D.; Echtermann, T.; Muentener, C.; Sidler, X. Agreement of Benchmarking High Antimicrobial Usage Farms Based on Either Animal Treatment Index or Number of National Defined Daily Doses. Front. Veter. Sci. 2020, 7. [Google Scholar] [CrossRef]
- Persoons, D.; Dewulf, J.; Smet, A.; Herman, L.; Heyndrickx, M.; Martel, A.; Catry, B.; Butaye, P.; Haesebrouck, F. Antimicrobial use in Belgian broiler production. Prev. Veter. Med. 2012, 105, 320–325. [Google Scholar] [CrossRef] [PubMed]
- Timmerman, T.; Dewulf, J.; Catry, B.; Feyen, B.; Opsomer, G.; de Kruif, A.; Maes, D. Quantification and evaluation of antimicrobial drug use in group treatments for fattening pigs in Belgium. Prev. Veter. Med. 2006, 74, 251–263. [Google Scholar] [CrossRef] [PubMed]
- Dorado-Garcia, A.; Mevius, D.J.; Jacobs, J.J.; Van Geijlswijk, I.M.; Mouton, J.W.; Wagenaar, J.A.; Heederik, D.J. Quantitative assessment of antimicrobial resistance in livestock during the course of a nationwide antimicrobial use reduction in the Netherlands. J. Antimicrob. Chemother. 2016, 71, 3607–3619. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Netherlands Veterinary Medicines Institute (SDa). SDa-Report ‘Usage of Antibiotics in Agricultural Livestock in the Netherlands in 2019’. Available online: https://www.autoriteitdiergeneesmiddelen.nl/en (accessed on 16 February 2021).
- DANMAP. DANMAP 2019-Use of Antimicrobial Agents and Occurrence of Antimicrobial Resistance in Bacteria from Food Animals, Food and Humans in Denmark. Available online: https://www.danmap.org/reports/2019 (accessed on 16 February 2021).
- Agunos, A.; Gow, S.P.; Léger, D.F.; Deckert, A.E.; Carson, C.A.; Bosman, A.L.; Kadykalo, S.; Reid-Smith, R.J. Antimicrobial Use Indices—The Value of Reporting Antimicrobial Use in Multiple Ways Using Data from Canadian Broiler Chicken and Turkey Farms. Front. Veter. Sci. 2020, 7, 567872. [Google Scholar] [CrossRef]
- Waret-Szkuta, A.; Coelho, V.; Collineau, L.; Hémonic, A.; Buy, C.; Treff, M.; Raboisson, D. How Input Parameters and Calculation Rules Influence On-Farm Antimicrobial Use Indicators in Animals. Front. Veter. Sci. 2019, 6, 438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chauvin, C.; Querrec, M.; Perot, A.; Guillemot, D.; Sanders, P. Impact of antimicrobial drug usage measures on the identification of heavy users, patterns of usage of the different antimicrobial classes and time-trends evolution. J. Veter. Pharmacol. Ther. 2008, 31, 301–311. [Google Scholar] [CrossRef] [PubMed]
- Kasabova, S.; Hartmann, M.; Werner, N.; Käsbohrer, A.; Kreienbrock, L. Used Daily Dose vs. Defined Daily Dose—Contrasting Two Different Methods to Measure Antibiotic Consumption at the Farm Level. Front. Veter. Sci. 2019, 6, 116. [Google Scholar] [CrossRef]
- Government of Canada. Canadian Integrated Program for Antimicrobial Resistance Surveillance (CIPARS) 2018: Design and Methods. Available online: https://www.canada.ca/en/public-health/services/surveillance/canadian-integrated-program-antimicrobial-resistance-surveillance-cipars/cipars-reports/2018-annual-report-design-methods.html (accessed on 26 July 2021).
- Agriculture and Agri-food Canada. Per Capita Consumption. Protein Disappearance of Poultry and Other Animal Protein Sources in Canada (Food Available Per Person, Per Year). Available online: https://www.agr.gc.ca/eng/canadas-agriculture-sectors/animal-industry/poultry-and-egg-market-information/industry-indicators/per-capita-disappearance/?id=1384971854413 (accessed on 1 June 2021).
- Agunos, A.; Gow, S.P.; Léger, D.F.; Carson, C.A.; Deckert, A.E.; Bosman, A.L.; Loest, D.; Irwin, R.J.; Reid-Smith, R.J. Antimicrobial Use and Antimicrobial Resistance Indicators—Integration of Farm-Level Surveillance Data From Broiler Chickens and Turkeys in British Columbia, Canada. Front. Veter. Sci. 2019, 6. [Google Scholar] [CrossRef]
- European Centre for Disease Control (ECDC)/European Food Safety Authority (EFSA)/European Medicines Agency (EMA). ECDC/EFSA/EMA Second Joint Report on the Integrated Analysis of the Consumption of Antimicrobial Agents and Occurrence of Antimicrobial Resistance in Bacterial from Humans and Food Producing Animals. Joint Interagency Antimicrobial Consumption and Resistance Analysis (JIACRA) Report. Available online: http://onlinelibrary.wiley.com/doi/10.2903/j.efsa.2017.4872/epdf (accessed on 7 February 2018).
- European Food Safety Authority and European Centre for Disease Prevention and Control. The European Union Summary Report on Antimicrobial Resistance in zoonotic and indicator bacteria from humans, animals and food in 2018/2019. EFSA J. 2021, 19, e06490. [Google Scholar] [CrossRef]
- University of Zaragoza, Spain. Win Epi Program. Available online: http://www.winepi.net/uk/index.htm (accessed on 7 October 2021).
- Government of Canada. FoodNet Canada Annual Report 2016. Available online: https://www.canada.ca/content/dam/phac-aspc/documents/services/surveillance/foodnet-canada/publications/foodnet-canada-annual-report-2016/pub1-eng.pdf (accessed on 16 July 2019).
- Chicken Farmers of Canada. Chicken Data Booklet 2017. Available online: https://www.chickenfarmers.ca/sections/data-booklet/ (accessed on 17 March 2019).
- Agunos, A.; Léger, D.F.; Carson, C.A.; Gow, S.P.; Bosman, A.; Irwin, R.J.; Reid-Smith, R.J. Antimicrobial use surveillance in broiler chicken flocks in Canada, 2013–2015. PLoS ONE 2017, 12, e0179384. [Google Scholar] [CrossRef] [PubMed]
- Turkey Farmers of Canada. Canadian Turkey Stats Donnees Sur Le Dindon Canadien 1974–2020. Available online: https://www.turkeyfarmersofcanada.ca/wp-content/uploads/2021/08/TURKEY-FACTBOOK-1974-to-2020.pdf (accessed on 23 August 2020).
- Government of Canada. Statistics Canada: Hogs Statistics, Number of Farms Reporting and Average Number of Hogs Per Farm, Semi-Annual. Available online: https://www150.statcan.gc.ca/t1/tbl1/en/tv.action?pid=3210020201 (accessed on 4 October 2021).
- Huber, L.; Agunos, A.; Gow, S.P.; Carson, C.A.; Van Boeckel, T.P. Reduction in Antimicrobial Use and Resistance to Salmonella, Campylobacter, and Escherichia coli in Broiler Chickens, Canada, 2013–2019. Emerg. Infect. Dis. 2021, 27, 2434–2444. [Google Scholar] [CrossRef] [PubMed]
- Dohoo, I.; Martin, W.; Stryhn, H. Veterinary Epidemiologic Research, 2nd ed.; VER Inc.: Charlottetown, PE, Canada, 2009; p. 865. [Google Scholar]
- Arsenault, R. Campylobacter and Salmonella Positive Commercial Broiler Chicken Farms in Ontario and Associated Risk Factors. MSc Thesis, Ontario Veterinary College, Guelph, ON, Canada, 2005. [Google Scholar]
- Roberts, T.; McEwen, S.; Reid-Smith, R.; Sargeant, J.; Agunos, A.; Léger, D.; Guerin, M.T. Prevalence, risk factors and profiles of resistant Salmonella isolates obtained from Ontario broiler chicken farms at chick placement and pre-harvest. In Proceedings of the 64th Western Poultry Disease Conference, Sacramento, CA, USA, 22–25 March 2015. [Google Scholar]
- Dunlop, R.H.; McEwen, S.A.; Meek, A.H.; Friendship, R.M.; Black, W.D.; Clarke, R.C. Sampling considerations for herd-level measurement of faecal Escherichia coli antimicrobial resistance in finisher pigs. Epidemiol. Infect. 1999, 122, 485–496. [Google Scholar] [CrossRef] [PubMed]
- United States Food and Drugs Administration. NARMS Methodology. Available online: https://www.fda.gov/animal-veterinary/national-antimicrobial-resistance-monitoring-system/resources (accessed on 4 October 2021).
- United States Food and Drugs Administration. NARMS Interpretative Criteria for Susceptibility Testing. Available online: https://www.fda.gov/animal-veterinary/national-antimicrobial-resistance-monitoring-system/resources (accessed on 4 October 2021).
- European Centre for Disease Control (ECDC)/European Food Safety Authority (EFSA)/European Medicines Agency (EMA). ECDC, EFSA and EMA Joint Scientific Opinion on a List of Outcome Indicators as Regards Surveillance of Antimicrobial Resistance and Antimicrobial Consumption in Humans and Food-Producing Animals. Available online: https://www.efsa.europa.eu/en/efsajournal/pub/5017 (accessed on 7 February 2018).
- United Kingdom Veterinary Medicines Directorate. UK VARSS Veterinary Antimicrobial Resistance and Sales Surveillance 2018. Available online: https://www.gov.uk/government/publications/veterinary-antimicrobial-resistance-and-sales-surveillance-2018 (accessed on 19 July 2019).
- United States Food and Drugs Administration. NARMS Interactive Data Displays. Available online: https://www.fda.gov/animal-veterinary/national-antimicrobial-resistance-monitoring-system/narms-now-integrated-data (accessed on 4 June 2021).
- Chicken Farmers of Canada. AMU Strategy, a Prescription for Change. Available online: http://www.chickenfarmers.ca/wp-content/uploads/2018/01/AMU-Magazine_ENG_web-2.pdf (accessed on 7 February 2018).
- Turkey Farmers of Canada. Guidelines for Antimicrobial Use in the Turkey Industry. Available online: https://www.amstewardship.ca/guidelines-for-antimicrobial-use-in-the-turkey-industry-published-by-the-turkey-farmers-of-canada/ (accessed on 13 April 2020).
- Agunos, A.; Waddell, L.; Léger, D.; Taboada, E. A Systematic Review Characterizing On-Farm Sources of Campylobacter spp. for Broiler Chickens. PLoS ONE 2014, 9, e104905. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aerts, M.; Battisti, A.; Hendriksen, R.; Kempf, I.; Teale, C.; Tenhagen, B.; Veldman, K.; Wasyl, D.; Guerra, B.; Liébana, E.; et al. Technical specifications on harmonised monitoring of antimicrobial resistance in zoonotic and indicator bacteria from food-producing animals and food. EFSA J. 2019, 17, e05709. [Google Scholar] [CrossRef] [Green Version]
- Bosman, A.L.; Loest, D.; Carson, C.A.; Agunos, A.; Collineau, L.; Léger, D.F. Developing Canadian Defined Daily Doses for Animals: A Metric to Quantify Antimicrobial Use. Front. Veter. Sci. 2019, 6, 220. [Google Scholar] [CrossRef] [Green Version]
- Bos, M.E.H.; Taverne, F.J.; van Geijlswijk, I.M.; Mouton, J.; Mevius, D.J.; Heederik, D.J.J. Consumption of Antimicrobials in Pigs, Veal Calves, and Broilers in The Netherlands: Quantitative Results of Nationwide Collection of Data in 2011. PLoS ONE 2013, 8, e77525. [Google Scholar] [CrossRef]
- AACTING Network on Quantification, Benchmarking and Reporting of Veterinary Antimicrobial Usage (AMU) at Farm Level. Monitoring Systems. Available online: https://aacting.org/monitoring-systems/ (accessed on 1 June 2018).
- Van Cuong, N.; Phu, D.H.; Van, N.T.B.; Truong, B.D.; Kiet, B.T.; Hien, B.V.; Thu, H.T.V.; Choisy, M.; Padungtod, P.; Thwaites, G.; et al. High-Resolution Monitoring of Antimicrobial Consumption in Vietnamese Small-Scale Chicken Farms Highlights Discrepancies Between Study Metrics. Front. Veter. Sci. 2019, 6, 174. [Google Scholar] [CrossRef] [Green Version]
- Government of Canada. Canadian Integrated Program for Antimicrobial Resistance Surveillance (CIPARS) 2018: Integrated Findings. Available online: https://www.canada.ca/en/public-health/services/surveillance/canadian-integrated-program-antimicrobial-resistance-surveillance-cipars/cipars-reports/2018-annual-report-integrated-findings.html (accessed on 16 February 2021).
- Tarakdjian, J.; Capello, K.; Pasqualin, D.; Santini, A.; Cunial, G.; Scollo, A.; Mannelli, A.; Tomao, P.; Vonesch, N.; Di Martino, G. Antimicrobial use on Italian Pig Farms and its Relationship with Husbandry Practices. Animals 2020, 10, 417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- MARAN. MARAN 2019 Monitoring of Antimicrobial Resistance and Antibiotic Usage in Animals in the Netherlands in 2018. Available online: https://rivm.openrepository.com/handle/10029/623134 (accessed on 4 August 2020).
- Callens, B.; Cargnel, M.; Sarrazin, S.; Dewulf, J.; Hoet, B.; Vermeersch, K.; Wattiau, P.; Welby, S. Associations between a decreased veterinary antimicrobial use and resistance in commensal Escherichia coli from Belgian livestock species (2011–2015). Prev. Vet. Med. 2018, 157, 50–58. [Google Scholar] [CrossRef] [Green Version]
- Chantziaras, I.; Boyen, F.; Callens, B.; Dewulf, J. Correlation between veterinary antimicrobial use and antimicrobial resistance in food-producing animals: A report on seven countries. J. Antimicrob. Chemother. 2013, 69, 827–834. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luiken, R.E.C.; Van Gompel, L.; Munk, P.; Sarrazin, S.; Joosten, P.; Dorado-García, A.; Hansen, R.B.; Knudsen, B.E.; Bossers, A.; Wagenaar, J.A.; et al. Associations between antimicrobial use and the faecal resistome on broiler farms from nine European countries. J. Antimicrob. Chemother. 2019, 74, 2596–2604. [Google Scholar] [CrossRef] [PubMed]
- Van Gompel, L.; Luiken, R.E.C.; Sarrazin, S.; Munk, P.; Knudsen, B.E.; Hansen, R.B.; Bossers, A.; Aarestrup, F.M.; Dewulf, J.; Wagenaar, J.A.; et al. The antimicrobial resistome in relation to antimicrobial use and biosecurity in pig farming, a metagenome-wide association study in nine European countries. J. Antimicrob. Chemother. 2019, 74, 865–876. [Google Scholar] [CrossRef] [PubMed]
- Ceccarelli, D.; Hesp, A.; van der Goot, J.; Joosten, P.; Sarrazin, S.; Wagenaar, J.A.; Dewulf, J.; Mevius, D.J.; on behalf of the EFFORT Consortium. Antimicrobial resistance prevalence in commensal Escherichia coli from broilers, fattening turkeys, fattening pigs and veal calves in European countries and association with antimicrobial usage at country level. J. Med. Microbiol. 2020, 69, 537–547. [Google Scholar] [CrossRef] [PubMed]
- Government of Canada. Canadian Integrated Program for Antimicrobial Resistance Surveillance. 2016 Annual Report. Available online: http://publications.gc.ca/collections/collection_2018/aspc-phac/HP2-4-2016-eng.pdf (accessed on 16 July 2019).
- Varga, C.; Rajić, A.; McFall, M.E.; Reid-Smith, R.J.; Deckert, A.E.; Checkley, S.; McEwen, S.A. Associations between reported on-farm antimicrobial use practices and observed antimicrobial resistance in generic fecal Escherichia coli isolated from Alberta finishing swine farms. Prev. Veter. Med. 2009, 88, 185–192. [Google Scholar] [CrossRef]
- Varga, C.; Rajić, A.; McFall, M.E.; Reid-Smith, R.J.; McEwen, S.A. Associations Among Antimicrobial Use and Antimicrobial Resistance of Salmonella spp. Isolates from 60 Alberta Finishing Swine Farms. Foodborne Pathog. Dis. 2009, 6, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Makita, K.; Goto, M.; Ozawa, M.; Kawanishi, M.; Koike, R.; Asai, T.; Tamura, Y. Multivariable Analysis of the Association Between Antimicrobial Use and Antimicrobial Resistance in Escherichia coli Isolated from Apparently Healthy Pigs in Japan. Microb. Drug Resist. 2016, 22, 28–39. [Google Scholar] [CrossRef] [PubMed]
- Persoons, D.; Haesebrouck, F.; Smet, A.; Herman, L.; Heyndrickx, M.; Martel, A.; Catry, B.; Berge, A.C.; Butaye, P.; Dewulf, J. Risk factors for ceftiofur resistance in Escherichia coli from Belgian broilers. Epidemiol. Infect. 2010, 139, 765–771. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control (ECDC); European Food Safety Authority (EFSA); European Medicines Agency (EMA). ECDC, EFSA and EMA Third Joint Inter-Agency Report on Integrated Analysis of Consumption of Antimicrobial Agents and Occurrence of Antimicrobial Resistance in Bacteria from Humans and Food-Producing Animals in the EU/EEA, JIACRA III. 2016–2018. 2021, JIACRA III, 1–164. Available online: https://www.ecdc.europa.eu/en/publications-data/third-joint-interagency-antimicrobial-consumption-and-resistance-analysis-report (accessed on 1 August 2021).
- Caffrey, N.; Nekouei, O.; Gow, S.; Agunos, A.; Checkley, S. Risk factors associated with the A2C resistance pattern among E. coli isolates from broiler flocks in Canada. Prev. Veter. Med. 2017, 148, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Caffrey, N.; Agunos, A.; Gow, S.; Liljebjelke, K.; Waldner, C.L.; Mainali, C.; Checkley, S.L. A cross-sectional study of the prevalence factors associated with fluoroquinolone resistant Campylobacter jejuni in broiler flocks in Canada. Prev. Veter. Med. 2020, 186, 105164. [Google Scholar] [CrossRef] [PubMed]
- Government of Canada Food and Drugs Act. Regulations Amending the Food and Drug Regulations (Veterinary Drugs—Antimicrobial Resistance). 2017, Vol. 151, No. 10. Available online: http://www.gazette.gc.ca/rp-pr/p2/2017/2017-05-17/html/sor-dors76-eng.php (accessed on 25 May 2017).
- Government of Canada. Antimicrobial Resistance and Animals-Actions. Available online: https://www.canada.ca/en/public-health/services/antibiotic-antimicrobial-resistance/animals/actions.html#a2 (accessed on 25 July 2021).
- Van Limbergen, T.; Sarrazin, S.; Chantziaras, I.; Dewulf, J.; Ducatelle, R.; Kyriazakis, I.; McMullin, P.; Méndez, J.; Niemi, J.K.; Papasolomontos, S.; et al. Risk factors for poor health and performance in European broiler production systems. BMC Veter. Res. 2020, 16, 1–13. [Google Scholar] [CrossRef]
- United Kingdom Government, Veterinary Medicines Directorate UK Veterinary Antibiotic Resistance and Sales Surveillance Report (UK-VARSS 2019). 2020. Available online: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/950126/UK-VARSS_2019_Report__2020-TPaccessible.pdf (accessed on 1 September 2021).
- Government of Canada. Canadian Integrated Program for Antimicrobial Resistance Surveillance (CIPARS), Surveillance Bulletin. Reductions in Antimicrobial Use and Resistance: Preliminary Evidence of the Effect of the Canadian Chicken Industry’s Elimination of Use of Antimicrobials of Very High Importance to Human Medicine. Available online: https://www.canada.ca/en/public-health/services/publications/drugs-health-products/canadian-integrated-program-antimicrobial-resistances-surveillance-bulletin.html (accessed on 6 February 2017).








| AMR Outcomes | AMU Outcomes | Considerations for Inclusion in Data Integration | |||
|---|---|---|---|---|---|
| Increased | Stable or Decreased | Increased | Stable or Decreased | ||
| SusceptibleEcoli|Total antimicrobials | Br *, GFP, Tk | Br, GFP *, Tk * | Primary indicators | ||
| ≥3 MCREcoli|Total antimicrobials | Br *, GFP *, Tk | Br, GFP *, Tk * | Complementary indicators | ||
| CRO-REcoli|3rd gen. cephalosporins | Tk | Br *, GFP, | GFP, (Br) | WHO’s HP-CIA; HC-VDD Cat.1 | |
| GEN-REcoli|Aminoglycosides | Br, GFP, Tk | Tk * | Br * | WHO’s CIA’s; HC-VDD Cat.2 | |
| SXT-REcoli|Trimethoprim-sulfas | Br | GFP, Tk | Br, GFP | Tk | WHO’s HIA’s; HC-VDD Cat.2 |
| TET-REcoli| Tetracyclines | Tk | Br *, GFP, | Tk | Br, GFP * | WHO’s HIA’s, HC-VDD Cat.3 |
| CIP-RCampy|Fluoroquinolones | Br *, GFP * | Tk | (Br/GFP/Tk) | WHO’s HP-CIA; HC-VDD Cat.1 | |
| AZM-RCampy|Macrolides | Br, GFP | Tk | Br *, GFP, Tk * | WHO’s HP-CIA’s; HC-VDD Cat.2 | |
| AMU Indicator | About This AMU Indicator |
|---|---|
| Count-based | |
| Frequency | Percentage of flocks using a particular antimicrobial, or any antimicrobials for a specific disease syndrome or etiologic agent. Temporal trends were used to assess in parallel with animal health data. |
| Quantitative, weight-based | |
| mg/PCU | This is the general AMU indicator routinely used by CIPARS for reporting of AMU data (farm, national sales and distribution data). The PCU is based on the population size and average weight at treatment using either ESVAC weights [14] or Canadian weights at treatment. |
| mg/kg animal biomass | This indicator is used by the OIE for reporting global AMU data [15]; however, for this study, the kg animal biomass pertains to live weights documented immediately prior to the expected slaughter date and may differ from other biomass estimation methodology. |
| Quantitative, dose-based | |
| nDDDvetCA/1000 animal days at risk or Treatment Incidence 1000 (TI1000): | This is the dose-based indicator routinely used by CIPARS. The TII000 is determined by species. This indicator expresses the number of doses a thousand animals would receive per day over the observation period. The observation period for CIPARS data is the grow-out period and denotes the days at risk. If instead of dividing by 1000, the nDDDvetCA is divided by 100 animal days at risk, the TI becomes TI100. This indicator is interpreted as the number of days an animal was treated with antimicrobials per 100 days, or the percentage of days treated [12,13]. The DDDvetCA values were developed for each of the species and the methodology and DDDvetCA standards are described elsewhere [29,53]. |
| nDDDvetCA/PCU | This indicator is the total number of DDDvetCA’s adjusted for PCU. Interpreted as the mg adjusted for DDDvetCA standard for every PCU. |
| nDDDvetCA/kg animal biomass | This indicator is similar to nDDDvetCA/PCU where PCU is replaced with the kg animal biomass, and is interpreted as the mg adjusted for DDDvetCA standardfor every kg of live pre-slaughter weight. An alternate data source for the denominator is the final slaughter live weights obtained from processing plants. |
| Diagnosis | Antimicrobial Use | ||||
|---|---|---|---|---|---|
| Broad Syndromic Classifications | Examples of Specific Syndromes or Agents | Increased | Decreased | Increased | Decreased |
| Poultry | |||||
| Neonatal | Yolksacculitis, early septicemia | Br, Tk * | Br *, Tk * | ||
| Respiratory | Airsacculitis, | Br *, Tk * | Br, Tk | ||
| Enteric | Necrotic enteritis, coccidiosis, nonspecific enteritis | Br, Tk * | Br *, Tk * | ||
| Miscellaneous bacterial diseases | Vertebral canal osteomyelitis, Staphylococcus spp. Infection, clostridial dermatitis | Br * | Tk | Br | |
| Swine | |||||
| Systemic | E. coli infections | GF pigs * | GF pigs | ||
| Lawsonia spp. | GF pigs * | GF pigs * | |||
| Streptococcus suis | GF pigs * | GF pigs | |||
| Respiratory | Swine Influenza | GF pigs * | GF pigs | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the Crown. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Agunos, A.; Gow, S.P.; Deckert, A.E.; Kuiper, G.; Léger, D.F. Informing Stewardship Measures in Canadian Food Animal Species through Integrated Reporting of Antimicrobial Use and Antimicrobial Resistance Surveillance Data—Part I, Methodology Development. Pathogens 2021, 10, 1492. https://doi.org/10.3390/pathogens10111492
Agunos A, Gow SP, Deckert AE, Kuiper G, Léger DF. Informing Stewardship Measures in Canadian Food Animal Species through Integrated Reporting of Antimicrobial Use and Antimicrobial Resistance Surveillance Data—Part I, Methodology Development. Pathogens. 2021; 10(11):1492. https://doi.org/10.3390/pathogens10111492
Chicago/Turabian StyleAgunos, Agnes, Sheryl P. Gow, Anne E. Deckert, Grace Kuiper, and David F. Léger. 2021. "Informing Stewardship Measures in Canadian Food Animal Species through Integrated Reporting of Antimicrobial Use and Antimicrobial Resistance Surveillance Data—Part I, Methodology Development" Pathogens 10, no. 11: 1492. https://doi.org/10.3390/pathogens10111492





