Targeting the Gut Microbiota to Relieve the Symptoms of Irritable Bowel Syndrome
Abstract
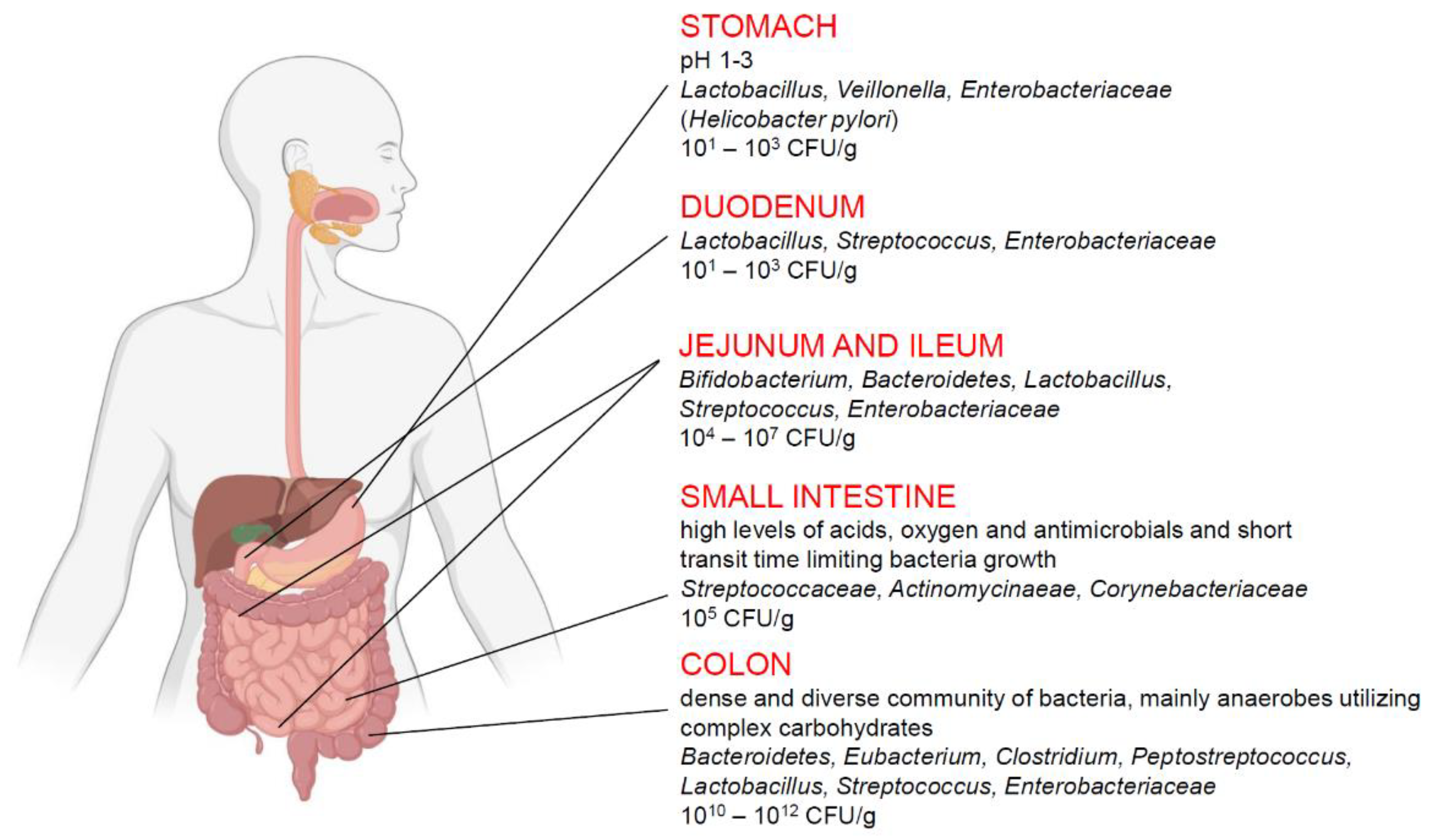
:1. Introduction
2. IBS diagnosis and Risk Factors
3. Available Ways to Manage IBS
4. The Gut Microbiome and IBS Pathogenesis
5. Small Intestinal Bacterial Overgrowth (SIBO)
6. Antibiotics as a Treatment Options for IBS
6.1. Neomycin
6.2. Rifaximin
7. Do diet or Probiotics Matter?
8. Fecal Microbiota Transplantation
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| IBS | irritable bowel syndrome |
| CNS | central nervous system |
| IBD | inflammatory bowel disease |
| SIBO | small intestinal bacterial overgrowth |
| IBS-D | IBS with diarrhea |
| IBS-C | IBS with constipation |
| FODMAP | fermentable oligosaccharides, disaccharides and monosaccharides and polyols |
| FMT | fecal microbiota transplantation |
| QOL | quality of life |
| NNT | number need to treat |
| NNH | number needed to harm |
| ATP | Adenosine triphosphate |
| 5-HT4 | 5-Hydroxytryptamine receptor 4 |
| 5-HT3 | 5-Hydroxytryptamine receptor 3 |
References
- Frändemark, Å.; Törnblom, H.; Jakobsson, S.; Simrén, M. Work Productivity and Activity Impairment in Irritable Bowel Syndrome (IBS): A Multifaceted Problem. Am. J. Gastroenterol. 2018, 113, 1540–1549. [Google Scholar] [CrossRef] [PubMed]
- Longstreth, G.F.; Thompson, W.G.; Chey, W.D.; Houghton, L.A.; Mearin, F.; Spiller, R.C. Functional bowel disorders. Gastroenterology 2006, 130, 1480–1491. [Google Scholar] [CrossRef] [PubMed]
- Flacco, M.E.; Manzoli, L.; De Giorgio, R.; Gasbarrini, A.; Cicchetti, A.; Bravi, F.; Altini, M.; Caio, G.P.; Ursini, F. Costs of irritable bowel syndrome in European countries with universal healthcare coverage: A meta-analysis. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 2986–3000. [Google Scholar] [PubMed]
- Zhang, F.; Xiang, W.; Li, C.-Y.; Li, S.-C. Economic burden of irritable bowel syndrome in China. World J. Gastroenterol. 2016, 22, 10450–10460. [Google Scholar] [CrossRef]
- Peery, A.F.; Crockett, S.D.; Murphy, C.C.; Lund, J.L.; Dellon, E.S.; Williams, J.L.; Jensen, E.T.; Shaheen, N.J.; Barritt, A.S.; Lieber, S.R.; et al. Burden and Cost of Gastrointestinal, Liver, and Pancreatic Diseases in the United States: Update 2018. Gastroenterology 2019, 156, 254–272.e11. [Google Scholar] [CrossRef] [Green Version]
- Ford, A.C.; Sperber, A.D.; Corsetti, M.; Camilleri, M. Irritable bowel syndrome. Lancet 2020, 396, 1675–1688. [Google Scholar] [CrossRef]
- Posserud, I.; Stotzer, P.-O.; Björnsson, E.S.; Abrahamsson, H.; Simrén, M. Small intestinal bacterial overgrowth in patients with irritable bowel syndrome. Gut 2007, 56, 802–808. [Google Scholar] [CrossRef] [Green Version]
- Ford, A.C.; Spiegel, B.M.; Talley, N.J.; Moayyedi, P. Small intestinal bacterial overgrowth in irritable bowel syndrome: Systematic review and meta-analysis. Clin. Gastroenterol. Hepatol. 2009, 7, 1279–1286. [Google Scholar] [CrossRef]
- Ahmad, O.F.; Akbar, A. Microbiome, antibiotics and irritable bowel syndrome. Br. Med. Bull. 2016, 120, 91–99. [Google Scholar] [CrossRef]
- Ford, A.; Moayyedi, P.; Lacy, B.E.; Lembo, A.J.; Saito, Y.A.; Schiller, L.R.; Soffer, E.E.; Spiegel, B.M.R.; Quigley, E.M.M. American College of Gastroenterology Monograph on the Management of Irritable Bowel Syndrome and Chronic Idiopathic Constipation. Am. J. Gastroenterol. 2014, 109, S2–S26. [Google Scholar] [CrossRef]
- Mearin, F.; Lacy, B.E.; Chang, L.; Chey, W.D.; Lembo, A.J.; Simren, M.; Spiller, R. Bowel Disorders. Gastroenterology 2016, S0016–5085, 00222–00225. [Google Scholar]
- Lewis, S.J.; Heaton, K.W. Stool Form Scale as a Useful Guide to Intestinal Transit Time. Scand. J. Gastroenterol. 1997, 32, 920–924. [Google Scholar] [CrossRef]
- Sperber, A.; Dumitrascu, D.; Fukudo, S.; Gerson, C.; Ghoshal, U.C.; Gwee, K.A.; Hungin, A.P.; Kang, J.-Y.; Minhu, C.; Schmulson, M.; et al. The global prevalence of IBS in adults remains elusive due to the heterogeneity of studies: A Rome Foundation working team literature review. Gut 2017, 66, 1075–1082. [Google Scholar] [CrossRef] [Green Version]
- Ringel, Y.; Sperber, A.D.; Drossman, D.A. Irritable bowel syndrome. Annu. Rev. Med. 2001, 52, 319–338. [Google Scholar] [CrossRef]
- Dunlop, S.P.; Jenkins, D.; Spiller, R.C. Distinctive clinical, psychological, and histological features of postinfective irritable bowel syndrome. Am. J. Gastroenterol. 2003, 98, 1578–1583. [Google Scholar] [CrossRef]
- Shulman, R.J.; Devaraj, S.; Heitkemper, M. Activation of the Innate Immune System in Children with Irritable Bowel Syndrome Evidenced by Increased Fecal Human β-Defensin-2. Clin. Gastroenterol. Hepatol. 2021, 19, 2121–2127. [Google Scholar] [CrossRef]
- Langhorst, J.; Junge, A.; Rueffer, A.; Wehkamp, J.; Foell, D.; Michalsen, A.; Musial, F.; Dobos, G.J. Elevated human beta-defensin-2 levels indicate an activation of the innate immune system in patients with irritable bowel syndrome. Am. J. Gastroenterol. 2009, 104, 404–410. [Google Scholar] [CrossRef]
- Wollny, T.; Piktel, E.; Durnaś, B.; Bucki, R. Regulation of Cationic Antimicrobial Peptides Expression in the Digestive Tract. In Antimicrobial Peptides in Gastrointestinal Diseases; Cho, C.H., Ed.; Academic Press: Cambridge, MA, USA, 2018; pp. 1–20. [Google Scholar]
- Wollny, T.; Wątek, M.; Durnaś, B.; Niemirowicz, K.; Piktel, E.; Żendzian-Piotrowska, M.; Góźdź, S.; Bucki, R. Sphingosine-1-Phosphate Metabolism and Its Role in the Development of Inflammatory Bowel Disease. Int. J. Mol. Sci. 2017, 18, 741. [Google Scholar] [CrossRef] [Green Version]
- Peyrin-Biroulet, L.; Christopher, R.; Behan, D.; Lassen, C. Modulation of sphingosine-1-phosphate in inflammatory bowel disease. Autoimmun. Rev. 2017, 16, 495–503. [Google Scholar] [CrossRef]
- Wollny, T.; Wątek, M.; Wnorowska, U.; Piktel, E.; Góźdź, S.; Kurek, K.; Wolak, P.; Król, G.; Żendzian-Piotrowska, M.; Bucki, R. Hypogelsolinemia and Decrease in Blood Plasma Sphingosine-1-Phosphate in Patients Diagnosed with Severe Acute Pancreatitis. Dig. Dis. Sci. 2021, in press. [Google Scholar] [CrossRef]
- Lovell, R.M.; Ford, A. Global Prevalence of and Risk Factors for Irritable Bowel Syndrome: A Meta-analysis. Clin. Gastroenterol. Hepatol. 2012, 10, 712–721.e4. [Google Scholar] [CrossRef] [PubMed]
- Petersen, M.W.; Schröder, A.; Jørgensen, T.; Ørnbøl, E.; Dantoft, T.M.; Eliasen, M.; Thuesen, B.H.; Fink, P. The unifying diagnostic construct of bodily distress syndrome (BDS) was confirmed in the general population. J. Psychosom. Res. 2020, 128, 109868. [Google Scholar] [CrossRef] [PubMed]
- Card, T.; Enck, P.; Barbara, G.; Boeckxstaens, G.E.; Santos, J.; Azpiroz, F.; Mearin, F.; Aziz, Q.; Marshall, J.; Spiller, R. Post-infectious IBS: Defining its clinical features and prognosis using an internet-based survey. United Eur. Gastroenterol. J. 2018, 6, 1245–1253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klem, F.; Wadhwa, A.; Prokop, L.J.; Sundt, W.J.; Farrugia, G.; Camilleri, M.; Singh, S.; Grover, M. Prevalence, Risk Factors, and Outcomes of Irritable Bowel Syndrome After Infectious Enteritis: A Systematic Review and Meta-analysis. Gastroenterology 2017, 152, 1042–1054.e1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marshall, J.K.; Thabane, M.; Garg, A.X.; Clark, W.F.; Moayyedi, P.; Collins, S.M. Eight year prognosis of postinfectious irritable bowel syndrome following waterborne bacterial dysentery. Gut 2010, 59, 605–611. [Google Scholar] [CrossRef]
- Ford, A.C.; Moayyedi, P. Meta-analysis: Factors affecting placebo response rate in the irritable bowel syndrome. Aliment. Pharmacol. Ther. 2010, 32, 144–158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chapman, R.W.; Stanghellini, V.; Geraint, M.; Halphen, M. Randomized clinical trial: Macrogol/PEG 3350 plus electrolytes for treatment of patients with constipation associated with irritable bowel syndrome. Am. J. Gastroenterol. 2013, 108, 1508–1515. [Google Scholar] [CrossRef]
- Ford, A.; Moayyedi, P.; Chey, W.D.; Harris, L.A.; Lacy, B.E.; Saito, Y.A.; Quigley, E.M.M. American College of Gastroenterology Monograph on Management of Irritable Bowel Syndrome. Am. J. Gastroenterol. 2018, 113 (Suppl. 2), 1–18. [Google Scholar] [CrossRef]
- Zheng, L.; Lai, Y.; Lu, W.; Li, B.; Fan, H.; Yan, Z.; Gong, C.; Wan, X.; Wu, J.; Huang, D.; et al. Pinaverium Reduces Symptoms of Irritable Bowel Syndrome in a Multicenter, Randomized, Controlled Trial. Clin. Gastroenterol. Hepatol. 2015, 13, 1285–1292.e1. [Google Scholar] [CrossRef]
- Weerts, Z.Z.R.; Masclee, A.A.; Witteman, B.J.; Clemens, C.H.; Winkens, B.; Brouwers, J.R.; Frijlink, H.W.; Muris, J.W.; De Wit, N.J.; Essers, B.A.; et al. Efficacy and Safety of Peppermint Oil in a Randomized, Double-Blind Trial of Patients with Irritable Bowel Syndrome. Gastroenterology 2020, 158, 123–136. [Google Scholar] [CrossRef]
- Gorard, D.A.; Libby, G.W.; Farthing, M.J.G. Effect of a tricyclic antidepressant on small intestinal motility in health and diarrhea-predominant irritable bowel syndrome. Dig. Dis. Sci. 1995, 40, 86–95. [Google Scholar] [CrossRef]
- Ford, A.C.; Lacy, B.E.; Harris, L.A.; Quigley, E.M.M.; Moayyedi, P. Effect of Antidepressants and Psychological Therapies in Irritable Bowel Syndrome: An Updated Systematic Review and Meta-Analysis. Am. J. Gastroenterol. 2019, 114, 21–39. [Google Scholar] [CrossRef]
- Saito, Y.A.; Almazar, A.E.; Tilkes, K.E.; Choung, R.S.; Van Norstrand, M.D.; Schleck, C.D.; Zinsmeister, A.R.; Talley, N.J. Randomised clinical trial: Pregabalin vs placebo for irritable bowel syndrome. Aliment. Pharmacol. Ther. 2019, 49, 389–397. [Google Scholar] [CrossRef]
- Ford, A.C.; Suares, N.C. Effect of laxatives and pharmacological therapies in chronic idiopathic constipation: Systematic review and meta-analysis. Gut 2011, 60, 209–218. [Google Scholar] [CrossRef] [Green Version]
- Brenner, D.M.; Fogel, R.; Dorn, S.D.; Krause, R.; Eng, P.; Kirshoff, R.; Nguyen, A.; Crozier, R.A.; Magnus, L.; Griffin, P.H. Efficacy, safety, and tolerability of plecanatide in patients with irritable bowel syndrome with constipation: Results of two phase 3 randomized clinical trials. Am. J. Gastroenterol. 2018, 113, 735–745. [Google Scholar] [CrossRef]
- Chey, W.D.; Lembo, A.J.; Lavins, B.J.; Shiff, S.J.; Kurtz, C.B.; Currie, M.G.; MacDougall, J.E.; Jia, X.D.; Shao, J.Z.; Fitch, D.A.; et al. Linaclotide for Irritable Bowel Syndrome with Constipation: A 26-Week, Randomized, Double-blind, Placebo-Controlled Trial to Evaluate Efficacy and Safety. Am. J. Gastroenterol. 2012, 107, 1702–1712. [Google Scholar] [CrossRef]
- Chey, W.D.; Lembo, A.J.; Rosenbaum, D. Tenapanor Treatment of Patients with Constipation-Predominant Irritable Bowel Syndrome: A Phase 2, Randomized, Placebo-Controlled Efficacy and Safety Trial. Am. J. Gastroenterol. 2017, 112, 763–774. [Google Scholar] [CrossRef] [Green Version]
- Drossman, D.A.; Chey, W.D.; Johanson, J.F.; Fass, R.; Scott, C.; Panas, R.; Ueno, R. Clinical trial: Lubiprostone in patients with constipation-associated irritable bowel syndrome--results of two randomized, placebo-controlled studies. Aliment. Pharmacol. Ther. 2009, 29, 329–341. [Google Scholar] [CrossRef]
- Black, C.; Burr, N.; Quigley, E.M.; Moayyedi, P.; Houghton, L.; Ford, A.C. Efficacy of Secretagogues in Patients with Irritable Bowel Syndrome with Constipation: Systematic Review and Network Meta-analysis. Gastroenterology 2018, 155, 1753–1763. [Google Scholar] [CrossRef] [Green Version]
- Fukudo, S.; Kinoshita, Y.; Okumura, T.; Ida, M.; Akiho, H.; Nakashima, Y.; Nishida, A.; Haruma, K. Ramosetron Reduces Symptoms of Irritable Bowel Syndrome with Diarrhea and Improves Quality of Life in Women. Gastroenterology 2016, 150, 358–366.e8. [Google Scholar] [CrossRef] [Green Version]
- Hecht, G. Innate mechanisms of epithelial host defense: Spotlight on intestine. Am. J. Physiol. Physiol. 1999, 277, C351–C358. [Google Scholar] [CrossRef]
- Johansson, M.E.V.; Sjövall, H.; Hansson, G.C. The gastrointestinal mucus system in health and disease. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 352–361. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johansson, M.E.; Phillipson, M.; Petersson, J.; Velcich, A.; Holm, L.; Hansson, G.C. The inner of the two Muc2 mucin-dependent mucus layers in colon is devoid of bacteria. Proc. Natl. Acad. Sci. USA 2008, 105, 15064–15069. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paone, P.; Cani, P.D. Mucus barrier, mucins and gut microbiota: The expected slimy partners? Gut 2020, 69, 2232–2243. [Google Scholar] [CrossRef]
- Thursby, E.; Juge, N. Introduction to the human gut microbiota. Biochem. J. 2017, 474, 1823–1836. [Google Scholar] [CrossRef]
- Ley, R.E.; Peterson, D.A.; Gordon, J.I. Ecological and Evolutionary Forces Shaping Microbial Diversity in the Human Intestine. Cell 2006, 124, 837–848. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palmer, C.; Bik, E.M.; DiGiulio, D.B.; Relman, D.A.; Brown, P.O. Development of the Human Infant Intestinal Microbiota. PLoS Biol. 2007, 5, e177. [Google Scholar] [CrossRef] [Green Version]
- Zeng, M.Y.; Inohara, N.; Nuñez, G. Mechanisms of inflammation-driven bacterial dysbiosis in the gut. Mucosal Immunol. 2017, 10, 18–26. [Google Scholar] [CrossRef] [Green Version]
- von Martels, J.Z.H.; Sadaghian Sadabad, M.; Bourgonje, A.R.; Blokzijl, T.; Dijkstra, G.; Faber, K.N.; Harmsen, H.J.M. The role of gut microbiota in health and disease: In vitro modeling of host-microbe interactions at the aerobe-anaerobe interphase of the human gut. Anaerobe 2017, 44, 3–12. [Google Scholar] [CrossRef] [Green Version]
- Wostmann, B.S.; Larkin, C.; Moriarty, A.; Bruckner-Kardoss, E. Dietary intake, energy metabolism, and excretory losses of adult male germfree Wistar rats. Lab. Anim. Sci. 1983, 33, 46–50. [Google Scholar]
- Xu, J.; Bjursell, M.K.; Himrod, J.; Deng, S.; Carmichael, L.K.; Chiang, H.C.; Hooper, L.V.; Gordon, J.I. A Genomic View of the Human- Bacteroides thetaiotaomicron Symbiosis. Science 2003, 299, 2074–2076. [Google Scholar] [CrossRef] [Green Version]
- Bäckhed, F.; Ding, H.; Wang, T.; Hooper, L.V.; Koh, G.Y.; Nagy, A.; Semenkovich, C.F.; Gordon, J.I. The gut microbiota as an environmental factor that regulates fat storage. Proc. Natl. Acad. Sci. USA 2004, 101, 15718–15723. [Google Scholar] [CrossRef] [Green Version]
- anaka, M.; Nakayama, J. Development of the gut microbiota in infancy and its impact on health in later life. Allergol. Int. 2017, 66, 515–522. [Google Scholar]
- Xu, J.; Gordon, J.I. Honor thy symbionts. Proc. Natl. Acad. Sci. USA 2003, 100, 10452–10459. [Google Scholar] [CrossRef] [Green Version]
- Frank, D.N.; St Amand, A.L.; Feldman, R.A.; Boedeker, E.C.; Harpaz, N.; Pace, N.R. Molecular-phylogenetic characterization of microbial community imbalances in human inflammatory bowel diseases. Proc. Natl. Acad. Sci. USA 2007, 104, 13780–13785. [Google Scholar] [CrossRef] [Green Version]
- Qin, J.; Li, R.; Raes, J.; Arumugam, M.; Burgdorf, K.; Manichanh, C.; Nielsen, T.; Pons, N.; Levenez, F.; Yamada, T.; et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature 2010, 464, 59–65. [Google Scholar] [CrossRef] [Green Version]
- Arumugam, M.; Raes, J.; Pelletier, E.; Le Paslier, D.; Yamada, T.; Mende, D.R.; Fernandes, G.R.; Tap, J.; Bruls, T.; Batto, J.M.; et al. Enterotypes of the human gut microbiome. Nature 2011, 473, 174–180. [Google Scholar] [CrossRef]
- O’Hara, A.M.; Shanahan, F. The gut flora as a forgotten organ. EMBO Rep. 2006, 7, 688–693. [Google Scholar] [CrossRef] [Green Version]
- Rinninella, E.; Raoul, P.; Cintoni, M.; Franceschi, F.; Miggiano, G.A.D.; Gasbarrini, A.; Mele, M.C. What is the Healthy Gut Microbiota Composition? A Changing Ecosystem across Age, Environment, Diet, and Diseases. Microorganisms 2019, 7, 14. [Google Scholar] [CrossRef] [Green Version]
- Swidsinski, A.; Loening-Baucke, V.; Lochs, H.; Hale, L.P. Spatial organization of bacterial flora in normal and inflamed intestine: A fluorescence in situ hybridization study in mice. World J. Gastroenterol. 2005, 11, 1131–1140. [Google Scholar] [CrossRef]
- Florin, T.H.; Zhu, G.; Kirk, K.M.; Martin, N.G. Shared and unique environmental factors determine the ecology of methanogens in humans and rats. Am. J. Gastroenterol. 2000, 95, 2872–2879. [Google Scholar] [CrossRef] [PubMed]
- Fricke, W.F.; Seedorf, H.; Henne, A.; Krüer, M.; Liesegang, H.; Hedderich, R.; Gottschalk, G.; Thauer, R.K. The genome sequence of Methanosphaera stadtmanae reveals why this human intestinal archaeon is restricted to methanol and H2 for methane formation and ATP synthesis. J. Bacteriol. 2006, 188, 642–658. [Google Scholar] [CrossRef] [Green Version]
- Eckburg, P.B.; Bik, E.M.; Bernstein, C.N.; Purdom, E.; Dethlefsen, L.; Sargent, M.; Gill, S.R.; Nelson, K.E.; Relman, D.A. Diversity of the Human Intestinal Microbial Flora. Science 2005, 308, 1635–1638. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mihajlovski, A.; Alric, M.; Brugère, J.-F. A putative new order of methanogenic Archaea inhabiting the human gut, as revealed by molecular analyses of the mcrA gene. Res. Microbiol. 2008, 159, 516–521. [Google Scholar] [CrossRef] [PubMed]
- Jensen, N.S.; Canale-Parola, E. Bacteroides pectinophilus sp. nov. and Bacteroides galacturonicus sp. nov.: Two pectinolytic bacteria from the human intestinal tract. Appl. Environ. Microbiol. 1986, 52, 880–887. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Faith, J.J.; Guruge, J.L.; Charbonneau, M.; Subramanian, S.; Seedorf, H.; Goodman, A.L.; Clemente, J.C.; Knight, R.; Heath, A.C.; Leibel, R.L.; et al. The Long-Term Stability of the Human Gut Microbiota. Science 2013, 341, 1237439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thabane, M.; Kottachchi, D.T.; Marshall, J. Systematic review and meta-analysis: The incidence and prognosis of post-infectious irritable bowel syndrome. Aliment. Pharmacol. Ther. 2007, 26, 535–544. [Google Scholar] [CrossRef] [PubMed]
- Jalanka-Tuovinen, J.; Salojärvi, J.; Salonen, A.; Immonen, O.; Garsed, K.; Kelly, F.M.; Zaitoun, A.; Palva, A.; Spiller, R.C.; de Vos, W.M. Faecal microbiota composition and host-microbe cross-talk following gastroenteritis and in postinfectious irritable bowel syndrome. Gut 2014, 63, 1737–1745. [Google Scholar] [CrossRef]
- Sundin, J.; Rangel, I.; Fuentes, S.; Heikamp-de Jong, I.; Hultgren-Hörnquist, E.; de Vos, W.M.; Brummer, R.J. Altered faecal and mucosal microbial composition in post-infectious irritable bowel syndrome patients correlates with mucosal lymphocyte phenotypes and psychological distress. Aliment. Pharmacol. Ther. 2015, 41, 342–351. [Google Scholar] [CrossRef]
- Pittayanon, R.; Lau, J.T.; Yuan, Y.; Leontiadis, G.I.; Tse, F.; Surette, M.; Moayyedi, P. Gut Microbiota in Patients with Irritable Bowel Syndrome—A Systematic Review. Gastroenterology 2019, 157, 97–108. [Google Scholar] [CrossRef] [Green Version]
- Rezaie, A.; Buresi, M.; Lembo, A.; Lin, H.; McCallum, R.; Rao, S.; Schmulson, M.; Valdovinos, M.; Zakko, S.; Pimentel, M. Hydrogen and Methane-Based Breath Testing in Gastrointestinal Disorders: The North American Consensus. Am. J. Gastroenterol. 2017, 112, 775–784. [Google Scholar] [CrossRef] [Green Version]
- Ghoshal, U.C.; Srivastava, D.; Ghoshal, U.; Misra, A. Breath tests in the diagnosis of small intestinal bacterial overgrowth in patients with irritable bowel syndrome in comparison with quantitative upper gut aspirate culture. Eur. J. Gastroenterol. Hepatol. 2014, 26, 753–760. [Google Scholar] [CrossRef]
- Pyleris, E.; Giamarellos-Bourboulis, E.J.; Tzivras, D.; Koussoulas, V.; Barbatzas, C.; Pimentel, M. The Prevalence of Overgrowth by Aerobic Bacteria in the Small Intestine by Small Bowel Culture: Relationship with Irritable Bowel Syndrome. Dig. Dis. Sci. 2012, 57, 1321–1329. [Google Scholar] [CrossRef]
- Choung, R.S.; Ruff, K.C.; Malhotra, A.; Herrick, L.; Locke, G.R., III; Harmsen, W.S.; Zinsmeister, A.R.; Talley, N.J.; Saito, Y.A. Clinical predictors of small intestinal bacterial overgrowth by duodenal aspirate culture. Aliment. Pharmacol. Ther. 2011, 33, 1059–1067. [Google Scholar] [CrossRef]
- Rao, S.S.C.; Bhagatwala, J. Small Intestinal Bacterial Overgrowth: Clinical Features and Therapeutic Management. Clin. Transl. Gastroenterol. 2019, 10, e00078. [Google Scholar] [CrossRef]
- Rana, S.; Sharma, S.K.; Kaur, J.; Sinha, S.; Singh, K. Comparison of Lactulose and Glucose Breath Test for Diagnosis of Small Intestinal Bacterial Overgrowth in Patients with Irritable Bowel Syndrome. Digestion 2012, 85, 243–247. [Google Scholar] [CrossRef]
- Scarpellini, E.; Giorgio, V.; Gabrielli, M.; Lauritano, E.; Pantanella, A.; Fundarò, C.; Gasbarrini, A. Prevalence of Small Intestinal Bacterial Overgrowth in Children with Irritable Bowel Syndrome: A Case-Control Study. J. Pediatr. 2009, 155, 416–420. [Google Scholar] [CrossRef]
- Shah, A.; Talley, N.J.; Jones, M.; Kendall, B.J.; Koloski, N.; Walker, M.M.; Morrison, M.; Holtmann, G.J. Small Intestinal Bacterial Overgrowth in Irritable Bowel Syndrome: A Systematic Review and Meta-Analysis of Case-Control Studies. Am. J. Gastroenterol. 2020, 115, 190–201. [Google Scholar] [CrossRef]
- Pimentel, M. Evaluating a bacterial hypothesis in IBS using a modification of Koch’s postulates: Part 1. Am. J. Gastroenterol. 2010, 105, 718–721. [Google Scholar] [CrossRef]
- Singh, V.V.; Toskes, P.P. Small bowel bacterial overgrowth: Presentation, diagnosis, and treatment. Curr. Gastroenterol. Rep. 2003, 5, 365–372. [Google Scholar] [CrossRef]
- Da Cunha, B.R.; Fonseca, L.P.; Calado, C.R.C. Antibiotic Discovery: Where Have We Come from, Where Do We Go? Antibiotics 2019, 8, 45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chmielewska, S.; Skłodowski, K.; Depciuch, J.; Deptuła, P.; Piktel, E.; Fiedoruk, K.; Kot, P.; Paprocka, P.; Fortunka, K.; Wollny, T.; et al. Bactericidal Properties of Rod-, Peanut-, and Star-Shaped Gold Nanoparticles Coated with Ceragenin CSA-131 against Multidrug-Resistant Bacterial Strains. Pharmaceutics 2021, 13, 425. [Google Scholar] [CrossRef] [PubMed]
- Van Citters, G.W.; Lin, H.C. Management of small intestinal bacterial overgrowth. Curr. Gastroenterol. Rep. 2005, 7, 317–320. [Google Scholar] [CrossRef] [PubMed]
- Baker, D.E. Rifaximin: A nonabsorbed oral antibiotic. Rev. Gastroenterol. Disord. 2005, 5, 19–30. [Google Scholar]
- Yang, J.; Lee, H.-R.; Low, K.; Chatterjee, S.; Pimentel, M. Rifaximin versus Other Antibiotics in the Primary Treatment and Retreatment of Bacterial Overgrowth in IBS. Dig. Dis. Sci. 2008, 53, 169–174. [Google Scholar] [CrossRef]
- Gerard, L.; Garey, K.W.; DuPont, H.L. Rifaximin: A nonabsorbable rifamycin antibiotic for use in nonsystemic gastrointestinal infections. Expert Rev. Anti. Infect. Ther. 2005, 3, 201–211. [Google Scholar] [CrossRef]
- Jiang, Z.D.; Dupont, H.L. Rifaximin: In vitro and in vivo Antibacterial Activity—A Review. Chemotherapy 2005, 51, 67–72. [Google Scholar] [CrossRef]
- Debbia, E.; Maioli, E.; Roveta, S.; Marchese, A. Effects of Rifaximin on Bacterial Virulence Mechanisms at Supra- and Sub-Inhibitory Concentrations. J. Chemother. 2008, 20, 186–194. [Google Scholar] [CrossRef]
- Pimentel, M.; Chow, E.J.; Lin, H.C. Normalization of lactulose breath testing correlates with symptom improvement in irritable bowel syndrome: A double-blind, randomized, placebo-controlled study. Am. J. Gastroenterol. 2003, 98, 412–419. [Google Scholar]
- Pimentel, M.; Park, S.; Mirocha, J.; Kane, S.V.; Kong, Y. The effect of a nonabsorbed oral antibiotic (rifaximin) on the symptoms of the irritable bowel syndrome: A randomized trial. Ann. Intern. Med. 2006, 145, 557–563. [Google Scholar] [CrossRef]
- Sharara, A.I.; Aoun, E.; Abdul-Baki, H.; Mounzer, R.; Sidani, S.; ElHajj, I. A Randomized Double-Blind Placebo-Controlled Trial of Rifaximin in Patients with Abdominal Bloating and Flatulence. Am. J. Gastroenterol. 2006, 101, 326–333. [Google Scholar] [CrossRef]
- Cuoco, L.; Salvagnini, M. Small intestine bacterial overgrowth in irritable bowel syndrome: A retrospective study with rifaximin. Minerva Gastroenterol. Dietol. 2006, 52, 89–95. [Google Scholar]
- Meyrat, P.; Safroneeva, E.; Schoepfer, A.M. Rifaximin treatment for the irritable bowel syndrome with a positive lactulose hydrogen breath test improves symptoms for at least 3 months. Aliment. Pharmacol. Ther. 2012, 36, 1084–1093. [Google Scholar] [CrossRef]
- Pimentel, M. Review article: Potential mechanisms of action of rifaximin in the management of irritable bowel syndrome with diarrhoea. Aliment. Pharmacol. Ther. 2016, 43, 37–49. [Google Scholar] [CrossRef] [Green Version]
- Pimentel, M.; Lembo, A.; Chey, W.D.; Zakko, S.; Ringel, Y.; Yu, J.; Mareya, S.M.; Shaw, A.L.; Bortey, E.; Forbes, W.P. Rifaximin Therapy for Patients with Irritable Bowel Syndrome without Constipation. N. Engl. J. Med. 2011, 364, 22–32. [Google Scholar] [CrossRef] [Green Version]
- Lembo, A.; Pimentel, M.; Rao, S.S.; Schoenfeld, P.; Cash, B.; Weinstock, L.B.; Paterson, C.; Bortey, E.; Forbes, W.P. Repeat Treatment with Rifaximin Is Safe and Effective in Patients with Diarrhea-Predominant Irritable Bowel Syndrome. Gastroenterology 2016, 151, 1113–1121. [Google Scholar] [CrossRef] [Green Version]
- Shah, E.; Kim, S.; Chong, K.; Lembo, A.; Pimentel, M. Evaluation of Harm in the Pharmacotherapy of Irritable Bowel Syndrome. Am. J. Med. 2012, 125, 381–393. [Google Scholar] [CrossRef]
- Pietrzak, A.; Skrzydło-Radomańska, B.; Mulak, A.; Lipiński, M.; Małecka-Panas, E.; Reguła, J.; Rydzewska, G. Guidelines on the management of irritable bowel syndrome: In memory of Professor Witold Bartnik. Prz. Gastroenterol. 2018, 13, 259–288. [Google Scholar]
- Mullin, G.E.; Shepherd, S.J.; Roland, B.C.; Ireton-Jones, C.; Matarese, L.E. Irritable bowel syndrome: Contemporary nutrition management strategies. JPEN J. Parenter. Enteral. Nutr. 2014, 38, 781–799. [Google Scholar] [CrossRef]
- Ford, A.C.; Lacy, B.E.; Talley, N.J. Irritable Bowel Syndrome. N. Engl. J. Med. 2017, 376, 2566–2578. [Google Scholar] [CrossRef] [Green Version]
- Gunn, D.; Abbas, Z.; Harris, H.C.; Major, G.; Hoad, C.; Gowland, P.; Marciani, L.; Gill, S.K.; Warren, F.J.; Rossi, M.; et al. Psyllium reduces inulin-induced colonic gas production in IBS: MRI and in vitro fermentation studies. Gut 2021, 0, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Shepherd, S.J.; Parker, F.C.; Muir, J.G.; Gibson, P.R. Dietary triggers of abdominal symptoms in patients with irritable bowel syndrome: Randomized placebo-controlled evidence. Clin. Gastroenterol. Hepatol. 2008, 6, 765–771. [Google Scholar] [CrossRef] [PubMed]
- Halmos, E.P.; Power, V.A.; Shepherd, S.J.; Gibson, P.R.; Muir, J.G. A Diet Low in FODMAPs Reduces Symptoms of Irritable Bowel Syndrome. Gastroenterology 2014, 146, 67–75.e5. [Google Scholar] [CrossRef] [PubMed]
- Böhn, L.; Störsrud, S.; Liljebo, T.; Collin, L.; Lindfors, P.; Törnblom, H.; Simrén, M. Diet Low in FODMAPs Reduces Symptoms of Irritable Bowel Syndrome as Well as Traditional Dietary Advice: A Randomized Controlled Trial. Gastroenterology 2015, 149, 1399–1407.e2. [Google Scholar] [CrossRef] [Green Version]
- Eswaran, S.L.; Chey, W.D.; Han-Markey, T.; Ball, S.; Jackson, K. A Randomized Controlled Trial Comparing the Low FODMAP Diet vs. Modified NICE Guidelines in US Adults with IBS-D. Am. J. Gastroenterol. 2016, 111, 1824–1832. [Google Scholar] [CrossRef]
- Halmos, E.; Christophersen, C.T.; Bird, A.; Shepherd, S.J.; Gibson, P.R.; Muir, J.G. Diets that differ in their FODMAP content alter the colonic luminal microenvironment. Gut 2015, 64, 93–100. [Google Scholar] [CrossRef]
- Dionne, J.; Ford, A.; Yuan, Y.; Chey, W.D.; Lacy, B.E.; Saito, Y.A.; Quigley, E.M.M.; Moayyedi, P. A Systematic Review and Meta-Analysis Evaluating the Efficacy of a Gluten-Free Diet and a Low FODMAPS Diet in Treating Symptoms of Irritable Bowel Syndrome. Am. J. Gastroenterol. 2018, 113, 1290–1300. [Google Scholar] [CrossRef] [Green Version]
- Hill, C.; Guarner, F.; Reid, G.; Gibson, G.R.; Merenstein, D.J.; Pot, B.; Morelli, L.; Canani, R.B.; Flint, H.J.; Salminen, S.; et al. Expert Consensus Document: The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 506–514. [Google Scholar] [CrossRef] [Green Version]
- Dale, H.F.; Rasmussen, S.H.; Asiller, Ö.Ö.; Lied, G.A. Probiotics in Irritable Bowel Syndrome: An Up-to-Date Systematic Review. Nutrients 2019, 11, 2048. [Google Scholar] [CrossRef] [Green Version]
- Cremon, C.; Barbaro, M.R.; Ventura, M.; Barbara, G. Pre- and probiotic overview. Curr. Opin. Pharmacol. 2018, 43, 87–92. [Google Scholar] [CrossRef]
- Asha, M.Z.; Khalil, S.F.H. Efficacy and Safety of Probiotics, Prebiotics and Synbiotics in the Treatment of Irritable Bowel Syndrome: A systematic review and meta-analysis. Sultan Qaboos Univ. Med. J. 2020, 20, e13–e24. [Google Scholar] [CrossRef] [Green Version]
- Mayorgas, A.; Dotti, I.; Salas, A. Microbial Metabolites, Postbiotics, and Intestinal Epithelial Function. Mol. Nutr. Food Res. 2021, 65, e2000188. [Google Scholar] [CrossRef]
- Wegh, C.A.M.; Geerlings, S.Y.; Knol, J.; Roeselers, G.; Belzer, C. Postbiotics and Their Potential Applications in Early Life Nutrition and Beyond. Int. J. Mol. Sci. 2019, 20, 4673. [Google Scholar] [CrossRef] [Green Version]
- Perez, M.; Astó, E.; Huedo, P.; Alcántara, C.; Buj, D.; Espadaler, J. Derived Postbiotics of a Multi-strain Probiotic Formula Clinically Validated for the Treatment of Irritable Bowel Syndrome. FASEB J. 2020, 34, 1. [Google Scholar] [CrossRef]
- Ianiro, G.; Eusebi, L.H.; Black, C.; Gasbarrini, A.; Cammarota, G.; Ford, A.C. Systematic review with meta-analysis: Efficacy of faecal microbiota transplantation for the treatment of irritable bowel syndrome. Aliment. Pharmacol. Ther. 2019, 50, 240–248. [Google Scholar] [CrossRef] [Green Version]
- Johnsen, P.H.; Hilpüsch, F.; Cavanagh, P.; Leikanger, I.S.; Kolstad, C.; Valle, P.C.; Goll, R. Faecal microbiota transplantation versus placebo for moderate-to-severe irritable bowel syndrome: A double-blind, randomised, placebo-controlled, parallel-group, single-centre trial. Lancet Gastroenterol. Hepatol. 2018, 3, 17–24. [Google Scholar] [CrossRef]
- El-Salhy, M.; Hatlebakk, J.G.; Gilja, O.H.; Kristoffersen, A.B.; Hausken, T. Efficacy of faecal microbiota transplantation for patients with irritable bowel syndrome in a randomised, double-blind, placebo-controlled study. Gut 2019, 69, 859–867. [Google Scholar] [CrossRef] [Green Version]

| Antibiotic | Efficiency in IBS treatment |
|---|---|
| Tetracycline, Amoxicilline clavulanate, Metronidazole, Norfloxacine | Moderate effect on IBS symptoms Possible systemic side effects Many cases of clinical resistance High risk of Clostridioides difficile infection |
| Neomycin | Rapid and durable clinical resistance Weak effect on IBS symptoms |
| Rifaximin | Effective in improvement of IBS symptoms including abdominal pain, bloating and diarrhea Weak effect on constipated patients Affects the composition of gut microbiota Direct anti-inflammatory actions Low possibility of clinical resistance or Clostridioides difficile infection |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wollny, T.; Daniluk, T.; Piktel, E.; Wnorowska, U.; Bukłaha, A.; Głuszek, K.; Durnaś, B.; Bucki, R. Targeting the Gut Microbiota to Relieve the Symptoms of Irritable Bowel Syndrome. Pathogens 2021, 10, 1545. https://doi.org/10.3390/pathogens10121545
Wollny T, Daniluk T, Piktel E, Wnorowska U, Bukłaha A, Głuszek K, Durnaś B, Bucki R. Targeting the Gut Microbiota to Relieve the Symptoms of Irritable Bowel Syndrome. Pathogens. 2021; 10(12):1545. https://doi.org/10.3390/pathogens10121545
Chicago/Turabian StyleWollny, Tomasz, Tamara Daniluk, Ewelina Piktel, Urszula Wnorowska, Anna Bukłaha, Katarzyna Głuszek, Bonita Durnaś, and Robert Bucki. 2021. "Targeting the Gut Microbiota to Relieve the Symptoms of Irritable Bowel Syndrome" Pathogens 10, no. 12: 1545. https://doi.org/10.3390/pathogens10121545
APA StyleWollny, T., Daniluk, T., Piktel, E., Wnorowska, U., Bukłaha, A., Głuszek, K., Durnaś, B., & Bucki, R. (2021). Targeting the Gut Microbiota to Relieve the Symptoms of Irritable Bowel Syndrome. Pathogens, 10(12), 1545. https://doi.org/10.3390/pathogens10121545








