The Neglected Angio-Neurotrophic Parasite Gurltia paralysans (Nematoda: Angiostrongylidae): Northernmost South American Distribution, Current Knowledge, and Future Perspectives
Abstract
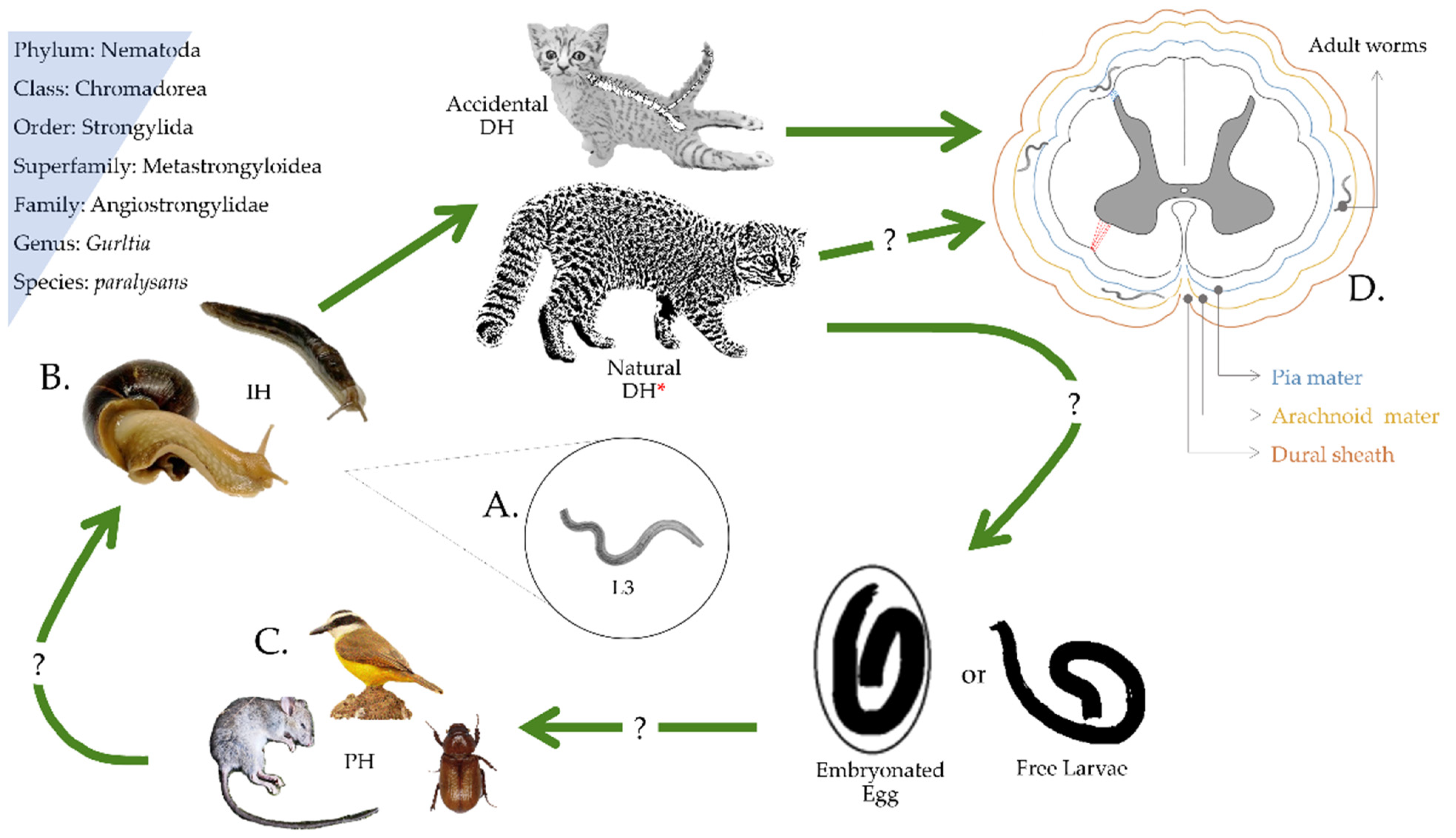
1. Introduction, Brief History, and the Enigmatic Life Cycle
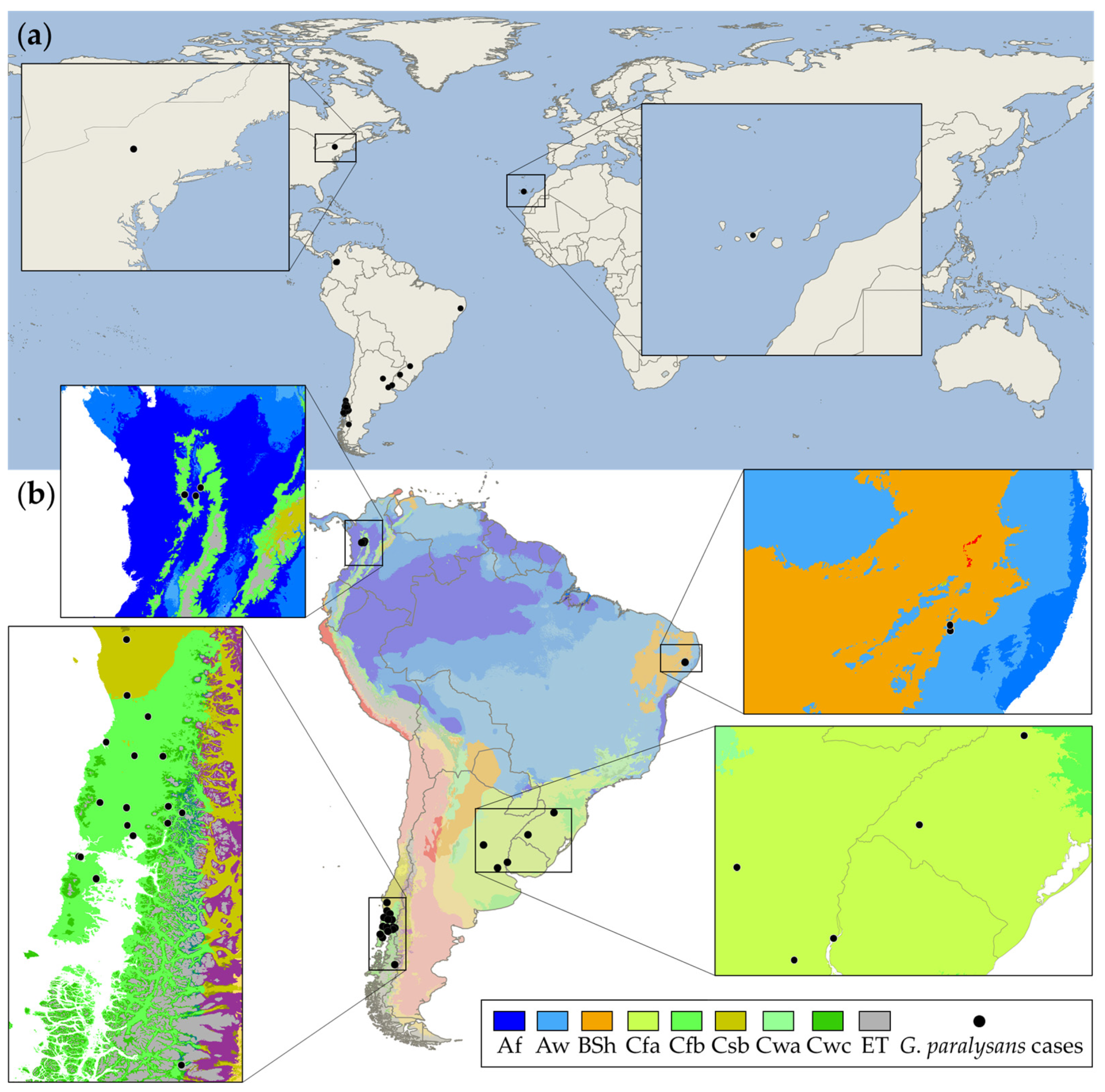
2. Worldwide Distribution Range of Gurltiosis
3. Clinical Signs and Diagnostic
4. Treatment
5. The Northernmost G. paralysans Case Report in South America
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mackey, T.K.; Liang, B.A.; Cuomo, R.; Hafen, R.; Brouwer, K.C.; Lee, D.E. Emerging and Reemerging Neglected Tropical Diseases: A Review of Key Characteristics, Risk Factors, and the Policy and Innovation Environment. Clin. Microbiol. Rev. 2014, 27, 949–979. [Google Scholar] [CrossRef]
- Gebreyes, W.A.; Dupouy-Camet, J.; Newport, M.J.; Oliveira, C.J.B.; Schlesinger, L.S.; Saif, Y.M.; Kariuki, S.; Saif, L.J.; Saville, W.; Wittum, T.; et al. The Global One Health Paradigm: Challenges and Opportunities for Tackling Infectious Diseases at the Human, Animal, and Environment Interface in Low-Resource Settings. PLoS Negl. Trop. Dis. 2014, 8, e3257. [Google Scholar] [CrossRef]
- Rohr, J.R.; Barrett, C.B.; Civitello, D.J.; Craft, M.E.; Delius, B.; DeLeo, G.A.; Hudson, P.J.; Jouanard, N.; Nguyen, K.H.; Ostfeld, R.S.; et al. Emerging human infectious diseases and the links to global food production. Nat. Sustain. 2019, 2, 445–456. [Google Scholar] [CrossRef]
- Lucio-Forster, A.; Eberhard, M.L.; Cama, V.A.; Jenks, M.H.; Jones, C.; Sanders, S.Y.; Pongratz, J.P.; Bowman, D.D. First report of Dracunculus insignis in two naturally infected cats from the northeastern USA. J. Feline Med. Surg. 2014, 16, 194–197. [Google Scholar] [CrossRef]
- Mallawarachchi, C.H.; Chandrasena, N.T.G.A.; Wickramasinghe, S.; Premaratna, R.; Gunawardane, N.Y.I.S.; Mallawarachchi, N.S.M.S.M.; de Silva, N.R. A preliminary survey of filarial parasites in dogs and cats in Sri Lanka. PLoS ONE 2018, 13, e0206633. [Google Scholar] [CrossRef] [PubMed]
- Mallawarachchi, C.H.; Chandrasena, T.G.A.N.; Withanage, G.P.; Premarathna, R.; Mallawarachchi, S.M.N.S.M.; Gunawardane, N.Y.; Dasanayake, R.S.; Gunarathna, D.; de Silva, N.R. Molecular Characterization of a Reemergent Brugia malayi Parasite in Sri Lanka, Suggestive of a Novel Strain. BioMed Res. Int. 2021, 2021, 1–8. [Google Scholar] [CrossRef]
- Otranto, D.; Mendoza-Roldan, J.A.; Dantas-Torres, F. Thelazia callipaeda. Trends Parasitol. 2021, 37, 263–264. [Google Scholar] [CrossRef] [PubMed]
- Hodžić, A.; Payer, A.; Duscher, G.G. The first autochthonous case of feline ocular thelaziosis in Austria. Parasitol. Res. 2019, 118, 1321–1324. [Google Scholar] [CrossRef] [PubMed]
- Schuster, R.K.; Thomas, K.; Sivakumar, S.; O’Donovan, D. The parasite fauna of stray domestic cats (Felis catus) in Dubai, United Arab Emirates. Parasitol. Res. 2009, 105, 125–134. [Google Scholar] [CrossRef]
- Nagamori, Y.; Payton, M.E.; Looper, E.; Apple, H.; Johnson, E.M. Retrospective survey of parasitism identified in feces of client-owned cats in North America from 2007 through 2018. Vet. Parasitol. 2020, 277, 109008. [Google Scholar] [CrossRef]
- Maharana, B.R.; Gupta, S.; Gupta, S.; Ganguly, A.; Kumar, B.; Chandratre, G.A.; Bisla, R.S. First report of molecular and phylogenetic analysis of Physaloptera praeputialis in naturally infected stray cats from India. Parasitol. Res. 2021, 120, 2047–2056. [Google Scholar] [CrossRef] [PubMed]
- Morelli, S.; Diakou, A.; Colombo, M.; Di Cesare, A.; Barlaam, A.; Dimzas, D.; Traversa, D. Cat Respiratory Nematodes: Current Knowledge, Novel Data and Warranted Studies on Clinical Features, Treatment and Control. Pathogens 2021, 10, 454. [Google Scholar] [CrossRef]
- Calvopiña, M.; Cevallos, W.; Atherton, R.; Saunders, M.; Small, A.; Kumazawa, H.; Sugiyama, H. High Prevalence of the Liver Fluke Amphimerus sp. in Domestic Cats and Dogs in an Area for Human Amphimeriasis in Ecuador. PLoS Negl. Trop. Dis. 2015, 9, e0003526. [Google Scholar] [CrossRef]
- Dahlem, D.; Bangoura, B.; Ludewig, E.; Glowienka, N.; Baldauf, K.; Stoeckel, F.; Burgener, I. Tetrathyridiosis in a domestic shorthair cat. J. Feline Med. Surg. Open Rep. 2015, 1, 205511691561559. [Google Scholar] [CrossRef] [PubMed]
- Barrios, N.; Gómez, M.; Zanelli, M.; Rojas-Barón, L.; Sepúlveda-García, P.; Alabí, A.; Adasme, M.; Müller, A.; Rosenfeld, C.; González-Lagos, C.; et al. A Molecular Survey on Neglected Gurltia paralysans and Aelurostrongylus abstrusus Infections in Domestic Cats (Felis catus) from Southern Chile. Pathogens 2021, 10, 1195. [Google Scholar] [CrossRef]
- Bowman, D.; Hendrix, C.; Lindsay, D.; Barr, S. Feline Clinical Parasitology; Bowman, D.D., Hendrix, C.M., Lindsay, D.S., Barr, S.C., Eds.; Iowa State University Press: Ames, IA, USA, 2002; ISBN 9780470376805. [Google Scholar]
- Pellegrino, F.C. Parasitic myelopathy by Gurltia Paralysans. Cienc. Vet. 2016, 18, 54–64. [Google Scholar] [CrossRef]
- Wolffhügel, K.W. Paraplegia cruralis parasitaria felis causada por Gurltia paralysans nov. gen., n. sp. Rev. Chil. Hist. Nat. 1933, 37, 190–192. [Google Scholar]
- Wolffhügel, K.W. Paraplegia cruralis parasitaria felis durch Gurltia paralysans nov. gen., nov. sp. (Nematoda). Infektkr Haustiere 1934, 46, 28–47. [Google Scholar]
- Dazzi, C.C.; Dos Santos, A.; Machado, T.P.; de Ataíde, M.W.; Rodriguez, R.; Pereira, A.M.; García, P.S.; da Motta, A.C. First case report of nematode parasitic myelopathy in a wild feline in Brazil. Rev. Bras. Parasitol. Vet. 2020, 29, 1–7. [Google Scholar] [CrossRef]
- Muñoz, P.; Hirzmann, J.; Rodriguez, E.; Moroni, M.; Taubert, A.; Gibbons, L.; Hermosilla, C.; Gómez, M. Redescription and first molecular characterization of the little known feline neurotropic nematode Gurltia paralysans (Nematoda: Metastrongyloidea). Vet. Parasitol. Reg. Stud. Rep. 2017, 10, 119–125. [Google Scholar] [CrossRef]
- Sepúlveda-García, P.; Gómez, M.; Moroni, M.; Muñoz, P.; Müller, A. Evaluation of terrestrial gastropods as possible intermediate hosts of Gurltia paralysans in southern Chile. Rev. Bras. Parasitol. Vet. 2021, 30, 1–6. [Google Scholar] [CrossRef]
- Moroni, M.; Muñoz, P.; Gómez, M.; Mieres, M.; Rojas, M.; Lillo, C.; Aguirre, F.; Acosta-Jamett, G.; Kaiser, M.; Lindsay, D.S. Gurltia paralysans (Wolffhügel, 1933): Description of adults and additional case reports of neurological diseases in three domestic cats from southern Chile. Vet. Parasitol. 2012, 184, 377–380. [Google Scholar] [CrossRef]
- Olsen, C.S.; Willesen, J.L.; Pipper, C.B.; Mejer, H. Occurrence of Aelurostrongylus abstrusus (Railliet, 1898) in Danish cats: A modified lung digestion method for isolating adult worms. Vet. Parasitol. 2015, 210, 32–39. [Google Scholar] [CrossRef]
- Nematoda. In Parasiticide Screening, Volume 2; Elsevier Inc.: London, UK, 2019; pp. 135–335.
- Rosen, L.; Ash, L.R.; Wallace, G.D. Life history of the canine lungworm Angiostrongylus vasorum (Baillet). Am. J. Vet. Res. 1970, 31, 131–143. [Google Scholar]
- Ferdushy, T.; Hasan, M.T. Angiostrongylus vasorum: The ‘French Heartworm’. Parasitol. Res. 2010, 107, 765–771. [Google Scholar] [CrossRef]
- Colella, V.; Lia, R.P.; Premont, J.; Gilmore, P.; Cervone, M.; Latrofa, M.S.; D’Anna, N.; Williams, D.; Otranto, D. Angiostrongylus vasorum in the eye: New case reports and a review of the literature. Parasit. Vectors 2016, 9, 161. [Google Scholar] [CrossRef]
- Olivieri, E.; Zanzani, S.A.; Gazzonis, A.L.; Giudice, C.; Brambilla, P.; Alberti, I.; Romussi, S.; Lombardo, R.; Mortellaro, C.M.; Banco, B.; et al. Angiostrongylus vasorum infection in dogs from a cardiopulmonary dirofilariosis endemic area of Northwestern Italy: A case study and a retrospective data analysis. BMC Vet. Res. 2017, 13, 165. [Google Scholar] [CrossRef] [PubMed]
- Gómez, M.; Moroni, M.; Muñoz, P.; Taubert, A.; Hermosilla, C.; Hirzmann, J.; Rojas, L. Gurltia paralysans: A neglected parasite of domestic cats. Austral J. Vet. Sci. 2021, 53, 33–45. [Google Scholar] [CrossRef]
- Gutiérrez, C.; Acuña, G.; Pérez, N. Monoplegia by Gurltia paralysans in a cat. Rev. Hosp. Vet. 2020, 12, 1–7. [Google Scholar]
- Gómez, G.A.; Taborda, D.A.; Andrés Alzate, G.; Gutiérrez, J.J.C. Domestic cat paraplegia compatible with Gurltia paralysans nematode. First cases reported in Colombia. Rev. Colomb. Cienc. Pecu. 2011, 24, 663–669. [Google Scholar]
- Udiz-Rodríguez, R.; Garcia-Livia, K.; Valladares-Salmerón, M.; Dorta-Almenar, M.N.; Martín-Carrillo, N.; Martin-Alonso, A.; Izquierdo-Rodriguez, E.; Feliu, C.; Valladares, B.; Foronda, P. First ocular report of Gurltia paralysans (Wolffhügel, 1933) in cat. Vet. Parasitol. 2018, 255, 74–77. [Google Scholar] [CrossRef]
- Gómez, M.; Mieres, M.; Moroni, M.; Mora, A.; Barrios, N.; Simeone, C.; Lindsay, D.S. Meningomyelitis due to nematode infection in four cats. Vet. Parasitol. 2010, 170, 327–330. [Google Scholar] [CrossRef]
- Rivero, R.; Matto, C.; de Adrien, M.L.; Nan, F.; Bell, T.; Gardiner, C. Parasite meningomyelitis in cats in Uruguay. Rev. Bras. Parasitol. Vet. 2011, 20, 259–261. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Guerrero, I.; Paludi, A.; Saumell, L. Primera Descripción en Argentina de Gurltia paralysans en un Felino Doméstico. DVM Thesis, Universidad Nacional del Centro de la Provincia de Buenas Aires, Tandil, Argentina, 2011. [Google Scholar]
- Mieres, M.; Gómez, M.A.; Lillo, C.; Rojas, M.A.; Moroni, M.; Muñoz, P.; Acosta-Jamett, G.; Wiegand, R. Clinical, imaging, and pathologic characteristics of gurltia paralysans myelopathy in domestic cats from chile. Vet. Radiol. Ultrasound 2013, 54, 237–244. [Google Scholar] [CrossRef] [PubMed]
- Togni, M.; Panziera, W.; Souza, T.M.; Oliveira Filho, J.C.; Mazzanti, A.; Barros, C.S.L.; Fighera, R.A. Aspectos epidemiológicos, clínicos e anatomopatológicos da infecção por Gurltia paralysans em gatos. Pesqui. Vet. Bras. 2013, 33, 363–371. [Google Scholar] [CrossRef][Green Version]
- Moroni, M.; Muñoz, P.; Mieres, M.; Gómez, M.A.; Vera, F. Severe myelopathy with thrombophlebitis caused by Gurltia paralysans infection in a cat. Vet. Rec. Case Rep. 2016, 4, 2–5. [Google Scholar] [CrossRef]
- Bono, M.F.; Orcellet, V.; Marengo, R.; Bosio, A.; Junkers, E.; Plaza, D.; Marini, M.R.; Sanchez, A.; Rubio, M.G.; Candiotti, V. A description of three cases of parasitic meningomyelitis in felines from the province of Santa Fé, Argentina. Parasitaria 2016, 74, 1–4. [Google Scholar]
- De Melo Neto, G.B.; Da Silva, L.R.; Alves, R.D.C.; Olinda, R.G.; Medeiros Dantas, A.F.; De Medeiros Torres, M.B.A. Gurltia paralysans Infection in Domestic Cats in the State of Pernambuco, Brazil. Acta Sci. Vet. 2019, 47, 1–6. [Google Scholar] [CrossRef]
- Gómez, M.; García, C.; Maldonado, I.; Pantchev, N.; Taubert, A.; Hermosilla, C.; Moroni, M.; Muñoz, P.; Duran, A.; Mieres, M.; et al. Intra vitam diagnosis of neglected Gurltia paralysans infections in domestic cats (Felis catus) by a commercial serology test for canine angiostrongylosis and insights into clinical and histopathological findings—Four-case report. Pathogens 2020, 9, 921. [Google Scholar] [CrossRef] [PubMed]
- López-Contreras, F.; Rojas-Barón, L.; Gómez, M.; Morera, F.; Sepúlveda, P.; Moroni, M.; Muñoz, P.; Acosta-Jammett, G.; Mieres, M.; Hirzmann, J.; et al. Molecular Detection of Gurltia paralysans by Semi-Nested PCR in Cerebrospinal Fluid and Serum Samples from Domestic Cats (Felis catus). Animals 2020, 10, 1169. [Google Scholar] [CrossRef]
- Cizauskas, C.A.; Carlson, C.J.; Burgio, K.R.; Clements, C.F.; Dougherty, E.R.; Harris, N.C.; Phillips, A.J. Parasite vulnerability to climate change: An evidence-based functional trait approach. R. Soc. Open Sci. 2016, 4, 160535. [Google Scholar] [CrossRef]
- Hermosilla, C.; Gómez, M.; Muñoz, P.; Moroni, M.; Mieres, M. Molekulare Charakterisierung von Gurltia paralysans und Etablierung einer Speziesspezifischen PCR. In Proceedings of the DVG Parasitologie Meeting, Giessen, Germany, 8–10 July 2013; p. 1. [Google Scholar]
- Rojas, M. Análisis clínico-patológico en 7 gatos domésticos (Felis catus) con paraparesis/plejia producida por Gurltia paralysans. DVM Thesis, Universidad Austral De Chile, Valdivia, Chile, 2011. [Google Scholar]
- Dantas-Torres, F.; Ketzis, J.; Mihalca, A.D.; Baneth, G.; Otranto, D.; Tort, G.P.; Watanabe, M.; Linh, B.K.; Inpankaew, T.; Jimenez Castro, P.D.; et al. TroCCAP recommendations for the diagnosis, prevention and treatment of parasitic infections in dogs and cats in the tropics. Vet. Parasitol. 2020, 283, 109167. [Google Scholar] [CrossRef] [PubMed]
- Gómez, M.; Mieres, M.; Lillo, C.; Rojas, M.; Muñoz, M. Clinical and imaging findings in feline myelopathy induced by Gurltia paralysans: A neglected neuroparasite in domestic cats. In Proceedings of the DVG Parasitologie Meeting, Hannover, Germany, 2–4 July 2012; p. 2. [Google Scholar]
- Bishop, B.; Bruce, C.; Evans, N.; Goudie, A.; Gration, K.A.; Gibson, S.; Pacey, M.; Perry, D.; Walshe, N.D.; Witty, M. Selamectin: A novel broad-spectrum endectocide for dogs and cats. Vet. Parasitol. 2000, 91, 163–176. [Google Scholar] [CrossRef]
- Plumb, D.C. Veterinary Drug Handbook, 9th ed.; Iowa State University Press: Ames, IA, USA, 2018; ISBN 978-1-119-34445-2. [Google Scholar]
- Lovell, R.A. Ivermectin and Piperazine Toxicoses in Dogs and Cats. Vet. Clin. N. Am. Small Anim. Pract. 1990, 20, 453–468. [Google Scholar] [CrossRef]
- Lynn, R.C. Drugs for the treatment of helminth infections. In Small Animal Clinical Pharmacology and Therapeutics; Saunders: Philadelphia, PA, USA, 2008; p. 594. ISBN 978 0 7020 2858 8. [Google Scholar]
- Tranquilli, W.J.; Paul, A.J.; Todd, K.S. Assessment of toxicosis induced by high-dose administration of milbemycin oxime in collies. Am. J. Vet. Res. 1991, 52, 1170–1172. [Google Scholar]
- Györke, A.; Dumitrache, M.O.; Kalmár, Z.; Paştiu, A.I.; Mircean, V. Molecular Survey of Metastrongyloid Lungworms in Domestic Cats (Felis silvestris catus) from Romania: A Retrospective Study (2008–2011). Pathogens 2020, 9, 80. [Google Scholar] [CrossRef] [PubMed]
- Penagos-Tabares, F.; Lange, M.K.; Vélez, J.; Hirzmann, J.; Gutiérrez-Arboleda, J.; Taubert, A.; Hermosilla, C.; Chaparro Gutiérrez, J.J. The invasive giant African snail Lissachatina fulica as natural intermediate host of Aelurostrongylus abstrusus, Angiostrongylus vasorum, Troglostrongylus brevior, and Crenosoma vulpis in Colombia. PLoS Negl. Trop. Dis. 2019, 13, e0007277. [Google Scholar] [CrossRef]
- Lopez-Osorio, S.; Navarro-Ruiz, J.L.; Rave, A.; Taubert, A.; Hermosilla, C.; Chaparro-Gutierrez, J.J. Aelurostrongylus abstrusus Infections in Domestic Cats (Felis silvestris catus) from Antioquia, Colombia. Pathogens 2021, 10, 337. [Google Scholar] [CrossRef]
- Uribe, M.; Payán, E.; Brabec, J.; Vélez, J.; Taubert, A.; Chaparro-Gutiérrez, J.J.; Hermosilla, C. Intestinal Parasites of Neotropical Wild Jaguars, Pumas, Ocelots, and Jaguarundis in Colombia: Old Friends Brought Back from Oblivion and New Insights. Pathogens 2021, 10, 822. [Google Scholar] [CrossRef]
- Schweizer, M.; Triebskorn, R.; Köhler, H. Snails in the sun: Strategies of terrestrial gastropods to cope with hot and dry conditions. Ecol. Evol. 2019, 9, 12940–12960. [Google Scholar] [CrossRef]

| G. paralysansa | Ael. Abstrususb | Ang. Vasorumc | |
|---|---|---|---|
| Male | |||
| Body length | 12,000–18,000 μm | 5440–7080 μm | 14,000–16,000 μm |
| Body width | 72–103 μm | 42.5–92.7 μm | – |
| Esophagus length | 360–432 μm | 219.4–360.9 μm | 219 μm |
| Spicule length | 650–816 μm | 103.7–138.9 μm | 360–490 μm |
| Gubernaculum length | 37–39 μm | 18.8–31 μm | 34–45 μm |
| Female | |||
| Body length | 20,500–30,000 μm | 7950–10,587 μm | 15,000–21,000 μm |
| Vulva to tail tip | 102–150 μm | 223.6 μm | – |
| Tail length | 30–50 μm | 27–29 µm | 27–29 µm |
| Eggs | 40–72 × 26–54 μm | 37.8–48.4 × 94.5–99.6 μm | 70–80 × 40–50 μm |
| Year | Location | Area | Host | (n) | Diagnosis Method | Reference |
|---|---|---|---|---|---|---|
| 1933 | Chile | Rural | Felis catus | - | M | [18] |
| 1933 | Chile | Rural | Leopardus guigna | - | - | [19] |
| 2010 | Chile | Rural | Felis catus | 4 | Ct, Hp, M | [34] |
| 2011 | Uruguay | Rural | Felis catus | 2 | Hp, M | [35] |
| 2011 | Colombia | Rural | Felis catus | 6 | Ct, Hp | [32] |
| 2011 | Argentina | Suburban | Felis catus | 1 | Hp | [36] |
| 2012 | Chile | Rural | Felis catus | 3 | Hp, M | [23] |
| 2013 | Chile | Rural | Felis catus | 9 | Ct | [37] |
| 2013 | Brazil | Rural | Felis catus | 4 | Hp | [38] |
| 2016 | Chile | Rural | Felis catus | 1 | Hp | [39] |
| 2016 | Argentina | Rural | Felis catus | 3 | Hp, M | [40] |
| 2017 | Chile | Suburban/Rural | Felis catus | - | SEM, phylogeny | [21] |
| 2018 | Spain | Suburban | Felis catus | 1 | M, phylogeny | [33] |
| 2019 | Brazil | Rural | Felis catus | 7 | Hp | [41] |
| 2020 | Chile | Rural | Felis catus | 4 | Serology, M | [42] |
| 2020 | Chile | Suburban | Felis catus | 1 | PCR | [31] |
| 2020 | Chile | Rural | Felis catus | 7 | PCR | [43] |
| 2020 | Brazil | Rural | Leopardus wiedii | 1 | M | [20] |
| 2021 | Chile | Urban | Felis catus | 93 | PCR | [15] |
| PCR Type & Gene | Primer Set (5′→3′) | Amplicon Size (bp) | Identification |
|---|---|---|---|
| semi-nested PCR 28S rDNA | AaGp28Sa1-R: AGGCATAGTTCACCATCT AaGp28Ss1-F: CGATRATATGTATGCCATT | 450 | Common sequence Metastrongyloidea |
| E1:Aa28Ss2-F: CGTTGATGTTGATGAGTATC E2:Gp28Sa3-R: TCTTGCCGCCATTATAGTA | 300 356 | A. abstrusus G. paralysans | |
| Endpoint-PCR rDNA Feline | F: AGCAGGAGGTGTTGGAAGAG R: AGGGAGAGAGCCTAATTCAAAGG | 100 | Internal control |
| Drug | Structure § | Dose † | Route and Frequency of Administration | Reference |
|---|---|---|---|---|
| Ivermectin a,* | 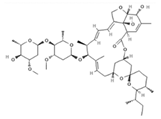 | 0.2–0.4 | PO, SID, 1× weekly, 4 doses | [30,32,48] |
| Selamectin b |  | 6 | PO, SID | [30,49] |
| Milbemycin oxime c | 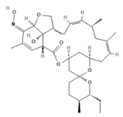 | 2 | PO, SID, 1× monthly | [30,50] |
| Ricobendazole d,* | 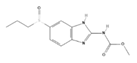 | 20 | PO, SID, 2× days | [30] |
| Fenbendazole e | 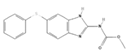 | 50 | PO, SID, 1× day, 3–5 doses | [30] |
| Genus | Species | Common Name | Conservation Status § |
|---|---|---|---|
| Herpailurus | yagouaroundi1 | Jaguarundi | LC |
| Leopardus | colocolo | Pampas cat | NT |
| Leopardus | geoffroyi2 | Geoffroy’s cat | LC |
| Leopardus | guigna2 | Kodkod | VU |
| Leopardus | guttulus | Southern tiger cat | VU |
| Leopardus | jacobita | Andean cat | EN |
| Leopardus | pardalis1 | Ocelot | LC |
| Leopardus | wiedii1,2 | Margay | NT |
| Leopardus | tigrinus1 | Northern tiger cat | VU |
| Panthera | onca1 | Jaguar | NT |
| Puma | concolor1 | Puma | LC |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uribe, M.; López-Osorio, S.; Chaparro-Gutiérrez, J.J. The Neglected Angio-Neurotrophic Parasite Gurltia paralysans (Nematoda: Angiostrongylidae): Northernmost South American Distribution, Current Knowledge, and Future Perspectives. Pathogens 2021, 10, 1601. https://doi.org/10.3390/pathogens10121601
Uribe M, López-Osorio S, Chaparro-Gutiérrez JJ. The Neglected Angio-Neurotrophic Parasite Gurltia paralysans (Nematoda: Angiostrongylidae): Northernmost South American Distribution, Current Knowledge, and Future Perspectives. Pathogens. 2021; 10(12):1601. https://doi.org/10.3390/pathogens10121601
Chicago/Turabian StyleUribe, Manuel, Sara López-Osorio, and Jenny J. Chaparro-Gutiérrez. 2021. "The Neglected Angio-Neurotrophic Parasite Gurltia paralysans (Nematoda: Angiostrongylidae): Northernmost South American Distribution, Current Knowledge, and Future Perspectives" Pathogens 10, no. 12: 1601. https://doi.org/10.3390/pathogens10121601
APA StyleUribe, M., López-Osorio, S., & Chaparro-Gutiérrez, J. J. (2021). The Neglected Angio-Neurotrophic Parasite Gurltia paralysans (Nematoda: Angiostrongylidae): Northernmost South American Distribution, Current Knowledge, and Future Perspectives. Pathogens, 10(12), 1601. https://doi.org/10.3390/pathogens10121601







